Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review
Abstract
:1. Introduction
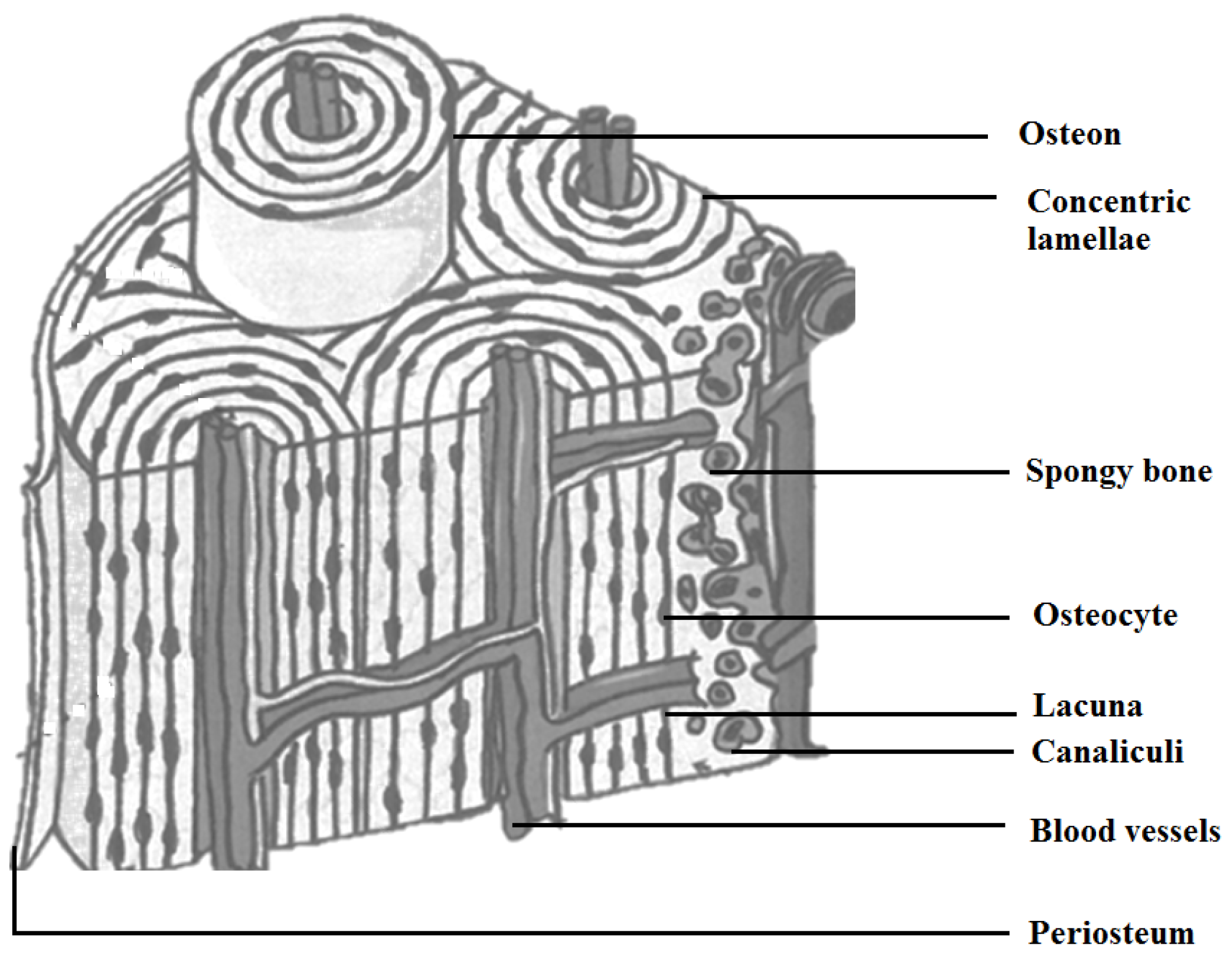
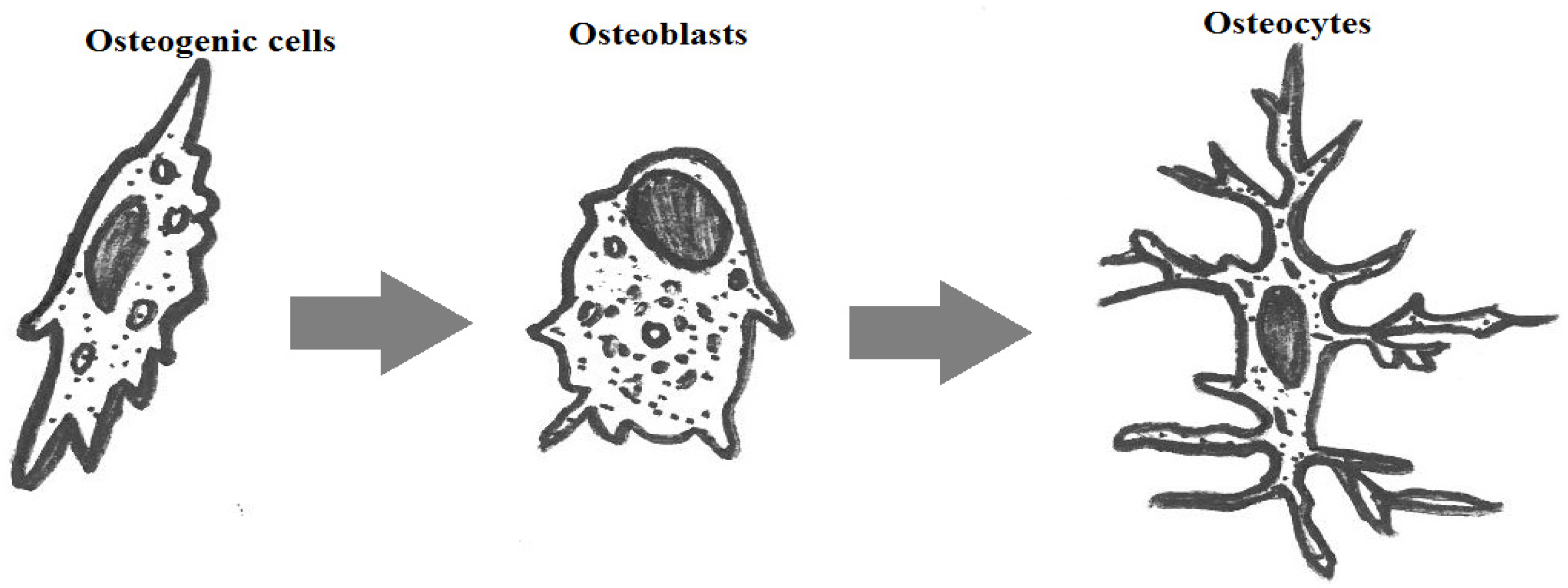
| Type of Bone | Compression Resistance (MPa) | Flexion Resistance (MPa) | Tension Resistance (MPa) | Modulus (GPa) | Porosity (%) |
|---|---|---|---|---|---|
| Cortical | 130–180 | 135–193 | 50–151 | 12–18 | 5–13 |
| Spongy | 4–12 | – | 1–5 | 0.1–0.5 | 30–90 |
| Ceramic | Chemical Formula | Usage |
|---|---|---|
| Alumina | Al2O3 | Bioinert |
| Zirconia | ZrO2 | |
| Pyrolytic carbon | Py-C | |
| Bioglass | Na2OCaOP2O3-SiO | Bioactive |
| Hydroxyapatite (sintered at high temperature) | Ca10(PO4)6(OH)2 | |
| Hydroxyapatite ( sintered at low temperature) | Ca10(PO4)6(OH)2 | Biodegradable |
| Tricalcium phosphate | Ca3(PO4)2 |
| Name | Young’s Modulus E (GPa) | Compressive Strength σ (MPa) | Tensile Strength σ (MPa) |
|---|---|---|---|
| Alumina | 380 | 4500 | 350 |
| Bioglass-ceramics | 22 | 500 | 56–83 |
| Calcium phosphates | 40–117 | 510–896 | 69–193 |
| Pyrolytic carbon | 18–28 | 517 | 280–560 |
2. Discussion on Dense Hydroxyapatites
2.1. Calcium Phosphates
| Name and Chemical Formula | Crystal Structure | Density | Usage |
|---|---|---|---|
| Monocalcium phosphate monohydrate Ca(H2PO4)2·H2O | Triclinic | 2.23 | In solution: as liquid phase in certain cements |
| Anhydrous monocalcium phosphate Ca(H2PO4)2 | Triclinic | 2.57 | In solution: as liquid phase in certain cements |
| Dicalcium phosphate dihydrate CaHPO4·2H2O | Monoclinic | 2.30 | Thin deposits, cements and composites |
| Dicalcium phosphate anhydrous CaHPO4 | Triclinic | 2.93 | Thin deposits, cements and composites |
| Amorphous Tricalcium phosphate Ca3(PO4)2·nH2O | Three polymorphs based on temperature | – | Thin deposits, cements and composites |
| Octocalcium phosphate Ca8(PO4)4(HPO4)2·5H2O | Triclinic | 2.67 | Cements |
| Tricalcium phosphate β Ca3(PO4)2 | Rhombohedral | 3.07 | Resorbable bioceramics, cements, composites |
| Tricalcium phosphate α Ca3(PO4)2 | Monoclinic | 2.86 | Resorbable bioceramics, cements, composites |
| Tetracalcium phosphate Ca4(PO4)2O | Monoclinic | 3.05 | Cements |
| Hydroxyapatite phospho-calcium Ca10(PO4)6(OH)2 | Hexagonal (the stoichiometric HAp is monoclinic at temperatures <212 °C, whereas in other cases, the small quantities of impurities lead to a change from monoclinic to hexagonal) | 3.16 | Cements, composites, ceramics and thin films |
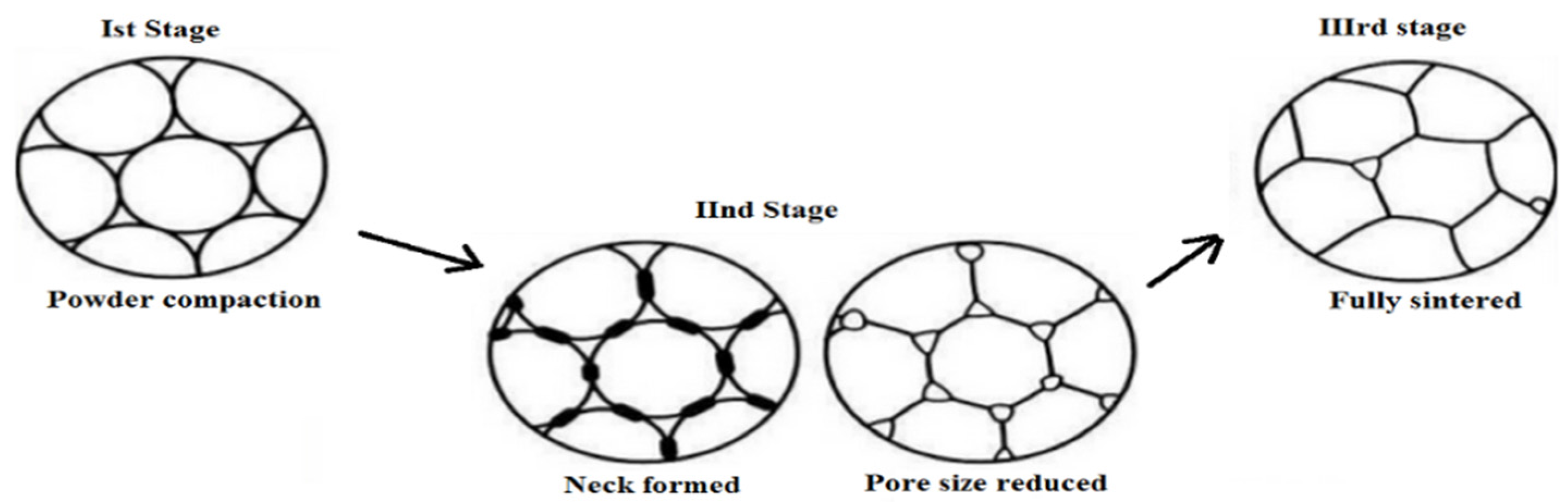
2.2. Sintering of Bioceramics
2.3. Nano-HAp
2.4. Porous Bioceramics
2.5. Bioactive Glasses
2.6. Metal Implants, Thin Films and Functionally-Gradient Materials of Bioceramics
2.7. Mechanical Properties
2.8. HAp Bio-Piezocomposites
2.9. Transparent Bioceramics
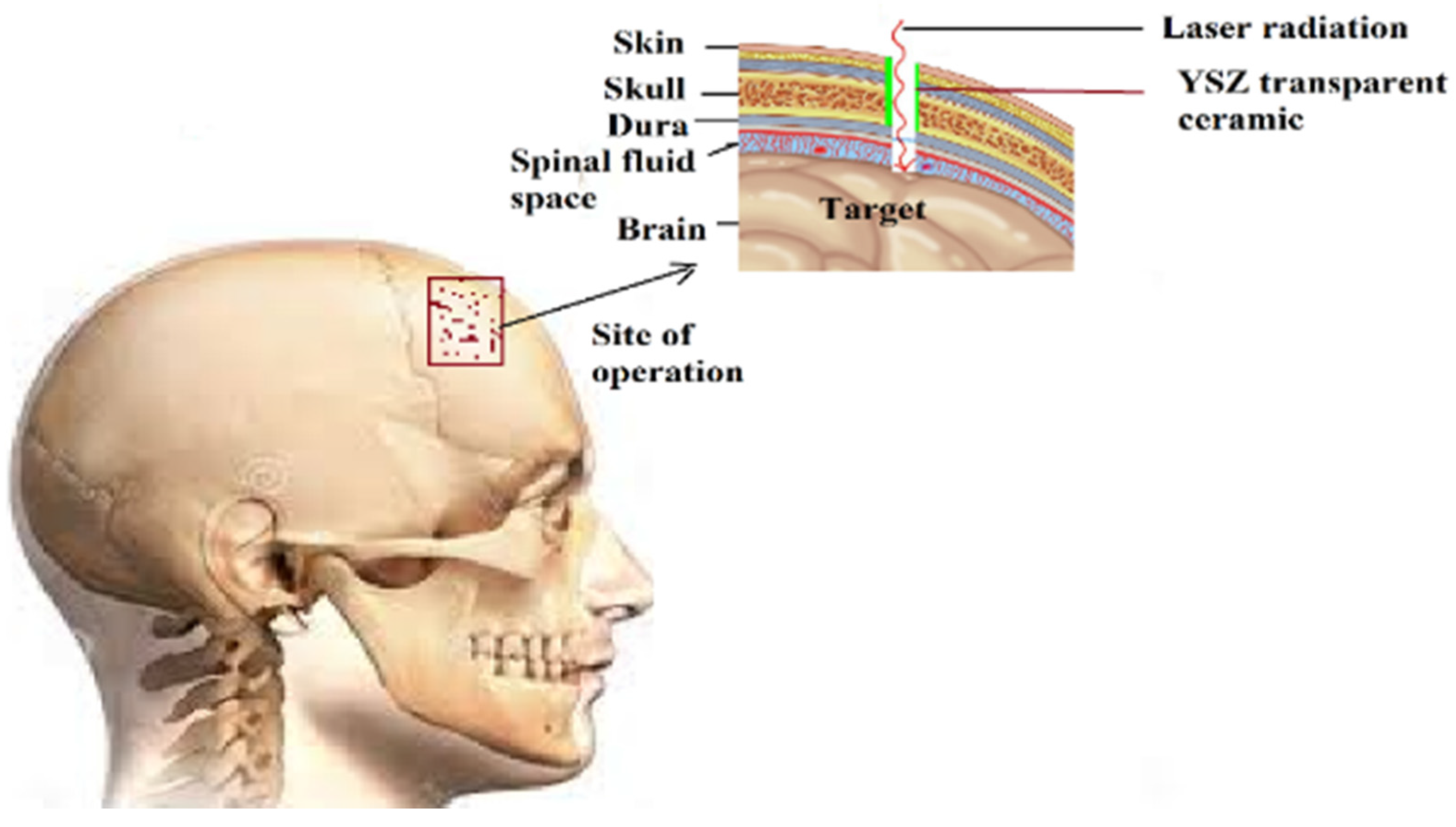
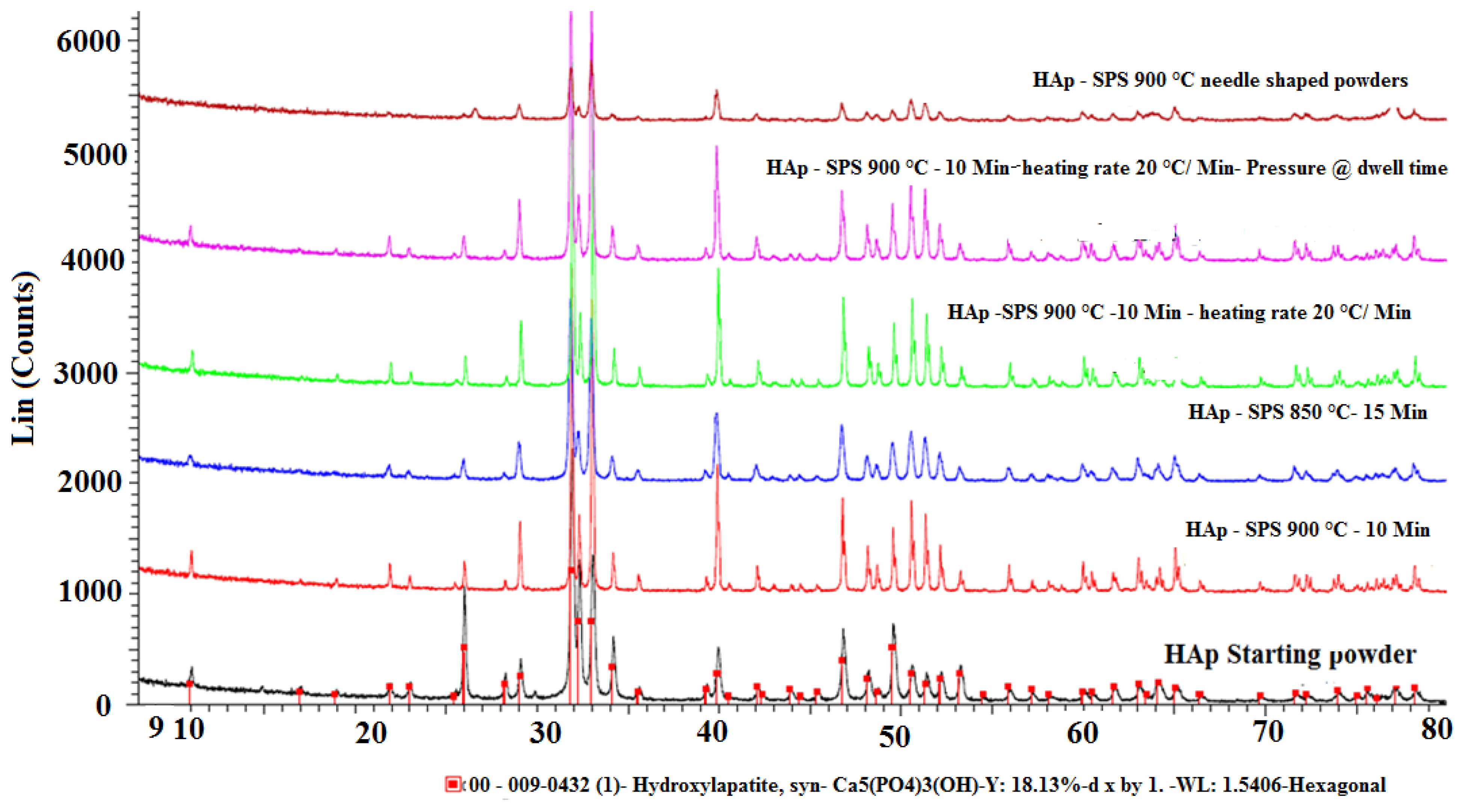

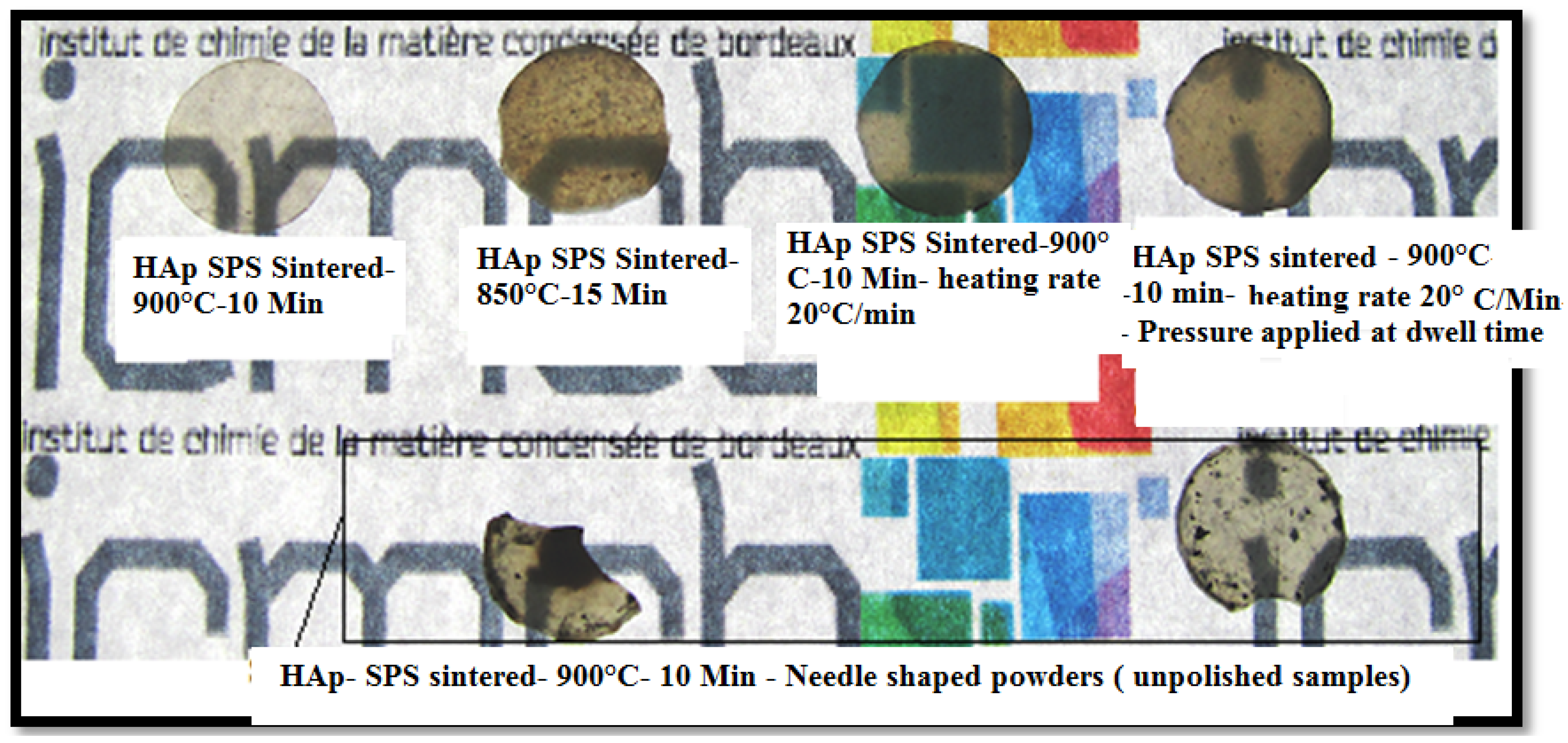
