Bionanomaterials or Nanobiomaterials: Differences in Definitions and Applications
Abstract
1. Introduction
2. Biomaterials
2.1. Types of Biomaterials
2.2. Methods of Producing Biomaterials
2.3. Applications of Biomaterials
3. Nanomaterials
3.1. Types of Nanomaterials
3.2. Methods of Producing Nanomaterials
3.3. Applications of Nanomaterials
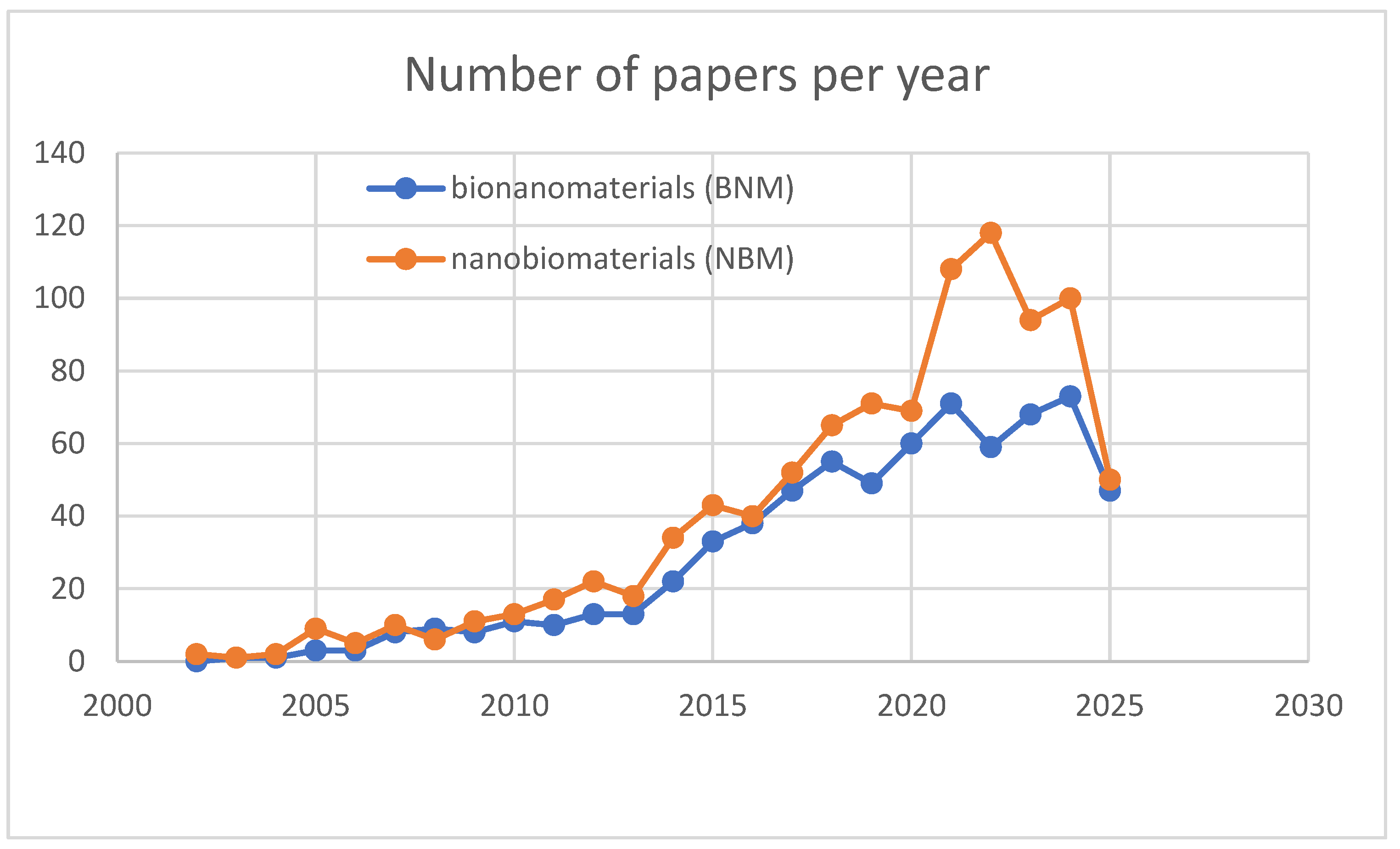
4. Nanobiomaterials and Bionanomaterials
4.1. Nanobiomaterials
4.2. Bionanomaterials
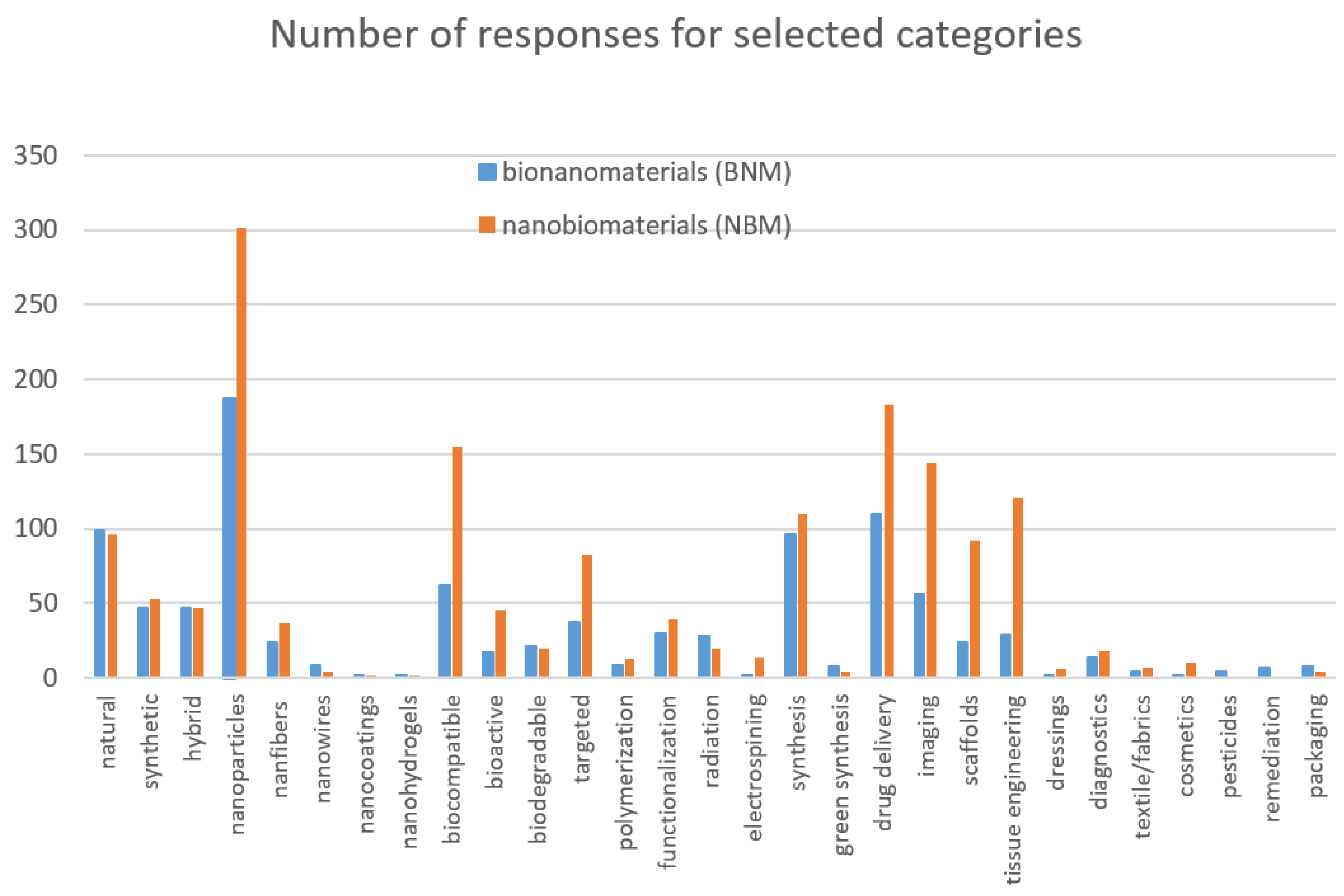
| Features of NBM and BNM | Nanobiomaterials | Bionanomaterials | ||
|---|---|---|---|---|
| Selected Examples | ||||
| types | origin | natural | bacterial extracellular vesicles as natural nanomaterials in disease diagnosis and therapeutics [94], microbial cellulose for healing of wounds [95], | bio-based nanomaterials [91], natural biopolymers [96], |
| synthetic | hydroxyapatite (HA) Al2O3–TiO2 [97], synthetic polymer-based nanocomposites [98], | synthetic biopolymers [96], synthetic polymer-based nanocomposites [98] | ||
| hybrid | hybrid nano hydrogel for bone regeneration [99], peroxidase-mimic GSF@AuNPs hybrid nanoparticles [100], | protein-based functional hybrid bionanomaterials [101], hybrid chitosan-cerium oxide nanoparticles [102], | ||
| structure | nanoparticles | TiO2 nanoparticles—a promising candidate for wound healing applications [103], collagen-I@AuNPs for treating skin injuries [104], | bioinspired nanoparticles emerge for medical use [105], protein corona formation on single nanoparticles for theranostic applications [106], | |
| nanofibers/nanowires | MnO2 nanofibers for selective and sensitive detection of biomolecules [107], nanowires for selective detection of chloramphenicol [108], | cellulose nanofibers and sodium alginate composite with antibacterial properties [109], ATP as building blocks for the self-assembly of excitonic nanowires [110], | ||
| nanocoatings | nanocoatings and their composites in dentistry [111], ZnO based nano-architectures, films and coatings for biomedical applications [112], | gentamicin loaded multilayers modified titanium coatings for prevention of implant infection [113], polysaccharide based coatings for fruit preservation [114], | ||
| nanohydrogels | halofuginone–silver thermosensitive nanohydrogels with antibacterial and anti-inflammatory properties [115], nanogels as promising nanosystems to treat a wide range of acute and chronic healthcare scenarios [116], | chitosan-inspired (nano)hydrogels [117] nanogels as carriers for medical applications [118], | ||
