Extracellular Matrix Molecules Facilitating Vascular Biointegration
Abstract
:1. Introduction
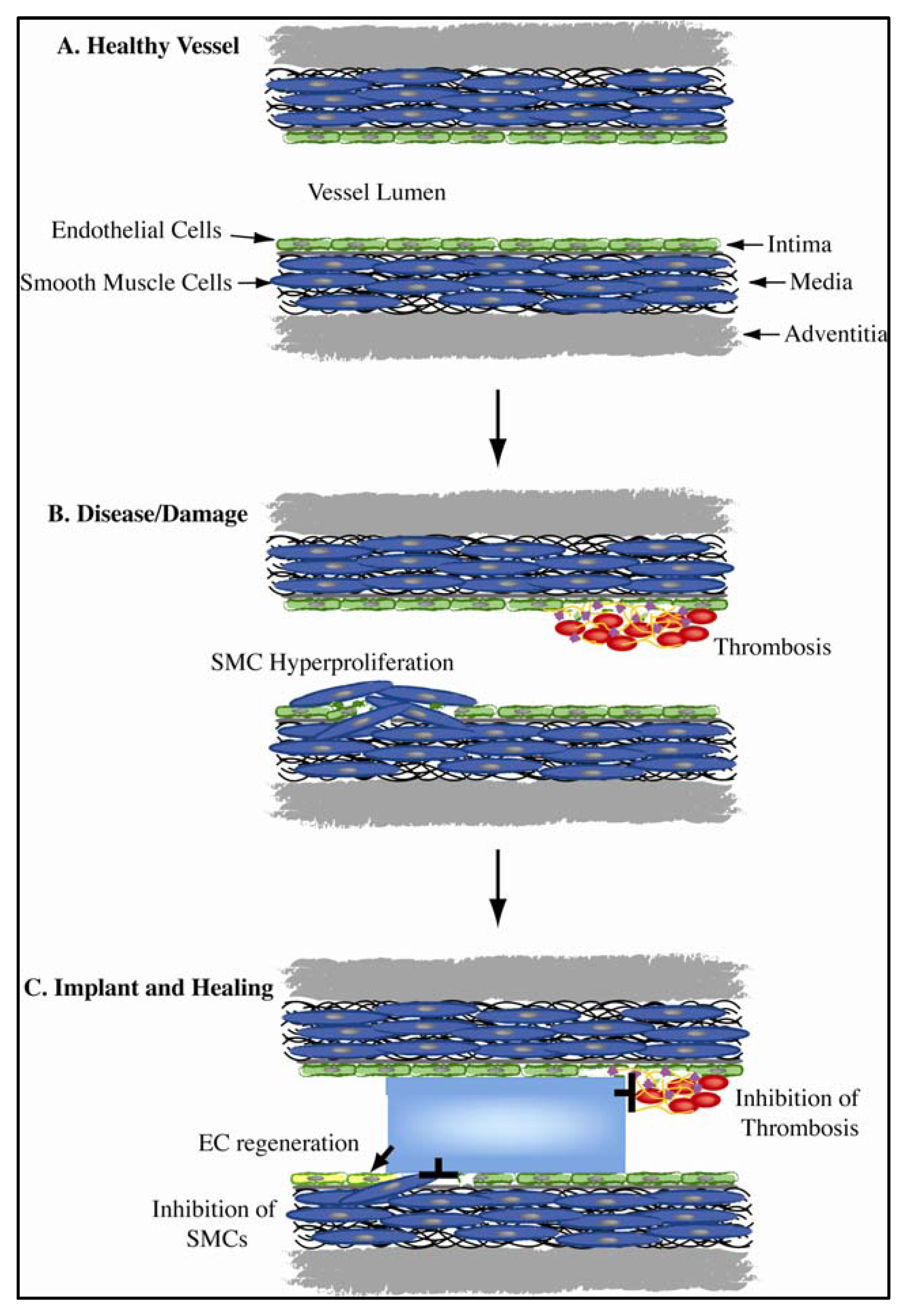
1.1. Vascular Biomimicry
1.2. Candidate Biomolecules from the Vessel Wall

2. Candidate Biomolecules
2.1. Fibrillin-1
2.2. Fibulin-5
2.3. Perlecan
2.4. Tropoelastin
3. Conclusions and Future Prospects
| Candidate | Effect on ECs | Effect on SMCs | Blood Compatibility | Translation to Date |
|---|---|---|---|---|
| Fibrillin-1 | Proliferation, enhanced | Proliferation, inhibited | Not yet tested | Enhanced fibroblast attachment to PU scaffold |
| Fibulin-5 | Attachment enhanced; apoptosis reduced | Proliferation, inhibited | Not yet tested | No translation |
| Tropoelastin | Attachment, proliferation, enhanced | Proliferation, inhibited | Hemocompatible; minimal activation of platelets | Improves EC binding, growth on steel; reduces thrombogenicity of catheters, ePTFE grafts |
| Perlecan | Proliferation, enhanced | Proliferation, hyperplasia (rat model) inhibited | Direct inhibition of thrombosis | Less thrombus present on ePTFE grafts, greater ECs; Stents show less neointima |
Conflict of Interest
Acknowledgments
References
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Hamilton, G.; Seifalian, A.M. Current status of prosthetic bypass grafts: A review. J. Biomed. Mater. Res. B 2005, 74, 570–581. [Google Scholar]
- Devine, C.; Hons, B.; McCollum, C. Heparin-bonded dacron or polytetrafluoroethylene for femoropopliteal bypass grafting: A multicenter trial. J. Vasc. Surg. 2001, 33, 533–539. [Google Scholar] [CrossRef]
- Devine, C.; McCollum, C. Heparin-bonded dacron or polytetrafluorethylene for femoropopliteal bypass: Five-year results of a prospective randomized multicenter clinical trial. J. Vasc. Surg. 2004, 40, 924–931. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Linde, T.; Krapiec, F.; Hamilton, K.; Steinseifer, U.; van Ryn, J.; Raaz, U.; Buerke, M.; Werdan, K.; Schlitt, A. In vitro comparison of dabigatran, unfractionated heparin, and low-molecular-weight heparin in preventing thrombus formation on mechanical heart valves. Thromb. Res. 2010, 126, e196–e200. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Baim, D.S.; Ho, K.K.; Popma, J.J.; Lansky, A.J.; Cohen, D.J.; Carrozza, J.P., Jr.; Chauhan, M.S.; Rodriguez, O.; Kuntz, R.E. Stent thrombosis in the modern era: A pooled analysis of multicenter coronary stent clinical trials. Circulation 2001, 103, 1967–1971. [Google Scholar]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 2006, 48, 193–202. [Google Scholar] [CrossRef]
- Lemesle, G.; Delhaye, C.; Bonello, L.; de Labriolle, A.; Waksman, R.; Pichard, A. Stent thrombosis in 2008: Definition, predictors, prognosis and treatment. Arch. Cardiovasc. Dis. 2008, 101, 769–777. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef]
- Daemen, J.; Wenaweser, P.; Tsuchida, K.; Abrecht, L.; Vaina, S.; Morger, C.; Kukreja, N.; Juni, P.; Sianos, G.; Hellige, G.; et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: Data from a large two-institutional cohort study. Lancet 2007, 369, 667–678. [Google Scholar]
