Silica as a Matrix for Encapsulating Proteins: Surface Effects on Protein Structure Assessed by Circular Dichroism Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Studies with Human Copper-Zinc Superoxide Dismutase
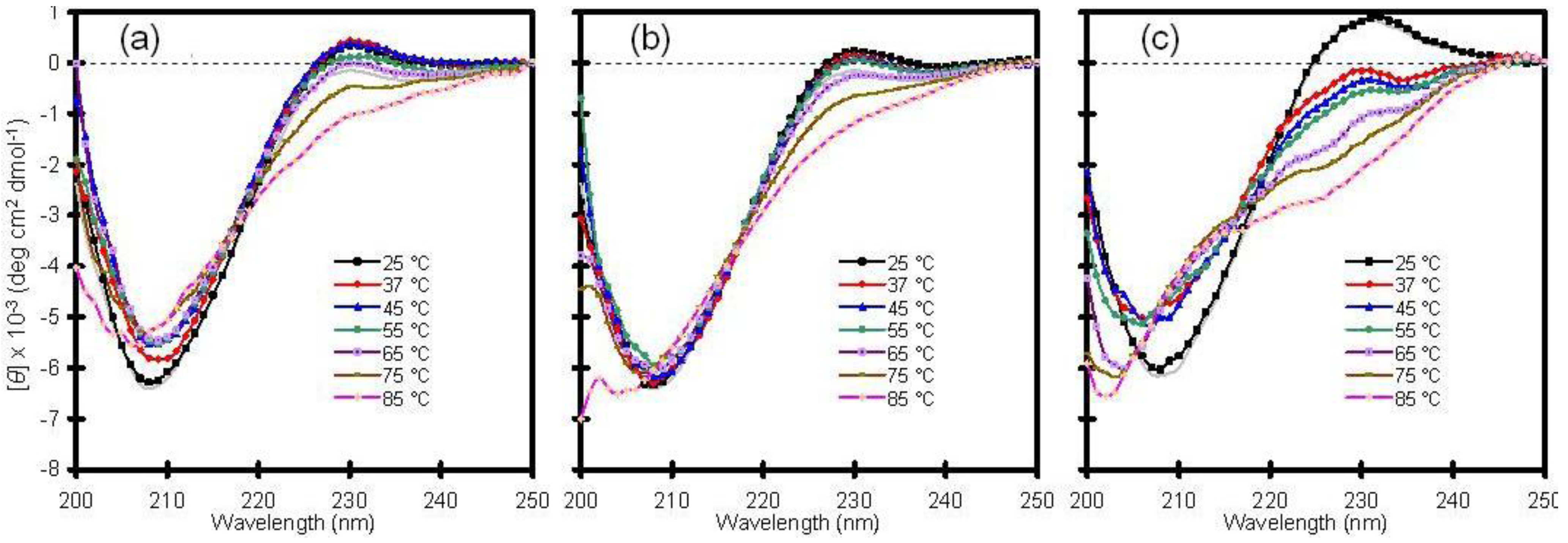
2.2. Xerogel-Encapsulated Type I Antifreeze Protein
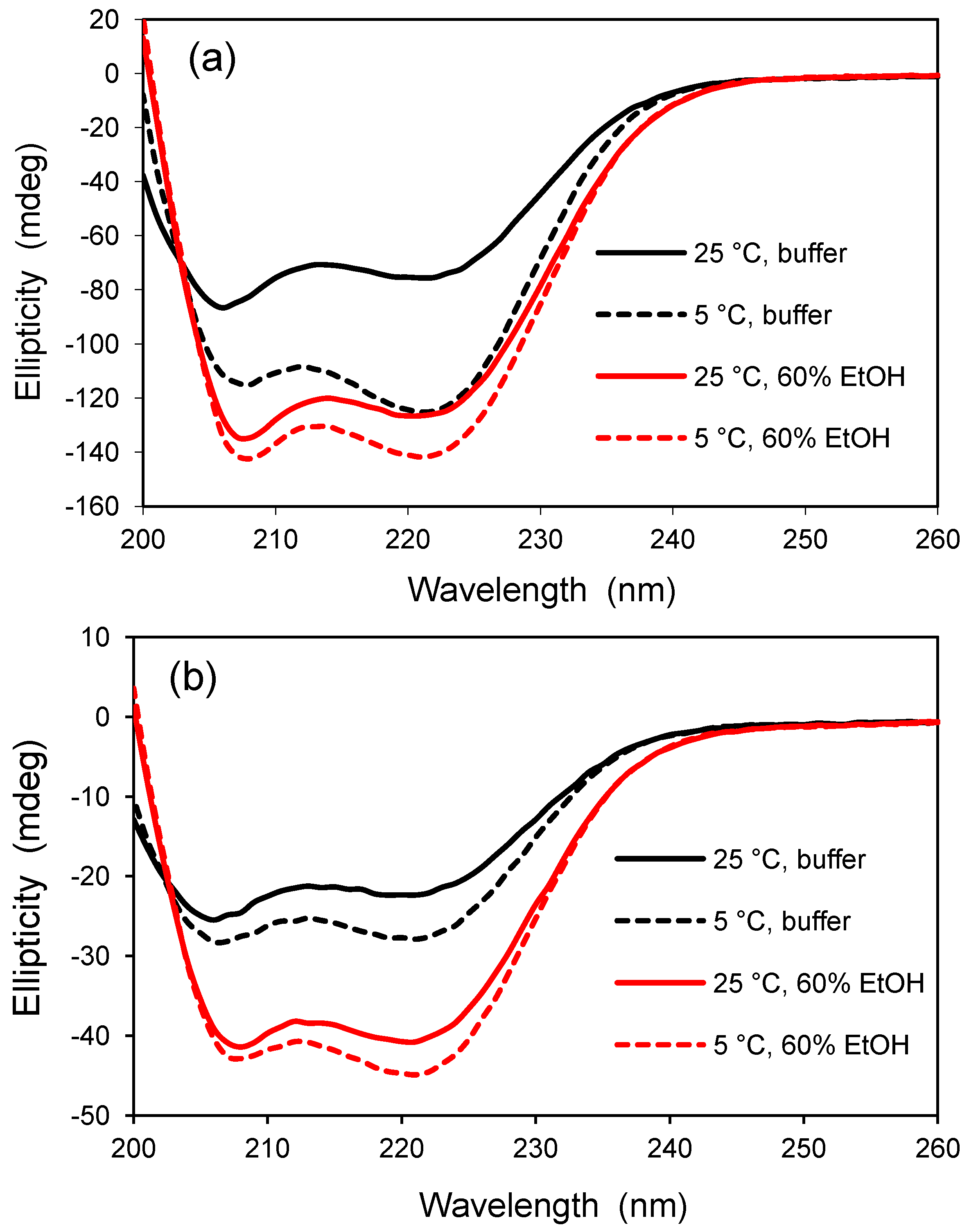
2.3. Entrapment of Polyglutamine, a Disease-Related Aggregation-prone Peptide
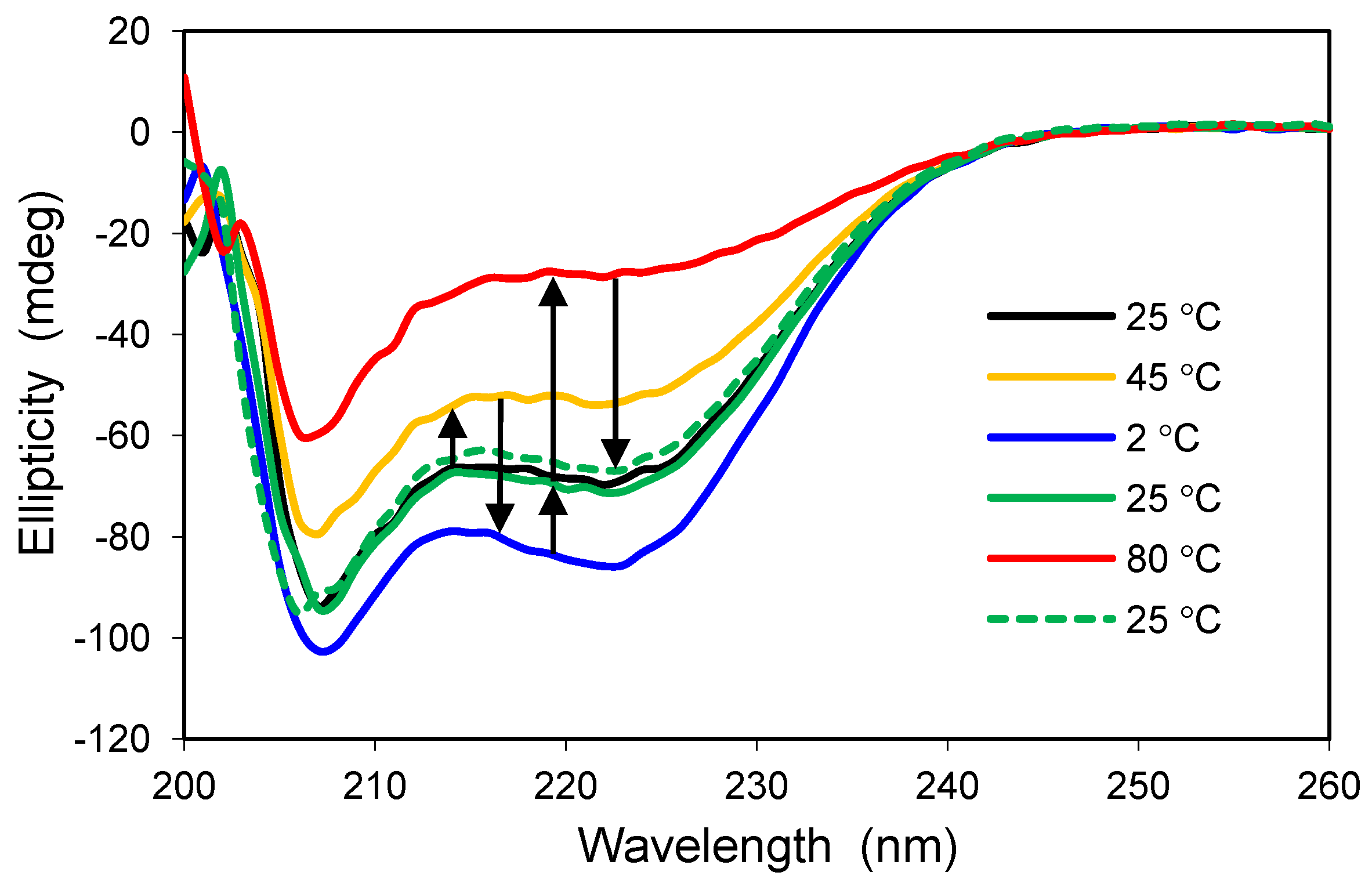
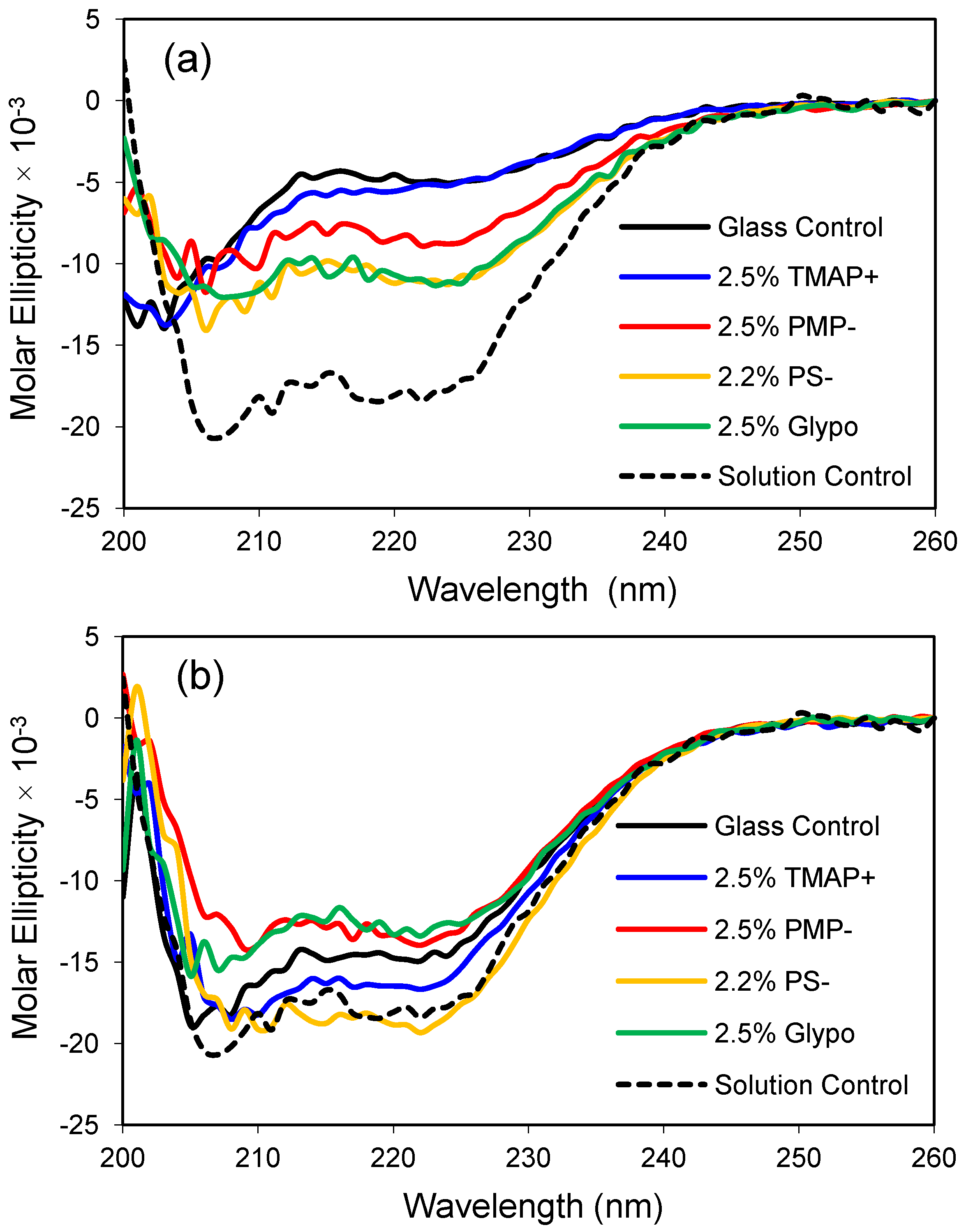
2.4. Effects of Hydrophilic Surface Modifications on Apomyoglobin Structure
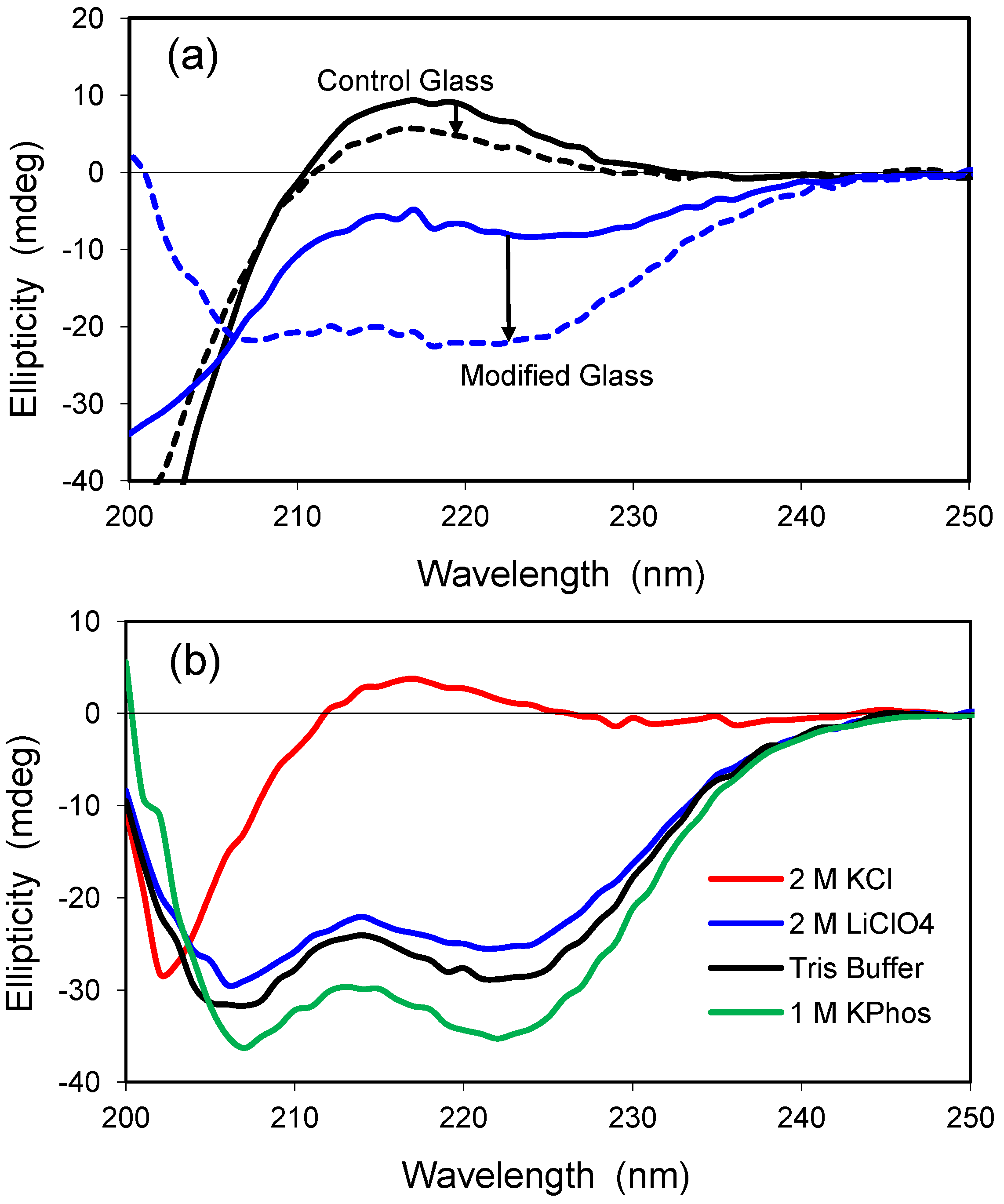
2.5. Confinement Effects on Polylysine
3. Experimental Section
3.1. Materials
3.2. Standard Glass Formation
3.3. Xerogels for AFP Entrapment
3.4. Polyglutamine Encapsulation in Fluoropropyl-modified Xerogel
3.5. Hydrophilic, Organically-modified Glasses
3.6. Circular Dichroism Spectroscopy
4. Conclusions
Acknowledgments
References
- Eggers, D.K.; Valentine, J.S. Crowding and hydration effects on protein conformation: A study with sol-gel encapsulated proteins. J. Mol. Biol. 2001, 314, 911–922. [Google Scholar] [CrossRef]
- Minton, A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef]
