Experimental and Theoretical Studies on the Adsorption Mechanisms of Uranium (VI) Ions on Chitosan
Abstract
:1. Introduction
2. Experimental and Theoretical Details
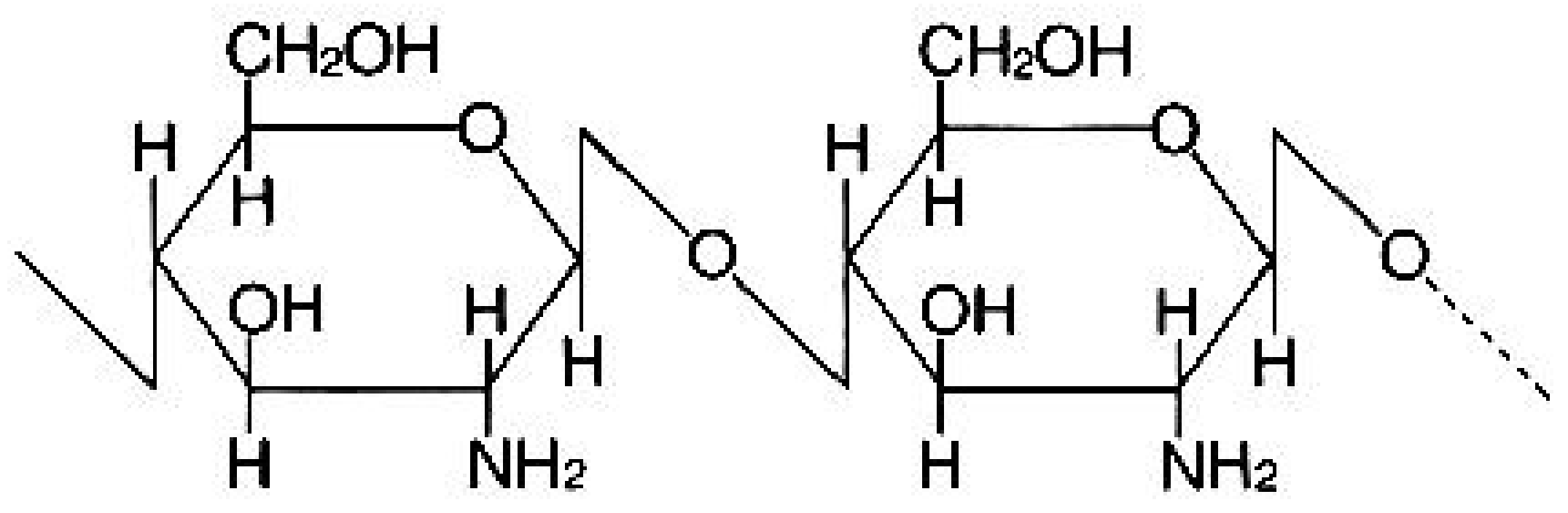
2.1. Materials
2.2. Adsorption Experiments
2.3. Quantum Chemistry Calculations
3. Results and Discussion
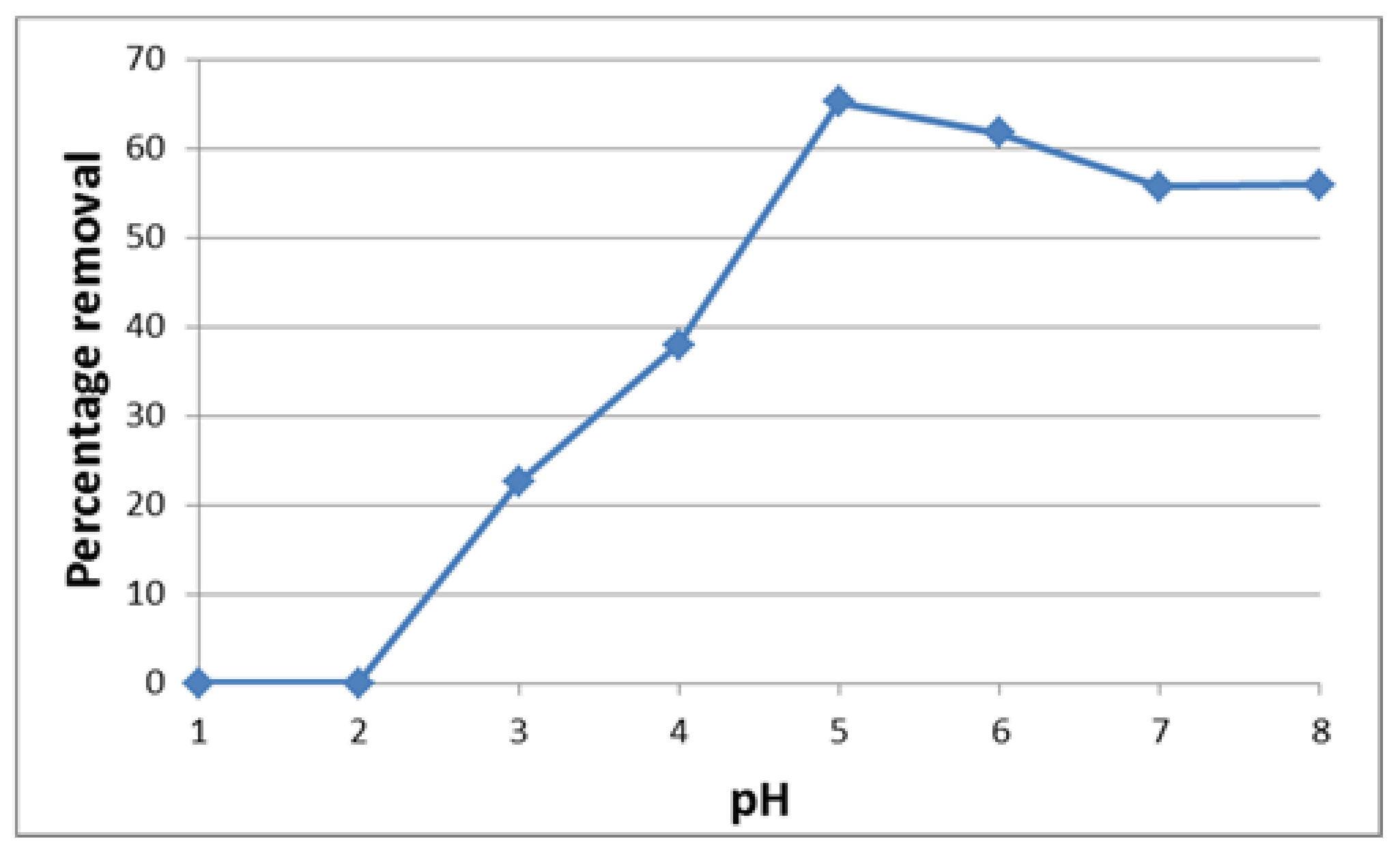
3.1. Influence of pH on the Adsorption of U(VI)
3.2. Adsorption Isotherms
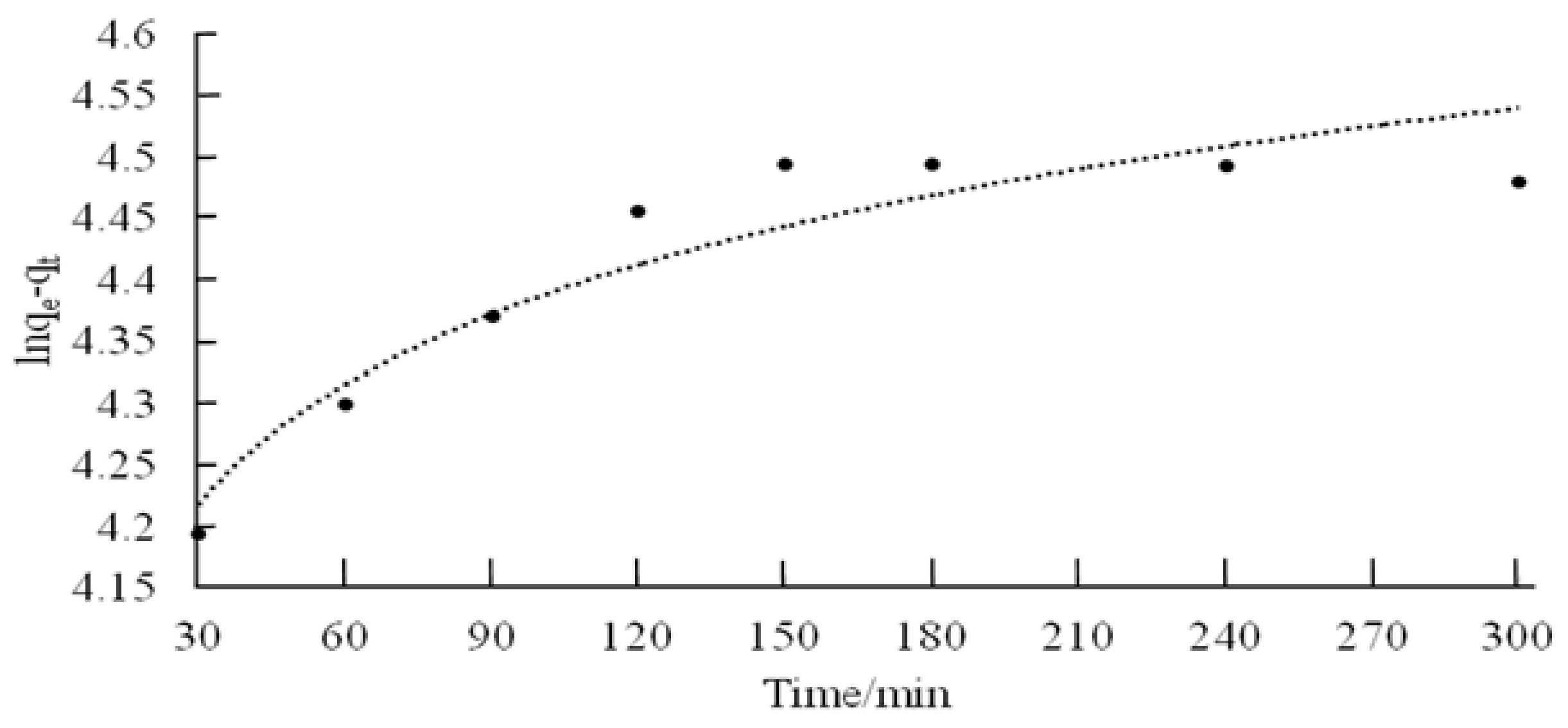
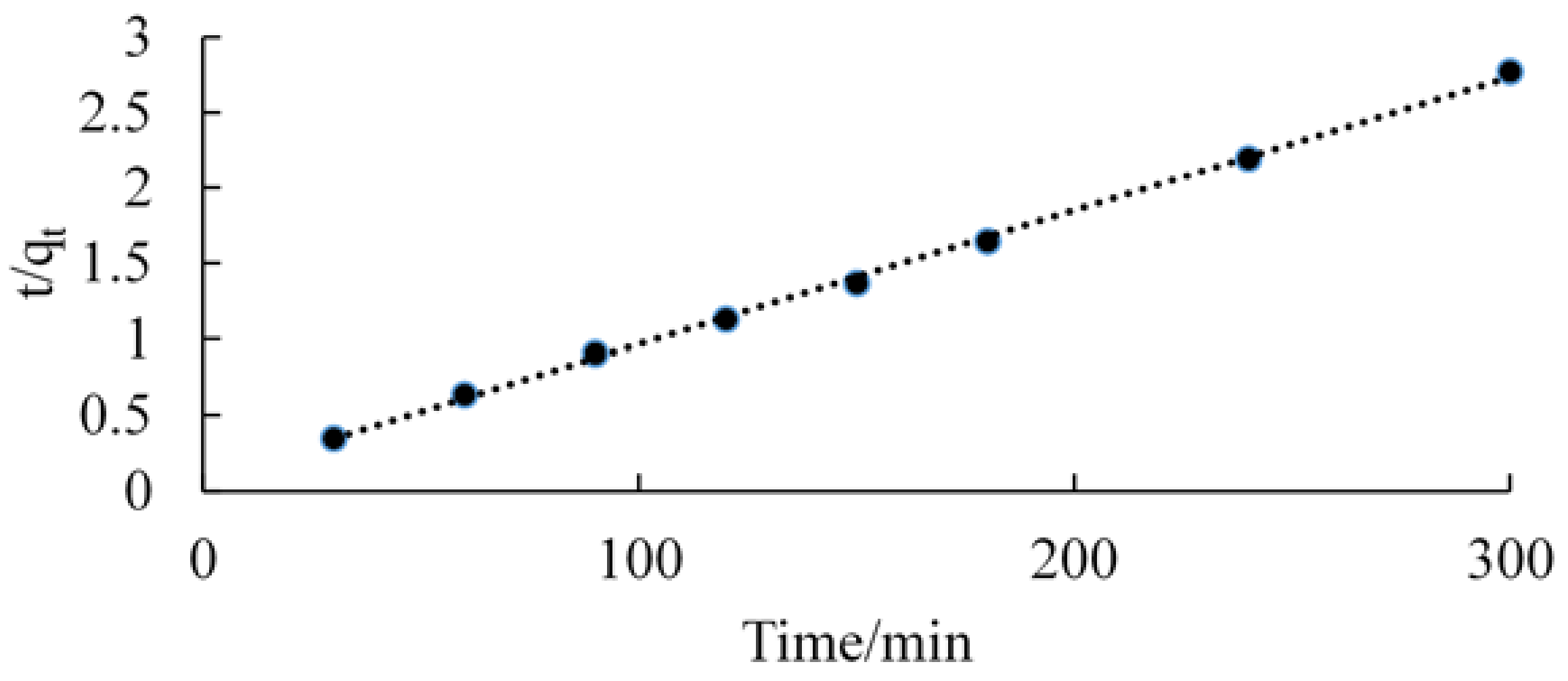
3.3. Kinetic Studies
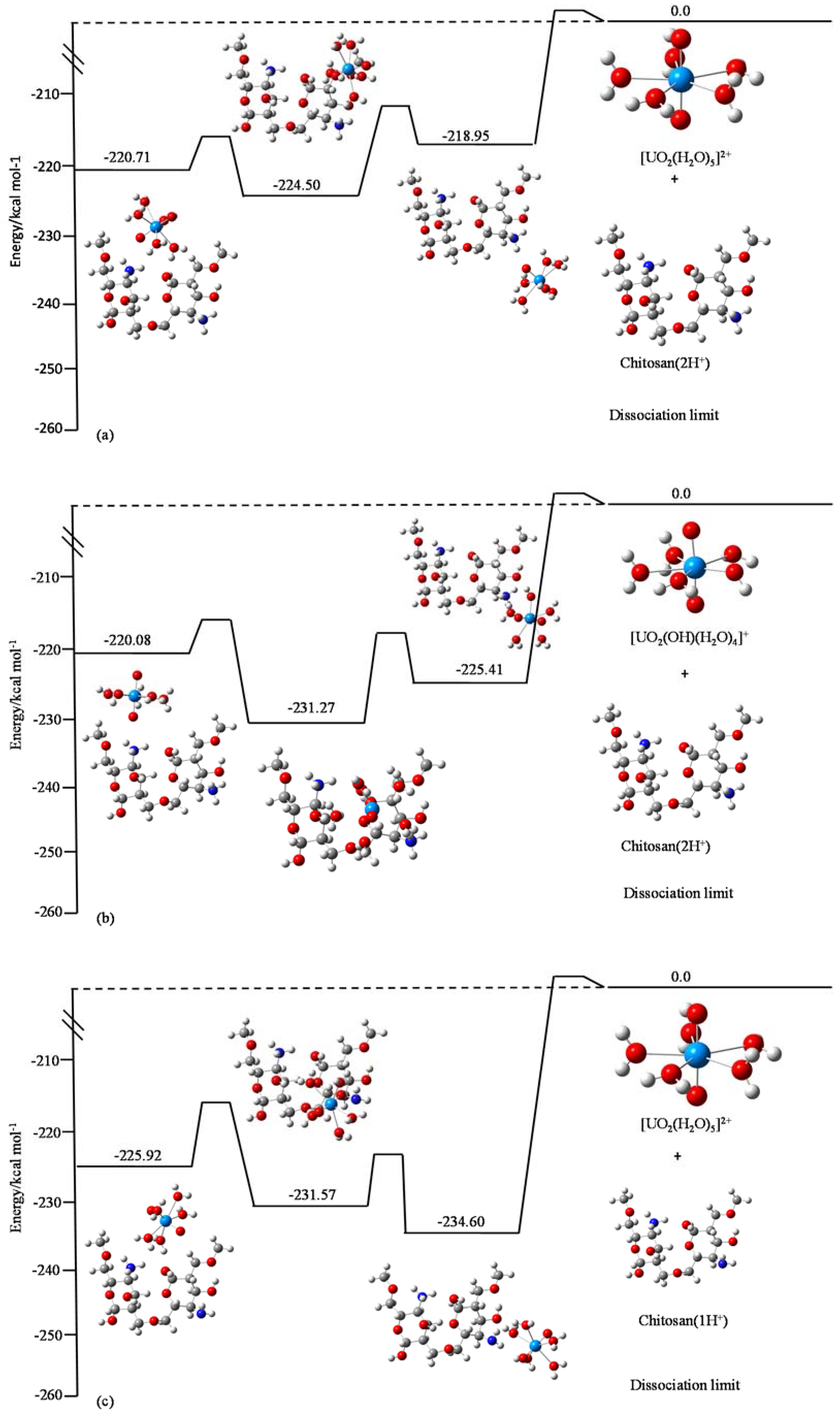
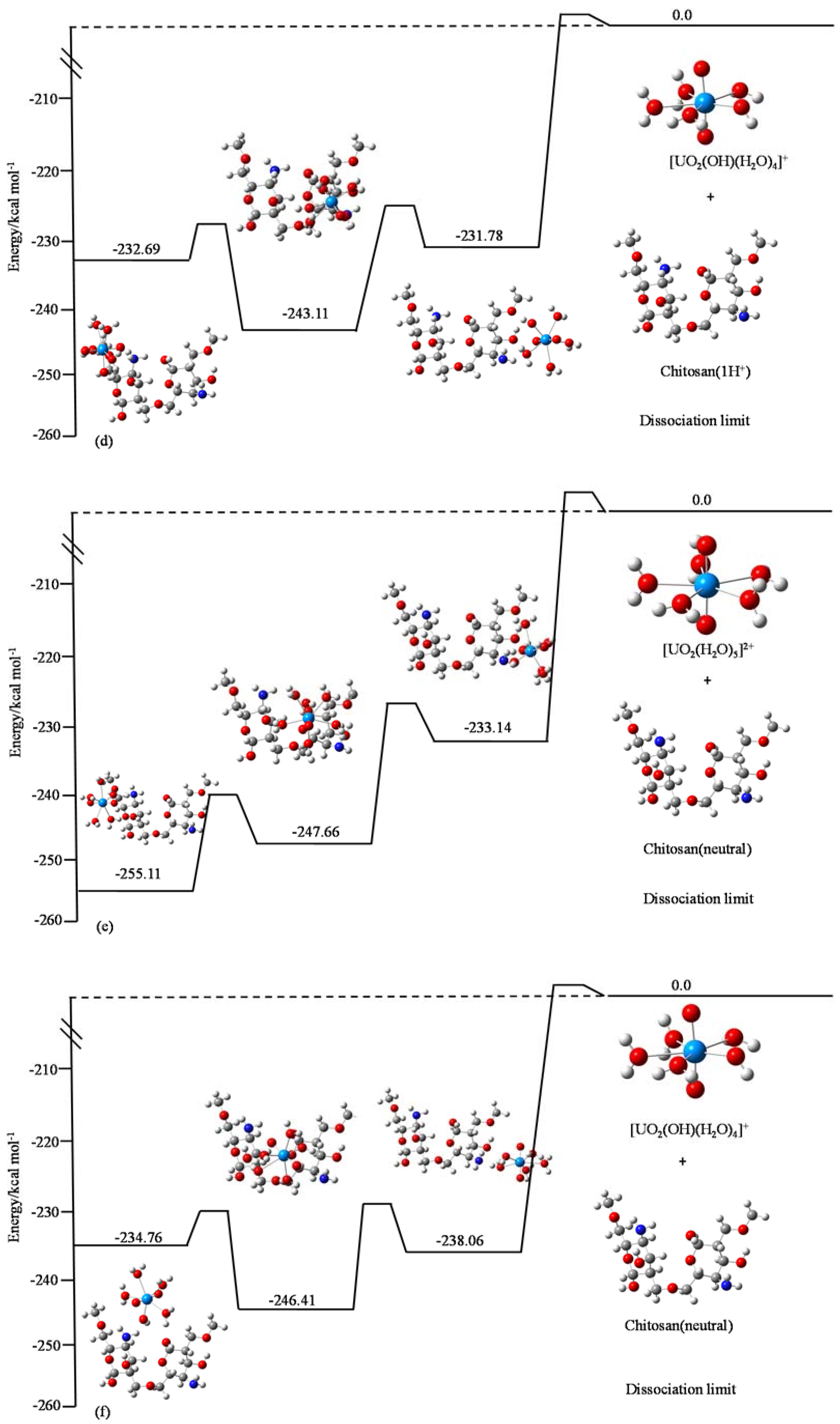
3.4. Quantum Chemistry Calculations of the Adsorption of U(VI) on Chitosan
