Mechanotransduction: Tuning Stem Cells Fate
Abstract
:1. Regenerative Medicine
2. Stem cells
2.1. Embryonic Stem Cells
2.2. Adult Stem Cells
2.3. Induced Pluripotent Stem Cells
3. Stem Cells and Biomaterial Interactions
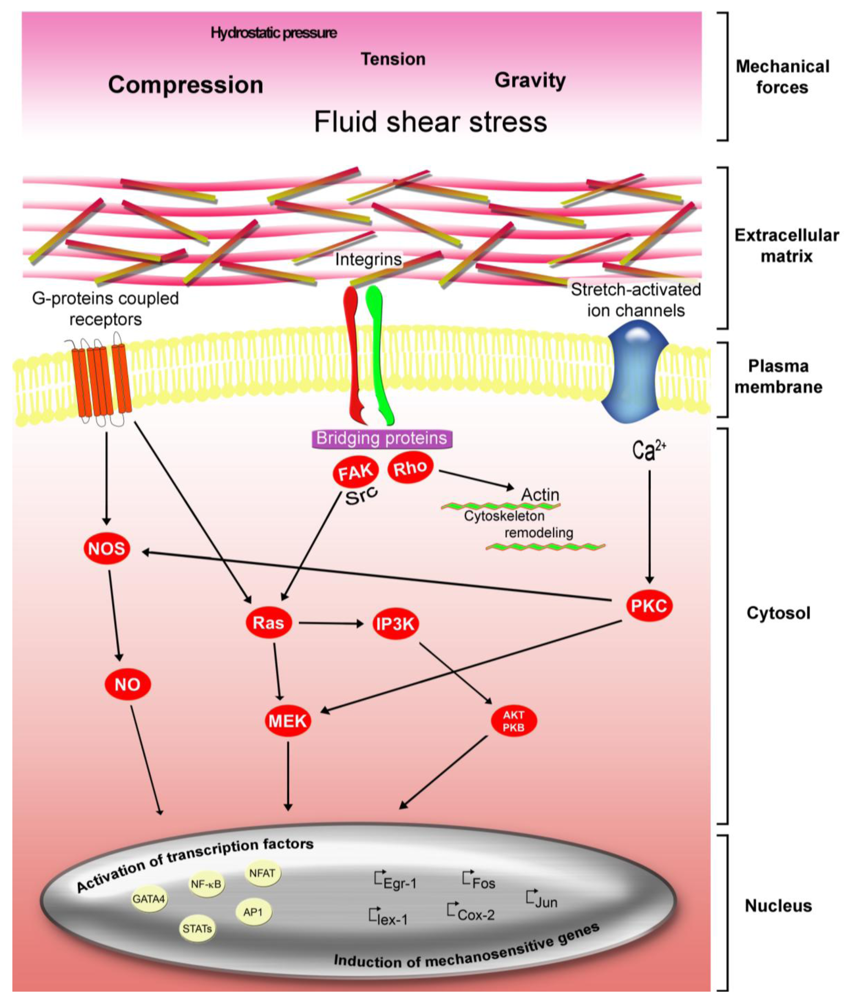
3.1. Integrins, Cytoskeleton Involvements in Mechanotransduction
3.2. Mechanical Forces with Asymmetrical Direction
3.3. Wnt and Beta-Catenin Involvement in Mechanotransduction
4. Future Perspectives
Acknowledgments
References
- Orlacchio, A.; Bernardi, G.; Orlacchio, A.; Martino, S. Stem cells and neurological diseases. Discov. Med. 2010, 9, 546–553. [Google Scholar]
- Lindvall, O.; Kokaia, Z. Stem cells in human neurodegenerative disorders-time for clinical translation? J. Clin. Invest. 2010, 120, 29–40. [Google Scholar]
- Martino, S.; Marconi, P.; Tancini, B.; Dolcetta, D.; De Angelis, M.G.; Montanucci, P.; Bregola, G.; Sandhoff, K.; Bordignon, C.; Orlacchio, A.; et al. A direct gene transfer strategy via brain internal capsule reverses the biochemical defect in Tay-Sachs disease. Hum. Mol. Genet. 2005, 14, 2113–2123. [Google Scholar]
- Martino, S.; di Girolamo, I.; Orlacchio, A.; Datti, A.; Orlacchio, A. MicroRNA implications across neurodevelopment and neuropathology. J. Biomed. Biotechnol. 2009, 2009, 654346. [Google Scholar]
- Martino, S.; di Girolamo, I.; Cavazzin, C.; Tiribuzi, R.; Galli, R.; Rivaroli, A.; Valsecchi, M.; Sandhoff, K.; Sonnino, S.; Vescovi, A.; et al. Neural precursor cell cultures from GM2 gangliosidosis animal models recapitulate the biochemical and molecular hallmarks of the brain pathology. J. Neurochem. 2009, 109, 135–147. [Google Scholar]
- Gentner, B.; Visigalli, I.; Hiramatsu, H.; Lechman, E.; Ungari, S.; Giustacchini, A.; Schira, G.; Amendola, M.; Quattrini, A.; Martino, S.; et al. Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy. Sci. Transl. Med. 2010, 2, 58–84. [Google Scholar]
- Lattanzi, A.; Neri, M.; Maderna, C.; di Girolamo, I.; Martino, S.; Orlacchio, A.; Amendola, M.; Naldini, L.; Gritti, A. Widespread enzymatic correction of CNS tissues by a single intracerebralinjection of therapeutic lentiviral vector in leukodystrophy mouse models. Hum. Mol. Genet. 2010, 19, 2208–2227. [Google Scholar]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; de Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl. J. Med. 2010, 363, 147–155. [Google Scholar]
- Rama, P.; Bonini, S.; Lambiase, A.; Golisano, O.; Paterna, P.; de Luca, M.; Pellegrini, G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 2001, 72, 1478–1485. [Google Scholar]
- Pellegrini, G.; Ranno, R.; Stracuzzi, G.; Bondanza, S.; Guerra, L.; Zambruno, G.; Micali, G.; de Luca, M. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation 1999, 68, 868–879. [Google Scholar]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P.; et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008, 372, 2023–2030. [Google Scholar]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; de Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar]
- Ali, R.R.; Sowden, J.C. Regenerative medicine: DIY eye. Nature 2011, 472, 42–43. [Google Scholar]
- Chen, J.; Jiang, H.; Dong, L.; Wang, Y.; Luo, C.; Zhou, M.; Zhang, W.; Huang, S.; Gu, X.; Qiu, W.; et al. Treatment of 2 children with mucopolysaccharidosis by allogeneic hematopoietic stem cell transplantation. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2008, 25, 675–677. [Google Scholar]
- Yeung, A.H.; Cowan, M.J.; Horn, B.; Rosbe, K.W. Airway management in children with mucopolysaccharidoses. Arch. Otolaryngol. Head Neck. Surg. 2009, 135, 73–79. [Google Scholar]