- Kedhi, E.; Joesoef, K.S.; McFadden, E.; Wassing, J.; van Mieghem, C.; Goedhart, D.; Smits, P.C. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (compare): A randomised trial. Lancet 2010, 375, 201–209. [Google Scholar]
- Pendyala, L.; Jabara, R.; Robinson, K.; Chronos, N. Passive and active polymer coatings for intracoronary stents: Novel devices to promote arterial healing. J. Int. Cardiol. 2009, 22, 37–48. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar]
- Campbell, J.H.; Efendy, J.L.; Campbell, G.R. Novel vascular graft grown within recipient’s own peritoneal cavity. Circ. Res. 1999, 85, 1173–1178. [Google Scholar]
- Yin, Y.; Wise, S.G.; Nosworthy, N.J.; Waterhouse, A.; Bax, D.V.; Youssef, H.; Byrom, M.J.; Bilek, M.M.; McKenzie, D.R.; Weiss, A.S.; et al. Covalent immobilisation of tropoelastin on a plasma deposited interface for enhancement of endothelialisation on metal surfaces. Biomaterials 2009, 30, 1675–1681. [Google Scholar] [CrossRef]
- Bennett, J.S.; Berger, B.W.; Billings, P.C. The structure and function of platelet integrins. J. Thromb. Haem. 2009, 7, 200–205. [Google Scholar] [CrossRef]
- Vyas, S.P.; Vaidya, B. Targeted delivery of thrombolytic agents: Role of integrin receptors. Expert Opin. Drug Deliv. 2009, 6, 499–508. [Google Scholar] [CrossRef]
- Maurer, L.M.; Tomasini-Johansson, B.R.; Mosher, D.F. Emerging roles of fibronectin in thrombosis. Thromb. Res. 2010, 125, 287–291. [Google Scholar] [CrossRef]
- Dufourcq, P.; Couffinhal, T.; Alzieu, P.; Daret, D.; Moreau, C.; Duplaa, C.; Bonnet, J. Vitronectin is upregulated after vascular injury and vitronectin blockade prevents neointima formation. Card. Res. 2002, 53, 952–962. [Google Scholar] [CrossRef]
- Nelson, P.R.; Yamamura, S.; Kent, K.C. Extracellular matrix proteins are potent agonists of human smooth muscle cell migration. J. Vasc. Surg. 1996, 24, 25–33. [Google Scholar] [CrossRef]
- Kushwaha, M.; Anderson, J.M.; Bosworth, C.A.; Andukuri, A.; Minor, W.P.; Lancaster, J.R., Jr.; Anderson, P.G.; Brott, B.C.; Jun, H.W. A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials 2010, 31, 1502–1508. [Google Scholar]
- De Mel, A.; Jell, G.; Stevens, M.M.; Seifalian, A.M. Biofunctionalization of biomaterials for accelerated in situ endothelialization: A review. Biomacromolecules 2008, 9, 2969–2979. [Google Scholar] [CrossRef]
- Lewis, A.L. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Coll. Surf. B Bioint. 2000, 18, 261–275. [Google Scholar] [CrossRef]
- Whelan, D.M.; van der Giessen, W.J.; Krabbendam, S.C.; van Vliet, E.A.; Verdouw, P.D.; Serruys, P.W.; van Beusekom, H.M. Biocompatibility of phosphorylcholine coated stents in normal porcine coronary arteries. Heart 2000, 83, 338–345. [Google Scholar] [CrossRef]
- Adams, J.C.; Watt, F.M. Regulation of development and differentiation by the extracellular matrix. Development 1993, 117, 1183–1198. [Google Scholar]
- Mecham, R. Overview of extracellular matrix. Curr. Prot. Cell Biol. 1998, 10, 1–14. [Google Scholar] [CrossRef]
- Juliano, R.L.; Haskill, S. Signal transduction from the extracellular matrix. J. Cell Biol. 1993, 120, 577–585. [Google Scholar] [CrossRef]
- Stephan, S.; Ball, S.G.; Williamson, M.; Bax, D.V.; Lomas, A.; Shuttleworth, C.A.; Kielty, C.M. Cell-matrix biology in vascular tissue engineering. J. Aant. 2006, 209, 495–502. [Google Scholar]
