Silicon Nitride Bioceramics with TiC Additives: Excellent Mechanical Properties, Cytocompatibility, and Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Physical Characterization of Materials
2.3. Cytotoxicity Test
2.4. Cell Adhesion and Morphology
2.5. Evaluation of Antibacterial Property
2.6. Statistical Analysis
3. Results
3.1. Characterization of Materials
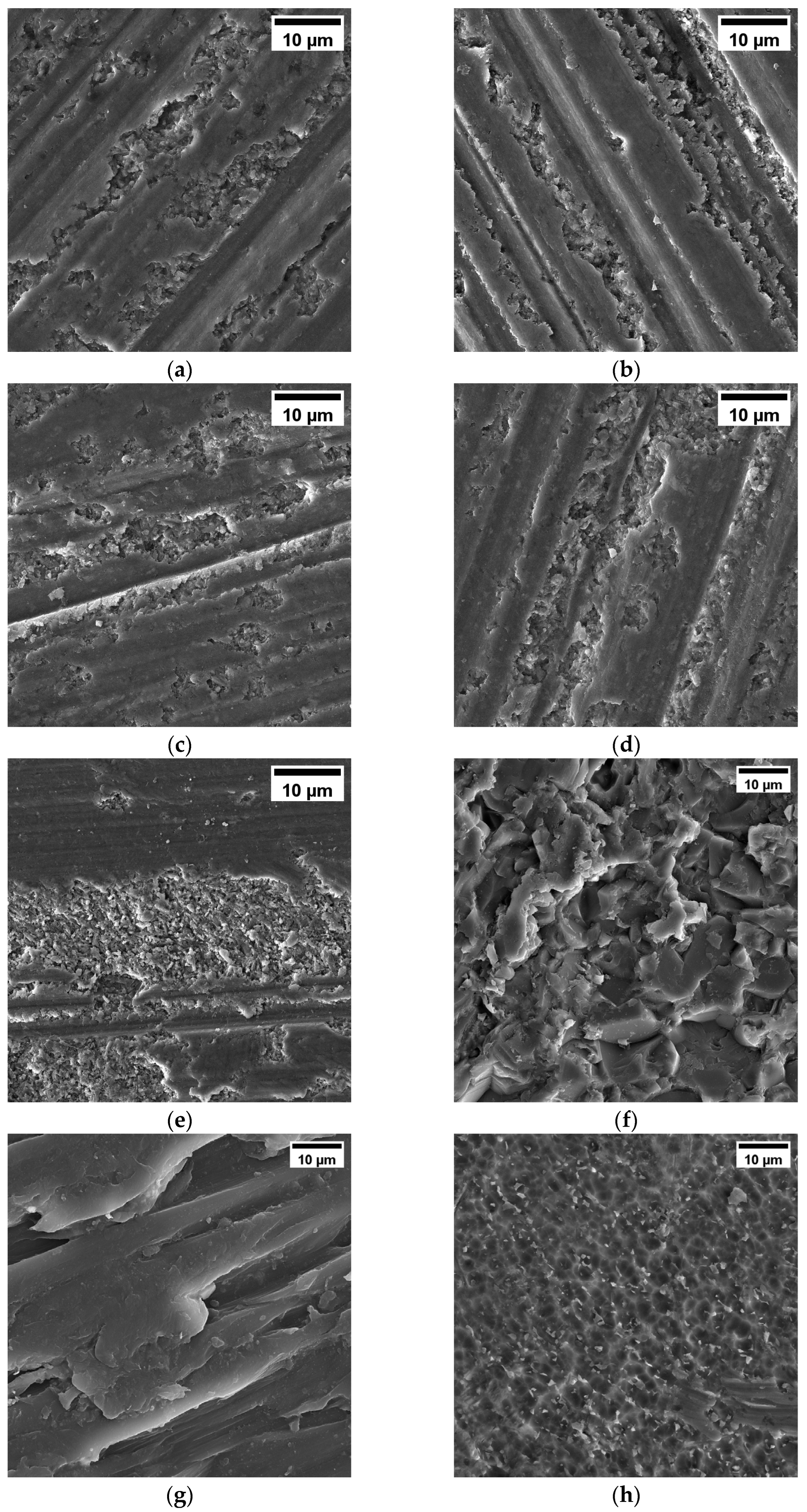
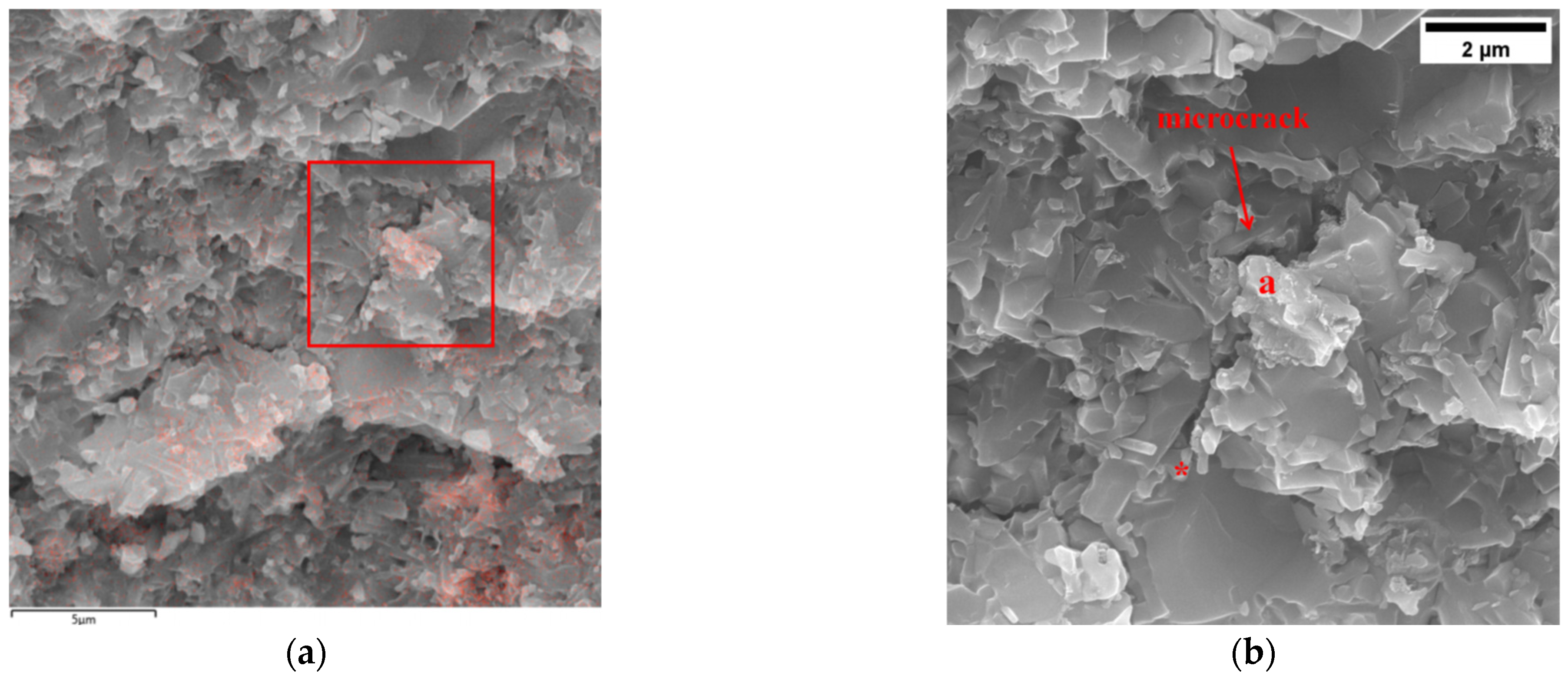
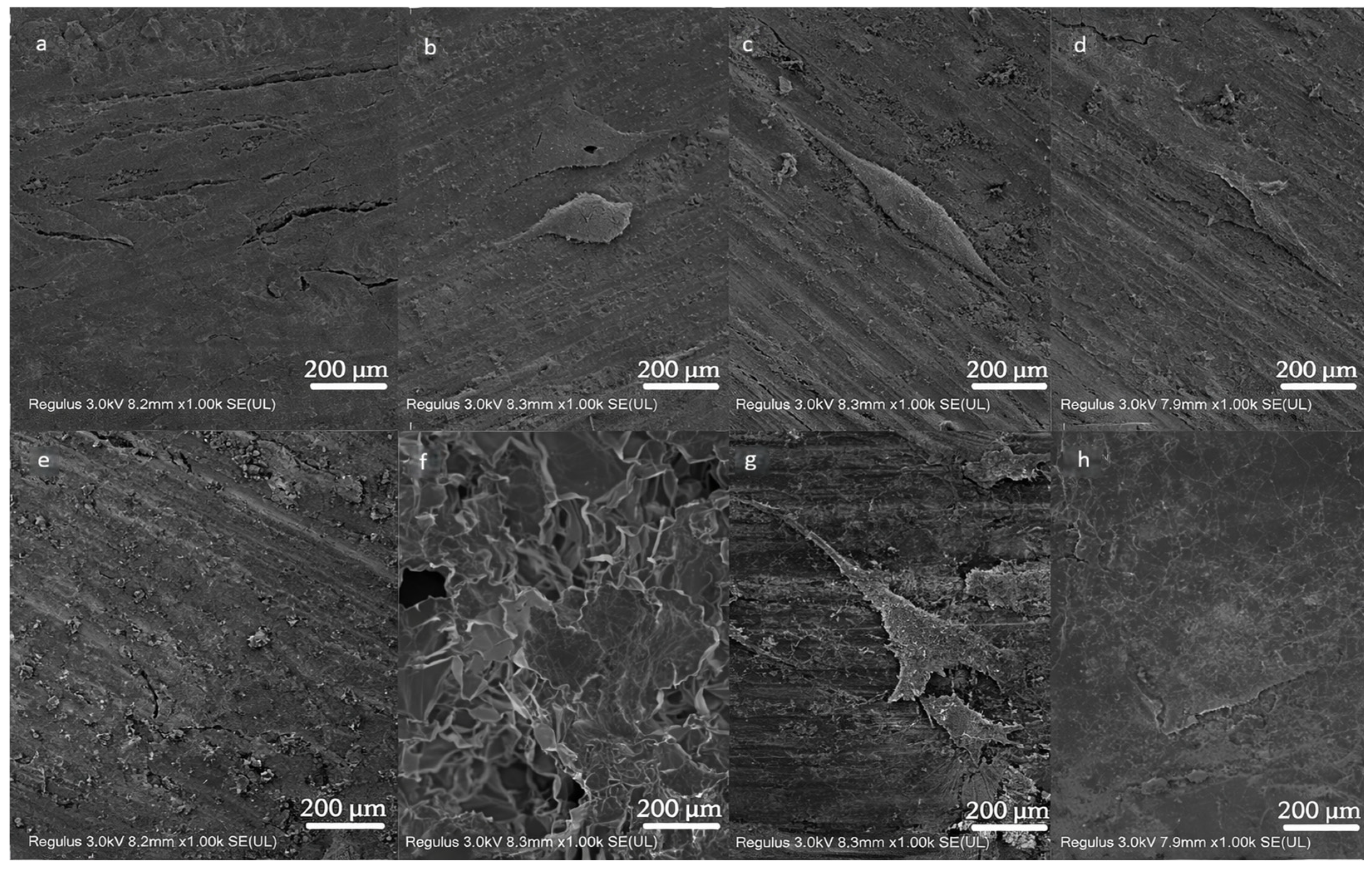
3.1.1. SEM Observation of Materials
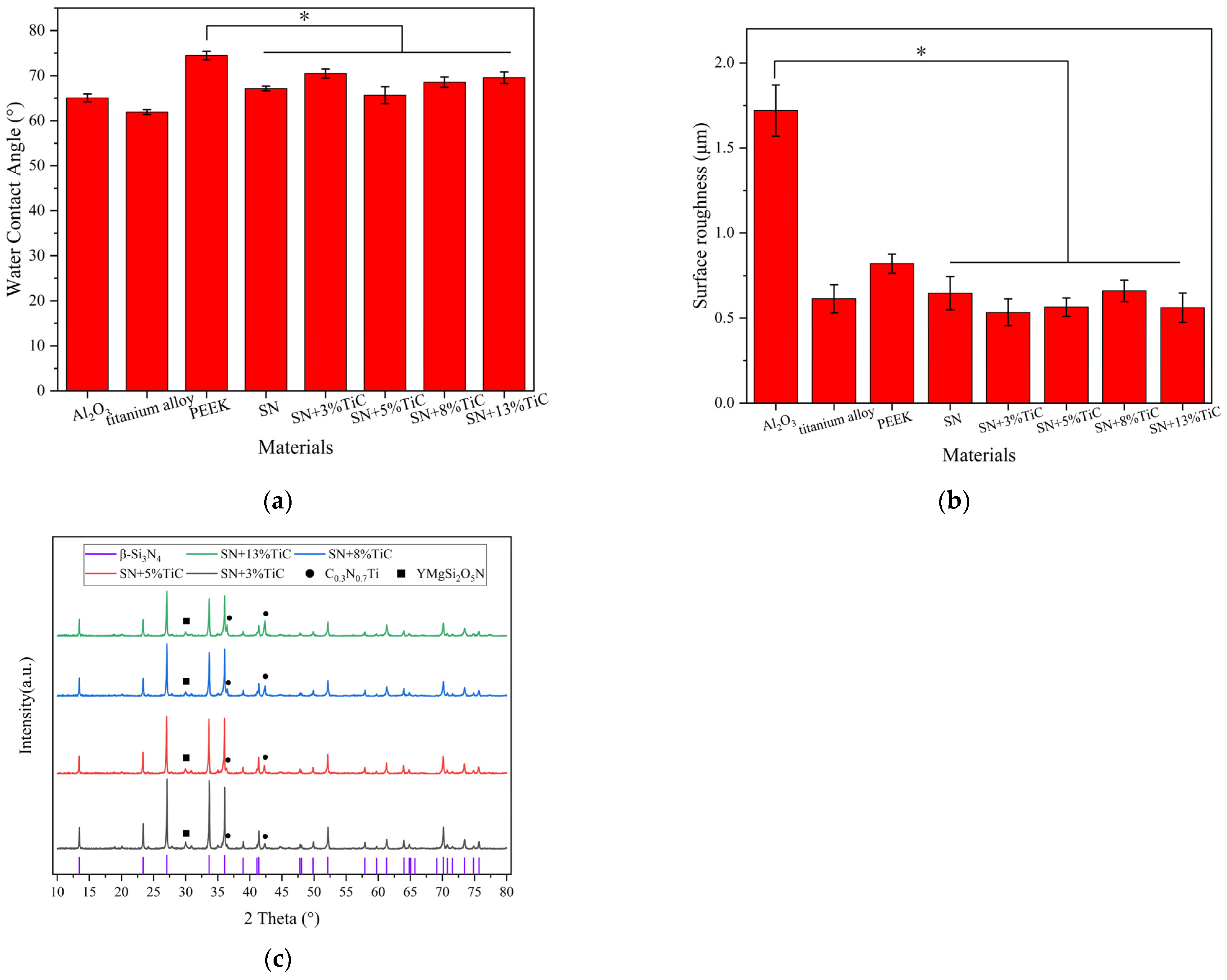
3.1.2. XRD of Silicon Nitride with TiC
3.1.3. Surface Roughness and Wettability of Materials
3.1.4. Si, Ti and Y Ions Dissolution Determination
3.2. Mechanical Properties of the Materials
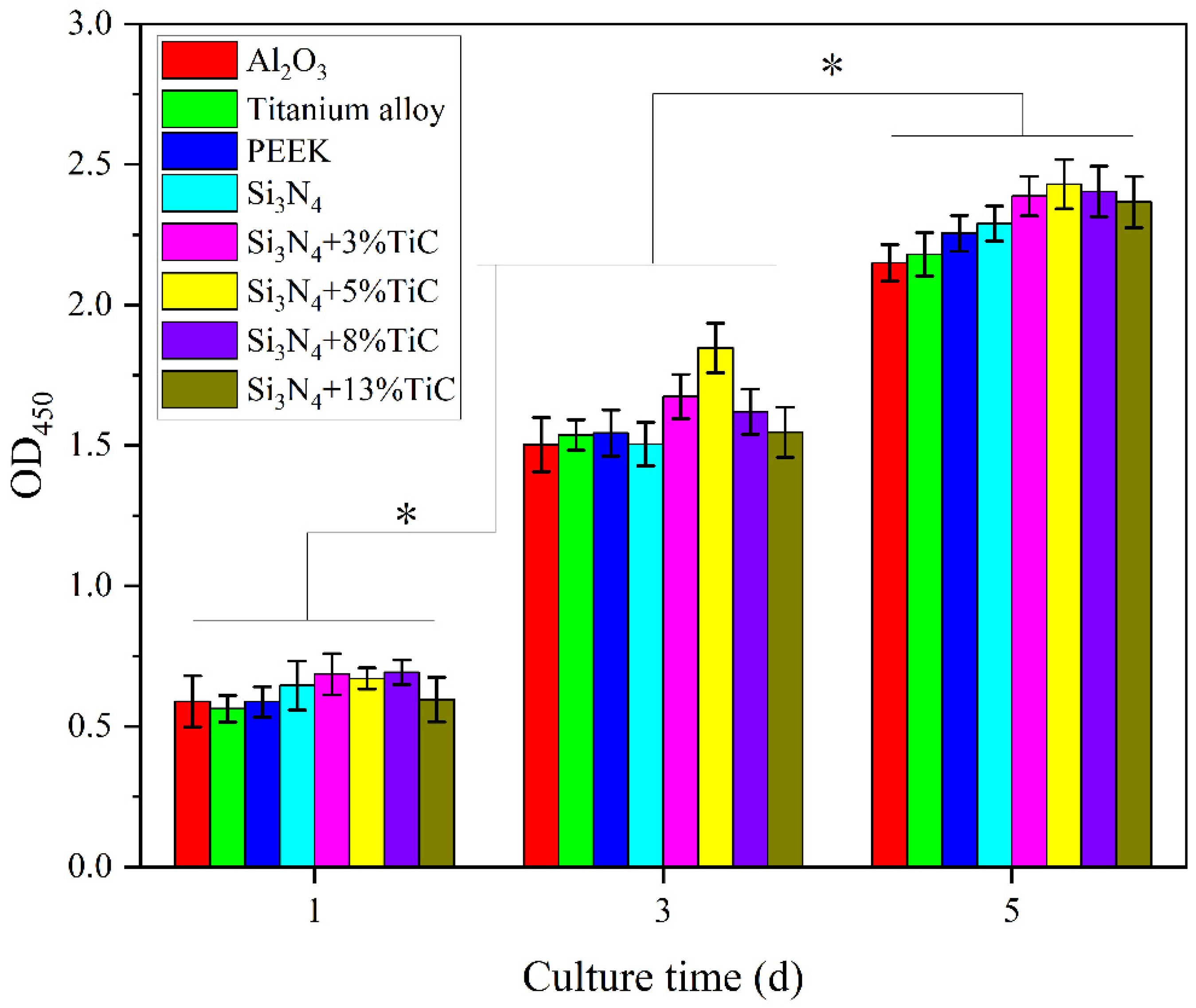
3.3. Evaluation of Cytotoxicity of the Silicon Nitrides with TiC
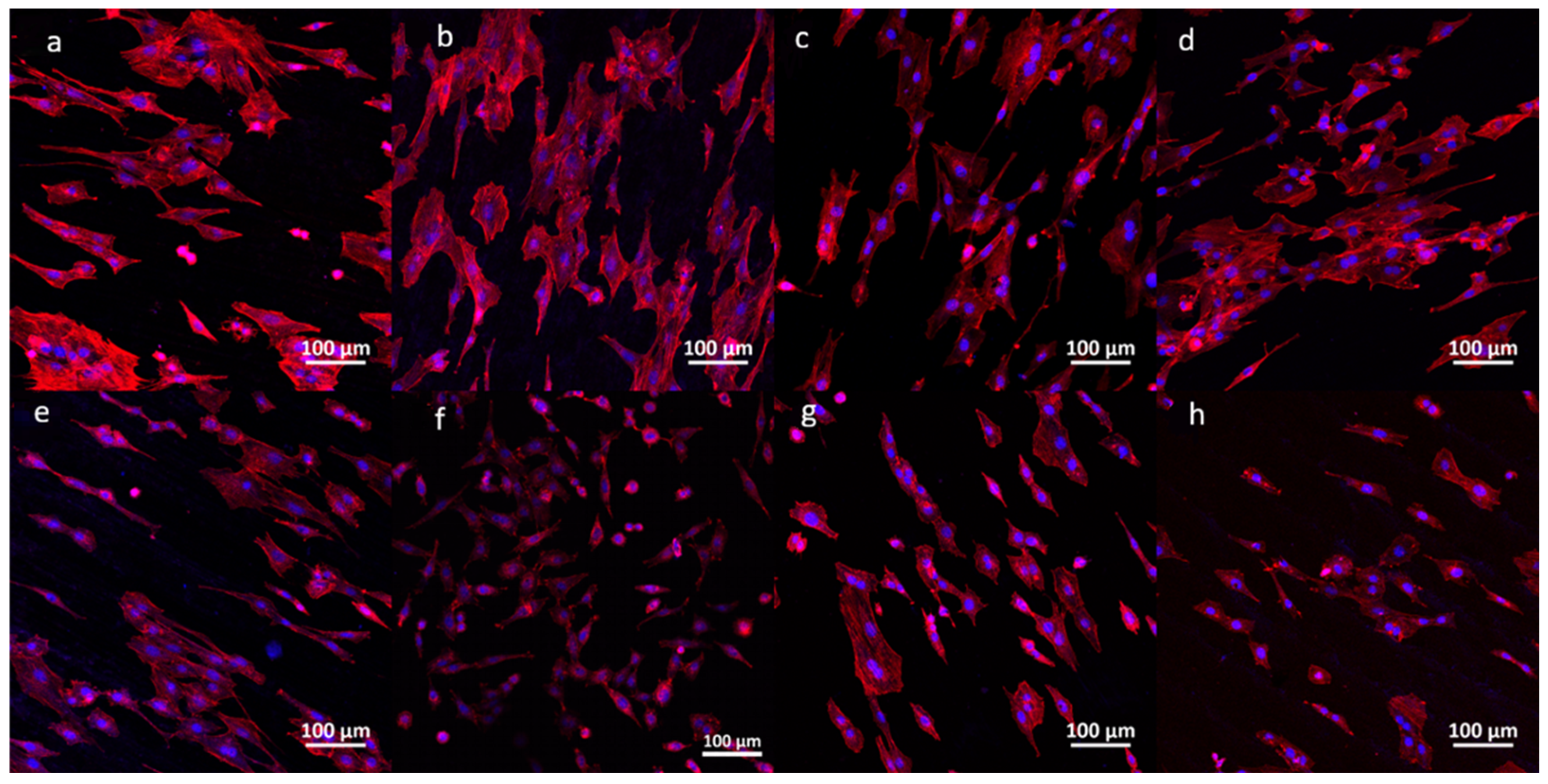
3.4. Cell Adhesion and Morphology of the Silicon Nitrides with TiC
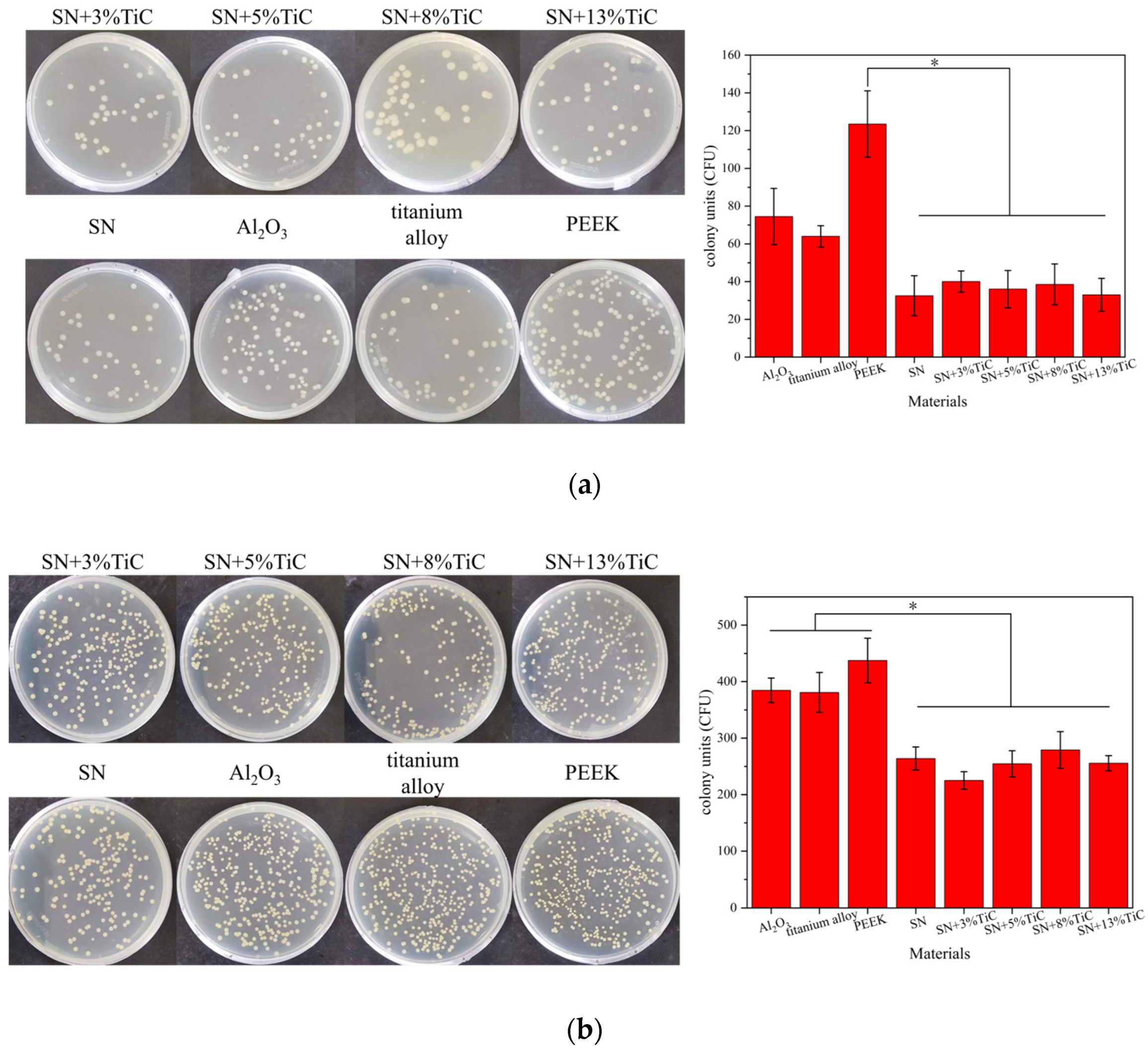
3.5. Evaluation of Antibacterial Property of the Silicon Nitrides with TiC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kohane, D.S.; Langer, R. Biocompatibility and drug delivery systems. Chem. Sci. 2010, 1, 441–446. [Google Scholar] [CrossRef]
- Mc Entire, B.J. Bioceramics: From Clinic to Concept. Bioceram. Dev. Appl. 2017, 7, 1000e108. [Google Scholar] [CrossRef]
- Rojbani, H.; Nyan, M.; Ohya, K.; Kasugai, S. Evaluation of the osteoconductivity of α-tricalcium phosphate, β-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J. Biomed. Mater. Res. Part A 2011, 98A, 488–498. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Treccani, L.; Klein, T.Y.; Meder, F.; Pardun, K.; Rezwan, K. Functionalized ceramics for biomedical, biotechnological and environmental applications. Acta Biomater. 2013, 9, 7115–7150. [Google Scholar] [CrossRef]
- Dion, I.; Bordenave, L.; Lefebvre, F.; Bareille, R.; Baquey, C.; Monties, J.R.; Havlik, P. Physico-chemistry and cytotoxicity of ceramics: Part II Cytotoxicity of ceramics. J. Mater. Sci. Mater. Med. 1994, 5, 18–24. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, D.; Kotobuki, N.; Maeda, M.; Hirose, M.; Ohgushi, H. Increased cell response to zirconia ceramics by surface modification. Appl. Phys. Lett. 2006, 89, 703. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Eur. Ceram. Soc. 2009, 29, 1245–1255. [Google Scholar] [CrossRef]