- Ellis, R.J. Macromolecular crowding: Obvious but underappreciated. Trends Biochem. Sci. 2001, 26, 597–604. [Google Scholar] [CrossRef]
- Elcock, A.H. Models of macromolecular crowding effects and the need for quantitative comparisons with experiment. Curr. Opin. Struct. Biol. 2010, 20, 196–206. [Google Scholar] [CrossRef]
- Arnaud, C.H. Close quarters: Crowded conditions such as those in cells can affect proteins’ structure, function and activity. Chem. Eng. News 2010, 88, 9–13. [Google Scholar]
- Crowley, P.B.; Chow, E.; Papkovskaia, T. Protein interactions in the Escherichia coli cytosol: An impediment to in-cell NMR spectroscopy. Chem. Bio. Chem. 2011, 12, 1043–1048. [Google Scholar]
- Schlesinger, A.P.; Wang, Y.; Tadeo, X.; Millet, O.; Pielak, G.J. Macromolecular crowding fails to fold a globular protein in cells. J. Am. Chem. Soc. 2011, 133, 8082–8085. [Google Scholar]
- Ellerby, L.M.; Nishida, C.R.; Nishida, F.; Yamanaka, S.A.; Dunn, B.; Valentine, J.S.; Zink, J.I. Encapsulation of proteins in transparent porous silicate glasses prepared by the sol–gel method. Science 1992, 255, 1113–1115. [Google Scholar]
