Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review
Abstract
:1. Introduction
2. Nanoparticles and Coating Materials for Mechanical-Property Improvement
2.1. Nanodiamond
2.2. Hidroxyapatite
2.3. Bioactive Glass Particles
2.4. Nano SiO2 and MgO Particles
2.5. Silver Nanoparticles
3. Conclusions and Recommendations
- Good affinity between nanoparticles and scaffold is the key to enhance the tensile strength. Additionally, the stronger interfacial bonding of the coating layer to the substrate can result in higher compressive strength and load transfer efficiency.
- Good dispersion of nanoparticles can result in a large interfacial area and thus significantly increases fracture energy and other mechanical properties.
- A thicker coating usually results in a mechanically stronger scaffold.
- Tensile testing requires gripping the scaffold; bioreactor grips could damage the sample, generating cracks before the measurement. It is a major issue for characterizing the mechanical property of porous ceramic scaffolds using conventional methods.
- The concentration-dependent effects of nanoparticles on the initiation and propagation of cracks due to scaffolds crystallinity need further, yet systematic, investigation.
- The influence of size and shape of nanoparticles either as a particles or embedded into coatings, on the mechanical properties of the scaffold is urged to be studied.
- It would be interesting to look into the relationship between the fiber diameter and the mechanical properties of fibrous scaffolds.
- It would be essential to investigate into how the chemical affinity between nanoparticles and scaffold materials affects the scaffold mechanical properties.
- Regarding to mechanical testing, it is necessary to observe in depth the propagation of cracks during compressive or tensile tests and to consider the distribution of nanoparticles (take into account possible agglomeration zones and zones with less concentration of nanoparticles), or other factors that might contribute to the mechanical performance of the scaffolds.
- Toughness measurement is essential since toughness is a key mechanical property.
- It could be beneficial to have a comprehensive understanding of how the viscosity and adhesion of the coating affect the coverage and thickness of the coatings and resulted mechanical properties of the scaffolds.
- Finally, it is important to study the mechanical properties under simulated in vivo environments. Testing mechanical properties in such an environment will allow one to gain insight into the mechanical performance of scaffolds once implanted into patients.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NDs | Nanodiamonds |
| PLLA | Poly(l-lactic acid) |
| ND-ODA | Octadecylamine-functionalized nanodiamond |
| HA | Hydroxyapatite |
| N6 | Nylon 6 |
| PHB | Poly(3-hydroxybutyrate) |
| nHA | Hydroxyapatite nanoparticle |
| PCL | Polycaprolactone |
| BCP | Biphasic calcium phosphate |
| nBG | Bioactive glass nanoparticles |
| β-TCP | β-tricalcium phosphate |
| AgNPs | Silver nanoparticles |
| TX | TritonX-100 |