- Roomans, G.M. Tissue engineering and the use of stem/progenitor cells for airway epithelium repair. Eur. Cell. Mater. 2010, 19, 284–299. [Google Scholar]
- Loffredo, F.S.; Steinhauser, M.L.; Gannon, J.; Lee, R.T. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell. Stem. Cell. 2011, 8, 389–398. [Google Scholar]
- Baumann, K. Dividing with symmetry. Nat. Rev. Mol. Cell. Biol. 2010, 11, 849–860. [Google Scholar]
- Williams, S.E.; Beronja, S.; Pasolli, H.A.; Fuchs, E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 2011, 470, 353–358. [Google Scholar]
- Snippert, H.J.; van der Flier, L.G.; Sato, T.; van Es, J.H.; van den Born, M.; Kroon-Veenboer, C.; Barker, N.; Klein, A.M.; van Rheenen, J.; Simons, B.D.; et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010, 143, 134–144. [Google Scholar]
- Lopez-Garcia, C.; Klein, A.M.; Simons, B.D.; Winton, D.J. Intestinal stem cell replacement follows a pattern of neutral drift. Science 2010, 330, 822–825. [Google Scholar]
- Fuchs, E.; Tumbar, T.; Guasch, G. Socializing with the neighbors: stem cells and their niche. Cell 2004, 116, 769–778. [Google Scholar]
- Conover, J.C.; Notti, R.Q. The neural stem cell niche. Cell Tissue Res. 2008, 331, 211–224. [Google Scholar]
- Anderson, M.F.; Aberg, M.A.; Nilsson, M.; Eriksson, P.S. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res. Dev. Brain Res. 2002, 134, 115–122. [Google Scholar]
- Conover, J.C.; Allen, R.L. The subventricular zone: new molecular and cellular developments. Cell Mol. Life Sci. 2002, 59, 2128–2135. [Google Scholar]
- Doetsch, F. A niche for adult neural stem cells. Curr. Opin. Genet Dev. 2003, 13, 543–550. [Google Scholar]
- Hagg, T. Molecular regulation of adult CNS neurogenesis: An integrated view. Trends Neurosci. 2005, 28, 589–595. [Google Scholar]
- Petersen, P.H.; Zou, K.; Hwang, J.K.; Jan, Y.N.; Zhong, W. Progenitor cell maintenance requires Numb and Numblike during mouse neurogenesis. Nature 2002, 419, 929–934. [Google Scholar]
- Temple, S. The development of neural stem cells. Nature 2001, 414, 112–117. [Google Scholar]
- Lim, D.A.; Alvarez-Buylla, A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl. Acad. Sci. USA. 1999, 96, 7526–7531. [Google Scholar]
- Lennington, J.B.; Yang, Z.; Conover, J.C. Neural stem cells and the regulation of adult neurogenesis. Reprod. Biol. Endocrinol. 2003, 1, 99–105. [Google Scholar]
- Maric, D.; Fiorio, Pla, A.; Chang, Y.H.; Barker, J.L. Self-renewing and differentiating properties of cortical neural stem cells are selectively regulated by basic fibroblast growth factor (FGF) signaling via specific FGF receptors. J. Neurosci. 2007, 27, 1836–1852. [Google Scholar]
- Brazel, C.Y.; Rao, M.S. Aging and neuronal replacement. Ageing Res. Rev. 2004, 3, 465–483. [Google Scholar]
- Enwere, E.; Shingo, T.; Gregg, C.; Fujikawa, H.; Ohta, S.; Weiss, S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004, 24, 8354–8365. [Google Scholar]
- Tropepe, V.; Craig, C.G.; Morshead, C.M.; van der Kooy, D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J. Neurosci. 1997, 17, 7850–7859. [Google Scholar]
- Caporaso, G.L.; Lim, D.A.; Alvarez-Buylla, A.; Chao, M.V. Telomerase activity in the subventricular zone of adult mice. Mol. Cell Neurosci. 2003, 23, 693–702. [Google Scholar]
- Blackburn, E.H. Switching and signaling at the telomere. Cell 2001, 106, 661–673. [Google Scholar]
- Smogorzewska, A.; de Lange, T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 2004, 73, 177–208. [Google Scholar]