- Betatti, S.; Pioselli, B.; Campanini, B.; Viappiani, C.; Mozzarelli, A. Protein-doped nanoporous silica gels. Encycl. Nanosci. Nanotechnol. 2004, 9, 81–103. [Google Scholar]
- Pierre, A.C. The sol-gel encapsulation of enzymes. Biocatal. Biotransform. 2004, 22, 145–170. [Google Scholar] [CrossRef]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent bio-applications of sol-gel materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhury, N.K. Entrapment of biomolecules in sol-gel matrix for applications in biosensors: Problems and future prospects. Biosens. Bioelectron. 2007, 22, 2387–2399. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Monton, M.R.N.; Forsberg, E.M.; Brennan, J.D. Tailoring sol-gel-derived silica materials for optical biosensing. Chem. Mater. 2012, 24, 796–811. [Google Scholar] [CrossRef]
- Valentine, J.S.; Doucette, P.A.; Potter, S.Z. Copper-Zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu. Rev. Biochem. 2005, 74, 563–593. [Google Scholar] [CrossRef]
- Khouderchah, N. Characterization of Human Copper/Zinc Superoxide Dismutase. M.Sc. Thesis, San José State University, San José, CA, USA, 2008. [Google Scholar]
- Rodriguez, J.A.; Valentine, J.S.; Eggers, D.K.; Roe, J.A.; Tiwari, A.; Brown, R.H.; Hayworth, L.J. Familial amyotrophic lateral sclerosis-associated mutations decrease the thermal stability of distinctly metallated species of human copper/zinc superoxide dismutase. J. Biol. Chem. 2002, 277, 15932–15937. [Google Scholar]
- Galaleldeen, A.; Strange, R.W.; Whitson, L.J.; Antonyuk, S.V.; Narayana, N.; Taylor, A.B.; Schuermann, J.P.; Holloway, S.P.; Hasnain, S.S.; Hart, P.J. Structural and biophysical properties of metal-free pathogenic SOD1 mutants A4V and G93A. Arch. Biochem. Biophys. 2009, 492, 40–47. [Google Scholar] [CrossRef]
- Ray, S.S.; Nowak, R.J.; Strokovich, K.; Brown, R.H.; Walz, T.; Lansbury, P.T. An intersubunit disulfide bond prevents in vitro aggregation of a superoxide dismutase-1 mutant linked to familial amyotrophic lateral sclerosis. Biochemistry 2004, 43, 4899–4905. [Google Scholar] [CrossRef]
- Davies, P.L.; Sykes, B.D. Antifreeze proteins. Curr. Opin. Struct. Biol. 1997, 7, 828–834. [Google Scholar] [CrossRef]
- Zemede, G.H. Sol-Gel Glass Encapsulation of Fish Antifreeze Proteins. M.Sc. Thesis, San José State University, San José, CA, USA, 2007. [Google Scholar]
- Takahashi, T.; Katada, S.; Onodera, O. Polyglutamine diseases: Where does toxicity come from? What is toxicity? Where are we going? J. Mol. Cell Biol. 2010, 2, 180–191. [Google Scholar] [CrossRef]