| functions | biocompatible | biocompatibility of nanobiomaterials [119], biocompatibility of chitosan-carbon nanocage hybrids for sustained drug release [120], | conjugation of nanoparticles with the biological molecules makes them more biocompatible [91], biocompatible bionanomaterials for food packing [121], | |
| bioactive | highly bioactive nanoparticle for activating osteogenesis [122], osteogenic nanometric bioactive glass particles [123], | advanced bioactive nanomaterials for diagnosis and treatment [124], bioactive polymers and nanobiomaterials composites [125], | ||
| biodegradable | bacterial cellulose—nanobiomaterial for biodegradable face masks [126], biodegradable PLA nanoplatforms as coatings for cardiovascular stents [127], | biodegradable poly(amino acid)-gold-magnetic complex for photothermal treatment [128], biodegradable PEG-dendritic block copolymers [129], | ||
| targeted | siRNA drug delivery strategies [130], extracellular vesicles in targeted delivery towards specific cells [131], | phage particles for targeted delivery of personalized neoantigen vaccines [132], molecularly targeted viral nanoparticles as tools for imaging cancer [133], | ||
| manufacturing methods | chemical | polymerization | on-surface polymerization in living cells [134], techniques for chemical and biological synthesis of polymeric nanoparticles [135], | techniques for chemical and biological synthesis of polymeric nanoparticles [135], microwave-assisted click polymerization of cyclic oligomers [136], |
| functionalization | surface functionalization of nanobiomaterials for tissue engineering and regenerative medicine [137], functionalization of tissue-specific bioinks [138], | functionalization of green synthesized bionanomaterials [139], lignocellulosic bionanomaterials for biosensor applications [140], | ||
| physicochemical | radiation | osteoconductive effect of a nanocomposite membrane treated with UV radiation [141], designing nanostructured Ti6Al4V with directed irradiation synthesis [142], | irradiation effects in polymer composites for their conversion into hybrids [143], surface modification of polymers by exposure to extreme ultraviolet radiation [144], | |
| electrospinning | polyvinyl alcohol/chitosan nanofibrous films by electrospinning method [145], application of electrospinning technology in the biomedical field [146], | electrospinning technology, machine learning, and control approaches [147], | ||