| PEG | Poly(ethylene) glycol |
| CS | Collagen scaffold |
References
- Little, C.J.; Bawolin, N.K.; Chen, X.B. Mechanical properties of natural cartilage and tissue engineered constructs. Tissue Eng. Part B 2011, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Bawolin, N.K.; Zhang, W.J.; Chen, X.B. A brief review of the modelling of the time dependent mechanical properties of tissue engineering scaffolds. J. Biomim. Biomater. Tissue Eng. 2010, 6, 19–33. [Google Scholar] [CrossRef]
- Bawolin, N.K.; Li, M.G.; Chen, X.B.; Zhang, W.J. Modeling material-degradation-induced elastic property of tissue engineering scaffolds. ASME J. Biomech. Eng. 2010. [Google Scholar] [CrossRef] [PubMed]
- Olubamiji, A.; Izadifar, Z.; Si, J.; Cooper, D.; Eames, F.; Chen, X.B. Modulating mechanical behavior of 3D-printed cartilage-mimetic PCL scaffolds: Influence of molecular weight & pore geometry. Biofabrication 2016, 8, 25020–25037. [Google Scholar]
- Bawolin, N.K.; Dolovich, A.T.; Zhang, C.W.; Chen, X.B. Characterization of mechanical properties of tissue scaffolds by phase contrast imaging and finite element modeling. ASME J. Biomech. Eng. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Chen, X.B. Effects of cell density on mechanical properties of alginate hydrogel tissue scaffolds. J. Biomim. Biomater. Tissue Eng. 2014, 19, 77–85. [Google Scholar] [CrossRef]
- You, F.; Wu, X.; Chen, X.B. 3D printing of alginate/gelatin hydrogel scaffolds and their mechanical-property characterization. Int. J. Polym. Mater. Polym. Biomater 2016, in press. [Google Scholar]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Hazeli, K.; Niu, J.; Kontsos, A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials 2012, 33, 5067–5075. [Google Scholar] [CrossRef] [PubMed]
- Karbushev, V.V.; Konstantinov, I.I.; Parsamyan, I.L.; Kulichikhin, V.G.; Popov, V.A.; George, T.F. Preparation of polymer-nanodiamond composites with improved properties. Adv. Mater. Res. 2009, 59, 275–278. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Finne-Wistrand, A.; Waag, T.; Xing, Z.; Yassin, M.; Yamamoto, A.; Mustafa, K.; Steinmüller-Nethl, D.; Krueger, A.; Albertsson, A.-C. Reinforced degradable biocomposite by homogenously distributed functionalized nanodiamond particles. Macromol. Mater. Eng. 2015, 300, 436–447. [Google Scholar] [CrossRef]
- Schrand, A.M.; Ciftan, H.S.A.; Shenderova, O.A. Nanodiamond particles: Properties and perspectives for bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Krüger, A.; Kataoka, F.; Ozawa, M.; Fujino, T.; Suzuki, Y.; Aleksenskii, A.E.; Vul’, A.Y.; Ōsawa, E. Unusually tight aggregation in detonation nanodiamond: Identification and disintegration. Carbon 2005, 43, 1722–1730. [Google Scholar] [CrossRef]
- Eidelman, E.D.; Siklitsky, V.I.; Sharonova, L.V.; Yagovkina, M.A.; Vul’, A.Y.; Takahashi, M.; Inakuma, M.; Ozawa, M.; Ōsawa, E. A stable suspension of single ultrananocrystalline diamond particles. Diam. Relat. Mater. 2005, 14, 1765–1769. [Google Scholar] [CrossRef]