- Mazzocchi, M.; Bellosi, A. On the possibility of silicon nitride as a ceramic for structural orthopaedic implants. Part I: Processing, microstructure, mechanical properties, cytotoxicity. J. Mater. Sci. Mater. Med. 2008, 19, 2881–2887. [Google Scholar] [CrossRef]
- Wang, W.; Hadfield, M.; Wereszczak, A.A. Surface strength of silicon nitride in relation to rolling contact performance measured on ball-on-rod and modified four-ball tests. Tribol. Int. 2010, 43, 423–432. [Google Scholar] [CrossRef]
- Hah, S.R.; Burk, C.B.; Fischer, T.E. Surface quality of tribochemically polished silicon nitride. J. Electrochem. Soc. 1999, 146, 1505–1509. [Google Scholar] [CrossRef]
- Neumann, A.; Reske, T.; Held, M.; Jahnke, K.; Ragoss, C.; Maier, H.R. Comparative investigation of the biocompatibility of various silicon nitride ceramic qualities in vitro. J. Mater. Sci.-Mater. Med. 2004, 15, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Cappi, B.; Neuss, S.; Salber, J.; Telle, R.; Knüchel, R.; Fischer, H. Cytocompatibility of high strength non-oxide ceramics. J. Biomed. Mater. Res. Part A 2010, 93A, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.G.; Higa, O.Z.; Bressiani, J.C. Cytotoxic evaluation of silicon nitride-based ceramics. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2004, 24, 643–646. [Google Scholar] [CrossRef]
- Fu, L.; Xiong, Y.; Carlsson, G.; Palmer, M.; Orn, S.; Zhu, W.; Weng, X.S.; Engqvist, H.; Xia, W. Biodegradable Si3N4 bioceramic sintered with Sr, Mg and Si for spinal fusion: Surface characterization and biological evaluation. Appl. Mater. Today 2018, 12, 260–275. [Google Scholar] [CrossRef]
- Pezzotti, G. Bioceramics are not bioinert. Mater. Today 2017, 20, 395–398. [Google Scholar] [CrossRef]
- Pezzotti, G. Silicon Nitride: A Bioceramic with a Gift. Acs Appl. Mater. Interfaces 2019, 11, 26619–26636. [Google Scholar] [CrossRef]
- Zanocco, M.; Marin, E.; Rondinella, A.; Boschetto, F.; Horiguchi, S.; Zhu, W.; McEntire, B.J.; Bock, R.M.; Bal, B.S.; Pezzotti, G. The role of nitrogen off-stoichiometry in the osteogenic behavior of silicon nitride bioceramics. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 105, 110053. [Google Scholar] [CrossRef]