| chemical/biological synthesis | synthesis/green synthesis | synthesis of copper nanobiomaterials [148], synthesis of biomimetic nano/submicro-fibrous tubes for potential small-diameter vascular grafts [149], | green synthesis of hydrogel scaffolds [150], green synthesis with root extract [151], self-assembling bionanomaterials [152], | |
| applications | medicine/pharmacy and textile technology | drug delivery | nanobiomaterials in spatio-temporal controlled drug delivery for lungs [153], rotavirus as a vector for heterologous peptides, drug delivery, and production of nanobiomaterials [154], | drug delivery with supramolecular amphiphilic macrocycle nanoparticles [155], plant virus nanoparticles as nanocarriers for drug delivery and imaging [156], |
| imaging | fluorescence bioimaging of molecular fluorophores [157], hyperspectral imaging for label free detection of nanobiomaterials [158], | bionanomaterials and systems for enhanced bioimaging in biomedical applications [159], viral nanoparticles for in vivo tumor imaging [160], | ||
| scaffolds/tissue engineering | 3D bioprinting and nanotechnology in tissue engineering scaffolds [161], surface modification by nanobiomaterials for vascular tissue engineering [162], | extending the versatility of bionanomaterial scaffolds [163], amyloid fibrils as a nanoscaffold for enzyme immobilization [164], | ||
| dressings | polyvinyl alcohol/chitosan nanofibrous films by electrospinning method for wound dressings [144], nanobiomaterials for vascular biology and wound management [165], | bionanomaterials for skin regeneration [166], bionanomaterials in wound dressings [167], | ||
| diagnostics | nanobiomaterials for point-of-care diagnostics [168], diagnostics strategies based on engineered nanobiomaterials [169], | clinical in vivo nanodiagnostics [170], bionanomaterials for diagnosis and therapy of SARS-CoV-2 [171], | ||
| textile/fabrics | composites based on CNTs and 2D material coated fabric sensors [172], silver plating on polyester and cotton blended fabric [173], | biomimicry in the field of textile technology [174], | ||
| cosmetology | cosmetics | dermal delivery of drugs and cosmetics [175], nanobiomaterials in cosmetics [176], | green synthesized nanomaterials for cosmetics [177], liposomes in cosmetics [178], | |
| agriculture and the environment | pesticides | bionanomaterials towards the environmental and agricultural domain [179], nanopesticide application in crop protection [180], | ||
| remediation | recent advances in nanoremediation [181], remediation of microplastics using bionanomaterials [182], | |||
