Multi-Composite Bioactive Osteogenic Sponges Featuring Mesenchymal Stem Cells, Platelet-Rich Plasma, Nanoporous Silicon Enclosures, and Peptide Amphiphiles for Rapid Bone Regeneration
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Mesenchymal Stem Cells for Bone Regeneration
2.3. BMP2-releasing Nanoporous Silicon Enclosures

2.4. Scaffold Fabrication

2.5. Pre-Mineralization of Scaffolds in Vitro
2.6. Scaffold Coatings and Injectable Carrier Gel for Cell and Microparticle Retention
| Material | Solution A | Solution B | Final Carrier |
|---|---|---|---|
| PRP | 20% | - | 10% |
| Osteogenic PA | 2% | - | 1% |
| Fibrinogen | 3 mg/mL | - | 1.5 mg/mL |
| CaCl2 | - | 100 mM | 50 mM |
| Thrombin (Human) | - | 100 units/mL | 50 units/mL |
| MSC | If applicable | ||
| GF-loaded Microparticles | If applicable |
2.7. In Vitro Mineralization-Injectable Gel Carrier
2.8. In Vitro Mineralization—Assembled Scaffolds
| Group Name | Cell Type | PRP | PA | NSE |
|---|---|---|---|---|
| 1. Scaffold | - | - | - | - |
| 2. PA only | - | - | X | - |
| 3. CB MSC | CB | X | X | X |
| 4. BM MSC | BM | X | X | X |
| 5. No PRP | CB | - | X | X |
| 6. No PA | CB | X | - | X |
| 7. No NSE | CB | X | X | - |
2.9. In Vivo Bone Formation and Angiogenesis
2.9.1. Implant Preparation and Loading Efficiency
2.9.2. Surgical Procedure and Implant Retrieval
3. Results and Discussion
3.1. Mesenchymal Stem Cells for Bone Regeneration

3.2. BMP2-Releasing Nanoporous Silicon Enclosures
3.3. Pre-Mineralization of Scaffolds in Vitro
3.4. Scaffold Coatings and Injectable Carrier Gel for Cell and Microparticle Retention


3.5. In Vitro Mineralization—Injectable Gel Carrier

3.6. In Vitro Mineralization—Assembled Scaffolds

| Experimental Group | PCL | Collagen |
|---|---|---|
| CB MSC (CB, PRP, PA, NSE) | 140.5 | 138.9 |
| BM MSC (BM, PRP, PA, NSE) | 119.6 | 110.5 |
| No PRP (CB, PA, NSE) | 132.2 | 148.1 |
| No PA (CB, PRP, NSE) | 113.4 | 94.9 |
| No NSE (CB, PRP, PA) | 133.1 | 141.5 |
3.7. In Vivo Bone Formation and Angiogenesis
3.7.1. Implant Preparation

3.7.2. In Vivo Bone Formation and Angiogenesis

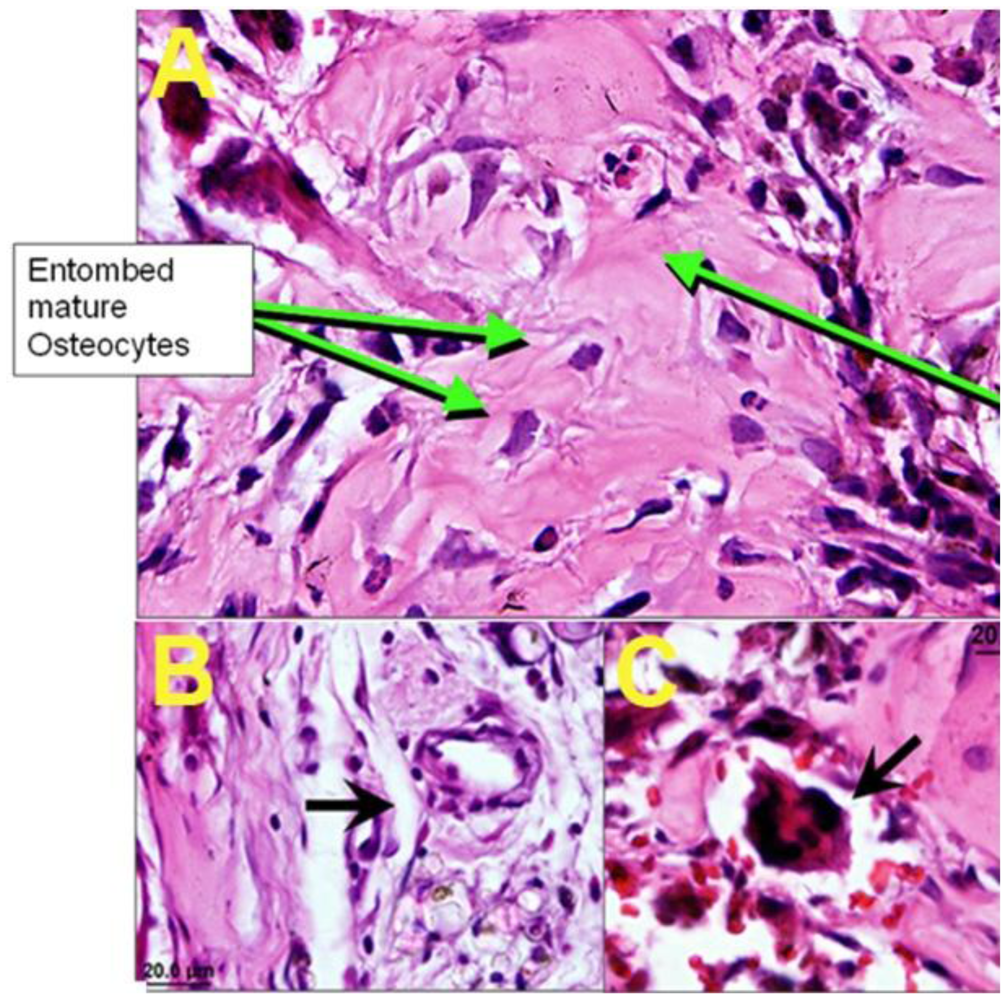
4. Conclusions
Acknowledgments
Appendix


References
- Goldstein, S.A.; Bonadio, J. Potential role for direct gene transfer in the enhancement of fracture healing. Clin Orthop Relat Res. 1998, 355, S154–S162. [Google Scholar]
- MDI, U.S. Markets for Biomaterials; Medical Data International, Inc.: Santa Ana, CA, USA, 2000. [Google Scholar]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar]
- Freed, L.E.; VunjakNovakovic, G. Tissue engineering bioreactors. In Principles of Tissue Engineering; Academic Press: San Diego, CA, USA, 2000; pp. 143–156. [Google Scholar]
- Garcia, A.J.; Ducheyne, P.; Boettiger, D. Effect of surface reaction stage on fibronectin-mediated adhesion of osteoblast-like cells to bioactive glass. J. Biomed. Mater. Res. 1998, 40, 48–56. [Google Scholar]