- Hu, G.F.; Zhu, Y.; Xu, F.Q.; Ye, J.K.; Guan, J.; Jiang, Y.Q.; Di, M.J.; Li, Z.N.; Guan, H.; Yao, X.C. Comparison of surface properties, cell behaviors, bone regeneration and osseointegration between nano tantalum/PEEK composite and nano silicon nitride/PEEK composite. J. Biomater. Sci.-Polym. Ed. 2022, 33, 35–56. [Google Scholar] [CrossRef]
- Lal, S.; Caseley, E.A.; Hall, R.M.; Tipper, J.L. Biological Impact of Silicon Nitride for Orthopaedic Applications: Role of Particle Size, Surface Composition and Donor Variation. Sci. Rep. 2018, 8, 9109. [Google Scholar] [CrossRef]
- Ishikawa, M.; Bentley, K.L.d.M.; McEntire, B.J.; Bal, B.S.; Schwarz, E.M.; Xie, C. Surface topography of silicon nitride affects antimicrobial and osseointegrative properties of tibial implants in a murine model. J. Biomed. Mater. Res. Part A 2017, 105, 3413–3421. [Google Scholar] [CrossRef]
- Howlett, C.R.; McCartney, E.; Ching, W. The effect of silicon nitride ceramic on rabbit skeletal cells and tissue. An in vitro and in vivo investigation. Clin. Orthop. Relat. Res. 1989, 244, 293–304. [Google Scholar] [CrossRef]
- Guedes e Silva, C.C.; Konig, B., Jr.; Carbonari, M.J.; Yoshimoto, M.; Allegrini, S., Jr.; Bressiani, J.C. Tissue response around silicon nitride implants in rabbits. J. Biomed. Mater. Res. Part A 2008, 84A, 337–343. [Google Scholar] [CrossRef]
- Dai, Y.; Guo, H.; Chu, L.Y.; He, Z.H.; Wang, M.Q.; Zhang, S.H.; Shang, X.F. Promoting osteoblasts responses in vitro and improving osteointegration in vivo through bioactive coating of nanosilicon nitride on polyetheretherketone. J. Orthop. Transl. 2020, 24, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, R.J.; Rao, P.J.; Phan, K.; Hardcastle, P.; Choy, W.J.; McCartney, E.R.; Druitt, R.K.; Mouatt, C.A.L.; Sorrell, C.C. Anterior Lumbar Interbody Fusion Using Reaction Bonded Silicon Nitride Implants: Long-Term Case Series of the First Synthetic Anterior Lumbar Interbody Fusion Spacer Implanted in Humans. World Neurosurg. 