- Perutz, M.F.; Johnson, T.; Suzuki, M.; Finch, J.T. Glutamine repeats as polar zippers: Their possible role in inherited neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 1994, 91, 5355–5358. [Google Scholar] [CrossRef]
- Chen, S.; Berthelier, V.; Yang, W.; Wetzel, R. Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity. J. Mol. Biol. 2001, 311, 173–182. [Google Scholar] [CrossRef]
- Altschuler, E.L.; Hud, N.V.; Mazrimas, J.A.; Rupp, B. Random coil conformation for extended polyglutamine stretches in aqueous soluble monomeric peptides. J. Peptide Res. 1997, 50, 73–75. [Google Scholar]
- Rocha, V.A.; Eggers, D.K. Hydrophobic, organically-modified silica gels enhance the secondary structure of encapsulated apomyoglobin. Chem. Commun. 2007, 1266–1268. [Google Scholar]
- Birtwhistle, N.J. Analysis of Sol-Gel Encapsulated Aggregate-Prone Peptides by Circular Dichroism. M.Sc. Thesis, San José State University, San José, CA, USA, 2012. [Google Scholar]
- Eggers, D.K.; Valentine, J.S. Molecular confinement influences protein structure and enhances thermal protein stability. Protein Sci. 2001, 10, 250–261. [Google Scholar] [CrossRef]