- Sarin, K.Y.; Cheung, P.; Gilison, D.; Lee, E.; Tennen, R.I.; Wang, E.; Artandi, M.K.; Oro, A.E.; Artandi, S.E. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 2005, 436, 1048–1052. [Google Scholar]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.Y.; Trounson, A.; Bongso, A. Embryonic stem cell lines from human blastocysts: Somatic, differentiation in vitro. Nat. Biotechnol. 2000, 18, 399–404. [Google Scholar]
- Reubinoff, B.E.; Itsykson, P.; Turetsky, T.; Pera, M.F.; Reinhartz, E.; Itzik, A.; Ben-Hur, T. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1134–1140. [Google Scholar]
- Schuldiner, M.; Eiges, R.; Eden, A.; Yanuka, O.; Itskovitz-Eldor, J.; Goldstein, R.S.; Benvenisty, N. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001, 913, 201–205. [Google Scholar]
- Schuldiner, M.; Yanuka, O.; Itskovitz-Eldor, J.; Melton, D.A.; Benvenisty, N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11307–11312. [Google Scholar]
- Zhang, S.C.; Wernig, M.; Duncan, ID.; Brustle, O.; Thomson, J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1129–1133. [Google Scholar]
- Kaufman, D.S.; Hanson, E.T.; Lewis, R.L.; Auerbach, R.; Thomson, J.A. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 10716–10721. [Google Scholar]
- Kehat, I.; Kenyagin-Karsenti, D.; Snir, M.; Segev, H.; Amit, M.; Gepstein, A.; Livne, E.; Binah, O.; Itskovitz-Eldor, J.; Gepstein, L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 2001, 108, 407–414. [Google Scholar]
- Levenberg, S.; Golub, J.S.; Amit, M.; Itskovitz-Eldor, J.; Langer, R. Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 4391–4396. [Google Scholar]
- Assady, S.; Maor, G.; Amit, M.; Itskovitz-Eldor, J.; Skorecki, K.L.; Tzukerman, M. Insulin production by human embryonic stem cells. Diabetes 2001, 50, 1691–1697. [Google Scholar]
- Fehrer, C.; Lepperdinger, G. Mesenchymal stem cell aging. Exp. Gerontol. 2005, 40, 926–930. [Google Scholar]
- Muraglia, A.; Cancedda, R.; Quarto, R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J. Cell Sci. 2000, 113, 1161–1166. [Google Scholar]
- Koh, C.J.; Atala, A. Tissue engineering, stem cells, and cloning: Opportunities for regenerative medicine. J. Am. Soc. Nephrol. 2004, 15, 1113–1125. [Google Scholar]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000, 6, 88–95. [Google Scholar]
- Zhang, W.W.; Zhang, X.J.; Liu, H.X.; Chen, J.; Ren, Y.H.; Huang, D.G.; Zou, X.H.; Xiao, W. Cdk1 is required for the self-renewal of mouse embryonic stem cells. J. Cell Biochem. 2011, 112, 942–948. [Google Scholar]
- Neganova, I.; Vilella, F.; Atkinson, S.P.; Lloret, M.; Passos, J.F.; von Zglinicki, T.; O'Connor, J.E.; Burks, D.; Jones, R.; Armstrong, L.; et al. An important role for CDK2 in G1 to S checkpoint activation and DNA damage response in human embryonic stem cells. Stem Cells 2011, 29, 651–659. [Google Scholar]
- Kamiya, D.; Banno, S.; Sasai, N.; Ohgushi, M.; Inomata, H.; Watanabe, K.; Kawada, M.; Yakura, R.; Kiyonari, H.; Nakao, K.; et al. Intrinsic transition of embryonic stem-cell differentiation into neural progenitors. Nature 2011, 470, 503–509. [Google Scholar]
- Jiang, Z.; Adams, GB.; Hanash, AM.; Scadden, DT.; Levy, RB. The contribution of cytotoxic and noncytotoxic function by donor T-cells that support engraftment after allogeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 2002, 8, 588–596. [Google Scholar]
- Bossolasco, P.; Montemurro, T.; Cova, L.; Zangrossi, S.; Calzarossa, C.; Buiatiotis, S.; Soligo, D.; Bosari, S.; Silani, V.; Deliliers, GL.; et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006, 16, 329–336. [Google Scholar]