- Holy, C.E.; Shoichet, M.S.; Davies, J.E. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: Investigating initial cell-seeding density and culture period. J. Biomed. Mater. Res. 2000, 51, 376–382. [Google Scholar]
- Ishaug, S.L.; Crane, G.M.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Bone formation bythree-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1997, 36, 17–28. [Google Scholar]
- Ma, P.X.; Zhang, R.Y.; Xiao, G.Z.; Franceschi, R. Engineering new bone tissue in vitro on highly porous poly(alpha-hydroxyl acids)/hydroxyapatite composite scaffolds. J. Biomed. Mater. Res. 2001, 54, 284–293. [Google Scholar]
- Marra, K.G.; Szem, J.W.; Kumta, P.N.; DiMilla, P.A.; Weiss, L.E. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. J. Biomed. Mater. Res. 1999, 47, 324–335. [Google Scholar]
- Schoeters, G.; Leppens, H.; Van Gorp, U.; Van Den Heuvel, R. Haemopoietic long-term bone marrow cultures from adult mice show osteogenic capacity in vitro on 3-dimensional collagen sponges. Cell Proliferation 1992, 25, 587–603. [Google Scholar]
- Xynos, I.D.; Hukkanen, M.V.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar]
- Crane, G.M.; Ishaug, S.L.; Mikos, A.G. Bone tissue engineering. Nat. Med. 1995, 1, 1322–1324. [Google Scholar]
- Murphy, M.B.; Blashki, D.; Buchanan, R.M.; Tasciotti, E. Engineering a better way to heal broken bones. Chem. Eng. Prog. 2010, 106, 37–43. [Google Scholar]
- Nimni, M.E.; Cheung, D.; Strates, B.; Kodama, M.; Sheikh, K. Chemically modified collagen: A natural biomaterial for tissue replacement. J. Biomed. Mater. Res. 1987, 21, 741–771. [Google Scholar]
- Kale, S.; Biermann, S.; Edwards, C.; Tarnowski, C.; Morris, M.; Long, M.W. Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat. Biotechnol. 2000, 18, 954–958. [Google Scholar]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar]
- Schliephake, H.; Tavassol, F.; Gelinsky, M.; Dard, M.; Sewing, A.; Pompe, W. Use of a mineralized collagen membrane to enhance repair of calvarial defects in rats. Clin. Oral Implants Res. 2004, 15, 112–118. [Google Scholar]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar]
- Ma, Z.W.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar]
- Sung, H.J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar]
- Yang, Y.Y.; Chung, T.S.; Ng, N.P. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials 2001, 22, 231–241. [Google Scholar]
- Sahoo, S.; Cho-Hong, J.G.; Siew-Lok, T. Development of hybrid polymer scaffolds for potential applications in ligament and tendon tissue engineering. Biomed. Mater. 2007, 2, 169–173. [Google Scholar]
- Yang, T.L.; Hsiao, Y.C.; Young, T.H. Comparison of plga, pcl, and chitosan in salivary gland branching morphogenesis. Biomed. Eng. Appl. Basis Commun. 2008, 20, 287–296. [Google Scholar]
- Baker, S.C.; Rohman, G.; Southgate, J.; Cameron, N.R. The relationship between the mechanical properties and cell behaviour on PLGA and PCL scaffolds for bladder tissue engineering. Biomaterials 2009, 30, 1321–1328. [Google Scholar]