- Menaa, B.; Herrero, M.; Rives, V.; Lavrenko, M.; Eggers, D.K. Favourable influence of hydrophobic surfaces on protein structure in porous organically-modified silica glasses. Biomaterials 2008, 29, 2710–2718. [Google Scholar] [CrossRef]
- Greenfield, N.; Fasman, G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 1969, 8, 4108–4116. [Google Scholar] [CrossRef]
- Bello, J. Helix formation in poly(N,N,N-trimethyl-L-lysine) and poly(L-lysine): Dependence on concentration and molecular weight. Biopolymers 1992, 32, 185–188. [Google Scholar] [CrossRef]
- Hapner, K.D.; Bradshaw, R.A.; Hartzell, C.R.; Gurd, F.R.N. Comparison of myoglobins from harbor seal, porpoise, and sperm whale. J. Biol. Chem. 1968, 243, 683–689. [Google Scholar]
- Chen, S.; Wetzel, R. Solubilization and disaggregation of polyglutamine peptides. Protein Sci. 2001, 10, 887–891. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Calabretta, P.J.; Chancellor, M.C.; Torres, C.; Abel, G.R., Jr.; Niehaus, C.; Birtwhistle, N.J.; Khouderchah, N.M.; Zemede, G.H.; Eggers, D.K. Silica as a Matrix for Encapsulating Proteins: Surface Effects on Protein Structure Assessed by Circular Dichroism Spectroscopy. J. Funct. Biomater. 2012, 3, 514-527. https://doi.org/10.3390/jfb3030514