- Williamson, M.R.; Shuttleworth, A.; Canfield, A.E.; Black, R.A.; Kielty, C.M. The role of endothelial cell attachment to elastic fibre molecules in the enhancement of monolayer formation and retention, and the inhibition of smooth muscle cell recruitment. Biomaterials 2007, 28, 5307–5318. [Google Scholar] [CrossRef]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Knox, S.M.; Whitelock, J.M. Perlecan: How does one molecule do so many things? Cell. Mol. Life Sci. 2006, 63, 2435–2445. [Google Scholar] [CrossRef]
- Nugent, M.A.; Nugent, H.M.; Iozzo, R.V.; Sanchack, K.; Edelman, E.R. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc. Natl. Acad. Sci. USA 2000, 97, 6722–6727. [Google Scholar]
- Robb, B.W.; Wachi, H.; Schaub, T.; Mecham, R.P.; Davis, E.C. Characterization of an in vitro model of elastic fiber assembly. Mol. Biol. Cell 1999, 10, 3595–3605. [Google Scholar]
- Mithieux, S.M.; Weiss, A.S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. [Google Scholar]
- Kielty, C.M.; Shuttleworth, C.A. Microfibrillar elements of the dermal matrix. Microsc. Res. Tech. 1997, 38, 413–427. [Google Scholar] [CrossRef]
- Davis, E.C.; Roth, R.A.; Heuser, J.E.; Mecham, R.P. Ultrastructural properties of ciliary zonule microfibrils. J. Struct. Biol. 2002, 139, 65–75. [Google Scholar] [CrossRef]
- Sabatier, L.; Miosge, N.; Hubmacher, D.; Lin, G.; Davis, E.C.; Reinhardt, D.P. Fibrillin-3 expression in human development. Matrix Biol. 2011, 30, 43–52. [Google Scholar] [CrossRef]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J. Cell Sci. 2002, 115, 2817–2828. [Google Scholar]
- Amadeu, T.P.; Braune, A.S.; Porto, L.C.; Desmouliere, A.; Costa, A.M. Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen. 2004, 12, 169–174. [Google Scholar] [CrossRef]
- Rock, M.J.; Cain, S.A.; Freeman, L.J.; Morgan, A.; Mellody, K.; Marson, A.; Shuttleworth, C.A.; Weiss, A.S.; Kielty, C.M. Molecular basis of elastic fiber formation: Critical interactions and a tropoelastin-fibrillin-1 cross-link. J. Biol. Chem. 2004, 279, 23748–23758. [Google Scholar]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J. Cell Sci. 2002, 115, 2817–2828. [Google Scholar]
- Dietz, H.C.; Cutting, G.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991, 352, 337–339. [Google Scholar]
- Charbonneau, N.L.; Carlson, E.J.; Tufa, S.; Sengle, G.; Manalo, E.C.; Carlberg, V.M.; Ramirez, F.; Keene, D.R.; Sakai, L.Y. In vivo studies of mutant fibrillin-1 microfibrils. J. Biol. Chem. 2010, 285, 24943–24955. [Google Scholar]
- Massam-Wu, T.; Chiu, M.; Choudhury, R.; Chaudhry, S.S.; Baldwin, A.K.; McGovern, A.; Baldock, C.; Shuttleworth, C.A.; Kielty, C.M. Assembly of fibrillin microfibrils governs extracellular deposition of latent tgf beta. J. Cell. Sci. 2010, 123, 3006–3018. [Google Scholar] [CrossRef]
- Rock, M.J.; Cain, S.A.; Freeman, L.J.; Morgan, A.; Mellody, K.; Marson, A.; Shuttleworth, C.A.; Weiss, A.S.; Kielty, C.M. Molecular basis of elastic fiber formation: Critical interactions and a tropoelastin-fibrillin-1 cross-link. J. Biol. Chem. 2004, 279, 23748–23758. [Google Scholar]