- Ilancheran, S.; Michalska, A.; Peh, G.; Wallace, E.M.; Pera, M.; Manuelpillai, U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol. Reprod. 2007, 77, 577–588. [Google Scholar]
- Lakshmipathy, U.; Verfaillie, C. Stem cell plasticity. Blood Rev. 2005, 19, 29–38. [Google Scholar]
- Cao, F.J.; Feng, S.Q. Human umbilical cord mesenchymal stem cells and the treatment of spinal cord injury. Chin. Med. J. (Engl). 2009, 122, 225–231. [Google Scholar]
- Tonlorenzi, R.; Della valle, A.; Schnapp, E.; Cossu, G.; Sampaolesi, M. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr. Protoc. Stem Cell Biol. 2007, 2. [Google Scholar] [CrossRef]
- Martino, S.; D'Angelo, F.; Armentano, I.; Tiribuzi, R.; Pennacchi, M.; Dottori, M.; Mattioli, S.; Caraffa, A.; Cerulli, G.G.; Kenny, J.M.; Orlacchio, A. Hydrogenated amorphous carbon nanopatterned film designs drive human bone marrow mesenchymal stem cell cytoskeleton architecture. Tissue Eng. Part A. 2009, 15, 3139–3149. [Google Scholar]
- Martino, S.; Tiribuzi, R.; Ciraci, E.; Makrypidi, G.; D'Angelo, F.; di Girolamo, I.; Gritti, A.; de Angelis, G.M.; Papaccio, G.; Sampaolesi, M.; et al. Coordinated involvement of cathepsins S, D and cystatin C in the commitment of hematopoietic stem cells to dendritic cells. Int. J. Biochem. Cell Biol. 2011, 43, 775–783. [Google Scholar]
- Vescovi, A.L.; Snyder, E.Y. Establishment and properties of neural stem cell clones: plasticity in vitro and in vivo. Brain Pathol. 1999, 9, 569–598. [Google Scholar]
- Lindvall, O.; Kokaia, Z.; Martinez-Serrano, A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004, 10, S42–S50. [Google Scholar]
- Gritti, A.; Galli, R.; Vescovi, A.L. Clonal analyses and cryopreservation of neural stem cell cultures. Methods Mol. Biol. 2008, 438, 173–184. [Google Scholar]
- Lee, P.H.; Park, H.J. Bone marrow-derived mesenchymal stem cell therapy as a candidate disease-modifying strategy in Parkinson's disease and multiple system atrophy. J. Clin. Neurol. 2009, 5, 1–10. [Google Scholar]
- Orlacchio, A.; Bernardi, G.; Orlacchio, A.; Martino, S. Stem cells: an overview of the current status of therapies for central and peripheral nervous system diseases. Curr. Med. Chem. 2010, 17, 595–608. [Google Scholar]
- Galli, R.; Borello, U.; Gritti, A.; Minasi, M.G.; Bjornson, C.; Coletta, M.; Mora, M.; De Angelis, M.G.; Fiocco, R.; Cossu, G.; et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat. Neurosci. 2000, 3, 986–991. [Google Scholar]
- Brittan, M.; Wright, N.A. Gastrointestinal stem cells. J. Pathol. 2002, 197, 492–509. [Google Scholar]
- Lemischka, I.R.; Moore, K.A. Stem cells: Interactive niches. Nature 2003, 425, 778–779. [Google Scholar]
- Kiger, A.A.; Jones, D.L.; Schulz, C.; Rogers, M.B.; Fuller, M.T. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 2001, 294, 2542–2545. [Google Scholar]
- Spradling, A.; Drummond-Barbosa, D.; Kai, T. Stem cells find their niche. Nature 2001, 414, 98–104. [Google Scholar]
- Cassano, M.; Quattrocelli, M.; Crippa, S.; Perini, I.; Ronzoni, F.; Sampaolesi, M. Cellular mechanisms and local progenitor activation to regulate skeletal muscle mass. J. Muscle. Res. Cell Motil. 2009, 30, 243–253. [Google Scholar]
- d'Aquino, R.; Tirino, V.; Desiderio, V.; Studer, M.; De Angelis, G.C.; Laino, L.; De Rosa, A.; Di Nucci, D.; Martino, S.; Paino, F.; et al. Human neural crest-derived postnatal cells exhibit remarkable embryonic attributes either in vitro or in vivo. Eur. Cell Mater. 2011, 21, 304–316. [Google Scholar]