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harmsen, K.; De Haan, F.A.M. Occurrence and Behaviour of Uranium and Thorium in Soil and Water. Neth. J. Agric. Sci. 1980, 28, 40–62. [Google Scholar]
- Duquène, L.; Tack, F.; Meers, E.; Baeten, J.; Wannijn, J.; Vandenhove, H. Effect of Biodegradable Amendments on Uranium Solubility in Contaminated Soils. Sci. Total Environ. 2008, 391, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Roos, P.; Zhu, Y.-G.; Jakobsen, I. Arbuscular Mycorrhizas Contribute to Phytostabilization of Uranium in Uranium Mining Tailings. J. Environ. Radioact. 2008, 99, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.M.S.C.; Silva, M.M.V.G.; Neiva, A.M.R. Pollution of Water and Stream Sediments Associated with the Vale de Abrutiga Uranium Mine, Central Portugal. Mine Water Environ. 2004, 23, 66–75. [Google Scholar] [CrossRef]
- Pinto, M.M.S.C.; Silva, M.M.V.; Neiva, A.M.R.; Guimarães, F.; Silva, P.B. Release, Migration, Sorption, and (Re) Precipitation of U during Peraluminous Granite Alteration under Oxidizing Conditions in Central Portugal. Geosciences 2018, 8, 95. [Google Scholar] [CrossRef]
- Pinto, M.; Cabral, M.S.; Silva, M.M. The Vale de Abrutiga Uranium Phosphates mine, Central Portugal. Chem. Erde 2007, 67, 251–252. [Google Scholar] [CrossRef]
- Pinto, C.; Marina, M.S.; Silva, M.V.G.; Neiva, A.M.R. Geochemistry of U-bearing Minerals at Vale de Abrutiga Uranium Mine, Central Portugal. Neues Jahrb. Mineral. Abh. 2008, 185, 183–198. [Google Scholar] [CrossRef]