| packaging | application of an organic silver-metal framework modified with sodium alginate in packaging [183], nanobiomaterials for food packaging sensor applications [184], | bionanomaterials for development of sustainable food packaging [157], (bio)nanotechnology in food science [185], | ||
4.3. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ISO/TS 80004-1:2023; Nanotechnologies—Vocabulary Part 1: Core vocabulary. ISO: Geneva, Switzerland, 2023.
- ISO/TS 80004-5:2011; Nanotechnologies—Vocabulary Part 5: Nano/bio interface. ISO: Geneva, Switzerland, 2011.
- ISO/TC 229; Nanotechnologies. ISO: Geneva, Switzerland, 2005.
- ISO/TS 13329:2024; Nanomaterials—Preparation of Safety Data Sheets (SDS), Second edition. ISO: Geneva, Switzerland, 2024.
- Standards for Nanotechnology. Available online: https://www.nano.gov/you/standards/ (accessed on 4 September 2025).
- PubMed®. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 4 September 2025).
- Erzen, B.; Karataş, M.; Orhan, R.; Aydogmus, E. Advancements and Challenges in Biomaterials: Innovations, Sustainability, and Future Prospects. J. Macromol. Sci. Part B 2025, 1–27. [Google Scholar] [CrossRef]
- Leonardi, F.; Simonazzi, B.; Martini, F.M.; D’Angelo, P.; Foresti, R.; Botti, M. Synthetic and Natural Biomaterials in Veterinary Medicine and Ophthalmology: A Review of Clinical Cases and Experimental Studies. Vet. Sci. 2024, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Kumar, A.; Mohammed, M.K.A.; Singh, S. Biomaterial types, properties, medical applications, and other factors: A recent review. J. Zhejiang Univ. Sci. 2023, 24, 1027–1042. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Gkekas, I.; Kastrinaki, G.; Prigione, A.; Zaspalis, V.T.; Petrakis, S. Biocompatibility of α-Al2O3 Ceramic Substrates with Human Neural Precursor Cells. J. Funct. Biomater. 2020, 11, 65. [Google Scholar] [CrossRef]
- Krishnan, L.; Chakrabarty, P.; Govarthanan, K.; Rao, S.; Santra, T.S. Bioglass and nano bioglass: A next-generation biomaterial for therapeutic and regenerative medicine applications. Int. J. Biol. Macromol. 2024, 277 Pt 2, 133073. [Google Scholar] [CrossRef]
- Montesissa, M.; Sassoni, E.; Boi, M.; Borciani, G.; Boanini, E.; Graziani, G. Synthetic or Natural (Bio-Based) Hydroxyapatite? A Systematic Comparison between Biomimetic Nanostructured Coatings Produced by Ionized Jet Deposition. Nanomaterials 2024, 14, 1332. [Google Scholar] [CrossRef]
- Haubold, A.D.; More, R.B.; Bokros, J.C. Carbons. In Handbook of Biomaterial Properties; Black, J., Hastings, G., Eds.; Springer: Boston, MA, USA, 1998. [Google Scholar]