- Bax, D.V.; Mahalingam, Y.; Cain, S.; Mellody, K.; Freeman, L.; Younger, K.; Shuttleworth, C.A.; Humphries, M.J.; Couchman, J.R.; Kielty, C.M. Cell adhesion to fibrillin-1: Identification of an arg-gly-asp-dependent synergy region and a heparin-binding site that regulates focal adhesion formation. J. Cell. Sci. 2007, 120, 1383–1392. [Google Scholar] [CrossRef]
- Bax, D.V.; Bernard, S.E.; Lomas, A.; Morgan, A.; Humphries, J.; Shuttleworth, C.A.; Humphries, M.J.; Kielty, C.M. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J. Biol. Chem. 2003, 278, 34605–34616. [Google Scholar]
- Mariko, B.; Ghandour, Z.; Raveaud, S.; Quentin, M.; Usson, Y.; Verdetti, J.; Huber, P.; Kielty, C.; Faury, G. Microfibrils and fibrillin-1 induce integrin-mediated signaling, proliferation and migration in human endothelial cells. Am. J. Phys. Cell Phys. 2010, 299, C977–C987. [Google Scholar] [CrossRef]
- Carta, L.; Pereira, L.; Arteaga-Solis, E.; Lee-Arteaga, S.Y.; Lenart, B.; Starcher, B.; Merkel, C.A.; Sukoyan, M.; Kerkis, A.; Hazeki, N.; et al. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 2006, 281, 8016–8023. [Google Scholar]
- Bunton, T.E.; Biery, N.J.; Myers, L.; Gayraud, B.; Ramirez, F.; Dietz, H.C. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of marfan syndrome. Circ. Res. 2001, 88, 37–43. [Google Scholar]
- Nataatmadja, M.; West, M.; West, J.; Summers, K.; Walker, P.; Nagata, M.; Watanabe, T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation 2003, 108 Suppl. 1, II329–II334. [Google Scholar]
- Baumgartner, H.R.; Muggli, R.; Tschopp, T.B.; Turitto, V.T. Platelet adhesion, release and aggregation in flowing blood: Effects of surface properties and platelet function. Thromb. Haem. 1976, 35, 124–138. [Google Scholar]
- Legrand, Y.; Karniguian, A.; Fauvel, F.; Gutman, N. The molecular interaction between platelet and vascular wall. Blood Cells 1983, 9, 263–274. [Google Scholar]
- Zheng, W.; Wang, Z.; Song, L.; Zhao, Q.; Zhang, J.; Li, D.; Wang, S.; Han, J.; Zheng, X.L.; Yang, Z.; et al. Endothelialization and patency of rgd-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials 2012, 33, 2880–2891. [Google Scholar]
- Jozwiak, A.B.; Kielty, C.M.; Black, R.A. Surface functionalization of polyurethane for the immobilization of bioactive moieties on tissue scaffolds. J. Mater. Chem. 2008, 18, 2240–2248. [Google Scholar]
- Yanagisawa, H.; Davis, E.C. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int. J. Biochem. Cell Biol. 2010, 42, 1084–1093. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Davis, E.C.; Starcher, B.C.; Ouchi, T.; Yanagisawa, M.; Richardson, J.A.; Olson, E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 2002, 415, 168–171. [Google Scholar]
- Lomas, A.C.; Mellody, K.T.; Freeman, L.J.; Bax, D.V.; Shuttleworth, C.A.; Kielty, C.M. Fibulin-5 binds human smooth-muscle cells through alpha5beta1 and alpha4beta1 integrins, but does not support receptor activation. Biochem. J. 2007, 405, 417–428. [Google Scholar] [CrossRef]
- Freeman, L.J.; Lomas, A.; Hodson, N.; Sherratt, M.J.; Mellody, K.T.; Weiss, A.S.; Shuttleworth, A.; Kielty, C.M. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem. J. 2005, 388, 1–5. [Google Scholar]