- Saito, E.; Kang, H.; Taboas, J.M.; Diggs, A.; Flanagan, C.L.; Hollister, S.J. Experimental and computational characterization of designed and fabricated 50:50 PLGA porous scaffolds for human trabecular bone applications. J. Mater. Sci.: Mater. Med. 2010, 21, 2371–2383. [Google Scholar]
- Caplan, A.I. The mesengenic process. Clin. Plast. Surg. 1994, 21, 429–435. [Google Scholar]
- Filshie, R.J.A.; Zannettino, A.C.W.; Makrynikola, V.; Gronthos, S.; Henniker, A.J.; Bendall, L.J.; Gottlieb, D.J.; Simmons, P.J.; Bradstock, K.F. MUC18, a member of the immunoglobulin superfamily, is expressed on bone marrow fibroblasts and a subset of hematological malignancies. Leukemia 1998, 12, 414–421. [Google Scholar]
- Gronthos, S.; Fitter, S.; Diamond, P.; Simmons, P.J.; Itescu, S.; Zannettin, A.C.W. A novel monoclonal antibody (STRO-3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev. 2007, 16, 953–963. [Google Scholar]
- Gronthos, S.; Graves, S.E.; Ohta, S.; Simmons, P.J. The Stro-1(+) Fraction of Adult Human Bone-Marrow Contains the Osteogenic Precursors. Blood 1994, 84, 4164–4173. [Google Scholar]
- Gronthos, S.; Simmons, P.J. The growth-factor requirements of stro-1-positive human bone-marrow stromal precursors under serum-deprived conditions in-vitro. Blood 1995, 85, 929–940. [Google Scholar]
- Gronthos, S.; Simmons, P.J.; Graves, S.E.; Robey, P.G. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone 2001, 28, 174–181. [Google Scholar]
- Gronthos, S.; Stewart, K.; Graves, S.E.; Hay, S.; Simmons, P.J. Integrin expression and function on human osteoblast-like cells. J. Bone Miner. Res. 1997, 12, 1189–1197. [Google Scholar]
- Gronthos, S.; Zannettino, A.C.W.; Hay, S.J.; Shi, S.T.; Graves, S.E.; Kortesidis, A.; Simmons, P.J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 2003, 116, 1827–1835. [Google Scholar]
- Lundberg, P.; Allison, S.J.; Lee, N.J.; Baldock, P.A.; Brouard, N.; Rost, S.; Enriquez, R.F.; Sainsbury, A.; Lamghari, M.; Simmons, P.; Eisman, J.A.; Gardiner, E.M.; Herzog, H. Greater bone formation of Y2 knockout mice is associated with increased osteoprogenitor numbers and altered Y1 receptor expression. J. Biol. Chem. 2007, 282, 19082–19091. [Google Scholar]
- Semerad, C.L.; Christopher, M.J.; Liu, F.L.; Short, B.; Simmons, P.J.; Winkler, I.; Levesque, J.P.; Chappel, J.; Ross, F.P.; Link, D.C. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood 2005, 106, 3020–3027. [Google Scholar]
- Simmons, P.J.; Torok-Storb, B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991, 78, 55–62. [Google Scholar] [Green Version]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [Green Version]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [Green Version]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [Green Version]