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- LeGeros, R.Z.; LeGeros, J.P. Dense Hydroxyapaite. In An Introduction to Bioceramics; Hench, L.L., Wilson, J., Eds.; World Scientific Publishing Co.: Singapore; River Edge, NJ, USA, 1993; pp. 139–179. [Google Scholar]
- Petit, R. The use of hydroxyapatite in orthopaedic surgery, a ten year review. Eur. J. Orthop. Surg. Traumatol. 1999, 9, 71–74. [Google Scholar] [CrossRef]
- Ramesh, S. Grain size—Properties correlation in polycrystalline hydroxypatite bioceramic. Malays. J. Chem. 2001, 3, 35–40. [Google Scholar]
- Sinha, A.; Mishra, T.; Ravishankar, N. Polymer assisted hydroxyapatite microspheres suitable for biomedical application. J. Mater. Sci. Mater. Med. 2008, 19, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.N.; Magalh, M.C.F.; Marques, P.A.A.P.; Senos, A.M.R. Wet synthesis and characterization of modified hydroxyapatite powders. J. Mater. Sci. Mater. Med. 1996, 7, 501–505. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics, past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Dubok, V.A. Bioceramics ¾ yesterday, today, tomorrow. Powder Metall. Metal Ceram. 2000, 39, 7–8. [Google Scholar] [CrossRef]
- Hench, L. Bioceramics, from concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hickman, K. Bioceramics. Ph.D. Thesis, 1999. http://www.csa.com/discoveryguides/archives/bceramics.php. [Google Scholar]
- Hulbert, S.F.; Bokros, J.C.; Hench, L.L.; Wilson, J.; Heimke, G. Ceramics in Clinical Applications Past, Present and Future; High Tech Ceramics; Elsevier: Amsterdam, The Netherlands, 1987; pp. 189–213. [Google Scholar]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.Z.C.; Zanger, K.; Krauspe, R. Significance of nano- and microtopography for cell-surface interactions in orthopaedic implants. J. Biomed. Biotech. 2007, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gaston, M.S.; Simpson, A.H.R.W. Inhibition of fracture healing. J. Bone Joint Surg. Br. 2007, 89B, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopedic biomaterials and the clinical need for bone engineering. Proc. IMechE H J. Eng. Med. 2010, 224, 1329–1343. [Google Scholar] [CrossRef]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef] [PubMed]
- Veljović, D.J.; Jokić, B.; Petrović, R.; Palcevskis, E.; Dindune, A.; Mihailescu, I.N.; Janaćković, D.J. Processing of dense nanostructured HAP ceramics by sintering and hot pressing. Ceram. Int. 2009, 35, 1407–1413. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate-containing biocomposites and hybrid biomaterials for biomedical applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef] [PubMed]
- Kurien, T.; Pearson, R.G.; Scammell, B.E. Bone graft substitutes currently available in orthopaedic practice, the evidence for their use. Bone Joint J. 2013, 95-B, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Structure of Bone Tissue. Available online: http://training.seer.cancer.gov/anatomy/skeletal/tissue.html (accessed on 10 December 2015).