- Parolini, O.; Caruso, M. Review: Preclinical studies on placenta-derived cells and amniotic membrane: an update. Placenta. 2011, 32 (Suppl. 2), S186–S195. [Google Scholar]
- Jiang, Y.; Lv, H.; Huang, S.; Tan, H.; Zhang, Y.; Li, H. Bone marrow mesenchymal stem cells can improve the motor function of a Huntington's disease rat model. Neurol Res. 2011, 33, 331–337. [Google Scholar]
- Marcus, A.J.; Woodbury, D. Fetal stem cells from extra-embryonic tissues: Do not discard. J. Cell Mol. Med. 2008, 12, 730–742. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar]
- Hu, K.; Yu, J.; Suknuntha, K.; Tian, S.; Montgomery, K.; Choi, K.D.; Stewart, R.; Thomson, J.A.; Slukvin, I.I. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood 2011, 117, 109–119. [Google Scholar]
- Montserrat, N.; Garreta Bahima, E.; Gonzalez, F.; Gutierrez, J.; Eguizabal, C.; Ramos Perez, V.; Borros, S.; Izpisua Belmonte, J.C. Simple generation of human induced Pluripotent stem cells using Poly({beta}-Amino Esters) as non-viral gene delivery system. J. Biol. Chem. 2011, 286, 12417–12428. [Google Scholar]
- Narsinh, K.H.; Sun, N.; Sanchez-Freire, V.; Lee, A.S.; Almeida, P.; Hu, S.; Jan, T.; Wilson, K.D.; Leong, D.; Rosenberg, J. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J. Clin. Invest. 2011, 121, 1217–1221. [Google Scholar]
- Pasi, C.E.; Dereli-Öz, A.; Negrini, S.; Friedli, M.; Fragola, G.; Lombardo, A.; van Houwe, G.; Naldini, L.; Casola, S.; Testa, G.; Halazonetis, T.D. Genomic instability in induced stem cells. Cell Death Differ. 2011, 18, 745–753. [Google Scholar]
- Yazawa, M.; Hsueh, B.; Jia, X.; Pasca, A.M.; Bernstein, J.A.; Hallmayer, J.; Dolmetsch, R.E. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 2011, 471, 230–234. [Google Scholar]
- Ye, Z.; Zhan, H.; Mali, P.; Dowey, S.; Williams, D.M.; Jang, Y.Y.; Dang, C.V.; Spivak, J.L.; Moliterno, A.R.; Cheng, L. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood 2009, 114, 5473–5480. [Google Scholar]
- Park, I.H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-specific induced pluripotent stem cells. Cell 2008, 134, 877–886. [Google Scholar]
- Ebert, A.D.; Yu, J.; Rose, F.F., Jr.; Mattis, V.B.; Lorson, C.L.; Thomson, J.A.; Svendsen, C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009, 457, 277–280. [Google Scholar]
- Vitale, A.M.; Wolvetang, E.; Mackay-Sim, A. Induced pluripotent stem cells: A new technology to study human diseases. Int. J. Biochem. Cell Biol. 2011, in press. [Google Scholar]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stabil. 2010, 95, 2126–2146. [Google Scholar]
- Wang, J.H.; Thampatty, B.P. Mechanobiology of adult and stem cells. Int. Rev. Cell Mol. Biol. 2008, 271, 301–304. [Google Scholar]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar]
- del Rio, A.; Perez-Jimenez, R.; Liu, R.; Roca-Cusachs, P.; Fernandez, J.M.; Sheetz, M.P. Stretching single talin rod molecules activates vinculin binding. Science 2009, 323, 638–641. [Google Scholar]
- Ingber, D.E. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar]
- Ingber, D.E. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008, 97, 163–179. [Google Scholar]
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signalling. Annu. Rev. Cell Dev. Biol. 1996, 12, 463–518. [Google Scholar]
- Ingberg, D. Integrins as mechanochemical transducers. Curr. Opin. Cell. Biol. 1991, 3, 841–848. [Google Scholar]