- Aleksenskiy, A.; Baidakova, M.; Osipov, V.; Vul’, A. The fundamental properties and characteristics of nanodiamonds. In Nanodiamonds Applications in Biology and Nanoscale Medicine, 1st ed.; Dean, H., Ed.; Springer-Verlag: Boston, MA, USA, 2010; pp. 55–77. [Google Scholar]
- Havlik, R.J. Hydroxyapatite safety and efficacy report. PSEF Data Comm. Indianap. Ind. US 2002. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Venegas, P.; Hamdy, A.S.; Engel, F.B.; Lim, J.H. Preparation and characterization of vertically arrayed hydroxyapatite nanoplates on electrospun nanofibers for bone tissue engineering. Chem. Eng. J. 2014, 254, 612–622. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.-I.; Nouri-Khorasani, S.; Lu, Z.; Appleyard, R.; Zreiqat, H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite–PCL composites. Biomaterials 2010, 31, 5498–5509. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, S.I.R.; Tavangarian, F.; Emadi, R. Nanostructured bioactive glass coating on porous hydroxyapatite scaffold for strength enhancement. Mater. Lett. 2008, 62, 3428–3430. [Google Scholar] [CrossRef]
- Esfahani, S.I.R.; Lu, Z.F.; Li, J.J.; Ellis-Behnke, R.; Kaplan, D.L.; Zreiqat, H. Effect of self-assembled nanofibrous silk/polycaprolactone layer on the osteoconductivity and mechanical properties of biphasic calcium phosphate scaffolds. Acta Biomater. 2012, 8, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, M.; Karbasi, S.; Ebrahimi-Kahrizsangi, R. Physical and mechanical properties of a poly-3-hydroxybutyrate-coated nanocrystalline hydroxyapatite scaffold for bone tissue engineering. J. Porous Mater. 2012, 19, 667–675. [Google Scholar] [CrossRef]
- Tetteh, G.; Khan, A.S.; Delaine-Smith, R.M.; Reilly, G.C.; Rehman, I.U. Electrospun polyurethane/hydroxyapatite bioactive Scaffolds for bone tissue engineering: The role of solvent and hydroxyapatite particles. J. Mech. Behav. Biomed. Mater. 2014, 39, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, J.; Zhang, Q.; Xiao, Y.; Lang, M. Improved mechanical properties of hydroxyapatite/poly (ε-caprolactone) scaffolds by surface modification of hydroxyapatite. Appl. Surf. Sci. 2010, 256, 6107–6112. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, X.; Duan, K.; Guo, L.Y.; Zhou, S.B.; Weng, J. Improving mechanical and biological properties of macroporous HA scaffolds through composite coatings. Colloids Surf. B Biointerfaces 2009, 74, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Nejati, E.; Mirzadeh, H.; Zandi, M. Synthesis and characterization of nano-hydroxyapatite rods/poly(l-lactide acid) composite scaffolds for bone tissue engineering. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1589–1596. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Z.-M.; He, C.L.; Han, X.J. Mechanical performance of laminated composites incorporated with nanofibrous membranes. Mater. Sci. Eng. A 2006, 435–436, 309–317. [Google Scholar] [CrossRef]
- Mehrabanian, M.; Nasr-Esfahani, M. HA/nylon 6,6 porous scaffolds fabricated by salt-leaching/solvent casting technique: Effect of nano-sized filler content on scaffold properties. Int. J. Nanomed. 2011, 6, 1651–1659. [Google Scholar]