- Baggett, J. Bone Tissue. Available online: http://slideplayer.com/slide/1704473/ (accessed on 10 December 2015).
- Lin, L.; Tong, A.; Zhang, H.; Hu, Q.; Fang, M. The Mechanical Properties of Bone Tissue Engineering Scaffold Fabricating via Selective Laser Sintering. In Life System Modeling and Simulation, Proceedings of the International Conference, LSMS 2007, Shanghai, China, 14–17 September, 2007; Li, K., Ed.; Springer: Berlin, Germnay, 2007; pp. 146–152. [Google Scholar]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Rho, J.-Y.; Tsui, T.Y.; Pharr, G.M. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials 1997, 18, 1325–1330. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Kong, Y.-M.; Kim, S.; Kim, H.-E.; Lee, I.-S. Reinforcement of hydroxyapatite bioceramic by addition of ZrO2 coated with Al2O3. J. Am. Ceram. Soc. 1999, 82, 2963–2968. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Kokubo, T. Bioactive glass ceramics, properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Bechtold, J.E.; Camisa, W.J.; Freeman, A.L.; Gustilo, R.B.; Sasing, J.L. Bone Compactor. Patent A61F5/00, 2012. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphates and human beings A historical perspective from the 1770s until 1940. Biomatter 2012, 2, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Nery, E.B.; LeGeros, R.Z.; Lynch, K.; Kalbfleisch, J. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/β-TCP in periodontal osseous defects. J. Periodontol. 1992, 63, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Gouin, F.; Delecrin, J.; Passuti, N.; Touchais, S.; Poirier, P.; Bainvel, J.V. Comblement osseux par céramique phosphocalcique biphasée macroporeuse, à propos de 23 cas. Rev. Chir. Orthop. 1995, 81, 59–65. [Google Scholar] [PubMed]
- Ransford, A.O.; Morley, T.; Edgar, M.A.; Webb, P.; Passuti, N.; Chopin, D.; Morin, C.; Michel, F.; Garin, C.; Pries, D. Synthetic porous ceramic compared with autograft in scoliosis surgery. A prospective, randomized study of 341 patients. J. Bone Joint Surg. Br. 1998, 80, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, R.; Daculsi, G.; Bouler, J.M. Macroporous calcium phosphate ceramic: A prospective study of 106 cases in lumbar spinal fusion. J. Long term Eff. Med. Implant. 1999, 9, 403–412. [Google Scholar]
- Soares, E.J.C.; Franca, V.P.; Wykrota, L.; Stumpf, S. Clinical Evaluation of A new Bioaceramic Ophthalmic Implant. In Bioceramics; LeGeros, R.Z., LeGeros, J.P., Eds.; World Scientific: Singapore, 1998; Volume 11, pp. 633–636. [Google Scholar]
- Wykrota, L.L.; Garrido, C.A.; Wykrota, F.H.I. Clinical Evaluation of Biphasic Calcium Phosphate Ceramic Use in Orthopaedic Lesions. In Bioceramics; LeGeros, R.Z., LeGeros, J.P., Eds.; World Scientific: Singapore, 1998; Volume 11, pp. 641–644. [Google Scholar]
- Malard, O.; Guicheux, J.; Bouler, J.M.; Gauthier, O.; Beauvillain de Montreuil, C.; Aguado, E.; Pilet, P.; LeGeros, R.; Daculsi, G. Calcium phosphate scaffold and bone marrow for bone reconstruction in irradiated area, a dog study. Bone 2005, 36, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Meffert, R.M.; Thomas, J.R.; Hamilton, K.M.; Brownstein, C.N. Hydroxylapatite as an alloplastic graft in the treatment of human periodontal osseous defects. J. Periodontol. 1985, 56, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Yukna, R.A.; Harrison, B.G.; Caudill, R.F.; Evans, G.H.; Mayer, E.T.; Miller, S. Evaluation of durapatite ceramic as an alloplastic implant in periodontal osseous defects. II. Twelve month reentry results. J. Periodontol. 1985, 56, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Piecuch, J.F. Augmentation of the atrophic edentulous ridge with porous replamine form hydroxyapatite (Interpore 200). Dent. Clin. N. Am. 1986, 30, 291–305. [Google Scholar] [PubMed]
- Munting, E.; Verhelpen, M.; Li, F.; Vincent, A. Contribution of Hydroxyapatite Coatings to Implant Fixations. In CRC Handbook of Bioactive Ceramics; Yamamuro, T., Hench, L.L., Wilson, J., Eds.; CRC Press: Boca Raton, FL, USA, 1990; Volume 2, pp. 143–148. [Google Scholar]
- Chang, E.; Chang, W.J.; Wang, B.C.; Yang, C.Y. Plasma spraying of zirconia reinforced hydroxyapatite composite coatings on titanium, Part I, Phase, microstructure and bonding strength. J. Mater. Sci. Mater. Med. 1997, 8, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Rigo, E.C.; Boschi, A.O.; Yoshimoto, M.; Allegrini, S., Jr.; Kong, B., Jr.; Corbani, M.J. Evaluation in vitro and in vivo of biomimetic hydroxyapatite coated on titanium dental implants. Mater. Sci. Eng. 2004, C24, 647. [Google Scholar] [CrossRef]
- Jean, A.; Kerebel, B.; Kerebel, L.M.; Legeros, R.Z.; Hamel, H. Effects of various calcium phosphate biomaterials on reparative dentin bridge formation. J. Endod. 1988, 14, 83–87. [Google Scholar] [CrossRef]
- Pissiotis, E.; Spangberg, L.S. Biological evaluation of collagen gels containing calcium hydroxide and hydroxyapatite. J. Endod. 1990, 16, 468–473. [Google Scholar] [CrossRef]
- Chohayeb, A.A.; Adrian, J.C.; Salamat, K. Pulpal response to tricalcium phosphate as a capping agent. Oral Surg. Oral Med. Oral Pathol. 1991, 71, 343–345. [Google Scholar] [CrossRef]
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cells Mater. 2006, 11, 43–56. [Google Scholar]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Rel. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Nakamura, S.; Takeda, H.; Yamashita, K. Proton transport polarization and depolarization of hydroxyapatite ceramics. J. Appl. Phys. 2001, 89, 5386–5392. [Google Scholar] [CrossRef]
- Gittings, J.P.; Bowen, C.R.; Turner, I.G.; Baxter, F.R.; Chaudhuri, J.B. Polarisation behaviour of calcium phosphate based ceramics. Mater. Sci. Forum 2008, 587–588, 91–95. [Google Scholar] [CrossRef]
- Kotobuki, N.; Kawagoe, D.; Nomura, D.; Katou, Y.; Muraki, K.; Fujimori, H.; Goto, S.; Ioku, K.; Ohgushi, H. Observation and quantitative analysis of rat bone marrow stromal cells cultured in vitro on newly formed transparent beta-tricalcium phosphate. J. Mater. Sci. Mater. Med. 2006, 17, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Weissman, J.L.; Snyderman, C.H.; Hirsch, B.E. Hydroxyapatite cement to repair skull base defects, radiologic appearance. Am. J. Neuroradiol. 1996, 17, 1569–1574. [Google Scholar] [PubMed]
- Hench, L.L. Bioactive Glasses and Glass-Ceramics. Mater. Sci. Forum 1998, 293, 37–64. [Google Scholar] [CrossRef]
- Asazuma, T.; Masuoka, K.; Motosuneya, T.; Tsuji, T.; Yasuoka, H.; Fujikawa, K. Posterior lumbar interbody fusion using dense hydroxyapatite blocks and autogenous iliac bone, clinical and radiographic examinations. J. Spinal Disord. Tech. 2005, 18, 41–47. [Google Scholar] [CrossRef]
- Davies, J.E. In vitro modeling of the bone/implant interface. Anat. Rec. 1996, 245, 426–445. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Rivera, E.M.; Araiza, M.; Brostow, W.; Castaño, V.M.; Díaz-Estrada, J.R.; Hernández, R.; Rodríguez, J.R. Synthesis of hydroxyapatite from eggshells. Mater. Lett. 1999, 41, 128–134. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, S.H. Fabrication of Calcium Phosphate bioceramics by using eggshell and phosphoric acid. Mater. Lett. 2003, 57, 4570–4574. [Google Scholar] [CrossRef]
- Balazsi, C.; Weber, F.; Kover, Z.; Horvath, E.; Nemeth, C. Preparation of Calcium-Phosphate bioceramics from natural resources. J. Eur. Ceram. Soc. 2007, 27, 1601–1606. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Crystallographic study of hydroxyapatite bioceramics derived from various sources. Cryst. Growth Des. 2005, 5, 111–112. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium Phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Lecomte, A.; Gautier, H.; Bouler, J.M.; Gouyette, A.; Pegon, Y.; Daculsi, G.; Merle, C. Biphasic Calcium Phosphate, A comparative study of interconnected porosity in two ceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tancret, F.; Bouler, J.M.; Chamousset, J.; Minois, L.M. Modelling the mechanical properties of microporous and macroporous biphasic Calcium Phosphate bioceramics. J. Eur. Ceram. Soc. 2006, 26, 3647–3656. [Google Scholar] [CrossRef]
- Bouler, J.M.; Trecant, M.; Delecrin, J.; Royer, J.; Passuti, N.; Daculsi, G. Macroporous biphasic Calcium Phosphate ceramics, Influence of five synthesis parameters on compressive strength. J. Biomed. Mater. Res. 1996, 32, 603–609. [Google Scholar] [CrossRef]
- O’Neill, W.C. The fallacy of the Calcium—Phosphorus product. Kidney Int. 2007, 72, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.V.; Bertran, C.A.; Kawachi, E.Y.; Camilli, J.A. Repair of cranial bone defects with Calcium Phosphate ceramic implant or autogenous bone graft. J. Craniofac. Surg. 2007, 18, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lorenzo, L.M.; Vallet-Regí, M.; Ferreira, J.M.F. Fabrication of hydroxyapatite bodies by uniaxial pressing from a precipitated powder. Biomaterials 2001, 22, 583–588. [Google Scholar] [CrossRef]
- Nazarpak, M.H.; Solati-Hashjin, M.; Moztarzadeh, F. Preparation of hydroxyapatite ceramics for biomedical applications. J. Ceram. Proc. Res. 2009, 10, 54–57. [Google Scholar]
- Itoh, H.; Wakisaka, Y.; Ohnuma, Y.; Kuboki, Y. A new porous hydroxyapatite ceramic prepared by cold isostatic pressing and sintering synthesized flaky powder. Dental Mater. 1994, 13, 25–35. [Google Scholar] [CrossRef]