2018, 120, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.M.; Jones, E.N.; Ray, D.A.; Bal, B.S.; Pezzotti, G.; McEntire, B.J. Bacteriostatic behavior of surface modulated silicon nitride in comparison to polyetheretherketone and titanium. J. Biomed. Mater. Res. Part A 2017, 105, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Bock, R.M.; McEntire, B.J.; Jones, E.; Boffelli, M.; Zhu, W.; Baggio, G.; Boschetto, F.; Puppulin, L.; Adachi, T.; et al. Silicon Nitride Bioceramics Induce Chemically Driven Lysis in Porphyromonas gingivalis. Langmuir 2016, 32, 3024–3035. [Google Scholar] [CrossRef]
- Webster, T.J.; Patel, A.A.; Rahaman, M.N.; Bal, B.S. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454. [Google Scholar] [CrossRef]
- Gorth, D.J.; Puckett, S.; Ercan, B.; Webster, T.J.; Rahaman, M.; Bal, B.S. Decreased bacteria activity on Si3N4 surfaces compared with PEEK or titanium. Int. J. Nanomed. 2012, 7, 4829–4840. [Google Scholar] [CrossRef]
- Patel, A.A.; Webster, T.J.; Lakshminarayanan, R. Decreased Bacterial Count and Biofilm Formation Associated with Silicon Nitride (Si3N4) Materials Used in Interbody Fusion Cages (IBF) Compared to Titanium and Polyetheretherketone (PEEK). Spine J. 2012, 12, S135–S136. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Zhang, H.; Wu, Y.; Chu, Z.; Wu, Q.; Lu, M.; Tang, C. Silicon nitride as a potential candidate for dental implants: Osteogenic activities and antibacterial properties. J. Mater. Res. 2021, 36, 1866–1882. [Google Scholar] [CrossRef]
- Rambo, W.M., Jr. Treatment of lumbar discitis using silicon nitride spinal spacers: A case series and literature review. Int. J. Surg. Case Rep. 2018, 43, 61–68. [Google Scholar] [CrossRef]
- Youssef, J.A. Radiographic Follow-up of Transforaminal Lumbar Fusion with Silicon Nitride Spacers: A case Report of Two Patients. J. Musculoskelet. Disord. Treat. 2019, 2, 009. [Google Scholar] [CrossRef]
- Lee, Y.H.; Ko, S.; Park, H.; Lee, D.; Shin, S.; Jo, I.; Lee, S.B.; Lee, S.K.; Kim, Y.; Cho, S. Effect of TiC particle size on high temperature oxidation behavior of TiC reinforced stainless steel. Appl. Surf. Sci. 2019, 480, 951–955. [Google Scholar] [CrossRef]
- Mao, J.J.; Li, S.S.; Zhang, Y.X.; Chu, X.L.; Yang, Z.X. The stability of TiC surfaces in the environment with various carbon chemical potential and surface defects. Appl. Surf. Sci. 2016, 386, 202–209. [Google Scholar] [CrossRef]
- Zikin, A.; Antonov, M.; Hussainova, I.; Katona, L.; Gavrilovic, A. High temperature wear of cermet particle reinforced NiCrBSi hardfacings. Tribol. Int. 2013, 68, 45–55. [Google Scholar] [CrossRef]
