A Pulsatile Bioreactor for Conditioning of Tissue-Engineered Cardiovascular Constructs under Endoscopic Visualization
Abstract
:1. Introduction
2. Results and Discussion
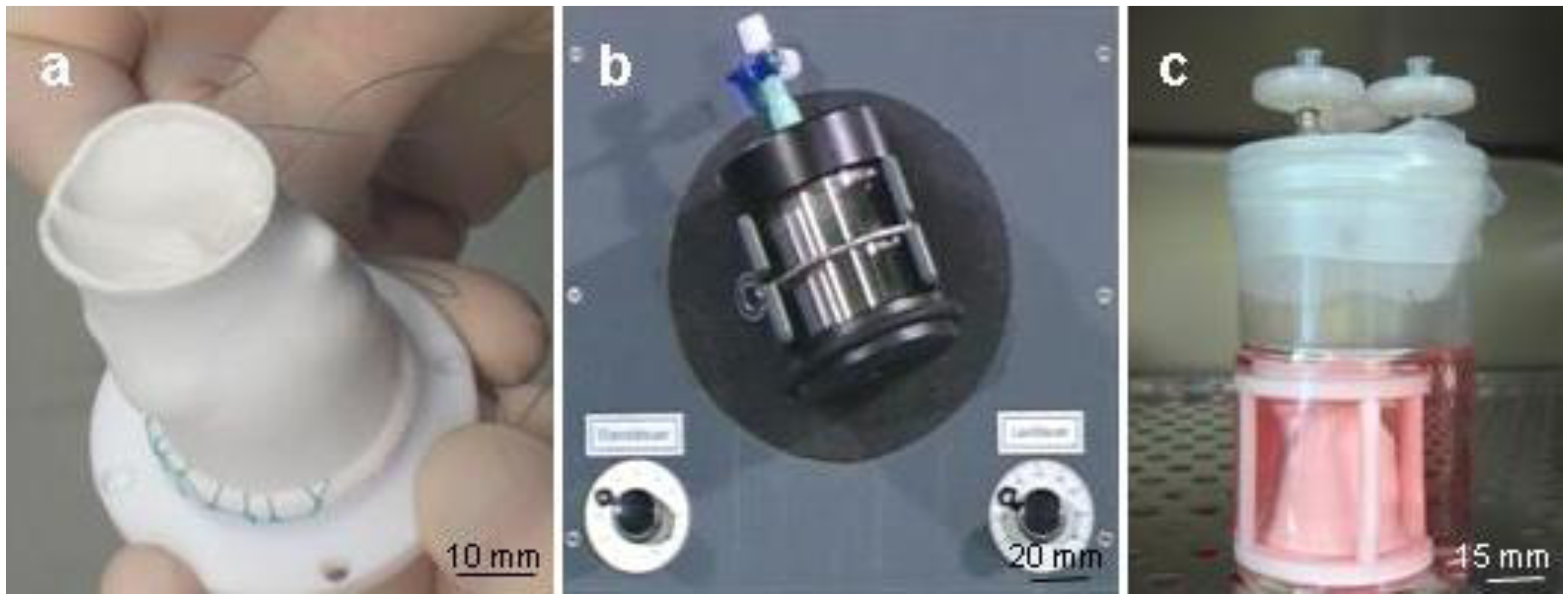
2.1. Bioreactor Assembly
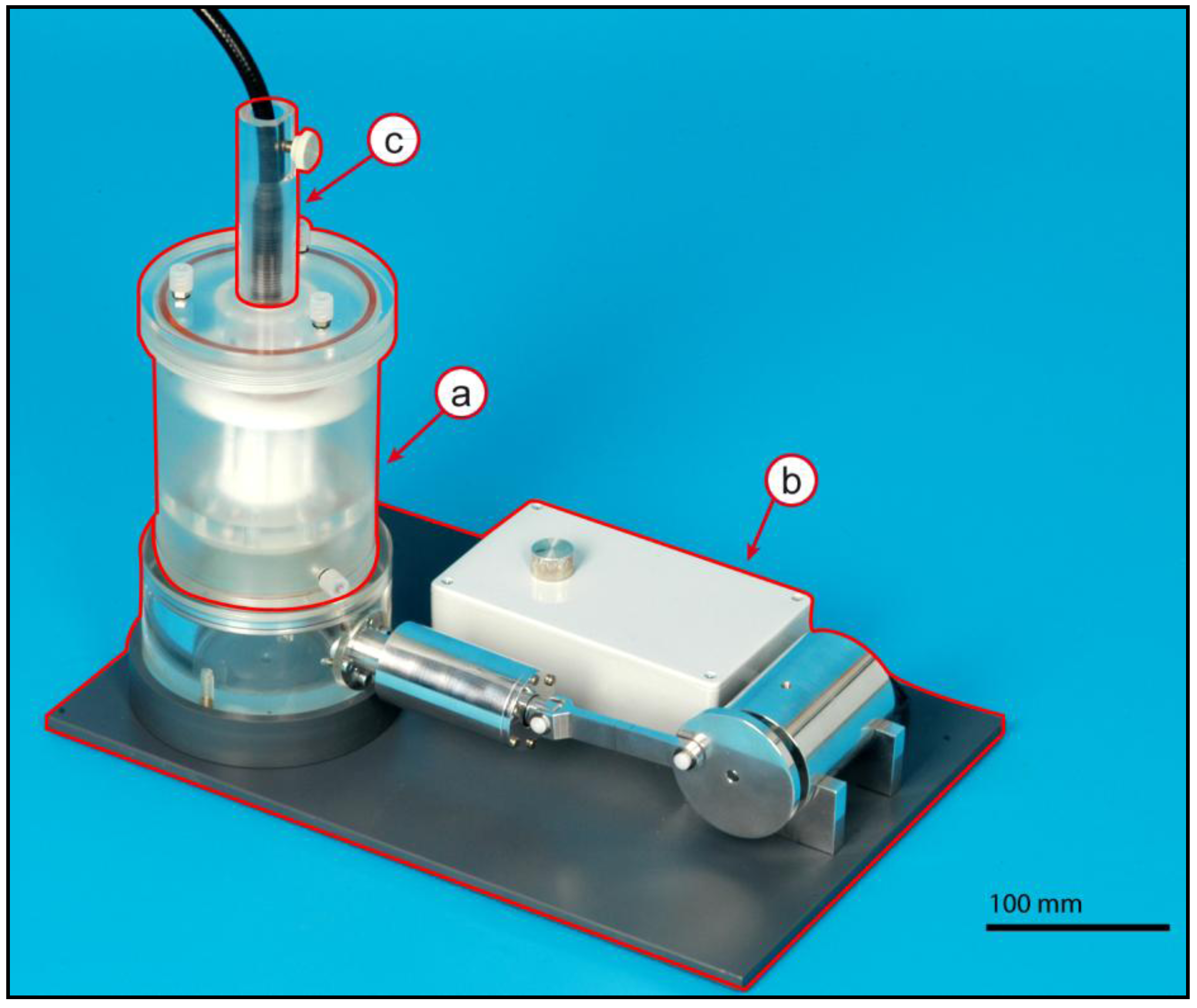
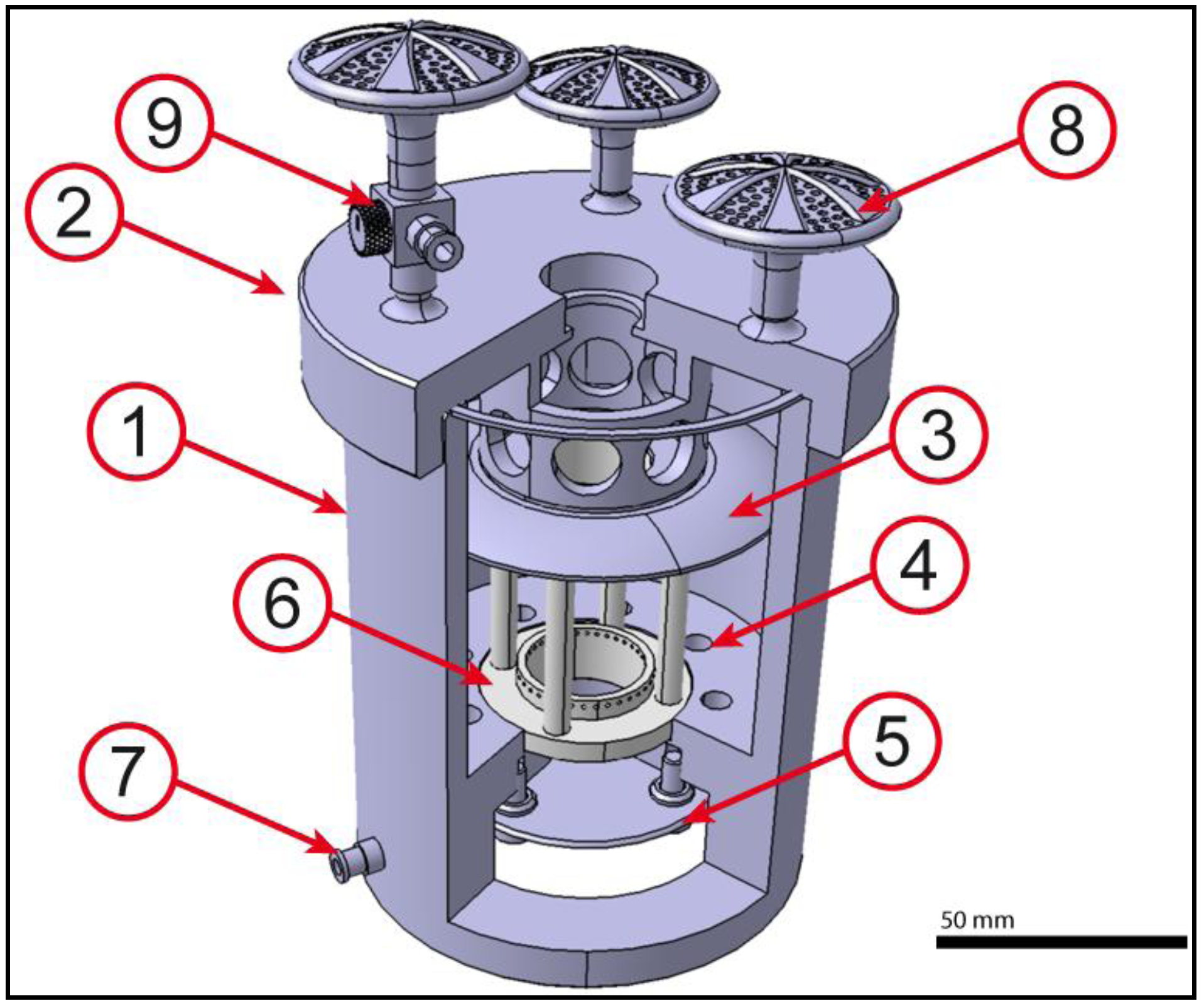
2.2. Core Unit

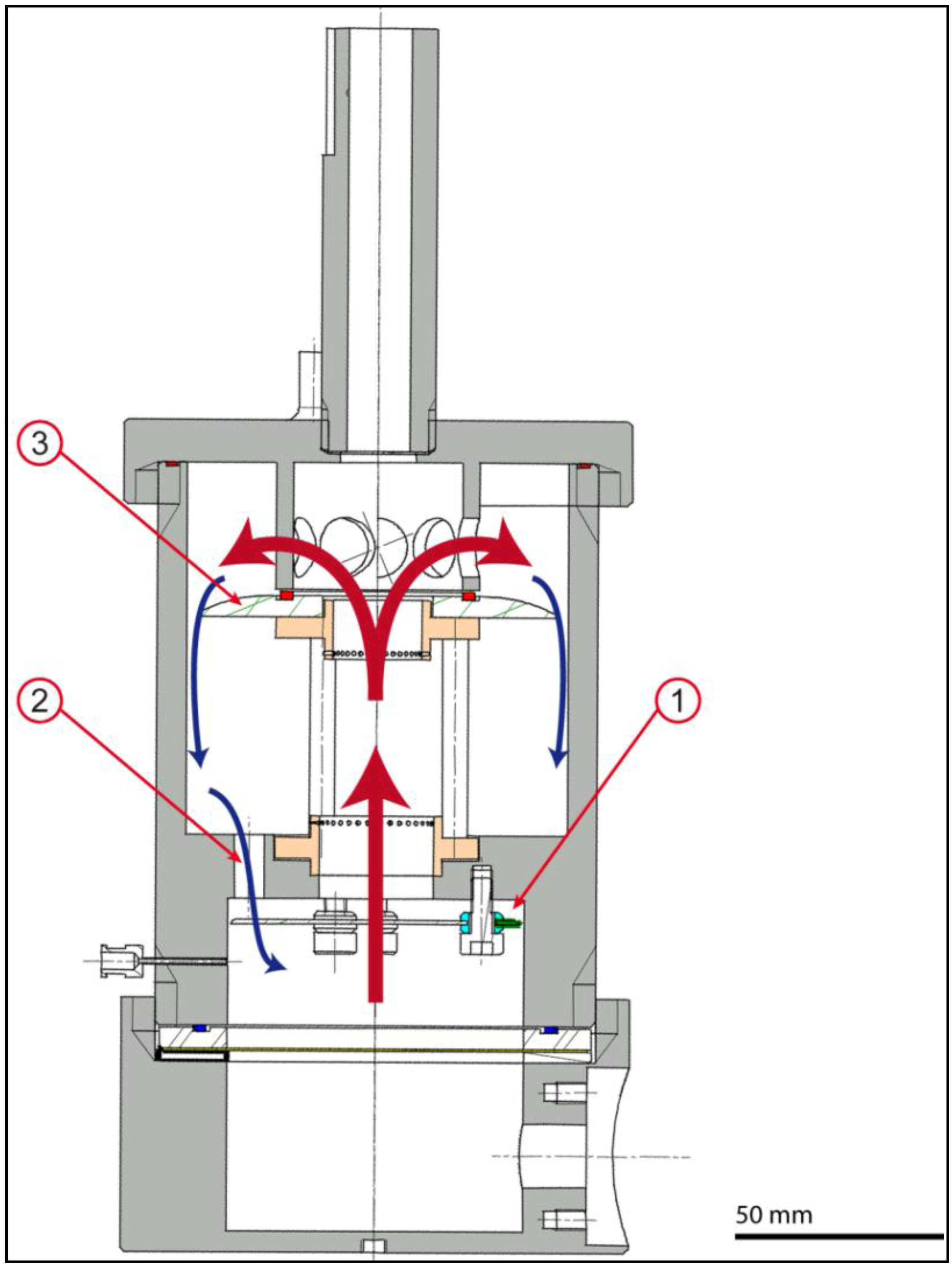
2.3. Actuation Unit
2.4. Monitoring Unit


2.5. Bioreactor Function
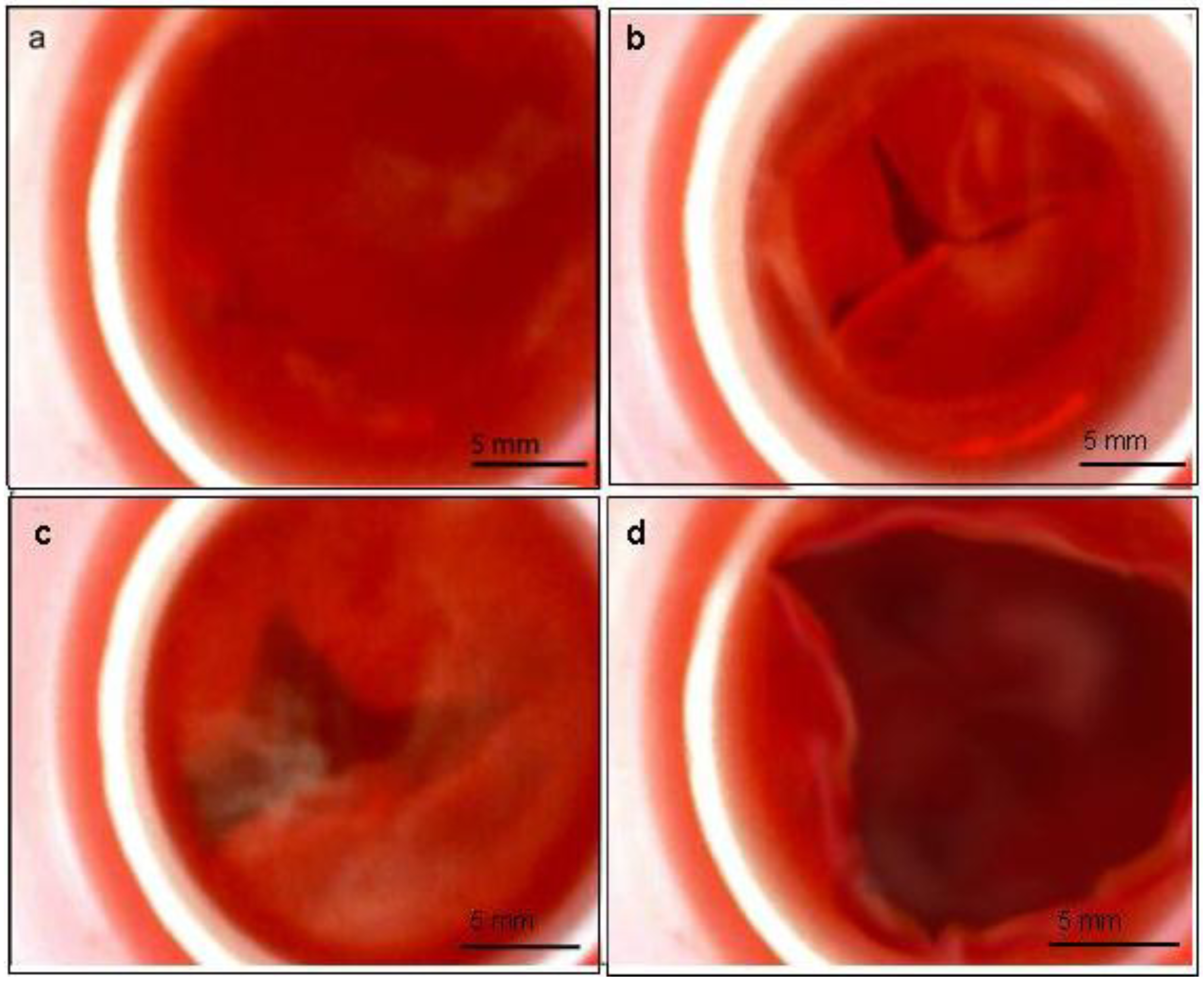
2.6. Bioreactor Sterility
2.7. Bioreactor Functionality
3. Experimental Section
3.1. Construction of the Bioreactor
| Group | Description | Components |
|---|---|---|
| A | Membrane Carrier | 2 aluminum coils, 4 fixing pins, 1 membrane, 1 seal ring |
| B | Valve | 1 valve membrane, 4 guiding rings |
| C | Core Unit | 1 housing, 1 air chamber, assembly A, assembly B, 1 lid, 1 Teflon® support |
| D | Monitoring Unit | 1 endoscope cylinder, glass plate, fixing screw |
| E | Actuator | 1 cylinder, piston, 2 seal rings, cylinder lid |
| F | Eccentric | 1 extender wheel, 1 piston rod, 2 securing pins, 2 plates |