- Abdal-hay, A.; Tijing, L.D.; Lim, J.K. Characterization of the surface biocompatibility of an electrospun nylon 6/CaP nanofiber scaffold using osteoblasts. Chem. Eng. J. 2013, 215–216, 57–64. [Google Scholar] [CrossRef]
- Kayvon, M.; Sina, E. Handbook of Polymer Applications in Medicine and Medical Devices. Available online: http://library.books24x7.com.cyber.usask.ca/toc.aspx?site=D7375&bookid=58853 (accessed on 8 July 2016).
- Ramier, J.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V.; Renard, E.; Albanese, P.; Grande, D. Biocomposite scaffolds based on electrospun poly(3-hydroxybutyrate) nanofibers and electrosprayed hydroxyapatite nanoparticles for bone tissue engineering applications. Mater. Sci. Eng. C 2014, 38, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.; Wheeler, D.; Greenspan, D. Molecular control of bioactivity in sol-gel glasses. J. Sol Gel Sci. Technol. 1998, 13, 245–250. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Preparation and characterization of nano-bioactive-glasses (NBG) by a quick alkali-mediated sol–gel method. Mater. Lett. 2007, 61, 3251–3253. [Google Scholar] [CrossRef]
- Esfahani, S.I.R.; Nouri-Khorasani, S.; Lu, Z.F.; Appleyard, R.C.; Zreiqat, H. Effects of bioactive glass nanoparticles on the mechanical and biological behavior of composite coated scaffolds. Acta Biomater. 2011, 7, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z.; Nooeaid, P.; Fathi, M.; Bagri, A.; Boccaccini, A.R. The effect of coating type on mechanical properties and controlled drug release of PCL/zein coated 45S5 bioactive glass scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 54, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, J.H.; Kim, Y.T.; Riu, D.H.; Jung, S.J.; Lee, Y.J.; Chung, S.C.; Kim, Y.H. Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors. Biomaterials 2003, 24, 1389–1398. [Google Scholar] [CrossRef]
- Golovanova, O.; Strunina, N.; Lemesheva, S.; Baisova, B. Determination of the elemental composition of human bone tissue by atomic emission spectral analysis. J. Appl. Spectrosc. 2011, 78, 145–148. [Google Scholar] [CrossRef]
- Gao, C.D.; Wei, P.P.; Feng, P.; Xiao, T.; Shuai, C.J.; Peng, S. Nano SiO2 and MgO improve the properties of porous β-TCP scaffolds via advanced manufacturing technology. Int. J. Mol. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-based crystalline nanoparticles, microbially fabricated. PNAS. 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Marsich, E.; Donati, I.; Paoletti, S. Silver nanocomposites and their biomedical applications. In Kumar CSSR, editor. Nanocomposites; Wiley-VCH: New York, NY, USA, 2010; pp. 81–127. [Google Scholar]
- Mandal, A.; Meda, V.; Zhang, W.J.; Farhan, M.K.; Gnanamani, A. Synthesis, characterization and comparison of antimicrobial activity of PEG/TritonX-100 capped silver nanoparticles on collagen scaffold. Colloids Surf. B Biointerfaces 2012, 90, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Al-Munajjed, A.A.; O’brien, F.J. Influence of a novel calcium-phosphate coating on the mechanical properties of highly porous collagen scaffolds for bone repair. J. Mech. Behav. Biomed. Mater. 2009, 2, 138–146. [Google Scholar] [CrossRef] [PubMed]