- Bai, L.; Ge, C.C.; Shen, W.P.; Mao, X.D.; Zhang, K. Densification, microstructure, and fracture behavior of TiC/Si3N4 composites by spark plasma sintering. Rare Met. 2008, 27, 315–319. [Google Scholar] [CrossRef]
- Tian, C.Y.; Liu, N.; Lu, M.H. Microstructure and mechanical properties of Si3N4-TiC nanocomposites. Mater. Sci. Technol. 2007, 23, 1032–1038. [Google Scholar] [CrossRef]
- Chen, T.; Deng, Z.X.; Liu, D.F.; Zhu, X.C.; Xiong, Y. Bioinert TiC ceramic coating prepared by laser cladding: Microstructures, wear resistance, and cytocompatibility of the coating. Surf. Coat. Technol. 2021, 423, 127635. [Google Scholar] [CrossRef]
- Chen, T.; Li, W.P.; Liu, D.F.; Xiong, Y.; Zhu, X.C. Effects of heat treatment on microstructure and mechanical properties of TiC/TiB composite bioinert ceramic coatings in-situ synthesized by laser cladding on Ti6Al4V. Ceram. Int. 2021, 47, 755–768. [Google Scholar] [CrossRef]
- Veronesi, F.; Giavaresi, G.; Fini, M.; Longo, G.; Ioannidu, C.A.; d’Abusco, A.S.; Superti, F.; Panzini, G.; Misiano, C.; Palattella, A.; et al. Osseointegration is improved by coating titanium implants with a nanostructured thin film with titanium carbide and titanium oxides clustered around graphitic carbon. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 70, 264–271. [Google Scholar] [CrossRef]
- Brama, M.; Rhodes, N.; Hunt, J.; Ricci, A.; Teghil, R.; Migliaccio, S.; Della Rocca, C.; Leccisotti, S.; Lioi, A.; Scandurra, M.; et al. Effect of titanium carbide coating on the osseointegration response in vitro and in vivo. Biomaterials 2007, 28, 595–608. [Google Scholar] [CrossRef]
- Choy, M.T.; Tang, C.Y.; Chen, L.; Wong, C.T.; Tsui, C.P. In vitro and in vivo performance of bioactive Ti6Al4V/TiC/HA implants fabricated by a rapid microwave sintering technique. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 42, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiao, H.; Yang, W.; Feng, J.; Wang, Q.; Zhao, K. TiCw/TiC ceramics composites: SPS preparation, thermal and mechanical properties. Mater. Sci. Eng. B 2025, 314, 118005. [Google Scholar] [CrossRef]