- Shah, R.; Pai, N.; Khandekar, R.; Aslam, R.; Wang, Q.; Yan, Z.; Rosenkranz, A. DLC coatings in biomedical applications—Review on current advantages, existing challenges, and future directions. Surf. Coat. Technol. 2024, 487, 131006. [Google Scholar] [CrossRef]
- Okroj, W.; Kaminska, M.; Klimek, L.; Szymanski, W.; Walkowiak, B. Blood platelets in contact with nanocrystalline diamond surface. Diam. Relat. Mater. 2006, 15, 1535–1539. [Google Scholar] [CrossRef]
- Komorowski, P.; Siatkowska, M.; Kamińska, M.; Jakubowski, W.; Walczyńska, M.; Walkowiak-Przybyło, M.; Szymański, W.; Piersa, K.; Wielowski, P.; Sokołowska, P.; et al. Comprehensive Biological Evaluation of Biomaterials Used in Spinal and Orthopedic Surgery. Materials 2020, 13, 4769. [Google Scholar] [CrossRef]
- Santos, G.A.D. The Importance of Metallic Materials as Biomaterials. Adv. Tissue Eng. Regen. Med. 2017, 3, 00054. [Google Scholar] [CrossRef]
- Moharil, S.; Reche, A.; Durge, K. Polyetheretherketone (PEEK) as a Biomaterial: An Overview. Cureus 2023, 15, e44307. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.C.; Allenby, M.C.; Lewis, P.M.; Woodruff, M.A. Biomedical applications of polyethylene. Eur. Polym. J. 2019, 118, 412–428. [Google Scholar] [CrossRef]
- Kelly, M.; Macdougall, K.; Olabisi, O.; McGuire, N. In vivo response to polypropylene following implantation in animal models: A review of biocompatibility. Int. Urogynecol. J. 2017, 28, 171–180. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Bhandari, P.; Singh, T.; Sharma, S.; Singh, J.; Singh, S. Promising Role of Polylactic Acid as an Ingenious Biomaterial in Scaffolds, Drug Delivery, Tissue Engineering, and Medical Implants: Research Developments, and Prospective Applications. Molecules 2023, 28, 485. [Google Scholar] [CrossRef] [PubMed]
- Avci-Adali, M.; Grözinger, G.; Cabane, V.; Schreve, M.; Wendel, H.P. Improving Bioactive Characteristics of Small Diameter Polytetrafluoroethylene Stent Grafts by Electrospinning: A Comparative Hemocompatibility Study. Bioengineering 2023, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Stodolak-Zych, E.; Cholewa-Kowalska, K. Biodegradable ceramic-polymer composites for biomedical applications: A review. Mater. Sci. Eng. C 2017, 71, 1175–1191. [Google Scholar] [CrossRef]
- Nayak, G.S.; Carradò, A.; Masson, P.; Pourroy, G.; Mouillard, F.; Migonney, V.; Falentin-Daudre, C.; Pereira, C.; Palkowski, H. Trends in Metal-Based Composite Biomaterials for Hard Tissue Applications. JOM 2022, 74, 102–125. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices Part 1: Evaluation and Testing Within a Risk Management Process. International Organization for Standardization: Vernier, Switzerland, 2018.