- Sadoshima, J.; Izumo, S. the cellular and molecular response of myocytes to mechanical stress. Annu. Rev. Physiol. 1997, 59, 551–571. [Google Scholar]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone away. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar]
- D'Angelo, F.; Armentano, I.; Mattioli, S.; Crispoltoni, L.; Tiribuzi, R.; Cerulli, G.G.; Palmerini, C.A.; Kenny, J.M.; Martino, S.; Orlacchio, A. Micropatterned hydrogenated amorphous carbon guides mesenchymal stem cells towards neuronal differentiation. Eur. Cell Mater. 2010, 20, 231–244. [Google Scholar]
- Biggs, M.J.; Richards, R.G.; Dalby, M.J. Nanotopographical modification: a regulator of cellular function through focal adhesions. Nanomedicine 2010, 6, 619–633. [Google Scholar]
- Sardella, E.; Detomaso, L.; Gristina, R.; Senesi, G.S.; Agheli, H.; Sutherland, D.S.; d'Agostino, R.; Favia, P. Nano-structured cell-adhesive and cell-repulsive plasma-deposited coatings: chemical and topographical effects on keratinocyte adhesion. Plasma Process. Polym. 2008, 5, 540–551. [Google Scholar]
- Armentano, I.; Ciapetti, G.; Pennacchi, M.; Dottori, M.; Devescovi, V.; Granchi, D.; Baldini, N.; Olalde, B.; Jurado, M.J.; Marquinez Alava, J.I.; et al. Role of PLLA Plasma Surface Modification in the Interaction with Human Marrow Stromal Cells. J. Appl. Polym. Sci. 2009, 114, 3602–3611. [Google Scholar]
- Di Mundo, R.; Gristina, R.; Sardella, E.; Intranuovo, F.; Nardulli, M.; Milella, A.; Palumbo, F.; d'Agostino, R.; Favia, P. Micro-/nanoscale structuring of cell-culture substrates with fluorocarbon plasmas. Plasma Process. Polym. 2010, 7, 212–223. [Google Scholar]
- Plant, A.L.; Bhadriraju, K.; Spurlin, T.A.; Elliott, J.T. Review Cell response to matrix mechanics: Focus on collagen. Biochim. Biophys. Acta. 2009, 1793, 893–902. [Google Scholar]
- Pek, Y.S.; Wan, A.C.A.; Ying, J.Y. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 2010, 31, 385–391. [Google Scholar]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005, 60, 24–34. [Google Scholar]
- Akhyari, P.; Fedak, P.W.; Weisel, R.D.; Lee, T.Y.; Verma, S.; Mickle, D.A.; Li, R.K. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation 2002, 106, 1137–1142. [Google Scholar]
- Garvin, J.; Qi, J.; Maloney, M.; Banes, A.J. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003, 9, 967–979. [Google Scholar]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiaiton using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar]
- Leipzig, N.D.; Shoichet, M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30, 6867–6878. [Google Scholar]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA. 2010, 107, 4872–4877. [Google Scholar]
- Ingber, D. Mechanical signaling. Ann. N Y Acad. Sci. 2002, 961, 162–163. [Google Scholar]
- Katsumi, A.; Orr, A.W.; Tzima, E.; Schwartz, M.A. Integrins in mechanotransduction. J. Biol. Chem. 2004, 279, 12001–12004. [Google Scholar]
- Schwartz, M.A.; Schaller, M.D.; Ginsberg, M.H. Integrins: Emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 1995, 11, 549–599. [Google Scholar]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar]
- Ingber, D.E. Cellular tensegrity: Defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993, 104, 613–627. [Google Scholar]
- Stupack, D.G. The biology of integrins. Oncology (Williston Park) 2007, 21, 6–12. [Google Scholar]
- Geiger, B.; Bershadsky, A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell 2002, 110, 139–142. [Google Scholar]
- Sawada, Y.; Sheetz, M.P. Force transduction by triton cytoskeletons. J. Cell Biol. 2002, 156, 609–615. [Google Scholar]