| G | Motor Unit | 2 stands, 1 motor housing, 1 motor |
| H | Base Unit | 1 base plate, 1 reactor stand |
3.2. Sterilization of the Bioreactor
3.3. Seeding of PUHV
3.4. Functionality Testing
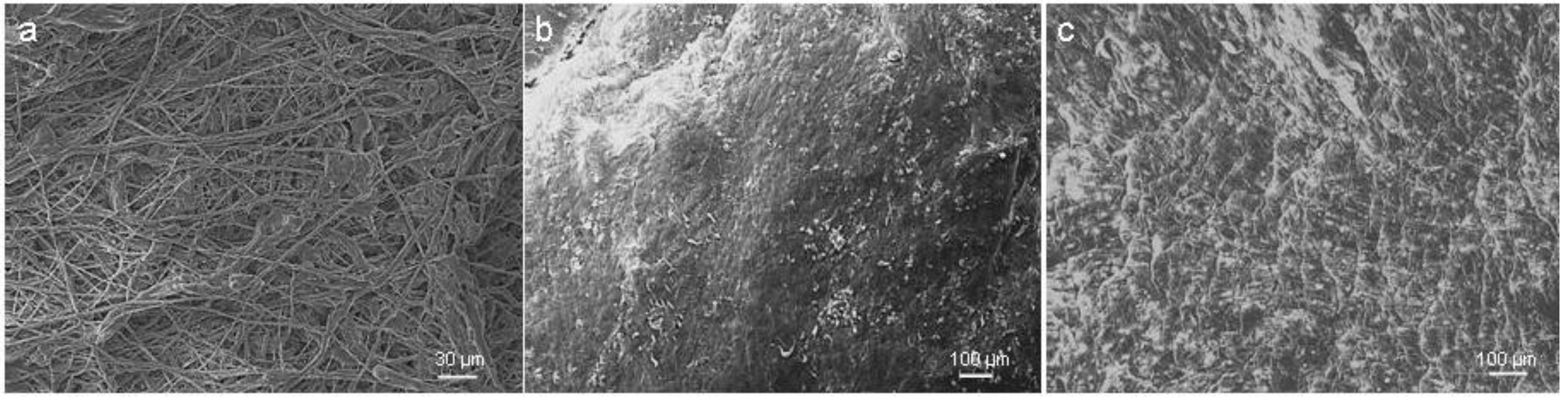
3.5. Scanning Electron Microscopy (SEM)
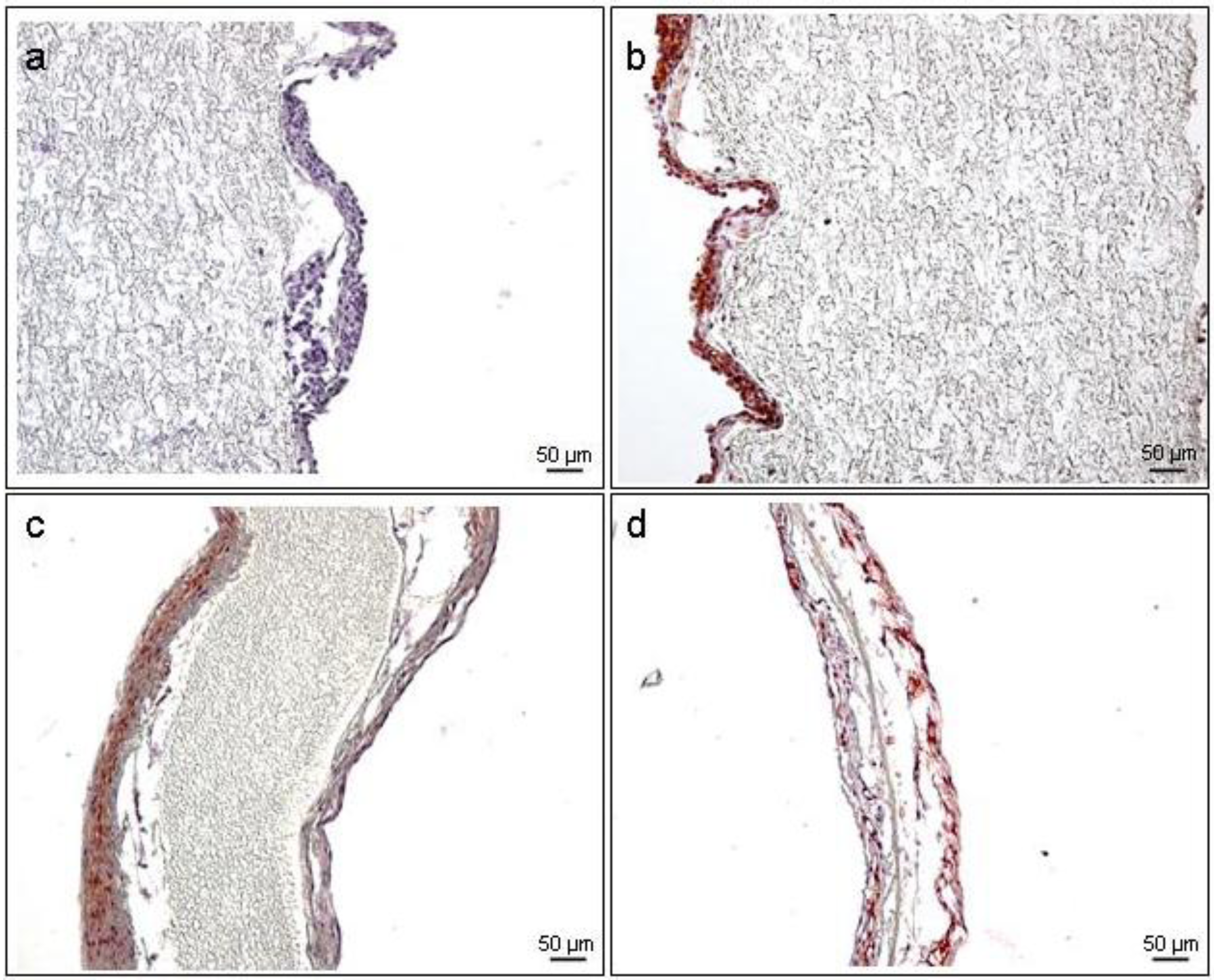
3.6. Immunohistochemistry (IHC)
4. Conclusions
Acknowledgments
References
- Rabkin, E.; Schoen, F.J. Cardiovascular tissue engineering. Cardiovasc. Pathol. 2002, 6, 305–317. [Google Scholar] [CrossRef]
- Schaefermeier, P.K.; Szymanski, D.; Weiss, F. Design and fabrication of three-dimensional scaffolds for tissue engineering of human heart valves. Eur. Surg. Res. 2009, 1, 49–53. [Google Scholar]
- Bilodeau, K.; Mantovani, D. Bioreactors for tissue engineering: Focus on mechanical constraints. A comparative review. Tissue Eng. 2006, 8, 2367–2383. [Google Scholar] [CrossRef]
- Keogh, B.E.; Kinsman, R. Fifth National Adult Cardiac Surgical Database Report; Dendrite Clinical Systems Ltd.: Bournemouth, UK, 2004; pp. 1–352. [Google Scholar]