- Spencer, J.A.; Hacker, S.L.; Davis, E.C.; Mecham, R.P.; Knutsen, R.H.; Li, D.Y.; Gerard, R.D.; Richardson, J.A.; Olson, E.N.; Yanagisawa, H. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc. Natl. Acad. Sci. USA 2005, 102, 2946–2951. [Google Scholar]
- Chapman, S.L.; Sicot, F.X.; Davis, E.C.; Huang, J.; Sasaki, T.; Chu, M.L.; Yanagisawa, H. Fibulin-2 and fibulin-5 cooperatively function to form the internal elastic lamina and protect from vascular injury. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 68–74. [Google Scholar]
- Yanagisawa, H.; Schluterman, M.K.; Brekken, R.A. Fibulin-5, an integrin-binding matricellular protein: Its function in development and disease. J. Cell Commun. Sig. 2009, 3, 337–347. [Google Scholar] [CrossRef]
- Nakamura, T.; Ruiz-Lozano, P.; Lindner, V.; Yabe, D.; Taniwaki, M.; Furukawa, Y.; Kobuke, K.; Tashiro, K.; Lu, Z.; Andon, N.L.; et al. Dance, a novel secreted rgd protein expressed in developing, atherosclerotic, and balloon-injured arteries. J. Biol. Chem. 1999, 274, 22476–22483. [Google Scholar]
- Preis, M.; Cohen, T.; Sarnatzki, Y.; Ben Yosef, Y.; Schneiderman, J.; Gluzman, Z.; Koren, B.; Lewis, B.S.; Shaul, Y.; Flugelman, M.Y. Effects of fibulin-5 on attachment, adhesion, and proliferation of primary human endothelial cells. Biochem. Biophys. Res. Commun. 2006, 348, 1024–1033. [Google Scholar]
- Guadall, A.; Orriols, M.; Rodriguez-Calvo, R.; Calvayrac, O.; Crespo, J.; Aledo, R.; Martinez-Gonzalez, J.; Rodriguez, C. Fibulin-5 is up-regulated by hypoxia in endothelial cells through a hypoxia-inducible factor-1 (hif-1alpha)-dependent mechanism. J. Biol. Chem. 2011, 286, 7093–7103. [Google Scholar]
- Whitelock, J.; Melrose, J. Heparan sulfate proteoglycans in healthy and diseased systems. Wiley Inter. Rev. Sys. Biol. Med. 2011, 3, 739–751. [Google Scholar] [CrossRef]
- Hayes, A.J.; Lord, M.S.; Smith, S.M.; Smith, M.M.; Whitelock, J.M.; Weiss, A.S.; Melrose, J. Colocalization in vivo and association in vitro of perlecan and elastin. Histo. Cell Biol. 2011, 136, 437–454. [Google Scholar]
- Whitelock, J.M.; Melrose, J.; Iozzo, R.V. Diverse cell signaling events modulated by perlecan. Biochemistry 2008, 47, 11174–11183. [Google Scholar]
- Alexopoulou, A.N.; Multhaupt, H.A.; Couchman, J.R. Syndecans in wound healing, inflammation and vascular biology. Int. J. Biochem. Cell Biol. 2007, 39, 505–528. [Google Scholar] [CrossRef]
- Kirkpatrick, C.A.; Selleck, S.B. Heparan sulfate proteoglycans at a glance. J. Cell. Sci. 2007, 120, 1829–1832. [Google Scholar] [CrossRef]
- Olsen, B.R. Life without perlecan has its problems. J. Cell Biol. 1999, 147, 909–912. [Google Scholar] [CrossRef]
- Arikawa-Hirasawa, E.; Watanabe, H.; Takami, H.; Hassell, J.R.; Yamada, Y. Perlecan is essential for cartilage and cephalic development. Nat. Genet. 1999, 23, 354–358. [Google Scholar] [CrossRef]
- Vikramadithyan, R.K.; Kako, Y.; Chen, G.; Hu, Y.; Arikawa-Hirasawa, E.; Yamada, Y.; Goldberg, I.J. Atherosclerosis in perlecan heterozygous mice. J. Lipid Res. 2004, 45, 1806–1812. [Google Scholar] [CrossRef]
- Costell, M.; Carmona, R.; Gustafsson, E.; Gonzalez-Iriarte, M.; Fassler, R.; Munoz-Chapuli, R. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ. Res. 2002, 91, 158–164. [Google Scholar] [CrossRef]