- Deng, Z.L.; Sharff, K.A.; Tang, N.; Song, W.X.; Luo, J.Y.; Luo, X.J.; Chen, J.; Bennett, E.; Reid, R.; Manning, D.; Xue, A.; Montag, A.G.; Luu, H.H.; Haydon, R.C.; He, T.C. Regulation of osteogenic differentiation during skeletal development. Front. Biosci. 2008, 13, 2001–2021. [Google Scholar] [Green Version]
- Colnot, C.; et al. Mechanisms of action of demineralized bone matrix in the repair of cortical bone defects. Clin. Orthop. Relat. Res. 2005, 435, 69–78. [Google Scholar] [Green Version]
- Cremer, T.; Thielemann, F.W. Role of vessels and perivascular tissue in matrix induced osteogenesis. J. Dent. Res. 1986, 65, 543–543. [Google Scholar] [Green Version]
- Schmid, J.; Wallkamm, B.; Hammerle, C.H.F.; Gogolewski, S.; Lang, N.P. The significance of angiogenesis in guided bone regeneration-A case report of a rabbit experiment. Clin. Oral Implants Res. 1997, 8, 244–248. [Google Scholar] [Green Version]
- Huang, Q.; Wang, Y.D.; Wu, T.; Jiang, S.; Hu, Y.L.; Pei, G.X. Preliminary separation of the growth factors in platelet-rich plasma: Effects on the proliferation of human marrow-derived mesenchymal stem cells. Chin. Med. J. (Engl.) 2009, 122, 83–87. [Google Scholar] [Green Version]
- Kasten, P.; Vogel, J.; Geiger, F.; Niemeyer, P.; Luginbuhl, R.; Szalay, K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials 2008, 29, 3983–3992. [Google Scholar] [Green Version]
- Hu, Z.; Peel, S.A.; Ho, S.K.; Sandor, G.K.; Clokie, C.M. Platelet-rich plasma induces mRNA expression of VEGF and PDGF in rat bone marrow stromal cell differentiation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 43–48. [Google Scholar] [Green Version]
- van den Dolder, J.; Mooren, R.; Vloon, A.P.; Stoelinga, P.J.; Jansen, J.A. Platelet-rich plasma: Quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006, 12, 3067–3073. [Google Scholar] [Green Version]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonne, A.; Delorme, B.; Herault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [Green Version]
- Ji, J.F.; He, B.P.; Dheen, S.T.; Tay, S.S. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 2004, 22, 415–427. [Google Scholar] [Green Version]
- Chamberlain, G.; Wright, K.; Rot, A.; Ashton, B.; Middleton, J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: Comparison with human. PLoS One 2008, 3, e2934. [Google Scholar] [Green Version]
- Wang, J.F.; Park, I.W.; Groopman, J.E. Stromal cell-derived factor-1alpha stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: Roles of phosphoinositide-3 kinase and protein kinase C. Blood 2000, 95, 2505–2513. [Google Scholar] [Green Version]
- Hall, M.P.; Band, P.A.; Meislin, R.J.; Jazrawi, L.M.; Cardone, D.A. Platelet-rich plasma: Current concepts and application in sports medicine. J. Am. Acad. Orthop. Surg. 2009, 17, 602–608. [Google Scholar] [Green Version]
- Kim, E.S.; Kim, J.J.; Park, E.J. Angiogenic factor-enriched platelet-rich plasma enhances in vivo bone formation around alloplastic graft material. J. Adv. Prosthodont 2010, 2, 7–13. [Google Scholar] [Green Version]