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Wendt, D.; Riboldi, S.A.; Cioffi, M. Bioreactors in tissue engineering: Scientific challenges and clinical perspectives. Adv. Biochem. Eng. Biot. 2009, 112, 1–27. [Google Scholar]
- Schopka, S.; Schmid, F.X.; Hirt, S. Recellularization of biological heart valves with human vascular cells: In vitro hemocompatibility assessment. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 1, 130–138. [Google Scholar]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2007, 7, 679–689. [Google Scholar]
- Meyer, U.; Meyer, T.; Handschel, J.; Wiesmann, H.P. Fundamentals of Tissue Engineering and Regenerative Medicine; Wiesmann, H.P., Lammers, L., Eds.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Wintermantel, E.; Ha, S.W. Medizintechnik: Life Science Engineering; Ha, S.W., Ed.; Springer: Berlin, Germany, 2009; Volume 5, pp. 230–254, Chapter 12. [Google Scholar]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 2, 80–86. [Google Scholar]
- Chen, H.C.; Hu, Y.C. Bioreactors for tissue engineering. Biotechnol. Lett. 2006, 18, 1415–1423. [Google Scholar] [CrossRef]
- Lee, D.J.; Steen, J.; Jordan, J.E. Endothelialization of heart valve matrix using a computer-assisted pulsatile bioreactor. Tissue Eng. Part A 2009, 4, 807–814. [Google Scholar]
- Mendelson, K.; Schoen, F.J. Heart valve tissue engineering: Concepts, approaches, progress and challange. Ann. Biomed. Eng. 2006, 12, 1799–1819. [Google Scholar] [CrossRef]
- Bjork, J.W.; Tranquillo, R.T. Transmural flow bioreactor for vascular tissue engineering. Biotechnol. Bioeng. 2009, 6, 1197–1206. [Google Scholar]
- Goldstein, A.S.; Christ, G. Functional tissue engineering requires bioreactor strategies. Tissue Eng. Part A 2009, 4, 739–740. [Google Scholar] [CrossRef]
- Portner, R.; Nagel-Heyer, S.; Goepfert, C. Bioreactor design for tissue engineering. J. Biosci. Bioeng. 2005, 3, 235–245. [Google Scholar]
- Sodian, R.; Lemke, T.; Loebe, M. New pulsatile bioreactor for fabrication of tissue-engineered patches. J. Biomed. Mater. Res. 2001, 4, 401–405. [Google Scholar]
- Zaucha, M.T.; Raykin, J.; Wan, W. A novel cylindrical biaxial computer-controlled bioreactor and biomechanical testing device for vascular tissue engineering. Tissue Eng. Part A 2009, 11, 174–183. [Google Scholar]