- Hayashi, K.; Madri, J.A.; Yurchenco, P.D. Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: An adhesion modulated by glycosaminoglycan. J. Cell Biol. 1992, 119, 945–959. [Google Scholar] [CrossRef]
- Whitelock, J.M.; Graham, L.D.; Melrose, J.; Murdoch, A.D.; Iozzo, R.V.; Underwood, P.A. Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 1999, 18, 163–178. [Google Scholar] [CrossRef]
- Segev, A.; Nili, N.; Strauss, B.H. The role of perlecan in arterial injury and angiogenesis. Cardiovasc. Res. 2004, 63, 603–610. [Google Scholar] [CrossRef]
- Weiser, M.C.; Belknap, J.K.; Grieshaber, S.S.; Kinsella, M.G.; Majack, R.A. Developmental regulation of perlecan gene expression in aortic smooth muscle cells. Matrix Biol. 1996, 15, 331–340. [Google Scholar] [CrossRef]
- Garl, P.J.; Wenzlau, J.M.; Walker, H.A.; Whitelock, J.M.; Costell, M.; Weiser-Evans, M.C. Perlecan-induced suppression of smooth muscle cell proliferation is mediated through increased activity of the tumor suppressor pten. Circ. Res. 2004, 94, 175–183. [Google Scholar] [CrossRef]
- Kinsella, M.G.; Tran, P.K.; Weiser-Evans, M.C.; Reidy, M.; Majack, R.A.; Wight, T.N. Changes in perlecan expression during vascular injury: Role in the inhibition of smooth muscle cell proliferation in the late lesion. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 608–614. [Google Scholar] [CrossRef]
- Tran, P.K.; Tran-Lundmark, K.; Soininen, R.; Tryggvason, K.; Thyberg, J.; Hedin, U. Increased intimal hyperplasia and smooth muscle cell proliferation in transgenic mice with heparan sulfate-deficient perlecan. Circ. Res. 2004, 94, 550–558. [Google Scholar] [CrossRef]
- Guyton, J.R.; Rosenberg, R.D.; Clowes, A.W.; Karnovsky, M.J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ. Res. 1980, 46, 625–634. [Google Scholar] [CrossRef]
- Mysliwiec, M.; Borawski, J.; Naumnik, B.; Rydzewska-Rosolowska, A. Endothelial dysfunction, atherosclerosis and thrombosis in uremia—possibilities of intervention. Rocz. Akad. Med. Bialymst. 2004, 49, 151–156. [Google Scholar]
- Lord, M.S.; Yu, W.; Cheng, B.; Simmons, A.; Poole-Warren, L.; Whitelock, J.M. The modulation of platelet and endothelial cell adhesion to vascular graft materials by perlecan. Biomaterials 2009, 30, 4898–4906. [Google Scholar]
- Segev, A.; Nili, N.; Osherov, A.B.; Qiang, B.; Wong, A.J.; Pillarisetti, S.; Strauss, B.H. A perlecan-inducing compound significantly inhibits smooth muscle cell function and in-stent intimal hyperplasia: Novel insights into the diverse biological effects of perlecan. EuroIntervention 2010, 6, 134–140. [Google Scholar] [CrossRef]
- Almine, J.F.; Bax, D.V.; Mithieux, S.M.; Nivison-Smith, L.; Rnjak, J.; Waterhouse, A.; Wise, S.G.; Weiss, A.S. Elastin-based materials. Chem. Soc. Rev. 2010, 39, 3371–3379. [Google Scholar]
- Kagan, H.M.; Li, W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 2003, 88, 660–672. [Google Scholar] [CrossRef]
- Baldock, C.; Oberhauser, A.F.; Ma, L.; Lammie, D.; Siegler, V.; Mithieux, S.M.; Tu, Y.; Chow, J.Y.; Suleman, F.; Malfois, M.; et al. Shape of tropoelastin, the highly extensible protein that controls human tissue elasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 4322–4327. [Google Scholar]