- Fischerová, Z.; Tlustoš, P.; Száková, J.; Šichorova, K. A Comparison of Phytoremediation Capability of Selected Plant Species for Given Trace Elements. Environ. Pollut. 2006, 144, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, S.; Sheng, G.; Guo, Z.; Wang, X. The removal of U(VI) from Aqueous Solution by Oxidized Multiwalled Carbon Nanotubes. J. Environ. Radioact. 2012, 105, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Balasubramanian, K. Theoretical Studies of UO2(OH)(H2O)n+, UO2(OH)2(H2O)n, NpO2(OH)(H2O)n, and PuO2(OH)(H2O)n+, (n ≤ 21) Complexes in Aqueous Solution. J. Chem. Phys. 2009, 131, 164504. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Saito, T.; Ishida, K.; Scheinost, A.C.; Tsuneda, T.; Nagasaki, S.; Tanaka, S. The Structures of Monomeric and Dimeric Uranyl Adsorption Complexes on Gibbsite: A Combined DFT and EXAFS Study. Geochim. Cosmochim. Acta 2009, 73, 5975–5988. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Lan, J.-H.; Wang, L.; Wu, Q.-Y.; Wang, C.-Z.; Bo, T.; Chai, Z.-F.; Shi, W.-Q. Adsorption of Uranyl Species on Hydroxylated Titanium Carbide Nanosheet: A First-Principle Study. J. Hazard. Mater. 2016, 308, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Du, X.; Sekiguchi, S.; Kano, N. Experimental and Theoretical Studies on the Adsorption and Desorption Mechanisms of Chromate Ions on Cross-Linked Chitosan. J. Funct. Binomater. 2017, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. GAUSSIAN09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Camm, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Hsi, C.K.D.; Langmuir, D. Adsorption of Uranyl onto