- Wang, L.; Wei, B.; Wang, D.; Chen, L.; Wang, Y. (Ti, Ta)C-(Ti, Ta)B2-SiC based ceramics with core-rim structure by in-situ reaction sintering at low temperature: Formation mechanism and enhanced mechanical properties. J. Mater. Sci. Technol. 2026, 246, 167–186. [Google Scholar] [CrossRef]
- Wang, H.L.; Li, J.B.; Liu, J.F.; Li, Y. Self-monitoring of fracture and strain in titanium carbide reinforced silicon nitride ceramics. J. Mater. Sci. 2000, 35, 609–612. [Google Scholar] [CrossRef]
- Jinmei, Y.; Yuhou, W.; Jian, S.; Tian, J.; Zhou, P.; Bao, Z.; Xia, Z.; Gao, L. Friction and wear characteristics of silicon nitride ceramics under dry friction condition. Mater. Res. Express 2021, 8, 035701. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X. Experimental Study of the Service Performance of Full Ceramic Silicon Nitride Ball Bearings. Lubricants 2025, 13, 461. [Google Scholar] [CrossRef]
- Manzoor, S.; Wani, M.F.; Saleem, S.S. Effect of load on the friction and wear behaviour of silicon nitride and silicon nitride titanium carbide ceramic composite. Mater. Today Proc. 2019, 19, 474–477. [Google Scholar] [CrossRef]
- Baldoni, J.G.; Huckabee, M.L.; Buljan, S.T. Mechanical Properties and Wear Resistance of Silicon Nitride-Titanium Carbide Composites. In Tailoring Multiphase and Composite Ceramics; Tressler, R.E., Messing, G.L., Pantano, C.G., Newnham, R.E., Eds.; Springer: Boston, MA, USA, 1986; pp. 329–345. [Google Scholar]
- Cai, B.; Tan, Y.; He, L.; Tan, H.; Gao, L. Tribological properties of TiC particles reinforced Ni-based alloy composite coatings. Trans. Nonferr. Met. Soc. China 2013, 23, 1681–1688. [Google Scholar] [CrossRef]
- Saravanan, C.; Subramanian, K.; Anandakrishnan, V.; Sathish, S. Tribological behavior of AA7075-TiC composites by powder metallurgy. Ind. Lubr. Tribol. 2018, 70, 1066–1071. [Google Scholar] [CrossRef]
- GB/T 16886.12-2005; Biological Evaluation of Medical Devices. Part 12: Sample Preparation and Reference Material. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2005.
- ISO 10993-8:2000; Biological Evaluation of Medical Devices. Part 8: Selection and Qualification of Reference Materials for Biological Tests. International Organization for Standardization: Geneva, Switzerland, 2000.
- GB/T 4740-2024; Test Method for Strength of Ceramic Materials. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2024.