- Vismara, R.; Soncini, M.; Talo, G.; Dainese, L.; Guarino, A.; Redaelli, A.; Fiore, G.B. A bioreactor with compliance monitoring for heart valve grafts. Ann. Biomed. Eng. 2010, 1, 100–108. [Google Scholar]
- Nawrat, Z. Review of research in cardiovascular devices. In Advances in Biomedical Engineering, 1st; Verdonck, P., Ed.; Elsevier: Oxford, UK, 2009; Volume 1, pp. 1–59. [Google Scholar]
- Ramaswamy, S.; Gottlieb, D.; Engelmayr, G.C.; Aikawa, E.; Schmidt, D.E.; Gaitan-Leon, D.M.; Sales, V.L.; Mayer, J.E.; Sacks, M.S. The role of organ level conditioning on the promotion of engineered heart valve tissue development in vitro using mesenchymal stem cells. Biomaterials 2010, 31, 1114–1125. [Google Scholar] [CrossRef]
- Hildebrand, D.K.; Wu, Z.J.; Mayer, J.E.; Sacks, M.S. Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves. Ann. Biomed. Eng. 2004, 32, 1039–1049. [Google Scholar] [CrossRef]
- Bjork, J.W.; Tranquillo, R.T. Transmural flow bioreactor for vascular tissue engineering. Biotechnol. Bioeng. 2009, 15, 1197–1206. [Google Scholar]