Ferric Oxyhydroxides: Application of the Surface Complexation Site-binding Model. Geochim. Cosmochim. Acta 1985, 49, 1931–1941. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A. Carbonate Effects and pH-dependence of Uranium Sorption onto Bacteriogenic Iron Oxides: Kinetic and Equilibrium Studies. J. Hazard. Mater. 2007, 139, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Misaelides, P.; Godelitsas, A.; Filippidis, A.; Charistos, D.; Anousis, I. Thorium and Uranium Uptake by Natural Zeolitic Materials. Sci. Total Environ. 1995, 173, 237–246. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D.D. The Dubinin-Radushkevich equation and underlying microscopic adsorption description. Carbon 2001, 39, 1327–2336. [Google Scholar] [CrossRef]
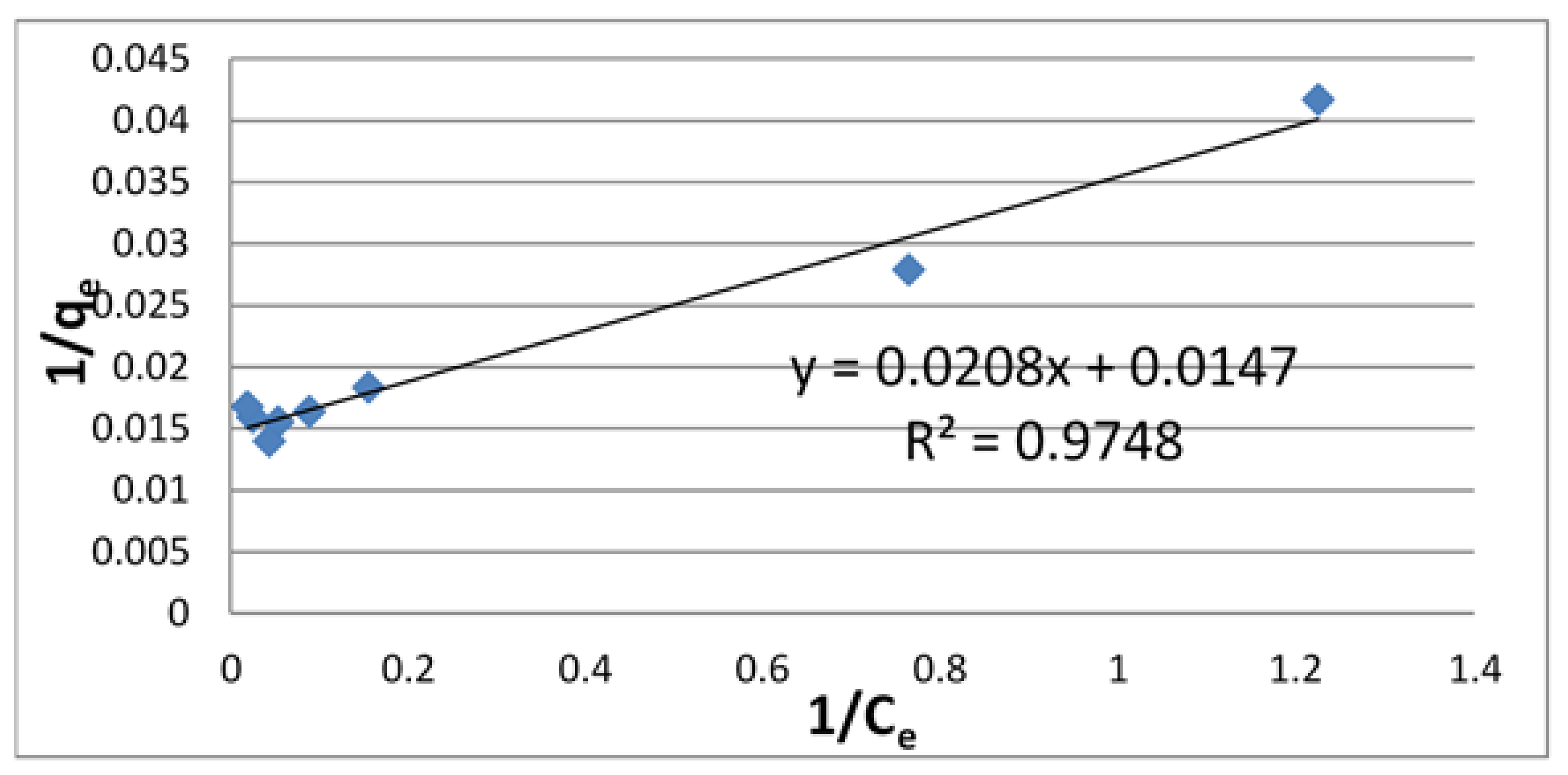
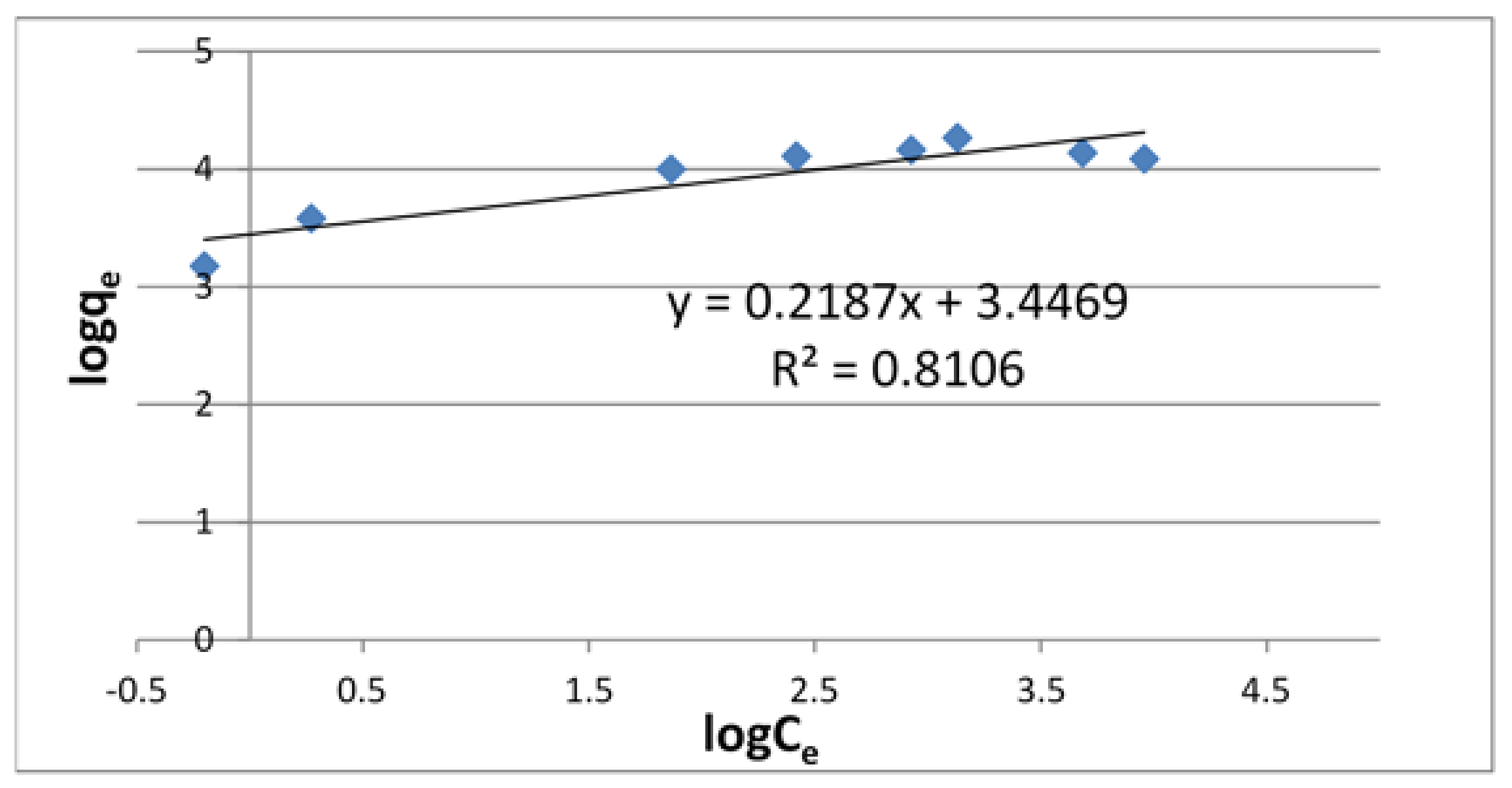
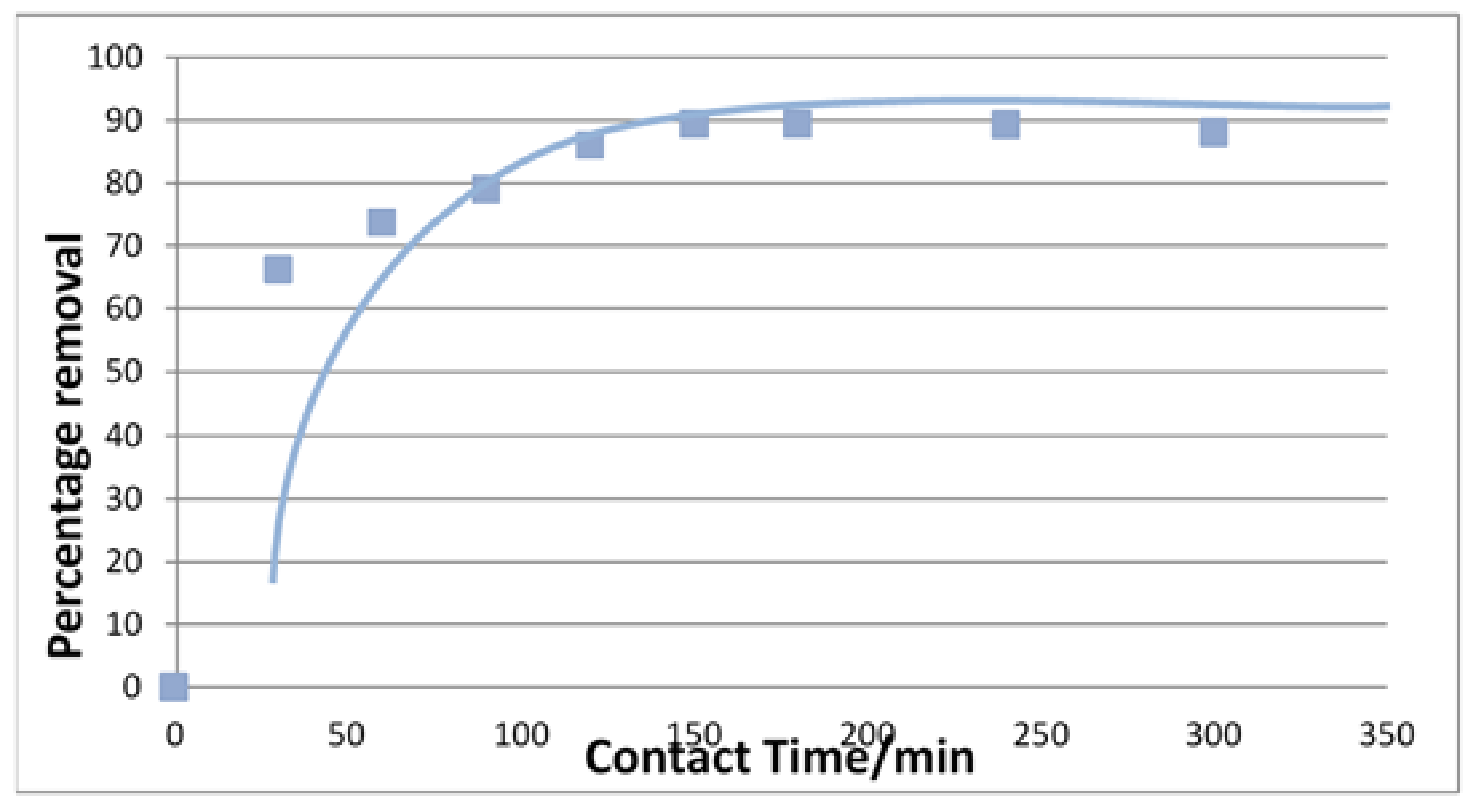
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| qmax | KL | R2 | KF | 1/n | R2 |
| 68.0 | 7.07 × 10−2 | 0.9748 | 31.4 | 0.219 | 0.8106 |
| Pseudo-First-Order Rate Equation | Pseudo-Second-Order Rate Equations | ||||
|---|---|---|---|---|---|
| K1 (min−1) | qe (mg/g) | R2 | K2 (min−1) | qe (mg/g) | R2 |
| 0.140 | 2.121 | 0.887 | 0.288 | 2.127 | 0.999 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishima, K.; Du, X.; Miyamoto, N.; Kano, N.; Imaizumi, H. Experimental and Theoretical Studies on the Adsorption Mechanisms of Uranium (VI) Ions on Chitosan. J. Funct. Biomater. 2018, 9, 49. https://doi.org/10.3390/jfb9030049
Mishima K, Du X, Miyamoto N, Kano N, Imaizumi H. Experimental and Theoretical Studies on the Adsorption Mechanisms of Uranium (VI) Ions on Chitosan. Journal of Functional Biomaterials. 2018; 9(3):49. https://doi.org/10.3390/jfb9030049
Chicago/Turabian StyleMishima, Kenji, Xiaoyu Du, Naoto Miyamoto, Naoki Kano, and Hiroshi Imaizumi. 2018. "Experimental and Theoretical Studies on the Adsorption Mechanisms of Uranium (VI) Ions on Chitosan" Journal of Functional Biomaterials 9, no. 3: 49. https://doi.org/10.3390/jfb9030049
APA StyleMishima, K., Du, X., Miyamoto, N., Kano, N., & Imaizumi, H. (2018). Experimental and Theoretical Studies on the Adsorption Mechanisms of Uranium (VI) Ions on Chitosan. Journal of Functional Biomaterials, 9(3), 49. https://doi.org/10.3390/jfb9030049