- GB/T 6569-2006; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Flexural Strength of Monolithic Ceramics at Room Temperature. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- GB/T 23806-2009; Fine Ceramics (Advanced Ceramics Advanced Technical Ceramics)—Test Method for Fracture Toughness of Monolithic Ceramics at Room Temperature by Single Edge Precracked Beam (SEPB) Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2009.
- Bal, B.S.; Rahaman, M.N. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, C.; Deng, L.W.; Li, Y.; Xiao, P.; Zhang, H.B. C0.3N0.7Ti-SiC Toughed Silicon Nitride Hybrids with Non-Oxide Additives Ti3SiC2. Materials 2020, 13, 1428. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Yang, J.; Zhu, S.Y.; Cheng, J.; Yu, Y.; Qiao, Z.H.; Liu, W.M. Temperature-driven wear behavior of Si3N4-based ceramic reinforced by in situ formed TiC0.3N0.7 particles. J. Am. Ceram. Soc. 2019, 102, 4333–4343. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Peñarrieta-Juanito, G.; Sordi, M.B.; Henriques, B.; Dotto, M.E.R.; Teughels, W.; Silva, F.S.; Magini, R.S.; Souza, J.C.M. Surface damage of dental implant systems and ions release after exposure to fluoride and hydrogen peroxide. J. Periodontal Res. 2019, 54, 46–52. [Google Scholar] [CrossRef]
- Moriyama, A.; Takahashi, U.; Mizuno, Y.; Takahashi, J.; Horie, M.; Iwahashi, H. The Truth of Toxicity Caused by Yttrium Oxide Nanoparticles to Yeast Cells. J. Nanosci. Nanotechnol. 2019, 19, 5418–5425. [Google Scholar] [CrossRef]
- Zhao, X.H.; Zhang, W.S.; He, Y.J.; Wang, L.Q.; Li, W.; Yang, L.W.; Xing, G.H. Phytotoxicity of Y2O3 nanoparticles and Y3+ ions on rice seedlings under hydroponic culture. Chemosphere 2021, 263, 127943. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Q.; Li, Y.F.; Ma, Y.Y.; Liu, Z.; Cao, L.L.; Wang, D.; Liu, S.D.; Xu, W.S.; Wang, W.Y. Size-dependent cytotoxicity of yttrium oxide nanoparticles on primary osteoblasts in vitro. J. Nanopart. Res. 2016, 18, 135. [Google Scholar] [CrossRef]
- German, R.; Suri, P.; Park, S. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Karacasulu, L. Cold sintering of ceramics and glasses: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100807. [Google Scholar] [CrossRef]
- Yang, J.F.; Zhang, G.J.; Ohji, T. Porosity and microstructure control of porous ceramics by partial hot pressing. J. Mater. Res. 2001, 16, 1916–1918. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, Y.D.; Zeng, X.F.; Tong, Y.; Liu, R.; Wang, K.; Shangguan, X.D.; Qiu, G.Z.; Sipaut, C.S. Silicon Nitride Bioceramics Sintered by Microwave Exhibit Excellent Mechanical Properties, Cytocompatibility In Vitro, and Anti-Bacterial Properties. J. Funct. Biomater. 2023, 14, 552. [Google Scholar] [CrossRef]
- Zeng, X.F.; Sipaut, C.S.; Ismail, N.M.; Liu, Y.D.; Farm, Y.Y.; He, J.Y. Mechanical properties and biological activity of 3D printed silicon nitride materials. Ceram. Int. 2024, 50, 16704–16713. [Google Scholar] [CrossRef]
- Kim, E.J.; Bu, S.Y.; Sung, M.K.; Choi, M.K. Effects of Silicon on Osteoblast Activity and Bone Mineralization of MC3T3-E1 Cells. Biol. Trace Elem. Res. 2013, 152, 105–112. [Google Scholar] [CrossRef]
- Yoshida, K.; Mishina, H.; Sasaki, S.; Morita, M.; Mabuchi, K. Mechanical properties of titanium cermets for joint prostheses. Mater. Trans. 2006, 47, 418–425. [Google Scholar] [CrossRef]
- Balázsi, K.; Vandrovcová, M.; Bacáková, L.; Balázsi, C. Structural and biocompatible characterization of TiC/a:C nanocomposite thin films. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 1671–1675. [Google Scholar] [CrossRef]
- Gyorgyey, A.; Ungvari, K.; Kecskemeti, G.; Kopniczky, J.; Hopp, B.; Oszko, A.; Pelsoczi, I.; Rakonczay, Z.; Nagy, K.; Turzo, K. Attachment and proliferation of human osteoblast-like cells (MG-63) on laser-ablated titanium implant material. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 4251–4259. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci.-Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef]
- Desrousseaux, C.; Sautou, V.; Descamps, S.; Traore, O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J. Hosp. Infect. 2013, 85, 87–93. [Google Scholar] [CrossRef]