- Yoshimi, R.; Yamada, Y.; Ito, K.; Nakamura, S.; Abe, A.; Nagasaka, T.; Okabe, K.; Kohgo, T.; Baba, S.; Ueda, M. Self-assembling peptide nanofiber scaffolds, platelet-rich plasma, and mesenchymal stem cells for injectable bone regeneration with tissue engineering. J. Craniofac. Surg. 2009, 20, 1523–1530. [Google Scholar] [Green Version]
- Sarkar, M.R.; Augat, P.; Shefelbine, S.J.; Schorlemmer, S.; Huber-Lang, M.; Claes, L.; Kinzl, L.; Ignatius, A. Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials 2006, 27, 1817–1823. [Google Scholar] [Green Version]
- Simsek, S.B.; Keles, G.C.; Baris, S.; Cetinkaya, B.O. Comparison of mesenchymal stem cells and autogenous cortical bone graft in the treatment of class II furcation defects in dogs. Clin. Oral Investig. 2010. [Google Scholar] [CrossRef]
- Schuckert, K.H.; Jopp, S.; Osadnik, M. Modern bone regeneration instead of bone transplantation: A combination of recombinant human bone morphogenetic protein-2 and platelet-rich plasma for the vertical augmentation of the maxillary bone-a single case report. Tissue Eng. Part C Methods 2010, 16, 1335–1346. [Google Scholar] [Green Version]
- Stendahl, J.C.; Rao, M.S.; Guler, M.O.; Stupp, S.I. Intermolecular forces in the self-assembly of peptide amphiphilic nanofibers. Adv. Funct. Mater. 2006, 16, 499–508. [Google Scholar] [Green Version]
- Behanna, H.A.; Donners, J.J.; Gordon, A.C.; Stupp, S.I. Coassembly of amphiphiles with opposite peptide polarities into nanofibers. J. Am. Chem. Soc. 2005, 127, 1193–2000. [Google Scholar] [Green Version]
- Claussen, R.C.; Rabatic, B.M.; Stupp, S.I. Aqueous self-assembly of unsymmetric peptide amphiphiles into nanofibers with hydrophilic cores and surfaces. J. Am. Chem. Soc. 2003, 125, 12680–12681. [Google Scholar] [Green Version]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [Green Version]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. USA 2002, 99, 5133–5138. [Google Scholar] [Green Version]
- Kapadia, M.R.; Chow, L.W.; Tsihlis, N.D.; Ahanchi, S.S.; Hrabie, J.A.; Murar, J.; Martinez, J.; Popowich, D.A.; Jiang, Q.; Hrabie, J.A.; Saavedra, J.E.; Keefer, L.K.; HUlvat, J.F.; Stupp, S.I.; Kibbe, M.P. Nitric oxide and nanotechnology: A novel approach to inhibit neointimal hyperplasia. J. Vasc.Surg. 2008, 47, 173–182. [Google Scholar] [Green Version]
- Rajangam, K.; Behanna, H.A.; Hui, M.J.; Han, X.; Hulvat, J.F.; Lomasney, J.W.; Stupp, S.I. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006, 6, 2086–2090. [Google Scholar] [Green Version]
- Sargeant, T.D.; Guler, M.O.; Oppenheimer, S.M.; Mata, A.; Satcher, R.L.; Dunand, D.C.; Stupp, S.I. Hybrid bone implants: Self-assembly of peptide amphiphile nanofibers within porous titanium. Biomaterials 2008, 29, 161–171. [Google Scholar] [Green Version]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 2004, 303, 1352–1355. [Google Scholar] [Green Version]
- Tysseling-Mattiace, V.M.; Sahni, V.; Niece, K.L.; Birch, D.; Czeisler, C.; Fehlings, M.G.; Stupp, S.I.; Kessler, J.A. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 2008, 28, 3814–3823. [Google Scholar] [Green Version]