- Gautier, H.; Merle, C.; Auget, J.L.; Daculsi, G. Isostatic compression, a new process for incorporating vancomycin into biphasic Calcium Phosphate, Comparison with a classical method. Biomaterials 2000, 21, 243–249. [Google Scholar] [CrossRef]
- Tadic, D.; Epple, M. Mechanically stable implants of synthetic bone mineral by cold isostatic pressing. Biomaterials 2003, 24, 4565–4571. [Google Scholar] [CrossRef]
- Pecqueux, F.; Tancret, F.; Payraudeau, N.; Bouler, J.M. Influence of microporosity and macroporosity on the mechanical properties of biphasic calcium phosphate bioceramics: Modelling and experiment. J. Eur. Ceram. Soc. 2010, 30, 819–829. [Google Scholar] [CrossRef]
- Viana, M.; Désiré, A.; Chevalier, E.; Champion, E.; Chotard, R.; Chulia, D. Interest of high shear wet granulation to produce drug loaded porous calcium phosphate pellets for bone filling. Key Eng. Mater. 2009, 396–398, 535–538. [Google Scholar] [CrossRef]
- Reikerås, O.; Johansson, C.B.; Sundfeldt, M. Bone ingrowths to press-fit and loose-fit implants, Comparisons between titanium and hydroxyapatite. J. Long Term Eff. Med. Implants 2006, 16, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Kannan, T.S. Dispersion and slip casting of hydroxyapatite. J. Am. Ceram. Soc. 2001, 84, 1710–1716. [Google Scholar] [CrossRef]
- Sakka, Y.; Takahashi, K.; Matsuda, N.; Suzuki, T.S. Effect of milling treatment on texture development of hydroxyapatite ceramics by slip casting in high magnetic field. Mater. Trans. 2007, 48, 2861–2866. [Google Scholar] [CrossRef]
- Zhang, Y.; Yokogawa, Y.; Feng, X.; Tao, Y.; Li, Y. Preparation and properties of bimodal porous apatite ceramics through slip casting using different hydroxyapatite powders. Ceram. Int. 2010, 36, 107–113. [Google Scholar] [CrossRef]
- Sepulveda, P.; Ortega, F.S.; Innocentini, M.D.M.; Pandolfelli, V.C. Properties of highly porous hydroxyapatite obtained by the gel casting of foams. J. Am. Ceram. Soc. 2000, 83, 3021–3024. [Google Scholar] [CrossRef]
- Padilla, S.; Vallet-Regí, M.; Ginebra, M.P.; Gil, F.J. Processing and mechanical properties of hydroxyapatite pieces obtained by the gel-casting method. J. Eur. Ceram. Soc. 2005, 25, 375–383. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Werner, J.; Vallet-Regí, M. Hierarchical pore structure of Calcium Phosphate scaffolds by a combination of gel-casting and multiple tape-casting methods. Acta Biomater. 2008, 4, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Fomin, A.S.; Barinov, S.M.; Ievlev, V.M.; Smirnov, V.V.; Mikhailov, B.P.; Belonogov, E.K.; Drozdova, N.A. Nanocrystalline hydroxyapatite ceramics produced by low-temperature sintering after high-pressure treatment. Dokl. Chem. 2008, 418, 22–25. [Google Scholar] [CrossRef]
- Kankawa, Y.; Kaneko, Y.; Saitou, K. Injection molding of highly-purified hydroxylapatite and TCP utilizing solid phase reaction method. J. Ceram. Soc. Jpn. 1991, 99, 438–442. [Google Scholar] [CrossRef]
- Kwon, S.H.; Jun, Y.K.; Hong, S.H.; Lee, I.S.; Kim, H.E.; Won, Y.Y. Calcium Phosphate bioceramics with various porosities and dissolution rates. J. Am. Ceram. Soc. 2002, 85, 3129–3131. [Google Scholar] [CrossRef]
- Fooki, A.C.B.M.; Aparecida, A.H.; Fideles, T.B.; Costa, R.C.; Fook, M.V.L. Porous hydroxyapatite scaffolds by polymer sponge method. Key Eng. Mater. 2009, 396–398, 703–706. [Google Scholar] [CrossRef]
- Sopyan, I.; Kaur, J. Preparation and characterization of porous hydroxyapatite through polymeric sponge method. Ceram. Int. 2009, 35, 3161–3168. [Google Scholar] [CrossRef]
- Velayudhan, S.; Ramesh, P.; Sunny, M.C.; Varma, H.K. Extrusion of hydroxyapatite to clinically significant shapes. Mater. Lett. 2000, 46, 142–146. [Google Scholar] [CrossRef]
- Yang, H.Y.; Thompson, I.; Yang, S.F.; Chi, X.P.; Evans, J.R.G.; Cook, R.J. Dissolution characteristics of extrusion freeformed hydroxyapatite—Tricalcium Phosphate scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 3345–3353. [Google Scholar] [CrossRef] [PubMed]
- Muthutantri, A.I.; Huang, J.; Edirisinghe, M.J.; Bretcanu, O.; Boccaccini, A.R. Dipping and electrospraying for the preparation of hydroxyapatite foams for bone tissue engineering. Biomed. Mater. 2008, 3, 25009–25022. [Google Scholar] [CrossRef] [PubMed]
- Roncari, E.; Galassi, C.; Pinasco, P. Tape casting of porous hydroxyapatite ceramics. J. Mater. Sci. Lett. 2000, 19, 33–35. [Google Scholar] [CrossRef]
- Tian, T.; Jiang, D.; Zhang, J.; Lin, Q. Aqueous tape casting process for hydroxyapatite. J. Eur. Ceram. Soc. 2007, 27, 2671–2677. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Yamada, M.; Shiota, M.; Yamashita, Y.; Kasugai, S. Histological and histomorphometrical comparative study of the degradation and osteoconductive characteristics of α- and β-tricalcium phosphate in block grafts. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82B, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Elliot, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier Science: Amsterdam, The Netherland, 1994; p. 34. [Google Scholar]
- Zhou, J.; Zhang, X.; Chen, J.; Zeng, S.; de groot, K. High temperature characteristics of synthetic hydroxyapatite. J. Mater. Sci. Mater. Med. 1993, 4, 83–85. [Google Scholar] [CrossRef]
- Famery, R.; Richard, N.; Boch, P. Preparation of α and β-tricalcium phosphate ceramics, with and without magnesium addition. Ceram. Int. 1994, 20, 327–336. [Google Scholar] [CrossRef]
- Chu, K.-T.; Ou, S.-F.; Chen, S.-Y.; Chiou, S.-Y.; Chou, H.-H.; Ou, K.-L. Research of phase transformation induced biodegradable properties on hydroxyapatite and tricalcium phosphate based bioceramic. Ceram. Int. 2013, 39, 1455–1462. [Google Scholar] [CrossRef]
- Ruseska, G.; Fidancevska, E.; Bossert, J. Mechanical and thermal-expansion characteristics of Ca10(PO4)6(OH)2-Ca3(PO4)2 composites. Sci. Sinter. 2006, 38, 245–253. [Google Scholar] [CrossRef]
- Patel, N.; Best, S.M.; Bonfield, W.; Gibson, I.R.; Hing, K.A.; Damien, E.; Revell, P.A. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med. 2002, 13, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-substituted hydroxyapatite, from synthesis to in vivo behavior. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Feng, Q.L.; Kim, J.O.; Wu, J.; Wang, H.; Chen, G.C.; Cui, F.Z. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Turner, I.G. Ceramics and Glasses. In Biomedical Materials; Narayanan, R., Ed.; Springer: New York, NY, USA, 2009; pp. 3–39. [Google Scholar]
- Bowen, P.; Carry, C. From powders to sintered pieces, forming, transformations and sintering of nanostructured ceramic oxides. Powder Technol. 2002, 128, 248–255. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Chen, I.-W.; Wang, X.H. Sintering dense nanocrystalline ceramics without final-stage grain growth. Nature 2000, 404, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Dynys, F.W.; Halloran, J.W. Influence of aggregates on sintering. J. Am. Ceram. Soc. 1984, 67, 596–601. [Google Scholar] [CrossRef]
- Tuan, W.H.; Gilbart, E.; Brook, R.J. Sintering of heterogeneous ceramic compacts. J. Mater. Sci. 1989, 24, 1062–1068. [Google Scholar] [CrossRef]
- Liu, D.-M.; Lin, J.-T. Influence of ceramic powders of different characteristics on particle packing structure and sintering behavior. J. Mater. Sci. 1999, 34, 1959–1972. [Google Scholar] [CrossRef]
- Chaim, R.; Levin, M.; Shlayer, A.; Estournès, C. Sintering and densification of nanocrystalline ceramic oxide powders: A review. Adv. Appl. Ceram. Struct. Funct. Bioceram. J. Adv. Psychiatr. Treat. 2008, 107, 159–169. [Google Scholar] [CrossRef]
- Arunachalam, V.S.; Sundaresan, R. Powder Metallurgy. In Materials Science and Technology; Cahn, R.W., Ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Coble, R.L. Sintering crystalline solids. I. Intermediate and final state diffusion models. J. Appl. Phys. 1961, 32, 787–792. [Google Scholar] [CrossRef]
- Braginsky, M.; Tikare, V.; Olevsky, E. Numerical simulation of solid state sintering. Int. J. Solids Struct. 2005, 42, 621–636. [Google Scholar] [CrossRef]
- Ji, S.; Gu, Q.; Xia, B. Porosity dependence of mechanical properties of solid materials. J. Mater. Sci. 2006, 41, 1757–1768. [Google Scholar] [CrossRef]
- Lynn Johnson, D. New method of obtaining volume, grain-boundary, and surface diffusion coefficients from sintering data. J. Appl. Phys. 1996, 40, 192–200. [Google Scholar] [CrossRef]
- Haberko, K.; Bućko, M.M.; Brzezińska-Miecznik, J.; Haberko, M.; Mozgawa, W.; Panz, T.; Pyda, A.; Zarebski, J. Natural hydroxyapatite—Its behaviour during heat treatment. J. Eur. Ceram. Soc. 2006, 26, 537–542. [Google Scholar] [CrossRef]
- Haberko, K.; Bućko, M.M.; Mozgawa, W.; Pyda, A.; Brzezińska-Miecznik, J.; Carpentier, J. Behaviour of bone origin hydroxyapatite at elevated temperatures and in O2 and CO2 atmospheres. Ceram. Int. 2009, 35, 2537–2540. [Google Scholar] [CrossRef]
- Janus, A.M.; Faryna, M.; Haberko, K.; Rakowska, A.; Panz, T. Chemical and microstructural characterization of natural hydroxyapatite derived from pig bones. Mikrochim. Acta 2008, 161, 349–353. [Google Scholar] [CrossRef]
- Bahrololoom, M.E.; Javidi, M.; Javadpour, S.; Ma, J. Characterisation of natural hydroxyapatite extracted from bovine cortical bone ash. J. Ceram. Proc. Res. 2009, 10, 129–138. [Google Scholar]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into biological apatite, physiochemical properties and preparation approaches. BioMed Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.Y. Characterization, thermal stability and sintering of hydroxyapatite powders prepared by different routes. Mater. Chem. Phys. 2005, 94, 333–341. [Google Scholar] [CrossRef]