- Ruel, J.; Lachance, G. A new bioreactor for the development of tissue-engineered heart valves. Ann. Biomed. Eng. 2009, 37, 674–681. [Google Scholar] [CrossRef]
- Sierad, L.N.; Simionescul, A.; Albers, C.; Chen, J.; Maivelett, J.; Tedder, M.E.; Liao, J.; Simionescu, D.T. Design and testing of a pulsatile conditioning system for dynamic endothelialization of polyphenol-stabilized tissue engineered heart valves. Cardiovasc. Eng. Technol. 2010, 1, 138–153. [Google Scholar] [CrossRef]
- Zeltinger, J.; Landeen, L.K.; Alexander, H.G.; Kidd, I.D.; Sibanda, B. Development and characterization of tissue-engineered aortic valves. Tissue Eng. 2011, 7, 9–22. [Google Scholar]
- Syedain, Z.H.; Meier, L.A.; Bjork, J.W.; Lee, A.; Tranquillo, R.T. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 2011, 32, 714–722. [Google Scholar] [CrossRef]
- Mol, A.; Driessen, N.J.B.; Rutten, M.C.M.; Hoerstrup, S.P.; Bouten, C.V.C.; Baaijens, F.P.T. Tissue engineering of human heart valve leaflets: A novel bioreactor for a strain-based conditioning approach. Ann. Biomed. Eng. 2005, 33, 1778–1788. [Google Scholar] [CrossRef]