- Behanna, H.A.; Donners, J.J.J.M.; Gordon, A.C.; Stupp, S.I. Coassembly of amphiphiles with opposite peptide polarities into nanofibers. J. Am. Chem. Soc. 2005, 127, 1193–1200. [Google Scholar] [Green Version]
- Bull, S.R.; Guler, M.O.; Bras, R.E.; Meade, T.J.; Stupp, S.I. Self-assembled peptide amphiphile nanofibers conjugated to MRI contrast agents. Nano Lett. 2005, 5, 1–4. [Google Scholar] [Green Version]
- Niece, K.L.; Hartgerink, J.D.; Donners, J.J.; Stupp, S.I. Self-assembly combining two bioactive peptide-amphiphile molecules into nanofibers by electrostatic attraction. J. Am. Chem. Soc. 2003, 125, 7146–7147. [Google Scholar] [Green Version]
- Guler, M.O.; Soukasene, S.; Hulvat, J.F.; Stupp, S.I. Presentation and recognition of biotin on nanofibers formed by branched peptide amphiphiles. Nano Lett. 2005, 5, 249–252. [Google Scholar] [Green Version]
- Sargeant, T.D.; Guler, M.O.; Oppenheimer, S.M.; Mata, A.; Satcher, R.L.; Dunand, D.C.; Stupp, S.I. Hybrid bone implants: Self-assembly of peptide amphiphile nanofibers within porous titanium. Biomaterials 2008, 29, 161–171. [Google Scholar] [Green Version]
- Sone, E.D.; Stupp, S.I. Semiconductor-encapsulated peptide-amphiphile nanofibers. J. Am. Chem. Soc. 2004, 126, 12756–12757. [Google Scholar] [Green Version]
- Ali, S.A.M.; Doherty, P.J.; Williams, D.F. The mechanisms of oxidative-degradation of biomedical polymers by free-radicals. J. Appl. Polym. Sci. 1994, 51, 1389–1398. [Google Scholar] [Green Version]
- Hsu, L.; Cvetanovich, G.L.; Stupp, S.I. Peptide amphiphile nanofibers with conjugated polydiacetylene backbones in their core. J. Am. Chem. Soc. 2008, 130, 3892–3899. [Google Scholar] [Green Version]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [Green Version]
- Ferrari, M. Nanogeometry: Beyond drug delivery. Nat. Nanotechnol 2008, 3, 131–132. [Google Scholar] [Green Version]
- Tasciotti, E.; Liu, X.; Bhavane, R.; Plant, K.; Leonard, A.D.; Price, B.K.; Cheng, M.M.; Decuzzi, P.; Tour, J.M.; Robertson, F.; Ferrari, M. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat.Nanotechnol 2008, 3, 151–157. [Google Scholar] [Green Version]
- Canham, L.T. Bioactive silicon structure fabrication through nanoetching techniques. Adv. Mater. 1995, 7, 1033–1037. [Google Scholar] [Green Version]
- Canham, L.T.; Newey, J.P.; Reeves, C.L.; Houlton, M.R.; Loni, A.; Simons, A.J.; Cox, T.I. The Effects of DC electric currents on the in-vitro calcification of bioactive silicon wafers. Adv. Mater. 1996, 8, 847–849. [Google Scholar] [Green Version]
- Canham, L.T.; Reeves, C.L.; Newey, J.P.; Houlton, M.R.; Cox, T.I.; Buriak, J.M.; Stewart, M.P. Derivatized mesoporous silicon with dramatically improved stability in simulated human blood plasma. Adv. Mater. 1999, 11, 1505–1507. [Google Scholar] [Green Version]