- Wise, S.G.; Weiss, A.S. Tropoelastin. Int. J. Biochem. Cell Biol. 2009, 41, 494–497. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Nerurkar, N.L.; Knutsen, R.H.; Okamoto, R.J.; Li, D.Y.; Mecham, R.P. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am. J. Phys. Heart Circ. Phys. 2005, 289, H1209–H1217. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Ciliberto, C.H.; Knutsen, R.H.; Levy, M.A.; Kovacs, A.; Mecham, R.P. Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ. Res. 2009, 104, 1217–1224. [Google Scholar] [CrossRef]
- Li, D.Y.; Brooke, B.; Davis, E.C.; Mecham, R.P.; Sorensen, L.K.; Boak, B.B.; Eichwald, E.; Keating, M.T. Elastin is an essential determinant of arterial morphogenesis. Nature 1998, 393, 276–280. [Google Scholar] [CrossRef]
- Sims, F.H.; Gavin, J.B.; Edgar, S.; Koelmeyer, T.D. Comparison of the endothelial surface and subjacent elastic lamina of anterior descending coronary arteries at the location of atheromatous lesions with internal thoracic arteries of the same subjects: A scanning electron microscopic study. Pathology 2002, 34, 433–441. [Google Scholar] [CrossRef]
- Ito, S.; Ishimaru, S.; Wilson, S.E. Effect of coacervated alpha-elastin on proliferation of vascular smooth muscle and endothelial cells. Angiology 1998, 49, 289–297. [Google Scholar] [CrossRef]
- Long, M.M.; King, V.J.; Prasad, K.U.; Freeman, B.A.; Urry, D.W. Elastin repeat peptides as chemoattractants for bovine aortic endothelial cells. J. Cell. Phys. 1989, 140, 512–518. [Google Scholar] [CrossRef]
- Robinet, A.; Fahem, A.; Cauchard, J.-H.; Huet, E.; Vincent, L.; Lorimier, S.; Antonicelli, F.; Soria, C.; Crepin, M.; Hornebeck, W.; et al. Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of mt1-mmp. J. Cell Sci. 2005, 118, 343–356. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoo, J.J.; Lim, G.J.; Atala, A.; Stitzel, J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J. Biomed. Mater. Res. A 2007, 83, 999–1008. [Google Scholar]
- Wise, S.G.; Byrom, M.J.; Waterhouse, A.; Bannon, P.G.; Ng, M.K.; Weiss, A.S. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 2011, 7, 295–303. [Google Scholar]
- Wilson, B.D.; Gibson, C.C.; Sorensen, L.K.; Guilhermier, M.Y.; Clinger, M.; Kelley, L.L.; Shiu, Y.T.; Li, D.Y. Novel approach for endothelializing vascular devices: Understanding and exploiting elastin-endothelial interactions. Ann. Biomed. Eng. 2010, 39, 337–346. [Google Scholar]
- Karnik, S.K.; Wythe, J.D.; Sorensen, L.; Brooke, B.S.; Urness, L.D.; Li, D.Y. Elastin induces myofibrillogenesis via a specific domain, vgvapg. Matrix Biol. 2003, 22, 409–425. [Google Scholar] [CrossRef]
- Leach, J.B.; Wolinsky, J.B.; Stone, P.J.; Wong, J.Y. Crosslinked alpha-elastin biomaterials: Towards a processable elastin mimetic scaffold. Acta Biomater. 2005, 1, 155–164. [Google Scholar] [CrossRef]
- Ito, S.; Ishimaru, S.; Wilson, S.E. Inhibitory effect of type 1 collagen gel containing alpha-elastin on proliferation and migration of vascular smooth muscle and endothelial cells. Cardiovasc. Surg. 1997, 5, 176–183. [Google Scholar] [CrossRef]