- Suchanek, W.; Yashima, M.; Kakihana, M.; Yoshimura, M. Hydroxyapatite ceramics with selected sintering additives. Biomaterials 1997, 18, 923–933. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bose, S.; Bandyopadhyay, A.; Hosick, H.L. Oxide based sintering additives for HAp ceramics. Ceram. Trans. 2003, 147, 63–72. [Google Scholar]
- Kalita, S.J.; Bose, S.; Hosick, H.L.; Bandyopadhyay, A. CaO-P2O5-Na2O-based sintering additives for hydroxyapatite (HAp) ceramics. Biomaterials 2004, 25, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Safronova, T.V.; Putlyaev, V.I.; Shekhirev, M.A.; Tretyakov, Y.D.; Kuznetsov, A.V.; Belyakov, A.V. Densification additives for hydroxyapatite ceramics. J. Eur. Ceram. Soc. 2009, 29, 1925–1932. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Kono, H.; Sassa, K.; Asai, S. Abnormal grain growth of hydroxyapatite ceramic sintered in a high magnetic field. J. Cryst. Growth 2010, 312, 323–326. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Cihlar, J.; Castkova, K. Direct synthesis of nanocrystalline hydroxyapatite by hydrothermal hydrolysis of alkylphosphates. Monatshefte. Chem. 2002, 133, 761–771. [Google Scholar] [CrossRef]
- Vijayalakshmi, U.; Rajeswari, S. Influence of process parameters on the sol-gel synthesis of nano hydroxyapatite using various phosphorus precursors. J. Sol-Gel Sci. Technol. 2012, 63, 45–55. [Google Scholar] [CrossRef]
- Costa, D.O.; Dixon, S.J.; Rizkalla, A.S. One- and three-dimensional growth of hydroxyapatite nanowires during sol-gel-hydrothermal synthesis. ACS Appl. Mater. Interfaces 2012, 4, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Tseng, Y.H.; Chan, J.C.C.; Mou, C.Y. Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to-single-crystal transformation. Adv. Funct. Mater. 2005, 15, 2005–2010. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J. The transformation of single-crystal calcium phosphate ribbon-like fibres to hydroxyapatite spheres assembled from nanorods. Nanotechnology 2008, 19, 155608–155618. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.X.; Bao, X. Influence of temperature, ripening time and calcination on the morphology and crystallinity of hydroxyapatite nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 1697–1704. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, J.; Yang, S.; Yu, Q.; Wang, Q.Z. Preparation of amino-acid-regulated hydroxyapatite particles by hydrothermal method. Mater. Lett. 2011, 65, 572–574. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Y.; Zhang, S.-F. Synthesis and characterization of microporous hydroxyapatite via hydrothermal method. Synth. React. Inorg. 2011, 41, 31–35. [Google Scholar]
- Fathi, M.H.; Zahrani, E.M. Fabrication and characterization of fluoridated hydroxyapatite nanopowders via mechanical alloying. J. Alloys Compd. 2009, 475, 408–414. [Google Scholar] [CrossRef]
- Silva, C.C.; Graça, M.P.F.; Valente, M.A.; Sombra, A.S.B. Crystallite size study of nanocrystalline hydroxyapatite and ceramic system with titanium oxide obtained by dry ball milling. J. Mater. Sci. 2007, 42, 3851–3855. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Bulk processing of hydroxyapatite nanopowder using radio frequency induction plasma. J. Am. Ceram. Soc. 2010, 93, 3720–3725. [Google Scholar] [CrossRef]
- Ruksudjarit, A.; Pengpat, K.; Rujijanagul, G.; Tunkasiri, T. Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone. Curr. Appl. Phys. 2008, 8, 270–272. [Google Scholar] [CrossRef]
- Mhin, S.W.; Ryu, J.H.; Kim, K.M. Simple synthetic route for hydroxyapatite colloidal nanoparticles via a Nd:YAG laser ablation in liquid medium. Appl. Phys. A 2009, 96, 435–440. [Google Scholar] [CrossRef]
- Musaev, O.R.; Dusevich, V.; Wieliecza, D.M.; Wrobel, J.M.; Kruger, M.B. Nanoparticle fabrication of hydroxyapatite by laser ablation in water. J. Appl. Phys. 2008, 104, 1–5. [Google Scholar] [CrossRef]
- Nelson, D.G.A.; Wefel Jongebloed, W.L.; Featherstone, J.D.B. Morphology, histology and crystallography of human dental enamel treated with pulsed low-energy infrared laser radiation. Caries Res. 1987, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Sridhar, T.M. Electrocrystallization of Hydroxyapatite and Its Dependence on Solution Conditions. Cryst. Growth Des. 2008, 8, 3965–3977. [Google Scholar] [CrossRef]
- Smolen, D.; Chudoba, T.; Malka, I.; Kedzierska, A.; Lojkowski, W.; Swieszkowski, W.; Kurzydlowski, K.J.; Mierzynska, M.K.; Szumiel, M.L. Highly biocompatible, nanocrystalline hydroxyapatite synthesized in a solvothermal process driven by high energy density microwave radiation. Int. J. Nanomed. 2013, 8, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hench, L.L.; Du, J.; Choy, K.-L.; Guo, J. Preparation of hydroxyapatite fibers by electrospinning technique. J. Am. Ceram. Soc. 2004, 87, 1988–1991. [Google Scholar] [CrossRef]
- Socol, G.; Macovei, A.M.; Miroiu, F.; Stefan, N.; Duta, L.; Dorcioman, G.; Mihailescu, I.N.; Petrescu, S.M.; Stan, G.E.; Marcov, D.A.; et al. Hydroxyapatite thin films synthesized by pulsed laser deposition and magnetron sputtering on PMMA substrates for medical applications. Mater. Sci. Eng. B 2010, 169, 159–168. [Google Scholar] [CrossRef]
- D’Elía, N.L.; Noel Gravina, A.; Ruso, J.M.; Laiuppa, J.A.; Santillán, G.E.; Messina, P.V. Manipulating the bioactivity of hydroxyapatite nano-rods structured networks, Effects on mineral coating morphology and growth kinetic. Biochim. Biophys. Acta 2013, 1830, 5014–5026. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, N.; Chinnakali, K.; Gnanam, F.D. The effect of powder processing on densification, microstructure and mechanical properties of hydroxyapatite. Ceram. Int. 2002, 28, 355–362. [Google Scholar] [CrossRef]
- Richard, R.; Alexander, B.; Eugene, Z.; Dan, H. Hydroxyapatite with Controllable Size and Morphology. WO2006083418 A2, 2006. [Google Scholar]
- Kim, H.W.; Kim, H.E.; Salih, V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials 2005, 26, 5221–30. [Google Scholar] [CrossRef] [PubMed]
- Mucalo, M. Hydroxyapatite (HAp) for Biomedical Applications, Technology & Engineering; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Du, C.; Cui, F.Z.; Feng, Q.L.; Zhu, X.D.; de Groot, K. Tissue response to nano hydroxyapatite/collagen composite implants in marrow cavity. J. Biomed. Mater. Res. 1998, 42, 540–548. [Google Scholar] [CrossRef]
- Müller-Mai, C.M.; Stupp, S.I.; Voigt, C.; Gross, U. Nanoapatite and organoapatite implants in bone: Histology and ultrastructure of the interface. J. Biomed. Mater. Res. 1995, 29, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Pielichowska, K.; Blazewicz, S. Bioactive polymer/hydroxyapatite (nano)composites for bone tissue regeneration. Biopolym. Adv. Polym. Sci. 2010, 232, 97–207. [Google Scholar]
- Hu, J.; Liu, Z.; Tang, S.; He, Y. Effect of hydroxyapatite nanoparticles on the growth and p53/c-Myc protein expression of implanted hepatic VX2 tumor in rabbits by intravenous injection. World J. Gastroenterol. 2007, 13, 2798–2802. [Google Scholar] [PubMed]
- Bauer, I.W.; Li, S.P.; Han, Y.C.; Yuan, L.; Yin, M.Z. Internalization of hydroxyapatite nanoparticles in liver cancer cells. J. Mater. Sci. Mater. Med. 2008, 19, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Pezzatini, S.; Solito, R.; Morbidelli, L.; Lamponi, S.; Boanini, E.; Bigi, A.; Ziche, M. The effect of hydroxyapatite nanocrystals on microvascular endothelial cell viability and functions. J. Biomed. Mater. Res. A 2006, 76, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, Y.; Yao, F.; Zhang, L.; Yao, K. Effect of nano- and micro-hydroxyapatite/chitosan- gelatin network film on human gastric cancer cells. Mater. Lett. 2008, 62, 3220–3223. [Google Scholar] [CrossRef]
- Cai, Y.R.; Tang, R.K. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787. [Google Scholar] [CrossRef]
- Sun, W.; Chu, C.; Wang, J.; Zhao, H. Comparison of periodontal ligament cells responses to dense and nanophase hydroxyapatite. J. Mater. Sci. Mater. Med. 2007, 18, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shaw, L.L. Morphology-Enhanced Low-Temperature Sintering of Nanocrystalline Hydroxyapatite. Adv. Mater. 2007, 19, 2364–2369. [Google Scholar] [CrossRef]
- Veljovic, Đ.; Zalite, I.; Palcevskis, E.; Smiciklas, I.; Petrovic, R.; Janackovic, Đ. Microwave Sintering of Fine Grained HAP and HAP/TCP Bioceramics. Ceram. Int. 2010, 36, 595–603. [Google Scholar] [CrossRef]
- Eriksson, M.; Liu, Y.; Hu, J.; Gao, L.; Nigren, M.; Shen, Z. Transparent Hydroxyapatite Ceramics with Nanograin Structure Prepared by High Pressure Spark Plasma Sintering at the Minimized Sintering Temperature. J. Eur. Ceram. Soc. 2011, 31, 1533–1540. [Google Scholar] [CrossRef]
- Lukic, M.J.; Veselinovic, L.; Stojanovic, Z.; Macek-Krzmanc, M.; Bracko, I.; Skapin, S.D.; Markovic, S.; Uskokovic, D. Peculiarities in Sintering Behavior of Ca-Deficient Hydroxyapatite Nanopowders. Mater. Lett. 2012, 68, 331–335. [Google Scholar] [CrossRef]
- Misiek, D.J.; Kent, J.N.; Carr, R.F. Soft tissue responses to hydroxylapatite particles of different shapes. J. Oral. Maxillofac. Surg. 1984, 42, 150–160. [Google Scholar] [CrossRef]
- Uskokovic, D.P.; Palmour, H., III; Spriggs, R.M. (Eds.) Science of Sintering, New Directions for Materials Processing and Microstructural Control; Plenum Press: New York, NY, USA; London, UK, 1989.