- Sun, W.; Puzas, J.E.; Sheu, T.J.; Fauchet, P.M. Porous silicon as a cell interface for bone tissue engineering. Phys. Status Solidi A. 2007, 204, 1429–1433. [Google Scholar] [Green Version]
- Low, S.P.; Williams, K.A.; Canham, L.T.; Voelcker, N.H. Evaluation of mammalian cell adhesion on surface-modified porous silicon. Biomaterials 2006, 27, 4538–4546. [Google Scholar] [Green Version]
- Gao, T.; Aro, H.T.; Ylanen, H.; Vuorio, E. Silica-based bioactive glasses modulate expression of bone morphogenetic protein-2 mRNA in Saos-2 osteoblasts in vitro. Biomaterials 2001, 22, 1475–1483. [Google Scholar] [Green Version]
- Ducheyne, P.; Qiu, Q. Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999, 20, 2287–2303. [Google Scholar] [Green Version]
- Amato, F.; Cosentino, C.; Pricl, S.; Ferrone, M.; Fermeglia, M.; Cheng, M.M.C.; Walczak, R.; Ferrari, M. Multiscale modeling of protein transport in silicon membrane nanochannels. Part 2. From molecular parameters to a predictive continuum diffusion model. Biomed. Microdevices 2006, 8, 291–298. [Google Scholar] [Green Version]
- Chu, T.M.; Warden, S.J.; Turner, C.H.; Stewart, R.L. Segmental bone regeneration using a load-bearing biodegradable carrier of bone morphogenetic protein-2. Biomaterials 2007, 28, 459–467. [Google Scholar] [Green Version]
- Zhang, M.Q.; Desai, T.; Ferrari, M. Proteins and cells on PEG immobilized silicon surfaces. Biomaterials 1998, 19, 953–960. [Google Scholar] [Green Version]
- Yamada, Y.; Ueda, M.; Naiki, T.; Takahashi, M.; Hata, K.; Nagasaka, T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: Tissue-engineered bone regeneration. Tissue Eng. 2004, 10, 955–964. [Google Scholar] [Green Version]
- Dallari, D.; Fini, M.; Stagni, C.; Torricelli, P.; Aldini, N.N.; Giavaresi, G.; Cenni, E.; Baldini, N.; Cenacchi, A.; Bassi, A.; Giardino, R.; Fornasari, P.M.; Giunti, A. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. J. Orthop. Res. 2006, 24, 877–888. [Google Scholar] [Green Version]
- Thorwarth, M.; Wehrhan, F.; Schultze-Mosgau, S.; Wiltfang, J.; Schlegel, K.A. PRP modulates expression of bone matrix proteins in vivo without long-term effects on bone formation. Bone 2006, 38, 30–40. [Google Scholar] [Green Version]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 2003, 24, 4375–4383. [Google Scholar] [Green Version]
- Liu, Y.; de Groot, K.; Hunziker, E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 2005, 36, 745–757. [Google Scholar] [Green Version]
- Kim, C.S.; Kim, J.I.; Kim, J.; Choi, S.H.; Chai, J.K.; Kim, C.K.; Cho, K.S. Ectopic bone formation associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials 2005, 26, 2501–1507. [Google Scholar] [Green Version]
- Kasten, P.; Vogel, J.; Luginbuhl, R.; Niemeyer, P.; Weiss, S.; Schneider, S.; Kramer, M.; Leo, A.; Richter, W. Influence of platelet-rich plasma on osteogenic differentiation of mesenchymal stem cells and ectopic bone formation in calcium phosphate ceramics. Cells Tissues Organs 2006, 183, 68–79. [Google Scholar] [Green Version]