- Miyamoto, K.; Atarashi, M.; Kadozono, H.; Shibata, M.; Koyama, Y.; Okai, M.; Inakuma, A.; Kitazono, E.; Kaneko, H.; Takebayashi, T.; et al. Creation of cross-linked electrospun isotypic-elastin fibers controlled cell-differentiation with new cross-linker. Int. J. Biol. Macromol. 2009, 45, 33–41. [Google Scholar] [CrossRef]
- Karnik, S.K.; Brooke, B.S.; Bayes-Genis, A.; Sorensen, L.; Wythe, J.D.; Schwartz, R.S.; Keating, M.T.; Li, D.Y. A critical role for elastin signaling in vascular morphogenesis and disease. Development 2003, 130, 411–423. [Google Scholar] [CrossRef]
- Waterhouse, A.; Wise, S.G.; Ng, M.K.; Weiss, A.S. Elastin as a nonthrombogenic biomaterial. Tissue Eng. Part B 2011, 17, 93–99. [Google Scholar]
- Waterhouse, A.; Yin, Y.B.; Wise, S.G.; Bax, D.V.; McKenzie, D.R.; Bilek, M.M.M.; Weiss, A.S.; Ng, M.K.C. The immobilization of recombinant human tropoelastin on metals using a plasma-activated coating to improve the biocompatibility of coronary stents. Biomaterials 2010, 31, 8332–8340. [Google Scholar]
- Hinds, M.T.; Rowe, R.C.; Ren, Z.; Teach, J.; Wu, P.-C.; Kirkpatrick, S.J.; Breneman, K.D.; Gregory, K.W; Courtman, D.W. Development of a reinforced porcine elastin composite vascular scaffold. J. Biomed. Mater. Res. A 2006, 77A, 458–469. [Google Scholar] [CrossRef]
- Woodhouse, K.A.; Klement, P.; Chen, V.; Gorbet, M.B.; Keeley, F.W.; Stahl, R.; Fromstein, J.D.; Bellingham, C.M. Investigation of recombinant human elastin polypeptides as non-thrombogenic coatings. Biomaterials 2004, 25, 4543–4553. [Google Scholar]
- Jordan, S.W.; Haller, C.A.; Sallach, R.E.; Apkarian, R.P.; Hanson, S.R.; Chaikof, E.L. The effect of a recombinant elastin-mimetic coating of an eptfe prosthesis on acute thrombogenicity in a baboon arteriovenous shunt. Biomaterials 2007, 28, 1191–1197. [Google Scholar] [CrossRef]
- Karnik, S.K.; Brooke, B.S.; Bayes-Genis, A.; Sorensen, L.; Wythe, J.D.; Schwartz, R.S.; Keating, M.T.; Li, D.Y. A critical role for elastin signaling in vascular morphogenesis and disease. Development 2003, 130, 411–423. [Google Scholar] [CrossRef]
- Bilek, M.M.; Bax, D.V.; Kondyurin, A.; Yin, Y.; Nosworthy, N.J.; Fisher, K.; Waterhouse, A.; Weiss, A.S.; dos Remedios, C.G.; McKenzie, D.R. Free radical functionalization of surfaces to prevent adverse responses to biomedical devices. Proc. Natl. Acad. Sci. USA 2011, 108, 14405–14410. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wise, S.G.; Waterhouse, A.; Michael, P.; Ng, M.K.C. Extracellular Matrix Molecules Facilitating Vascular Biointegration. J. Funct. Biomater. 2012, 3, 569-587. https://doi.org/10.3390/jfb3030569
Wise SG, Waterhouse A, Michael P, Ng MKC. Extracellular Matrix Molecules Facilitating Vascular Biointegration. Journal of Functional Biomaterials. 2012; 3(3):569-587. https://doi.org/10.3390/jfb3030569
Chicago/Turabian StyleWise, Steven G., Anna Waterhouse, Praveesuda Michael, and Martin K.C. Ng. 2012. "Extracellular Matrix Molecules Facilitating Vascular Biointegration" Journal of Functional Biomaterials 3, no. 3: 569-587. https://doi.org/10.3390/jfb3030569
APA StyleWise, S. G., Waterhouse, A., Michael, P., & Ng, M. K. C. (2012). Extracellular Matrix Molecules Facilitating Vascular Biointegration. Journal of Functional Biomaterials, 3(3), 569-587. https://doi.org/10.3390/jfb3030569