- Lenka, R.K.; Patro, P.K.; Mahata, T. Shape Forming and Sintering of Ceramics. In Handbook on Synthesis Strategies for Advanced Materials; Tyagi, A.K., Ningthoujam, R.S., Eds.; Indian Institute of Metals Series; Springer: Singapore, 2022. [Google Scholar]
- Davis, R.; Singh, A.; Jackson, J.M.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; Ribeiro da Silva, L.R.; Lawrence, A.A. A comprehensive review on metallic implant biomaterials and their subtractive manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 1473–1530. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Additive manufacturing of metallic biomaterials and its biocompatibility. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Xia, D.; Chen, J.; Zhang, Z.; Dong, M. Emerging polymeric biomaterials and manufacturing techniques in regenerative medicine. Aggregate 2022, 3, e176. [Google Scholar] [CrossRef]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Das, B.; Paul, S.; Sharma, H.K. A Review on BioPolymers Derived from Animal Sources with Special Reference to their Potential Applications. J. Drug Deliv. Ther. 2021, 11, 209–223. [Google Scholar] [CrossRef]
- Drishya, P.K.; Reddy, M.V.; Mohanakrishna, G.; Sarkar, O.; Isha; Rohit, M.V.; Patel, A.; Chang, Y.-C. Advances in Microbial and Plant-Based Biopolymers: Synthesis and Applications in Next-Generation Materials. Macromol 2025, 5, 21. [Google Scholar] [CrossRef]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Nanostructured Biomaterials for Regeneration. Adv. Funct. Mater. 2008, 18, 3568–3582. [Google Scholar] [CrossRef] [PubMed]
- Juma, T.; Wang, H.; Cao, X.; Wang, Q.; Wang, H.; Yu, B.; Bao, X.; Rong, W.; Tian, H.; Cao, Y. Novel biocompatible magnetronsputtered silver coating for enhanced antibacterial properties and osteogenesis in vitro. Sci. Rep. 2024, 14, 28599. [Google Scholar] [CrossRef]
- Kheradmand, Z.; Mohammadi, T.; Mukete, G.I.T. Biocompatible coatings for composite medical implants: Enhancing integration and performance. J. Compos. Compd. 2023, 5, 1–6. [Google Scholar] [CrossRef]
- Jastrzębski, K.; Grabarczyk, J.; Niedzielski, P.; Jędrzejczak, A.; Sobczyk-Guzenda, A.; Szymański, W.; Kamińska, M.; Skibska, B. The Doping of a Carbon Coating with Phosphorus as a Potential Way of Improving the Biological Properties of Diamond-Like Carbon. Materials 2024, 17, 5859. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343–2387. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Chu, C.; Xue, F.; Li, J.; Bai, J. Structure-function integrated biodegradable Mg/polymer composites: Design, manufacturing, properties, and biomedical applications. Bioact. Mater. 2024, 39, 74–105. [Google Scholar] [CrossRef]
- Palmer, R. Introduction to dental implants. Br. Dent. J. 1999, 187, 127–132. [Google Scholar] [PubMed][Green Version]
- Barreto, M.; Srivastava, S.; Mittal, H. Design, Materials and Biomechanics of Orthopaedic Implants: A Narrative Review. J. Clin. Diagn. Res. 2024, 18, RE01–RE07. [Google Scholar] [CrossRef]
- Decker, M.; Fleischer, T. Contacting the brain—Aspects of a technology assessment of neural implants. Biotechnol. J. 2008, 3, 1502–1510. [Google Scholar] [CrossRef]
- Sattari, S.A.; Xia, Y.; Azad, T.D.; Caraway, C.A.; Chang, L. Advances in Implant Technologies for Spine Surgery. Neurosurg. Clin. N. Am. 2024, 35, 217–227. [Google Scholar] [CrossRef]
- Kowalska-Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 9, 527–534. [Google Scholar] [CrossRef]
- Scribbick, F.W.; Scribbick, A.T. Ophthalmic Implants and Explants. Emerg. Med. Clin. N. Am. 1994, 12, 793–800. [Google Scholar] [CrossRef]
- Blume, C.; Kraus, X.; Heene, S.; Loewner, S.; Stanislawski, N.; Cholewa, F.; Blume, H. Vascular implants—New aspects for in situ tissue engineering. Eng. Life Sci. 2022, 22, 344–360. [Google Scholar] [CrossRef]
- Chung, J.S.; Emerson, D.; Megna, D.; Arabia, F.A. Total artificial heart: Surgical technique in the patient with normal cardiac anatomy. Ann. Cardiothorac. Surg. 2020, 9, 81–88. [Google Scholar] [CrossRef]