- Balachandran, K.; Sucosky, P.; Jo, H.; Yoganathan, A.P. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am. J. Pathol. 2010, 177, 49–57. [Google Scholar] [CrossRef]
- Tschoeke, B.; Flanagan, T.C.; Cornelissen, A.; Koch, S.; Roehl, A.; Sriharwoko, M.; Sachweh, J.S.; Gries, T.; Schmitz-Rode, T.; Jockenhoevel, S. Development of a composite degradable/nondegradable tissue-engineered vascular graft. Artif. Organs 2008, 32, 800–809. [Google Scholar]
- Stachelek, S.J.; Alferiev, I.; Conolly, J.M.; Sacks, M.; Hebbel, R.P.; Bianco, R.; Levy, R.J. Cholesterol-modified polyurethane valve cusps demonstrate blood outgrowth endothelial cell adhesion post-seeding in vitro and in vivo. Ann. Thorac. Surg. 2006, 81, 47–55. [Google Scholar]
- Gulbins, H.; Goldemund, A.; Anderson, I.; Haas, U.; Uhlig, A.; Meiser, B.; Reichart, B. Preseeding with autologous fibroblasts improves endothelialization of glutaraldehyde-fixed porcine aortic valves. J. Thorac. Cardiovasc. Surg. 2003, 125, 592–601. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