- Kim, H.W.; Kong, Y.M.; Koh, Y.H.; Kim, H.; Kim, H.M.; Ko, J.S. Pressureless Sintering and Mechanical and Biological Properties of Fluor-hydroxyapatite Composites with Zirconia. J. Am. Ceram. Soc. 2003, 86, 2019–2026. [Google Scholar] [CrossRef]
- Bernache-Assollant, D.; Ababou, A.; Champion, E.; Heughebaert, M. Sintering of calcium phosphate hydroxyapatite Ca10(PO4)6(OH)2 I. Calcination and particle growth. J. Eur. Ceram. Soc. 2003, 23, 229–241. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kawai, W.; Wakayama, S. The effect of pressure during sintering on the strength and the fracture toughness of hydroxyapatite ceramics. J. Mater. Sci. Mater. Med. 2006, 17, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Wang, B. A new approach for toughening of ceramics. Mater. Lett. 1997, 33, 237–240. [Google Scholar] [CrossRef]
- Kasuga, T.; Ota, Y.; Tsuji, K.; Abe, Y. Preparation of high-strength Calcium Phosphate ceramics with low modulus of elasticity containing β-Ca(PO3)2 fibers. J. Am. Ceram. Soc. 1996, 79, 1821–1824. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Yoshimura, M. Preparation of fibrous, porous hydroxyapatite ceramics from hydroxyapatite whiskers. J. Am. Ceram. Soc. 1998, 81, 765–767. [Google Scholar] [CrossRef]
- Li, J.G.; Hashida, T. In situ formation of hydroxyapatite-whisker ceramics by hydrothermal hot-pressing method. J. Am. Ceram. Soc. 2006, 89, 3544–3546. [Google Scholar] [CrossRef]
- Onoki, T.; Hashida, T. New method for hydroxyapatite coating of titanium by the hydrothermal hot isostatic pressing technique. Surf. Coat. Technol. 2006, 200, 6801–6807. [Google Scholar] [CrossRef]
- Uematsu, K.; Takagi, M.; Honda, T.; Uchida, N.; Saito, K. Transparent hydroxyapatite prepared by hot isostatic pressing of filter cake. J. Am. Ceram. Soc. 1989, 72, 1476–1478. [Google Scholar] [CrossRef]
- Nakahira, A.; Murakami, T.; Onoki, T.; Hashida, T.; Hosoi, K. Fabrication of porous hydroxyapatite using hydrothermal hot pressing and post-sintering. J. Am. Ceram. Soc. 2005, 88, 1334–1336. [Google Scholar] [CrossRef]
- Auger, M.A.; Savoini, B.; Muñoz, A.; Leguey, T.; Monge, M.A.; Pareja, R.; Victoria, J. Mechanical characteristics of porous hydroxyapatite/oxide composites produced by post-sintering hot isostatic pressing. Ceram. Int. 2009, 35, 2373–2380. [Google Scholar] [CrossRef]
- Nath, S.; Basu, B.; Sinha, A. A comparative study of conventional sintering with microwave sintering of hydroxyapatite synthesized by chemical route. Trends Biomater. Artif. Organs 2006, 19, 93–98. [Google Scholar]
- Ramesh, S.; Tan, C.Y.; Bhaduri, S.B.; Teng, W.D. Rapid densification of nanocrystalline hydroxyapatite for biomedical applications. Ceram. Int. 2007, 33, 1363–1367. [Google Scholar] [CrossRef]
- Silva, C.C.; Graça, M.P.F.; Sombra, A.S.B.; Valente, M.A. Structural and electrical study of Calcium Phosphate obtained by a microwave radiation assisted procedure. Phys. Rev. B Condens. Matter. 2009, 404, 1503–1508. [Google Scholar] [CrossRef]
- Gu, Y.W.; Loh, N.H.; Khor, K.A.; Tor, S.B.; Cheang, P. Spark plasma sintering of hydroxyapatite powders. Biomaterials 2002, 23, 37–43. [Google Scholar] [CrossRef]
- Guo, X.; Xiao, P.; Liu, J.; Shen, Z. Fabrication of nanostructured hydroxyapatite via hydrothermal synthesis and spark plasma sintering. J. Am. Ceram. Soc. 2005, 88, 1026–1029. [Google Scholar] [CrossRef]
- Drouet, C.; Largeot, C.; Raimbeaux, G.; Estournès, C.; Dechambre, G.; Combes, C.; Rey, C. Bioceramics, spark plasma sintering (SPS) of calcium phosphates. Adv. Sci. Technol. 2006, 49, 45–50. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Z. Dehydroxylation of hydroxyapatite in dense bulk ceramics sintered by spark plasma sintering. J. Eur. Ceram. Soc. 2012, 32, 2691–2696. [Google Scholar] [CrossRef]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Ramesh, S.; Tan, C.Y.; Sopyan, I.; Hamdi, M.; Teng, W.D. Consolidation of nanocrystalline hydroxyapatite powder. Sci. Technol. Adv. Mater. 2007, 8, 124–130. [Google Scholar] [CrossRef]
- Tancred, D.C.; McCormack, B.A.; Carr, A.J. A synthetic bone implant macroscopically identical to cancellous bone. Biomaterials 1998, 19, 2303–2311. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishnan, S. Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Sopyan, I.; Mel, M.; Ramesh, S.; Khalid, K.A. Porous hydroxyapatite for artificial bone applications. Sci. Technol. Adv. Mater. 2007, 8, 116–123. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, R.W.; Carlton, A.; Holmes, R. Interporous hydroxyapatite as a bone graft substitute in tibial plateau fractures. Clin. Orthop. 1989, 240, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.X.; Flautre, B.; Anselme, K.; Hardouin, P.; Gallur, A.; Descamps, M.; Thierry, B. Role of interconnections in porous bioceramics on bone recolonization in vitro and in vivo. J. Mater. Sci. Mater. Med. 1999, 10, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Arns, C.H.; Sheppard, A.P.; Hutmacher, D.W.; Milthorpe, B.K.; Knackstedt, M.A. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 2007, 28, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- Ayers, R.A.; Simske, S.J.; Nunes, C.R.; Wolford, L.M. Long-term bone ingrowth and residual microhardness of porous block hydroxyapatite implants in humans. J. Oral Maxillof. Surg. 1998, 56, 1297–1302. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Weiss, P.; Bosco, J.; Daculsi, G.; Aguado, E. Kinetic study of bone ingrowth and ceramic resorption associated with the implantation of different injectable calcium-phosphate bone substitutes. J. Biomed. Mater. Res. 1999, 47, 28–35. [Google Scholar] [CrossRef]
- Hing, K.A.; Best, S.M.; Bonfield, W. Characterization of porous hydroxyapatite. J. Mater. Sci. Mater. Med. 1999, 10, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, G.; Spagnuolo, G.; Ambrosio, L.; Nicolais, L. Macroporous hydroxyapatite as alloplastic material for dental applications. J. Mater. Sci. Mater. Med. 1999, 10, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Charriere, E.; Lemaitre, J.; Zysset, P. Hydroxyapatite cement scaffolds with controlled macroporosity: Fabrication protocol and mechanical properties. Biomaterials 2003, 24, 809–817. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. Engl. 2004, 43, 5980–5984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, D.; Huang, W.; Yao, A.; Kamitakahara, M.; Ioku, K. Preparation and characterization of periodic porous frame of hydroxyapatite. J. Ceram. Soc. Jpn. 2009, 117, 521–524. [Google Scholar] [CrossRef]
- Munch, E.; Franco, J.; Deville, S.; Hunger, P.; Saiz, E.; Tomsia, A.P. Porous ceramic scaffolds with complex architectures. JOM 2008, 60, 54–59. [Google Scholar] [CrossRef]
- Chen, C.W.; Riman, R.E.; Ten Huisen, K.S.; Brown, K. Mechanochemical-hydrothemal synthesis of hydroxyapatite from no ionic surfactant emulsion precursors. J. Cryst. Growth 2004, 270, 615–623. [Google Scholar] [CrossRef]
- Nagase, M.; Baker, D.G.; Schumacher, H.R. Prolonged inflammatory reactions induced by artificial ceramics in the rat pouch model. J. Rheumatol. 1988, 15, 1334–1338. [Google Scholar] [PubMed]
- Rooney, T.; Berman, S.; Indersano, A.T. Evaluation of porous block hydroxylapatite for augmentation of alveolar ridges. J. Oral Maxillof. Surg. 1988, 46, 15–18. [Google Scholar] [CrossRef]
- Prudhommeaux, F.; Schiltz, C.; Lioté, F.; Hina, A.; Champy, R.; Bucki, B.; Ortiz-Bravo, E.; Meunier, A.; Rey, C.; Bardin, T. Variation in the inflammatory properties of basic Calcium Phosphate crystals according to crystal type. Arthritis Rheumatol. 1996, 39, 1319–1326. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Rel. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Aguado, E.; Pilet, P.; Daculsi, G. Macroporous biphasic calcium phosphate ceramics: Influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials 1998, 19, 133–139. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamamuro, T.; Nakamura, T.; Kotani, S.; Ohtsuki, C.; Kokubo, T. The bonding behavior of calcite to bone. J. Biomed. Mater. Res. 1991, 25, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Evans, M.E.; Jones, I.A.; Rudd, C.D.; Scotchford, C.A.; Walker, G.S. Preparation of poly(E-caprolactone)/continuous bioglass fibre composite using monomer transfer moulding for bone implant. Biomaterials 2005, 26, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, M.; Kay, J.F.; Kennenth, I.; Gumaer, K.I.; Doremus, R.H.; Drobeck, H.P. Tissue cellular and subcellular events at a bone-ceramic hydroxyapatite interface. J. Bioeng. 1977, 1, 79–92. [Google Scholar] [PubMed]
- Driskell, T.D.; Hassler, C.R.; Tennery, V.J.; McCoy, I.R.; Clarke, W.J. Calcium phosphate resorbable ceramic: A potential alternative for bone grafting. J. Dent. Res. 1973, 52, 123–131. [Google Scholar]
- Lutz-Christian, G.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar]
- Vallet-Regí, M.; Balas, F. Silica materials for medical applications. Open Biomed. Eng. J. 2008, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, N.A.; Alqap, A.S.F.; Sopyan, I. Recent progress on hydroxyapatite-based dense biomaterials for load bearing bone substitutes. Recent Pat. Mater. Sci. 2011, 4, 63–80. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Ruiz-González, L.; Doadrio, J.C.; González-Calbet, J.M.; Vallet-Regí, M. Tissue regeneration: A new property of mesoporous materials. Solid State Sci. 2005, 7, 983–989. [Google Scholar] [CrossRef]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R.P. Metals for Biomedical Applications. In Biomedical Engineering—From Theory to Applications; Fazel, R., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Heimke, G.; Griss, P.; Jentschura, G.; Werner, E. Direct anchorage of Al2O3-ceramic hip components: Three years of clinical experience and results of further animal studies. J. Biomed. Mater. Res. 1979, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ehrl, P.A.; Reuther, J.; Frenkel, G. Al2O3-ceramic as material for dental implants, experimental and clinical study for the development of screw- and extension-implants. Int. J. Oral Surg. 1981, 10, 93–98. [Google Scholar] [PubMed]
- Zweymüller, K.; Semlitsch, M. Concept and material properties of a cementless hip prosthesis system with Al2O3 ceramic ball heads and wrought Ti-6Al-4V stems. Arch. Orthop. Trauma. Surg. 1982, 100, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, G.; Lucioli, N.; Malagoli, A.; Calderoli, C.; Cassese, F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int. Orthop. 2011, 35, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Maccauro, G.; Iommetti, P.R.; Raffaelli, L.; Manicone, P.F. Alumina and Zirconia Ceramic for Orthopaedic and Dental Devices. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Rettig, R.; Virtanen, S. Time-dependent electrochemical characterization of the corrosion of a magnesium rare-earth alloy in simulated body fluids. J. Biomed. Mater. Res. A 2008, 85, 167–175. [Google Scholar] [CrossRef] [PubMed]
- De Aza, H.; Chevalier, J.; Fantozzi, G.; Schehl, M.; Torrecillas, R. Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramics for joint prostheses. Biomaterials 2002, 23, 937–945. [Google Scholar] [CrossRef]
- Gamal, G.A.; Al-Mufadi, F.A.; Said, A.H. Effect of iron additives on the microstructure of hydroxyapatite. ETASR Eng. Technol. Appl. Sci. Res. 2013, 3, 532–539. [Google Scholar]