- Asou, Y.; Rittling, S.R.; Yoshitake, H.; Tsuji, K.; Shinomiya, K.; Nifuji, A.; Denhardt, D.T.; Noda, M. Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology 2001, 142, 1325–1332. [Google Scholar] [Green Version]
- Yaffe, A.; Kollerman, R.; Bahar, H.; Binderman, I. The influence of alendronate on bone formation and resorption in a rat ectopic bone development model. J. Periodontol. 2003, 74, 44–50. [Google Scholar] [Green Version]
- Yuan, H.; van Blitterswijk, C.A.; de Groot, K.; de Bruijn, J.D. Cross-species comparison of ectopic bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) scaffolds. Tissue Eng. 2006, 12, 1607–1615. [Google Scholar] [Green Version]
- Bruder, S.P.; Fox, B.S. Tissue engineering of bone. Cell based strategies. Clin. Orthop. Relat. Res. 1999, 367, S68–S83. [Google Scholar] [Green Version]
- Kneser, U.; Schaefer, D.J.; Polykandriotis, E.; Horch, R.E. Tissue engineering of bone: The reconstructive surgeon's point of view. J. Cell. Mol. Med. 2006, 10, 7–19. [Google Scholar] [Green Version]
- Mistry, A.S.; Mikos, A.G. Tissue engineering strategies for bone regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 94, 1–22. [Google Scholar] [Green Version]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murphy, M.B.; Blashki, D.; Buchanan, R.M.; Fan, D.; De Rosa, E.; Shah, R.N.; Stupp, S.I.; Weiner, B.K.; Simmons, P.J.; Ferrari, M.; et al. Multi-Composite Bioactive Osteogenic Sponges Featuring Mesenchymal Stem Cells, Platelet-Rich Plasma, Nanoporous Silicon Enclosures, and Peptide Amphiphiles for Rapid Bone Regeneration. J. Funct. Biomater. 2011, 2, 39-66. https://doi.org/10.3390/jfb2020039
Murphy MB, Blashki D, Buchanan RM, Fan D, De Rosa E, Shah RN, Stupp SI, Weiner BK, Simmons PJ, Ferrari M, et al. Multi-Composite Bioactive Osteogenic Sponges Featuring Mesenchymal Stem Cells, Platelet-Rich Plasma, Nanoporous Silicon Enclosures, and Peptide Amphiphiles for Rapid Bone Regeneration. Journal of Functional Biomaterials. 2011; 2(2):39-66. https://doi.org/10.3390/jfb2020039
Chicago/Turabian StyleMurphy, Matthew B., Daniel Blashki, Rachel M. Buchanan, Dongmei Fan, Enrica De Rosa, Ramille N. Shah, Samuel I. Stupp, Bradley K. Weiner, Paul J. Simmons, Mauro Ferrari, and et al. 2011. "Multi-Composite Bioactive Osteogenic Sponges Featuring Mesenchymal Stem Cells, Platelet-Rich Plasma, Nanoporous Silicon Enclosures, and Peptide Amphiphiles for Rapid Bone Regeneration" Journal of Functional Biomaterials 2, no. 2: 39-66. https://doi.org/10.3390/jfb2020039
APA StyleMurphy, M. B., Blashki, D., Buchanan, R. M., Fan, D., De Rosa, E., Shah, R. N., Stupp, S. I., Weiner, B. K., Simmons, P. J., Ferrari, M., & Tasciotti, E. (2011). Multi-Composite Bioactive Osteogenic Sponges Featuring Mesenchymal Stem Cells, Platelet-Rich Plasma, Nanoporous Silicon Enclosures, and Peptide Amphiphiles for Rapid Bone Regeneration. Journal of Functional Biomaterials, 2(2), 39-66. https://doi.org/10.3390/jfb2020039