| Reference | Nanoparticle/Coating | Scaffold | Ratio | Test | Results |
|---|---|---|---|---|---|
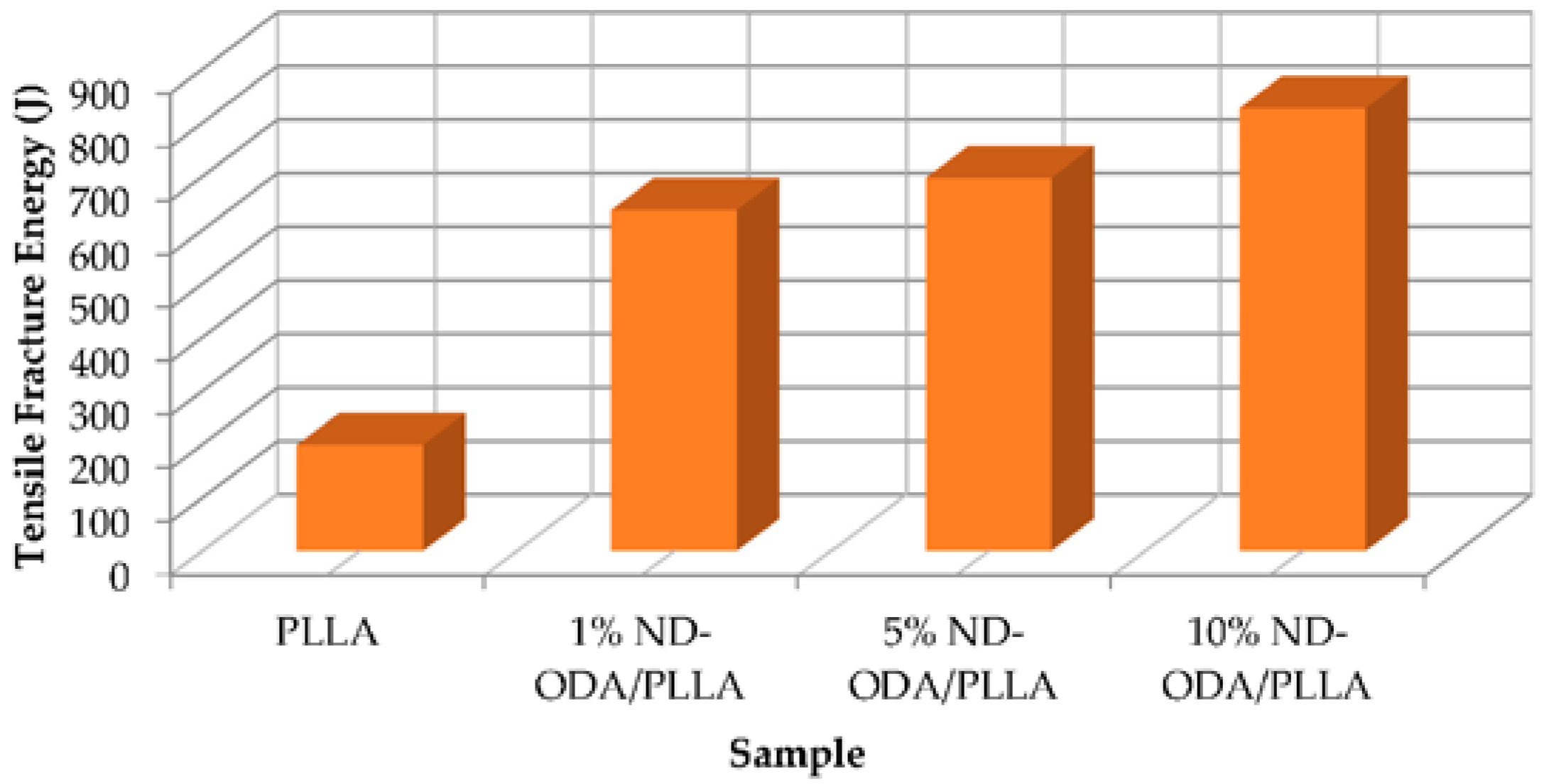
| Zhang et al. (2012) [8]. | Nanoparticle octadecylamine-functionalized nanodiamond (ND-ODA) | poly(l-lactic acid) (PLLA) | 10% wt ND-ODA/PLLA | Compression MTS servo-controlled hydraulic system, (MTS Systems Co., Eden Prairie, MN, USA) strain rate of 1 mm/min. Tension Instron Testing system, (Instron Co., Norwood, MA, USA) strain rate of 1 mm/min | Strain increase 280% at failure and a 310% increase in fracture energy in tensile tests. |
| Sun et al. (2015) [11]. | Nanodiamond (n-DP) | (l-lactide-co-e-caprolactone) (poly(LLA-co-CL)) | 10 wt %. n-DP-PLA | Tension Instron 5566 instrument (Instron, UK) with the crosshead speed of 100 mm/min | Increasing E-modulus by about six times (313.6 MPa). |
| Abdal-hay et al. (2014) [17]. | Coating Hydroxyapatite (HA) nanoplates | Nylon N6 nanofibers | N6 nanofibers immersed in a suspension solution of HA powder of 0.5% wt | Tension Tabletop tensile tester (Instron LLOYD Instruments, LR5K Plus, UK) speed 10 mm/min | The Young’s modulus of scaffold was improved by about 225% (average) and the tensile strength was also improved by about 71.8% (average) scaffold samples. |
| Ramier et al. (2014) [30]. | Nanoparticle Hydroxyapatite nanoparticle (nHA) | Poly(3-hydroxybutyrate) (PHB) | 14% (wt/vol) nHA/PHB | Tension Instron 5965 (Instron, Norwood, MA, USA) speed of 1mm/min | The mechanical properties of PHB mats with an increase of 67% of the elastic modulus and 51% of the tensile strength at break. |
| Esfahani et al. (2011) [33]. | Bioactive glass nanoparticles (nBG) Composition: 58 mol % SiO2, 38 mol % CaO and 4 mol % P2O5 | Biphasic calcium phosphate (BCP) scaffold | 30 wt % of nBG in BCP scaffold | Compression Universal Testing Machine (Instron 8874, UK) with a ramp rate of 0.5 mm/min. | The maximum compressive strength (increased aprox. 14 times) and modulus (increased aprox. 3 times) were achieved when 30 wt % nBG was added, compared with BCP scaffolds. |
| Esfahani et al. (2010) [18]. | Composite coating of Hydroxyapatite (HA) and polycaprolactone (PCL) | Biphasic calcium phosphate (BCP) scaffold | 3/10% wt. HA/PCL, Nano HA(Needle shape) | Compression Universal Testing Machine (Endura TEC, ELE 3400, Bose,, Eden Prairie, MN, USA) ramp rate of 0.5 mm/min. | The highest strength value was 2.1 MPa with a value 20 times higher than that of pure HA (0.1 MPa). |
| Gao et al. (2015) [37]. | Nano SiO2 and MgO particles | β-tricalcium phosphate (β-TCP) scaffolds | 0.5 wt % SiO2/β-TCP, 1.0 wt % MgO/β-TCP, 0.5 wt % SiO2 + 1.0 wt % MgO/β-TCP | Compression Mechanical tester (WD-D1, Shanghai Zhuoji Instruments Co., Shanghai, China) with a constant cross-head speed of 0.4 mm/min. | Improvement from 3.12 ± 0.36 MPa (β-TCP) to 5.74 ± 0.62 MPa (β-TCP/SiO2), 9.02 ± 0.55 MPa (β-TCP/MgO), and 10.43 ± 0.28 MPa (β-TCP/SiO2/MgO). |