- Habibovic, P.; Barrere, F.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Biomimetic hydroxyapatite coating on metal implants. J. Am. Ceram. Soc. 2002, 85, 517–522. [Google Scholar] [CrossRef]
- Kobayashi, T.; Itoh, S.; Nakamura, S.; Nakamura, M.; Shinomiya, K.; Yamashita, K. Enhanced bone bonding of hydroxyapatite-coated titanium implants by electrical polarization. J. Biomed. Mater. Res. A 2007, 82, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ramaswamy, Y.; Gale, D.; Yang, W.; Xiao, K.; Zhang, L.; Yin, Y.; Zreiqat, H. Novel sphene coatings on Ti–6Al–4V for orthopedic implants using sol–gel method. Acta Biomater. 2008, 4, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Ergun, C.; Webster, T.J.; Bizios, R.; Doremus, R.H. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. I. Structure and microstructure. J. Biomed. Mater. Res. 2002, 59, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Bizios, R. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. II. Mechanisms of osteoblast adhesion. J. Biomed. Mater. Res. 2002, 59, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.W.; Khor, K.A.; Cheang, P. In vitro studies of plasma-sprayed hydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid (SBF). Biomaterials 2003, 24, 1603–1611. [Google Scholar] [CrossRef]
- Rizzi, S.C.; Heath, D.J.; Coombes, A.G.; Bock, N.; Textor, M.; Downes, S. Biodegradable polymer/hydroxyapatite composites, surface analysis and initial attachment of human osteoblasts. J. Biomed. Mater. Res. 2001, 55, 475–486. [Google Scholar] [CrossRef]
- Furuzano, T.; Kisida, A.; Tanaka, J.; Matsuda, A. Hydroxyapatite Composite and Manufacturing Method Thereof, Medical Material Using Hydroxyapatite Complex. Patent US7473731, 2009. [Google Scholar]
- Verma, D.; Katti, K.; Katti, D. Bioactivity in in situ hydroxyapatite-polycaprolactone composites. J. Biomed. Mater. Res. A 2006, 78, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Developing bioactive composite materials for tissue replacement. Biomaterials 2003, 24, 2133–2151. [Google Scholar] [CrossRef]
- Jui, C.; Sanghamitra, B.; Kumar, S.M.; Debabrata, B. Process for the Preparation of Protein Mediated Calcium Hydroxyapatite (HAp) Coating on Metal Substrate. Patent US20,090,181,161, 2009. [Google Scholar]
- Zhang, Y.; Regeros, R.; Kim, J.-W. Bioactive Graded Zirconia Based Structure. Patent US20,090,118,114, 2009. [Google Scholar]
- Akao, M.; Aoki, H.; Kato, K. Mechanical properties of sintered hydroxyapatite for prosthetic applications. J. Mater. Sci. 1981, 16, 809–812. [Google Scholar] [CrossRef]
- Itatani, K.; Tsuchiya, K.; Sakka, Y.; Davies, I.J.; Koda, S. Superplastic deformation of hydroxyapatite ceramics with B2O3 or Na2O addition fabricated by pulse current pressure sintering. J. Eur. Ceram. Soc. 2011, 31, 2641–2648. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Nakamoto, S.; Miyashita, Y.; Ichino, R.; Okido, M. Osteoinductivity of HAp films with different surface morphologies coated by the thermal substrate method in aqueous solutions. Mater. Trans. 2006, 47, 1391–1394. [Google Scholar] [CrossRef]
- Sivaprasad, K.; Siva Rama Krishna, D.; Sampath Kumar, T.S. Microwave processing of functionally graded bioactive materials. Mater. Lett. 2003, 57, 2716–2721. [Google Scholar]
- Galea, L.; Alexeev, D.; Bohner, M.; Doebelin, N.; Studart, A.R.; Aneziris, C.G.; Graule, T. Textured and hierarchically structured calcium phosphate ceramic blocks through hydrothermal treatment. Biomaterials 2015, 67, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Galea, L.; Bohner, M.; Thuering, J.; Doebelin, N.; Ring, T.A.; Aneziris, C.G.; Graule, T. Growth kinetics of hexagonal sub-micrometric β-tricalcium phosphate particles in ethylene glycol. Acta Biomater. 2014, 10, 3922–3930. [Google Scholar] [CrossRef] [PubMed]
- Galea, L.; Bohner, M.; Thuering, J.; Doebelin, N.; Aneziris, C.G.; Graule, T. Control of the size, shape and composition of highly uniform, non-agglomerated, sub-micrometer β-tricalcium phosphate and dicalcium phosphate platelets. Biomaterials 2013, 34, 6388–6401. [Google Scholar] [CrossRef] [PubMed]
- Luz, G.M.; Mano, J.F. Biomimetic design of materials and biomaterials inspired by the structure of nacre. Phil. Trans. R. Soc. A 2009, 367, 1587–1605. [Google Scholar] [CrossRef] [PubMed]
- Finnemore, A.; Cunha, P.; Shean, T.; Vignolini, S.; Guldin, S.; Oyen, M.; Steiner, U. Biomimetic layer-by-layer assembly of artificial nacre. Nat. Commun. 2012, 3, 966. [Google Scholar] [CrossRef] [PubMed]
- Halouani, R.; Bernache-Assolant, D.; Champion, E.; Ababou, A. Microstructure and related mechanical properties of hot pressed hydroxyapatite ceramics. J. Mater. Sci. Mater. Med. 1994, 5, 563–568. [Google Scholar] [CrossRef]
- Viswanath, B.; Ravishankar, N. Interfacial reactions in hydroxyapatite/alumina nanocomposites. Scr. Mater. 2006, 55, 863–866. [Google Scholar] [CrossRef]
- Pezzotti, G.; Sakakura, S. Study of the toughening mechanisms in bone and biomimetic hydroxyapatite materials using Raman microprobe spectroscopy. J. Biomed. Mater. Res. A 2003, 65, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mobasherpour, I.; Hashjin, M.S.; Toosi, S.S.R.; Kamachali, R.D. Effect of the addition ZrO2-Al2O3 on nanocrystalline hydroxyapatite bending strength and fracture toughness. Ceram. Int. 2009, 35, 1569–1574. [Google Scholar] [CrossRef]
- Nath, S.; Biswas, K.; Basu, B. Phase stability and microstructure development in hydroxyapatite-mullite system. Scr. Mater. 2008, 58, 1054–1057. [Google Scholar] [CrossRef]
- Aminzare, M.; Eskandari, A.; Barooniand, M.H.; Berenov, A.; Razavi Hesabi, Z.; Taheri, M.; Sadrnezhaad, S.K. Hydroxyapatite nanocomposites: Synthesis, sintering and mechanical properties. Ceram. Int. 2013, 39, 2197–2206. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sudo, A. In vivo wear performance of highly cross-linked polyethylene vs. yttria stabilized zirconia and alumina stabilized zirconia at a mean seven-year follow-up. BMC Musculoskelet. Disord. 2013, 14, 154–160. [Google Scholar] [CrossRef] [PubMed]
- White, A.A.; Kinloch, I.A.; Windle, A.H.; Best, S.M. Optimization of the sintering atmosphere for high-density hydroxyapatite-carbon nanotube composites. J. R. Soc. Interface 2010, 7, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Gittings, J.P.; Bowen, C.R.; Turner, I.G.; Dent, A.C.E.; Baxter, F.R.; Chaudhuri, J.B. Dielectric properties of hydroxyapatite based ceramics. Acta Biomater. 2009, 5, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nagai, A.; Tanaka, Y.; Sekijima, Y.; Yamashita, K. Polarized hydroxyapatite promotes spread and motility of osteoblastic cells. J. Biomed. Mater. Res. A 2010, 92, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Tofail, S.A.M.; Baldisserri, C.; Haverty, D.; McMonagle, J.B.; Erhart, J. Pyroelectric surface charge in hydroxyapatite ceramics. J. Appl. Phys. 2009, 106, 106104–106107. [Google Scholar] [CrossRef]
- Yamashita, K.; Oikawa, N.; Umegaki, T. Acceleration and deceleration of bone like crystal growth on ceramic hydroxyapatite by electric poling. Chem. Mater. 1996, 8, 2697–2700. [Google Scholar] [CrossRef]
- Teng, N.C.; Nakamura, S.; Takagi, Y.; Yamashita, Y.; Ohgaki, M.; Yamashita, K. A new approach to enhancement of bone formation by electrically polarized hydroxyapatite. J. Dent. Res. 2001, 80, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nakamura, S.; Yamashita, K. Enhanced osteobonding by negative surface charges of electrically polarized hydroxyapatite. J. Biomed. Mater. Res. 2001, 57, 477–484. [Google Scholar] [CrossRef]
- Park, Y.J.; Hwang, K.S.; Song, J.E.; Ong, J.L.; Rawls, H.R. Growth of calcium phosphate on poling treated ferroelectric BaTiO3 ceramics. Biomaterials 2002, 23, 3859–3864. [Google Scholar] [CrossRef]
- Nakamura, S.; Kobayashi, T.; Yamashita, K. Extended bioactivity in the proximity of hydroxyapatite ceramic surfaces induced by polarization charges. J. Biomed. Mater. Res. 2002, 61, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Itoh, S.; Tanaka, Y.; Nagai, A.; Yamashita, K. Comparison of enhancement of bone ingrowth into hydroxyapatite ceramics with highly and poorly interconnected pores by electrical polarization. Acta Biomater. 2009, 5, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Niwa, K.; Nakamura, S.; Sekijima, Y.; Yamashita, K. Interaction of a blood coagulation factor on electrically polarized hydroxyapatite surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82B, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Tanaka, Y.; Nakamura, M.; Nagai, A.; Hashimoto, K.; Toda, Y.; Katayama, K.; Yamashita, K. Rate of bonelike apatite formation accelerated on polarized porous hydroxyapatite. J. Am. Ceram. Soc. 2008, 91, 3943–3949. [Google Scholar] [CrossRef]
- Itoh, S.; Nakamura, S.; Kobayashi, T.; Shinomiya, K.; Yamashita, K. Enhanced bone ingrowth into hydroxyapatite with interconnected pores by electrical polarization. Biomaterials 2006, 27, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K. Enhanced bioactivity of electrically poled hydroxyapatite ceramics and coatings. Mater. Sci. Forum 2003, 426–432, 3237–3242. [Google Scholar] [CrossRef]
- Sayer, M.; Stratilatov, A.D.; Reid, J.W.; Calderin, L.; Stott, M.J.; Yin, X.; MacKenzie, M.; Smith, T.J.N.; Hendry, J.A.; Langstaff, S.D. Structure and composition of silicon-stabilized tricalcium phosphate. Biomaterials 2003, 24, 369–382. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, Z.; Li, Y.; Zhang, Z.; de Bruijn, J.D.; de Groot, K. Osteoinduction by calcium phosphate biomaterials. J. Mater. Sci. Mater. Med. 1998, 9, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kobayashi, T.; Yamashita, K. Highly orientated calcification in newly formed bones on negatively charged hydroxyapatite electrets. Key Eng. Mater. 2005, 284–286, 897–900. [Google Scholar] [CrossRef]
- Boyde, A.; Corsi, A.; Quarto, R.; Cancedda, R.; Bianco, P. Osteoconduction in large macroporous hydroxyapatite ceramic implants: Evidence for a complementary integration and disintegration mechanism. Bone 1999, 24, 579–589. [Google Scholar] [CrossRef]
- Hwang, K.S.; Song, J.E.; Jo, J.W.; Yang, H.S.; Park, Y.J.; Ong, J.L.; Rawls, H.R. Effect of poling conditions on growth of calcium phosphate crystal in ferroelectric BaTiO3 ceramics. J. Mater. Sci. Mater. Med. 2002, 13, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Sadanaga, E.; Hirose, T. Dependence of the crystal structure on particle size in barium titanate. J. Am. Ceram. Soc. 1989, 72, 1555–1558. [Google Scholar] [CrossRef]
- Kerman, K.; Abazari, M.; Marandian-Hagh, N.; Akdogan, E.K. Lead free (K,Na)NbO3-based piezoelectric ceramics and transducers. IEEE Appl. Ferroelectr. 2008, 3, 1–3. [Google Scholar]
- Xu, J.Y.; Fan, S.J.; Xu, X.W. Ferroelectric potassium lithium niobate crystals grown by the vertical Bridgman method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2001, 85, 50–54. [Google Scholar] [CrossRef]
- Dubey, A.K.; Anumol, E.A.; Balani, K.; Basu, B. Multifunctional properties of multistage spark plasma sintered HA–BaTiO3-based piezobiocomposites for bone replacement applications. J. Am. Ceram. Soc. 2013, 96, 3753–3759. [Google Scholar] [CrossRef]
- Wang, J.; Shaw, L.L. Transparent nanocrystalline hydroxyapatite by pressure-assisted sintering. Scr. Mater. 2010, 63, 593–596. [Google Scholar] [CrossRef]