- Wang, N.; Naruse, K.; Stamenović, D.; Fredberg, J.J.; Mijailovich, S.M.; Tolić-Nørrelykke, I.M.; Polte, T.; Mannix, R.; Ingber, D.E. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA. 2001, 98, 7765–7770. [Google Scholar]
- Kamm, K.E.; Stull, J.T. Dedicated myosin light chain kinases with diverse cellular functions. J. Biol. Chem. 2001, 276, 4527–4530. [Google Scholar]
- Pfitzer, G. Invited review: Regulation of myosin phosphorylation in smooth muscle. J. Appl. Physiol. 2001, 91, 497–503. [Google Scholar]
- Chrzanowska-Wodnicka, M.; Burridge, K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996, 133, 1403–1415. [Google Scholar]
- Bershadsky, A.D.; Ballestrem, C.; Carramusa, L.; Zilberman, Y.; Gilquin, B.; Khochbin, S.; Alexandrova, A.Y.; Verkhovsky, A.B.; Shemesh, T.; Kozlov, M.M. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur. J. Cell Biol. 2006, 85, 165–173. [Google Scholar]
- Geiger, P.C.; Bailey, J.P.; Mantilla, C.B.; Zhan, W.Z.; Sieck, G.C. Mechanisms underlying myosin heavy chain expression during development of the rat diaphragm muscle. J. Appl. Physiol. 2006, 101, 1546–1555. [Google Scholar]
- Ingber, D.E. Mechanobiology and diseases of mechanotransduction. Ann. Med. 2003, 35, 564–577. [Google Scholar]
- Kumar, S.; Maxwell, I.Z.; Heisterkamp, A.; Polte, T.R.; Lele, T.P.; Salanga, M.; Mazur, E.; Ingber, D.E. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 2006, 90, 3762–3773. [Google Scholar]
- Brangwynne, C.P.; MacKintosh, F.C.; Kumar, S.; Geisse, N.A.; Talbot, J.; Mahadevan, L.; Parker, K.K.; Ingber, D.E.; Weitz, D.A. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J. Cell Biol. 2006, 173, 733–741. [Google Scholar]
- Vikstrom, K.L.; Lim, S.S.; Goldman, R.D.; Borisy, G.G. Steady state dynamics of intermediate filament networks. J. Cell Biol. 1992, 118, 121–129. [Google Scholar]
- Fey, E.G.; Wan, K.M.; Penman, S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: Three-dimensional organization and protein composition. J. Cell Biol. 1984, 98, 1973–1984. [Google Scholar]
- Maniotis, A.J.; Chen, C.S.; Ingber, D.E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA 1997, 94, 849–854. [Google Scholar]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar]
- Kang, Y.G.; Nam, J.H.; Kim, K.H.; Lee, K.S. FAK pathway regulates PGE production in compressed periodontal ligament cells. J. Dent Res. 2010, 89, 1444–1449. [Google Scholar]
- Ziegler, N.; Alonso, A.; Steinberg, T.; Woodnutt, D.; Kohl, A.; Müssig, E.; Schulz, S.; Tomakidi, P. Mechano-transduction in periodontal ligament cells identifies activated states of MAP-kinases p42/44 and p38-stress kinase as a mechanism for MMP-13 expression. BMC Cell Biol. 2010, 11, 10. [Google Scholar]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Keely, P.J. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 2009, 28, 4326–4343. [Google Scholar]
- Wu, Z.; Wong, K.; Glogauer, M.; Ellen, R.P.; McCulloch, C.A. Regulation of stretch-activated intracellular calcium transients by actin filaments. Biochem. Biophys. Res. Commun. 1999, 261, 419–425. [Google Scholar]
- Iqbal, J.; Zaidi, M. Molecular regulation of mechanotransduction. Biochem. Biophys. Res. Commun. 2005, 328, 751–755. [Google Scholar]
- Rosen, L.B.; Greenberg, M.E. Stimulation of growth factor receptor signal transduction by activation of voltage-sensitive calcium channels. Proc. Natl. Acad. Sci. USA. 1996, 93, 1113–1118. [Google Scholar]
- Sadoshima, J.; Izumo, S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 1997, 59, 551–571. [Google Scholar]