- Guambo, M.P.R.; Spencer, L.; Vispo, N.S.; Vizuete, K.; Debut, A.; Whitehead, D.C.; Santos-Oliveira, R.; Alexis, F. Natural Cellulose Fibers for Surgical Suture Applications. Polymers 2020, 12, 3042. [Google Scholar] [CrossRef] [PubMed]
- Özant, A.; Arslan, K. Synthetic Meshes in Hernia Surgery. Cyprus J. Med. Sci. 2023, 8, 1–7. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Labanca, M. Biomaterials in Maxillofacial Surgery: Membranes and Grafts. Int. J. Biomed. Sci. 2011, 7, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Passos, E.C.F.; Idalgo, F.A.; dos Santos Pereira, S.A.; Nunes, A.G.; Kassis, E.N.; Cicareli, A.J. Biomaterials, surgical processes and bone formation for dental implants: A systematic review. MedNEXT J. Med. Health Sci. 2023, 4 (Suppl. S2), 1–10. [Google Scholar] [CrossRef]
- Long, Y.; Xu, W.; Huang, F.; Peng, L.; Duan, Y.; Su, D.; Zhu, Z. Spontaneous chitosan hydrogel formation through carbonic acid modulation: A crosslinker-free, efficient preparation for dressings. Int. J. Biol. Macromol. 2025, 319, 145618. [Google Scholar] [CrossRef]
- Li, H.; Wen, H.; Zhang, H.; Cao, X.; Li, L.; Hu, X.; Zhang, Y.; Shen, X.; Shubhra, Q.T.; Yang, H.; et al. A multifunctional dihydromyricetin-loaded hydrogel for the sequential modulation of diabetic wound healing and glycemic control. Burn. Trauma 2025, 13, tkaf024. [Google Scholar] [CrossRef]
- Todd, E.A.; Mirsky, N.A.; Silva, B.L.G.; Shinde, A.R.; Arakelians, A.R.L.; Nayak, V.V.; Marcantonio, R.A.C.; Gupta, N.; Witek, L.; Coelho, P.G. Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions. J. Funct. Biomater. 2024, 15, 280. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Y.; Wang, Q.; Ma, G.; Liu, J. Liquid metal biomaterials for biomedical imaging. J. Mater. Chem. B 2022, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Recommendation of 10 June 2022 on the Definition of Nanomaterial (Text with EEA Relevance) (2022/C 229/01). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022H0614(01) (accessed on 4 September 2025).
- Rauscher, H.; Kestens, V.; Rasmussen, K.; Linsinger, T.; Stefaniak, E. Guidance on the Implementation of the Commission Recommendation 2022/C 229/01 on the Definition of Nanomaterial; JRC Science for Policy Report; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- Pozzi, M.; Dutta, S.J.; Kuntze, M.; Bading, J.; Rüßbült, J.S.; Fabig, C.; Langfeldt, M.; Schulz, F.; Horcajada, P.; Parak, W.J. Visualization of the High Surface-to-Volume Ratio of Nanomaterials and Its Consequences. J. Chem. Educ. 2024, 101, 3146–3155. [Google Scholar] [CrossRef] [PubMed]
- Alwan, S.H.; Omran, A.A.; Naser, D.K.; Ramadan, M.F. A Mini-Review on Graphene: Exploration of Synthesis Methods and Multifaceted Properties. Eng. Proc. 2023, 59, 226. [Google Scholar]
- Heinrich, A.J.; Oliver, W.D.; Vandersypen, L.M.K.; Ardavan, A.; Sessoli, R.; Loss, D.; Bleszynski Jayich, A.; Fernandez-Rossier, J.; Laucht, A.; Morello, A. Quantum-coherent nanoscience. Nat. Nanotechnol. 2021, 16, 1318–1329. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A Comprehensive Review of Nanomaterials: Types, Synthesis, Characterization, and Applications. Biointerface Res. Appl. Chem. 2023, 13, 41. [Google Scholar]
- Egwu, C.O.; Aloke, C.; Onwe, K.T.; Umoke, C.I.; Nwafor, J.; Eyo, R.A.; Chukwu, J.A.; Ufebe, G.O.; Ladokun, J.; Audu, D.T.; et al. Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules 2024, 29, 2584. [Google Scholar] [CrossRef] [PubMed]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How nanomaterials are transforming drug delivery, bio-imaging, and diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; El-Sayed Marei, H. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef]
- Rezić, I.; Meštrović, E. Characterization of Nanoparticles in Antimicrobial Coatings for Medical Applications—A Review. Coatings 2023, 13, 1830. [Google Scholar] [CrossRef]
- Sun, P.; Wei, N.; Zhang, P.; Yang, Y.; Zhu, M.; Shi, H.; Peng, L.-M.; Zhang, Z. How to build good inverters from nanomaterial-based transistors. Nano Res. 2023, 16, 12594–12600. [Google Scholar] [CrossRef]