- Dante, R.C.; Kajdas, C.K. A review and a fundamental theory of silicon nitride tribochemistry. Wear 2012, 288, 27–38. [Google Scholar] [CrossRef]
- Bock, R.M.; McEntire, B.J.; Bal, B.S.; Rahaman, M.N.; Boffelli, M.; Pezzotti, G. Surface modulation of silicon nitride ceramics for orthopaedic applications. Acta Biomater. 2015, 26, 318–330. [Google Scholar] [CrossRef]
- Boschetto, F.; Toyama, N.; Horiguchi, S.; Bock, R.M.; McEntire, B.J.; Adachi, T.; Marin, E.; Zhu, W.L.; Mazda, O.; Bal, B.S.; et al. In vitro antibacterial activity of oxide and non-oxide bioceramics for arthroplastic devices: II. Fourier transform infrared spectroscopy. Analyst 2018, 143, 2128–2140. [Google Scholar] [CrossRef]
- Boschetto, F.; Adachi, T.; Horiguchi, S.; Marina, E.; Paccotti, N.; Asai, T.; Zhu, W.L.; McEntire, B.J.; Yamamoto, T.; Kanamura, N.; et al. In situ molecular vibration insights into the antibacterial behavior of silicon nitride bioceramic versus gram-negative Escherichia coli. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2019, 223, 117299. [Google Scholar] [CrossRef]
- Pezzotti, G.; Bock, R.M.; McEntire, B.J.; Adachi, T.; Marin, E.; Boschetto, F.; Zhu, W.L.; Mazda, O.; Bal, S.B. In vitro antibacterial activity of oxide and non-oxide bioceramics for arthroplastic devices: I. In situ time-lapse Raman spectroscopy. Analyst 2018, 143, 3708–3721. [Google Scholar] [CrossRef] [PubMed]
- Boschetto, F.; Adachi, T.; Horiguchi, S.; Fainozzi, D.; Parmigiani, F.; Marin, E.; Zhu, W.L.; McEntire, B.J.; Yamamoto, T.; Kanamura, N.; et al. Monitoring metabolic reactions in Staphylococcus epidermidis exposed to silicon nitride using in situ time-lapse Raman spectroscopy. J. Biomed. Opt. 2018, 23, 056002. [Google Scholar] [CrossRef] [PubMed]
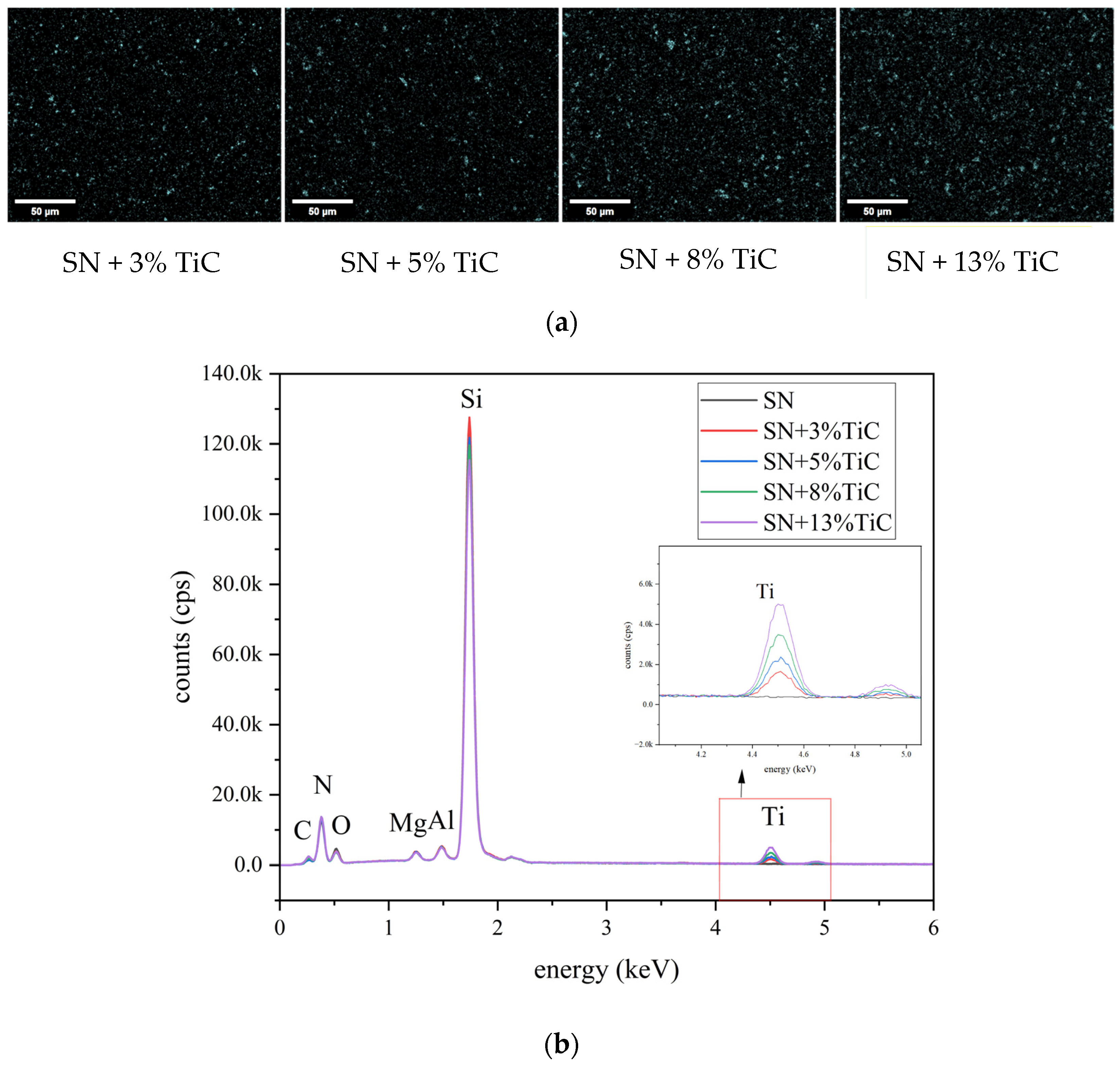

| Materials | C (cps) | N (cps) | O (cps) | Mg (cps) | Al (cps) | Si (cps) | Ti (cps) |
|---|---|---|---|---|---|---|---|
| SN + 3% TiC | 1.51 k | 12.77 k | 4.69 k | 3.74 k | 5.26 k | 125.80 k | 0.44 k |
| SN + 5% TiC | 1.89 k | 13.16 k | 3.90 k | 3.84 k | 5.41 k | 123.58 k | 1.66 k |
| SN + 8% TiC | 1.94 k | 13.48 k | 3.73 k | 3.78 k | 5.22 k | 121.78 k | 2.38 k |
| SN + 13% TiC | 1.98 k | 13.75 k | 3.68 k | 3.55 k | 5.07 k | 119.68 k | 3.45 k |
| Materials | Vickers Hardness (GPa) | Flexural Strength (MPa) | Compressive Strength (MPa) | Fracture Toughness (MPa·m1/2) |
|---|---|---|---|---|
| SN | 14.73 ± 0.3 | 523 ± 31.9 | 2465 ± 131.4 | 7.47 ± 0.583 |
| SN + 3% TiC | 15.02 ± 0.28 | 571 ± 29.3 | 2537 ± 132.9 | 8.35 ± 0.622 |
| SN + 5% TiC | 15.23 ± 0.33 | 532 ± 30.9 | 2431 ± 133.9 | 8.53 ± 0.663 |
| SN + 8% TiC | 14.51 ± 0.31 | 482 ± 32.7 | 2315 ± 138.4 | 7.66 ± 0.642 |
| SN + 13% TiC | 14.13 ± 0.39 | 435 ± 31.0 | 2114 ± 131.9 | 6.55 ± 0.601 |
| Alumina [58] | 14.00–16.00 | 300–500 | 2000–3000 | 4–5 |
| Titanium alloy [58] | 3.40 | / | 950–990 | 75 |
| PEEK [58] | / | 160–180 | 130–140 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Lou, Z.; He, J.; Liu, Y.; Zhu, H.; Zeng, X.; Abid, Z. Silicon Nitride Bioceramics with TiC Additives: Excellent Mechanical Properties, Cytocompatibility, and Antibacterial Properties. J. Funct. Biomater. 2026, 17, 20. https://doi.org/10.3390/jfb17010020
Lou Z, He J, Liu Y, Zhu H, Zeng X, Abid Z. Silicon Nitride Bioceramics with TiC Additives: Excellent Mechanical Properties, Cytocompatibility, and Antibacterial Properties. Journal of Functional Biomaterials. 2026; 17(1):20. https://doi.org/10.3390/jfb17010020
Chicago/Turabian StyleLou, Zhebin, Jiayu He, Yuandong Liu, Hanxu Zhu, Xiaofeng Zeng, and Zulaikha Abid. 2026. "Silicon Nitride Bioceramics with TiC Additives: Excellent Mechanical Properties, Cytocompatibility, and Antibacterial Properties" Journal of Functional Biomaterials 17, no. 1: 20. https://doi.org/10.3390/jfb17010020
APA StyleLou, Z., He, J., Liu, Y., Zhu, H., Zeng, X., & Abid, Z. (2026). Silicon Nitride Bioceramics with TiC Additives: Excellent Mechanical Properties, Cytocompatibility, and Antibacterial Properties. Journal of Functional Biomaterials, 17(1), 20. https://doi.org/10.3390/jfb17010020