- Sarasa-Renedo, A.; Chiquet, M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand. J. Med. Sci. Sports. 2005, 15, 223–230. [Google Scholar]
- White, C.R.; Frangos, J.A. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos. Trans. R Soc. Lond. B Biol. Sci. 2007, 362, 1459–1467. [Google Scholar]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar]
- Chien, S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1209–H1224. [Google Scholar]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53–62. [Google Scholar]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar]
- Shi, Z.D.; Wang, H.; Tarbell, J.M. Heparan sulfate proteoglycans mediate interstitial flow mechanotransduction regulating MMP-13 expression and cell motility via FAK-ERK in 3D collagen. PLoS One. 2011, 6, 15956:1–15956:10. [Google Scholar]
- Sinha, B.; Köster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; Morone, N.; Parton, R.G.; Raposo, G.; Sens, P.; Lamaze, C.; Nassoy, P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011, 144, 402–413. [Google Scholar]
- Wang, Y.; Maciejewski, B.S.; Drouillard, D.; Santos, M.; Hokenson, M.A.; Hawwa, R.L.; Huang, Z.; Sanchez-Esteban, J. A role for caveolin-1 in mechanotransduction of fetal type II epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, 775–783. [Google Scholar]
- Yang, Z.; Bidwell, J.P.; Young, S.R.; Gerard-O'Riley, R.; Wang, H.; Pavalko, F.M. Nmp4/CIZ inhibits mechanically induced beta-catenin signaling activity in osteoblasts. J. Cell Physiol. 2010, 223, 435–441. [Google Scholar]
- Jansen, J.H.; Eijken, M.; Jahr, H.; Chiba, H.; Verhaar, J.A.; van Leeuwen, J.P.; Weinans, H. Stretch-induced inhibition of Wnt/beta-catenin signaling in mineralizing osteoblasts. J. Orthop. Res. 2010, 28, 390–396. [Google Scholar]
- Santos, A.; Bakker, A.D.; Zandieh-Doulabi, B.; de Blieck-Hogervorst, J.M.; Klein-Nulend, J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem. Biophys. Res. Commun. 2010, 391, 364–369. [Google Scholar]
- Liedert, A.; Wagner, L.; Seefried, L.; Ebert, R.; Jakob, F.; Ignatius, A. Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem. Biophys. Res. Commun. 2010, 394, 755–759. [Google Scholar]
- Subbaiah, R.; Thomas, B. Efficacy of a bioactive alloplast, in the treatment of human periodontal osseous defects-a clinical study. Med. Oral Patol. Oral Cir. Bucal. 2011, 16, 239–244. [Google Scholar]
- Sendtner, M. Stem cells: Tailor-made diseased neurons. Nature 2009, 457, 269–270. [Google Scholar]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 2009, 136, 964–977. [Google Scholar]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 2008, 321, 1218–1221. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
D’Angelo, F.; Tiribuzi, R.; Armentano, I.; Kenny, J.M.; Martino, S.; Orlacchio, A. Mechanotransduction: Tuning Stem Cells Fate. J. Funct. Biomater. 2011, 2, 67-87. https://doi.org/10.3390/jfb2020067
D’Angelo F, Tiribuzi R, Armentano I, Kenny JM, Martino S, Orlacchio A. Mechanotransduction: Tuning Stem Cells Fate. Journal of Functional Biomaterials. 2011; 2(2):67-87. https://doi.org/10.3390/jfb2020067
Chicago/Turabian StyleD’Angelo, Francesco, Roberto Tiribuzi, Ilaria Armentano, Josè Maria Kenny, Sabata Martino, and Aldo Orlacchio. 2011. "Mechanotransduction: Tuning Stem Cells Fate" Journal of Functional Biomaterials 2, no. 2: 67-87. https://doi.org/10.3390/jfb2020067
APA StyleD’Angelo, F., Tiribuzi, R., Armentano, I., Kenny, J. M., Martino, S., & Orlacchio, A. (2011). Mechanotransduction: Tuning Stem Cells Fate. Journal of Functional Biomaterials, 2(2), 67-87. https://doi.org/10.3390/jfb2020067