Calabretta PJ, Chancellor MC, Torres C, Abel GR Jr., Niehaus C, Birtwhistle NJ, Khouderchah NM, Zemede GH, Eggers DK. Silica as a Matrix for Encapsulating Proteins: Surface Effects on Protein Structure Assessed by Circular Dichroism Spectroscopy. Journal of Functional Biomaterials. 2012; 3(3):514-527. https://doi.org/10.3390/jfb3030514
Chicago/Turabian StyleCalabretta, Phillip J., Mitchell C. Chancellor, Carlos Torres, Gary R. Abel, Jr., Clayton Niehaus, Nathan J. Birtwhistle, Nada M. Khouderchah, Genet H. Zemede, and Daryl K. Eggers. 2012. "Silica as a Matrix for Encapsulating Proteins: Surface Effects on Protein Structure Assessed by Circular Dichroism Spectroscopy" Journal of Functional Biomaterials 3, no. 3: 514-527. https://doi.org/10.3390/jfb3030514
APA StyleCalabretta, P. J., Chancellor, M. C., Torres, C., Abel, G. R., Jr., Niehaus, C., Birtwhistle, N. J., Khouderchah, N. M., Zemede, G. H., & Eggers, D. K. (2012). Silica as a Matrix for Encapsulating Proteins: Surface Effects on Protein Structure Assessed by Circular Dichroism Spectroscopy. Journal of Functional Biomaterials, 3(3), 514-527. https://doi.org/10.3390/jfb3030514