- Dubey, A.K.; Basu, B.; Balani, K.; Guo, R.; Bhalla, A.S. Dielectric and pyroelectric properties of HAp-BaTiO3 composites. Ferroelectrics 2011, 423, 63–76. [Google Scholar] [CrossRef]
- Suárez, M.; Fernández, A.; Torrecillas, R.; Menéndez, J.L. Sintering to Transparency of Polycrystalline Ceramic Materials. In Sintering of Ceramics—New Emerging Techniques; Lakshmanan, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 527–552. [Google Scholar]
- Kanzaki, N.; Onuma, K.; Ito, A.; Teraoka, K.; Tateishi, T.; Tsutsumi, S. Direct growth rate measurement of hydroxyapatite single crystal by moire phase shift interferometry. J. Phys. Chem. B 1998, 102, 6471–6476. [Google Scholar] [CrossRef]
- Gupta, T.K.; Lange, F.F.; Bechtold, J.H. Effect of stress-induced phase transformation on the properties of polycrystalline zirconia containing metastable tetragonal phase. J. Mater. Sci. 1978, 13, 1464–1470. [Google Scholar] [CrossRef]
- Ioku, K.J. Tailored bioceramics of calcium phosphates for regenerative medicine. Ceram. Soc. Jpn. 2010, 118, 775–783. [Google Scholar] [CrossRef]
- Klimke, J.; Trunec, M.; Krell, A. Transparent tetragonal yttria-stabilized zirconia ceramics: Influence of scattering caused by birefringence. J. Am. Ceram. Soc. 2011, 94, 1850–1858. [Google Scholar] [CrossRef]
- Fang, Y.; Agrawal, D.K.; Roy, D.M.; Roy, R. Fabrication of transparent hydroxyapatite ceramics by ambient-pressure sintering. Mater. Lett. 1995, 23, 147–151. [Google Scholar] [CrossRef]
- Damestani, Y.; Reynolds, C.L.; Szu, J.; Hsu, M.S.; Kodera, Y.; Binder, D.K.; Park, B.H.; Garay, J.E.; Rao, M.P.; Aguilar, G. Transparent nanocrystalline yttria-stabilized-zirconia calvarium prosthesis. Nanomed. Nanotechnol. Boil. 2013, 9, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Thamaraiselvi, T.V.; Rajeswari, S. Biological evaluation of bioceramic materials—A review. Trends Biomater. Artif. Organs 2004, 18, 9–17. [Google Scholar]
- Kotobuki, N.; Ioku, K.; Kawagoe, D.; Fujimori, H.; Goto, S.; Ohgushi, H. Observation of osteogenic differentiation cascade of living mesenchymal stem cells on transparent hydroxyapatite ceramics. Biomaterials 2005, 26, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ikoma, T.; Monkawa, A.; Suetsugu, Y.; Yamada, H.; Tanaka, J.; Moriyoshi, Y. Fabrication of transparent hydroxyapatite sintered body with high crystal orientation by pulse electric current sintering. J. Am. Ceram. Soc. 2005, 88, 243–245. [Google Scholar] [CrossRef]
- Varma, H.; Vijayan, S.P.; Babu, S.S. Transparent hydroxyapatite ceramics through gelcasting and low-temperature sintering. J. Am. Ceram. Soc. 2002, 85, 493–495. [Google Scholar] [CrossRef]
- Takikawa, K.; Akao, M. Fabrication of transparent hydroxyapatite and application to bone marrow derived cell/hydroxyapatite interaction observation in vivo. J. Mater. Sci. Mater. Med. 1996, 7, 439–445. [Google Scholar] [CrossRef]
- Boilet, L.; Descamps, M.; Rguiti, E.; Tricoteaux, A.; Lu, J.; Petit, F.; Lardot, V.; Cambier, F.; Leriche, A. Processing and properties of transparent hydroxyapatite and β tricalcium phosphate obtained by HIP process. Ceram. Int. 2013, 39, 283–288. [Google Scholar] [CrossRef]
- Ahn, E.S.; Gleason, N.J.; Nakahira, A.; Ying, J.Y. Nanostructure processing of hydroxyapatite-based bioceramics. Nano Lett. 2001, 1, 149–153. [Google Scholar] [CrossRef]
- Kotobuki, N.; Kawagoe, D.; Fujimori, H.; Goto, S.; Ioku, K.; Ohgushi, H. In vitro osteogenic activity of rat bone marrow derived mesenchymal stem cells cultured on transparent hydroxyapatite ceramics. Key Eng. Mater. 2003, 254–256, 1055–1058. [Google Scholar] [CrossRef]
- Tan, N.; Kou, Z.; Ding, Y.; Leng, Y.; Liu, C.; He, D. Novel substantial reductions in sintering temperatures for preparation of transparent hydroxyapatite bioceramics under ultrahigh pressure. Scr. Mater. 2011, 65, 819–822. [Google Scholar] [CrossRef]
- Kawagoe, D.; Koga, Y.; Ishida, E.H.; Kotobuki, N.; Ohgushi, H.; Ioku, K. Preparation of transparent hydroxyapatite ceramics by spark plasma sintering and cell culture test. Phosphorus Res. Bull. 2006, 20, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Barralet, J.E.; Fleming, G.J.P.; Campion, C.; Harris, J.J.; Wright, A.J. Formation of translucent hydroxyapatite ceramics by sintering in carbon dioxide atmospheres. J. Mater. Sci. 2003, 38, 3979–3993. [Google Scholar] [CrossRef]
- Okada, M.; Furuzono, T. Low-temperature synthesis of nanoparticle-assembled, transparent, and low-crystallized hydroxyapatite blocks. J. Colloid Interface Sci. 2011, 360, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, B.; Gawronski, J.; Blaszczyk, T. Effect of carbon interlayer on protective properties of hydroxyapatite coating deposited on 316L stainless steel by sol-gel method. Powder Metall. Metal. Ceram. 2010, 49, 468–473. [Google Scholar] [CrossRef]
- Marcelo, T.M.; Livramento, V.; Oliveira, M.V.; Carvalho, M.H. Microstructural characterization and interactions in Ti- and TiH2-hydroxyapatite vacuum sintered composites. Mater. Res. 2006, 9, 65–71. [Google Scholar] [CrossRef]
- Yun, J.; Son, H.; Prajatelistia, E.; Han, Y.-H.; Kim, S.; Kim, B.-N. Characterisation of transparent hydroxyapatite nanoceramics prepared by spark plasma sintering. Adv. Appl. Ceram. 2014, 113, 67–72. [Google Scholar] [CrossRef]
- Kim, B.-N.; Prajatelistia, E.; Han, Y.-H.; Son, H.W.; Sakka, Y.; Kim, S. Transparent hydroxyapatite ceramics consolidated by spark plasma sintering. Scr. Mater. 2013, 69, 366–369. [Google Scholar] [CrossRef]
- Majling, J.; Kremničan, V.; Ďurovčíkova, R.; Svetík, Š. Sintering of hydroxyapatite ceramics, with the aid of optical transmittance—Temperature spectra. J. Therm. Anal. Calorim. 1999, 57, 587–590. [Google Scholar] [CrossRef]
- Agrawal, D.K.; Fang, Y.; Roy, D.M.; Roy, R. Fabrication of hydroxyapatite ceramics by microwave processing. MRS Proc. 1992, 269. [Google Scholar] [CrossRef]
- Cuccu, A.; Montinaro, S.; Orrù, R.; Cao, G.; Bellucci, D.; Sola, A.; Cannillo, V. Consolidation of different hydroxyapatite powders by SPS: Optimization of the sintering conditions and characterization of the obtained bulk products. Ceram. Int. 2015, 41, 725–736. [Google Scholar] [CrossRef]
- Munir, Z.A.; Quach, D.V.; Ohyanagi, M. Sintering, Mechanisms of Convention Nanodensification and Field Assisted Processes; Castro, R., van Benthem, K., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 2012; Volume 7, pp. 137–158. [Google Scholar]
- Suárez, M.; Fernández, A.; Menéndez, J.L.; Torrecillas, R.; Kessel, H.U.; Hennicke, J.; Kirchner, R.; Kessel, T. Sintering Applications; Ertu, B., Ed.; InTech: Rijeka, Croatia, 2013; Volume 13, pp. 319–342. [Google Scholar]
- Gentilman, R. Polycrystalline material for laser applications. In Proceedings of the 46th Army Sagamore Materials Research Conference, St. Michaels, MD, USA, 9–12 May 2005.
- Quarles, G.J.; Castillo, V.K.; Dumm, J.Q.; Messing, S.G.; Lee, L.-H. Comparison of optical, mechanical and thermo-optical properties of oxide polycrystalline laser gain materials with single crystals. In Proceedings of the Frontiers in Optics, OSA Technical Digest (CD), Optical Society of America, Rochester, NY, USA, 2006.
- Ikesue, A.; Aung, Y.L. Ceramic laser materials. Nat. Photonics 2008, 2, 721–727. [Google Scholar] [CrossRef]
- Maca, K.; Trunec, M.; Chmelik, R. Processing and properties of fine-grained processing and properties of fine grained transparent MgAl2O4 ceramics. Ceram. Silik. 2007, 51, 94–97. [Google Scholar]
- Jarcho, M. Retrospective Analysis of hydroxyapatite development for oral implant applications. In The Dental Clinics of North America, Hydroxyapatite-Coated Implants; Sendax, V.I., Ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 1992; Volume 36. [Google Scholar]
- Fang, Y.; Agrawal, DK.; Roy, D.M.; Roy, R. Microwave sintering of hydroxyapatite ceramics. J. Mater. Res. 1994, 9, 180–187. [Google Scholar] [CrossRef]
- Li, S.; Izui, H.; Okano, M.; Watanabe, T. The Effects of Sintering Temperature and Pressure on the Sintering Behavior of Hydroxyapatite Powder Prepared by Spark Plasma Sintering. J. Biomech. Sci. Eng. 2008, 3, 1–12. [Google Scholar] [CrossRef]
- Nakahira, A.; Tamai, M.; Eguchi, K.; Nakamura, S.; Yamashita, K. Preparation and evaluation of dense hydroxyapatite by PECS method. Key Eng. Mater. 2003, 240–242, 551–554. [Google Scholar] [CrossRef]
- Majling, J.; Znaik, P.; Palova, A.; Svetik, S. Sintering of the ultrahigh pressure densified hydroxyapatite monolithic xerogels. J. Mater. Res. 1997, 12, 198–202. [Google Scholar] [CrossRef]
- Benaqqa, C.; Chevalier, J.; Saädaoui, M.; Fantozzi, G. Slow crack growth behaviour of hydroxyapatite ceramics. Biomaterials 2005, 26, 6106–6112. [Google Scholar] [CrossRef] [PubMed]
- Ioku, K.; Yamamoto, K.; Yanagisawa, K.; Yamasaki, N. Low temperature sintering of hydroxyapatite by hydrothermal hot pressing. Phosphorus Res. Bull. 1994, 4, 65–70. [Google Scholar] [CrossRef]
- Gandhi, A.A.; Gunning, R.D.; Ryan, K.M.; Tofail, S.A.M. The role of texturing and densification on optical transmittance of hydroxyapatite ceramics. J. Am. Ceram. Soc. 2010, 93, 3773–3777. [Google Scholar] [CrossRef]
- Uehira, M.; Okada, M.; Takeda, S.; Matsumoto, N. Preparation and characterization of low-crystallized hydroxyapatite nanoporous plates and granules. Appl. Surf. Sci. 2013, 287, 195–202. [Google Scholar] [CrossRef]
- Zhong, L.; Khor, K.A. Transparent hydroxyapatite obtained through spark plasma sintering, optical and mechanical properties. Key Eng. Mater. 2014, 631, 51–56. [Google Scholar] [CrossRef]
- Aoki, H. Medical Applications of Hydroxyapatite; Ishiyaku EuroAmerica, Inc.: Tokyo, Japan; St. Louis, MO, USA, 1994. [Google Scholar]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Pintar, F.A.; Yoganandan, N.; Myers, T.; Elhagediab, A.; Sances, A., Jr. Biomechanical properties of human lumbar spine ligaments. J. Biomech. 1992, 25, 1351–1356. [Google Scholar] [CrossRef]
- Currey, J.D. Mechanical properties of bone tissues with greatly differing functions. J. Biomech. 1979, 12, 313–319. [Google Scholar] [CrossRef]
- Rigaldie, Y.; Lemagnen, G.; Largeteau, A.; Larrouture, D.; Abba, M.; Durandeau, C.; Vallayer, B.; Grislain, L.; Demazeau, G. High hydrostatic pressure (HHP): An alternative method of sterilisation and decontamination of fragile drugs? Eur. J. Parenter. Sci. 2001, 6, 73–78. [Google Scholar]
- Rigaldie, Y.; Largeteau, A.; Lemagnen, G.; Ibalot, F.; Pardon, P.; Demazeau, G.; Grislain, L. Effects of high hydrostatic pressure on several sensitive therapeutic molecules and a soft nanodispersed drug delivery system. Pharm. Res. 2003, 20, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. J. Funct. Biomater. 2015, 6, 1099-1140. https://doi.org/10.3390/jfb6041099
Prakasam M, Locs J, Salma-Ancane K, Loca D, Largeteau A, Berzina-Cimdina L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. Journal of Functional Biomaterials. 2015; 6(4):1099-1140. https://doi.org/10.3390/jfb6041099
Chicago/Turabian StylePrakasam, Mythili, Janis Locs, Kristine Salma-Ancane, Dagnija Loca, Alain Largeteau, and Liga Berzina-Cimdina. 2015. "Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review" Journal of Functional Biomaterials 6, no. 4: 1099-1140. https://doi.org/10.3390/jfb6041099
APA StylePrakasam, M., Locs, J., Salma-Ancane, K., Loca, D., Largeteau, A., & Berzina-Cimdina, L. (2015). Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. Journal of Functional Biomaterials, 6(4), 1099-1140. https://doi.org/10.3390/jfb6041099