| Al-Munajjed et al. (2008) [42]. | Calcium-phosphate coating | Collagen | 0.5 M concentration of the coating, 22 h immersing time | Compression Uniaxial testing system (Zwick Z005 with a 5 N load cell) in phosphate buffered saline (PBS) | Increasing from 0.3 kPa (pure collagen scaffold) to up to 90 kPa (coated scaffold). |
| Koshkaki et al. (2013) [26]. | Beta tricalcium phosphate (b-TCP) | Gelatin | From 10 and 20 wt % of b-TCP nanoparticles | Compression Testing machine (DTM, Zwick-roell, HCT 400/25, Ulm, Germany) at a constant rate of 1 mm min-1 in dry condition. | The Gelatine scaffold had a compressive modulus of 265.8 ± 14. By adding 10 and 20 wt % nano b-TCP, the modulus values increased to 272.6 ± 48 and 429.1 ± 62.2 MPa. |
| Foroughi et al. [21]. | poly-3-hydroxybutyrate (P3HB) | 50% wt Hydroxyapatite (HAp) | 0.6 g P3HB g/10 mL chloroform, HAp scaffolds were immersed in the polymer solution for 30 s. | Compression tester (SANTAM-Eng. Design Co. Ltd.). The crosshead speed was set at 0.5 mm/min. | The compressive strength without polymer coating was 0.11 MPa, while the compressive strength level of HAp scaffolds with polymer coating was 1.46 MPa. |
| Esfahani et al. [19] | Bioactive powder, composition: 58 mol % SiO2, 38 mol % CaO and 4 mol % P2O5 | Hydroxyapatite (HA) | Bioactive glass coating on HA and sintering at 1000 °C for 2 h. | Compression universal testing machine (AG-400NL, Shimadzu Co.,Kyoto, Japan) at a crosshead speed of 0.5 mm/min. | From 0.22 to 1.49 MPa. |
| Esfahani et al. [20] | Nanofibrous structured silk over a thin poly(e-caprolactone) (PCL) layer | 40% wt Hydroxyapatite(HA)/60% wt Biphasic calcium phosphate (BCP) scaffold | 7 wt % silk/HA/β-TCP | Compression Universal testing machine (Instron 8874, UK) with a ramp rate of 0.5 mm/min | The compressive strength and modulus of the modified scaffolds reached 0.42 MPa (compared with 0.07 MPa for BCP) and ≈25 MPa (compared with 5 MPa for BCP), respectively. |
| Mandal et al. [41] | Coating of poly(ethylene) glycol (PEG) and TritonX-100 (TX) over nanoparticles of silver | Collagen | 0.9 mM PEG + 0.9 mM TX | Tension testing machine (SATRA Co., UK, Model No. TM-43 at 20 °C with 65% relative humidity). | Maximum percentage elongation of 46.67%. Application: Implants, catheters and wound dressing materials. |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona-Gomez, J.; Chen, X.; Yang, Q. Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review. J. Funct. Biomater. 2016, 7, 18. https://doi.org/10.3390/jfb7030018
Corona-Gomez J, Chen X, Yang Q. Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review. Journal of Functional Biomaterials. 2016; 7(3):18. https://doi.org/10.3390/jfb7030018
Chicago/Turabian StyleCorona-Gomez, Jesus, Xiongbiao Chen, and Qiaoqin Yang. 2016. "Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review" Journal of Functional Biomaterials 7, no. 3: 18. https://doi.org/10.3390/jfb7030018
APA StyleCorona-Gomez, J., Chen, X., & Yang, Q. (2016). Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review. Journal of Functional Biomaterials, 7(3), 18. https://doi.org/10.3390/jfb7030018