König, F.; Hollweck, T.; Pfeifer, S.; Reichart, B.; Wintermantel, E.; Hagl, C.; Akra, B. A Pulsatile Bioreactor for Conditioning of Tissue-Engineered Cardiovascular Constructs under Endoscopic Visualization. J. Funct. Biomater. 2012, 3, 480-496. https://doi.org/10.3390/jfb3030480
König F, Hollweck T, Pfeifer S, Reichart B, Wintermantel E, Hagl C, Akra B. A Pulsatile Bioreactor for Conditioning of Tissue-Engineered Cardiovascular Constructs under Endoscopic Visualization. Journal of Functional Biomaterials. 2012; 3(3):480-496. https://doi.org/10.3390/jfb3030480
Chicago/Turabian StyleKönig, Fabian, Trixi Hollweck, Stefan Pfeifer, Bruno Reichart, Erich Wintermantel, Christian Hagl, and Bassil Akra. 2012. "A Pulsatile Bioreactor for Conditioning of Tissue-Engineered Cardiovascular Constructs under Endoscopic Visualization" Journal of Functional Biomaterials 3, no. 3: 480-496. https://doi.org/10.3390/jfb3030480
APA StyleKönig, F., Hollweck, T., Pfeifer, S., Reichart, B., Wintermantel, E., Hagl, C., & Akra, B. (2012). A Pulsatile Bioreactor for Conditioning of Tissue-Engineered Cardiovascular Constructs under Endoscopic Visualization. Journal of Functional Biomaterials, 3(3), 480-496. https://doi.org/10.3390/jfb3030480