- Bouzouita, M.; Pathak, S.; Zayer, F.; Belgacem, H.; Tzouvadaki, I. Advanced memristive architectures based on nanomaterials for biomedical applications: A mini review. Front. Nanotechnol. 2025, 7, 1558743. [Google Scholar] [CrossRef]
- Sasikumar, S.; Sivaram, K.; Sreejisha, N.; Murugesan, S. Nanomaterials-based Field Effect Transistor biosensor for cancer therapy. Next Nanotechnol. 2025, 8, 100170. [Google Scholar] [CrossRef]
- Triana, M.A.; Hsiang, E.-L.; Zhang, C.; Dong, Y.; Wu, S.-T. Luminescent Nanomaterials for Energy-Efficient Display and Healthcare. Luminescent Nanomaterials for Energy-Efficient Display and Healthcare. ACS Energy Lett. 2022, 7, 1001–1020. [Google Scholar] [CrossRef]
- Courel, M. State-of-the-Art Nanomaterials for Solar Cells. Nanomaterials 2025, 15, 508. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B.; Kotnala, S.; Kukretee, N.; Chaudhary, P.; Tripathy, A.R.; Ghai, K.; Mudliar, S.L. Nanomaterials for supercapacitors as energy storage application: Focus on its characteristics and limitations. Mater. Today Proc. 2023, 73, 227–232. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Gournis, D.P.; Karakassides, M.A. Nanomaterials in Catalysis Applications. Catalysts 2023, 13, 627. [Google Scholar] [CrossRef]
- Mohite, D.D.; Chaturvedi, V.; De, S.; Jadhav, V.S. Nanomaterials in Automotive Applications: A Review and its Technical Aspects. Int. J. Contemp. Archit. New ARCH 2021, 8, 1450–1460. [Google Scholar]
- Macías-Silva, M.A.; Cedeno-Munoz, J.S.; Morales-Paredes, C.A.; Tinizaray-Castillo, R.; Perero-Espinoza, G.A.; Rodríguez-Díaz, J.M.; Jarre-Castro, C.M. Nanomaterials in construction industry: An overview of their properties and contributions in building house. Case Stud. Chem. Environ. Eng. 2024, 10, 100863. [Google Scholar] [CrossRef]
- Hafeez, M. Recent Progress and Overview of Nanocomposites. In Nanocomposite Materials for Biomedical and Energy Storage Applications; Sharma, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Vidales-Herrera, J.; López, I. Chapter 3—Nanomaterials in coatings: An industrial point of view. In Micro and Nano Technologies, Handbook of Nanomaterials for Manufacturing Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–77. [Google Scholar]
- Saleem, S.; Zaidi, S.J. Sustainable Use of Nanomaterials in Textiles and Their Environmental Impact. Materials 2020, 13, 5134. [Google Scholar] [CrossRef]
- Khalid, S.A.; Hashem, H.M.; Elokle, A.A. Nanotechnology in active packaging system for quality and safety of foods. Anim. Health Res. J. 2024, 9, 55–63. [Google Scholar]
- Shruti, A.; Bage, N.; Kar, P. Nanomaterials based sensors for analysis of food safety. Food Chem. 2024, 433, 137284. [Google Scholar] [CrossRef]
- Ferreira, L.; Pires, P.C.; Fonseca, M.; Costa, G.; Giram, P.S.; Mazzola, P.G.; Bell, V.; Mascarenhas-Melo, F.; Veiga, F.; Paiva-Santos, A.C. Nanomaterials in Cosmetics: An Outlook for European Regulatory Requirements and a Step Forward in Sustainability. Cosmetics 2023, 10, 53. [Google Scholar] [CrossRef]
- Dekkers, S.; Prud’homme De Lodder, L.C.H.; de Winter, R.; Sips, A.J.A.M.; de Jong, W.H. Inventory of Consumer Products Containing Nanomaterials; RIVM Letter Report 340120001; SIR Advisory Report 11124; RIVM: Bilthoven, The Netherlands, 2007. [Google Scholar]
- El-Kady, M.M.; Ansari, I.; Arora, C.; Rai, N.; Soni, S.; Verma, D.K.; Singh, P.; El Din Mahmoud, A. Nanomaterials: A comprehensive review of applications, toxicity, impact, and fate to environment. J. Mol. Liq. 2023, 370, 121046. [Google Scholar] [CrossRef]
- Cheng, G.X.; Cai, Z.J. Nano-structured scaffold materials used in tissue engineering. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2002, 24, 207–210. (In Chinese) [Google Scholar] [PubMed]
- Zhang, Q.Q.; Liang, Y. The application of nanotechnology in biomedicine. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2002, 24, 197–202. (In Chinese) [Google Scholar] [PubMed]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Tian, L.; Ramakrishna, S. Nanobiomaterials: State of the Art. In Nanobiomaterials: Classification, Fabrication and Biomedical Applications, 1st ed.; Wang, X.M., Ramalingam, M., Kong, X., Zhao, L., Eds.; Wiley-VCH: Weinheim, Germany, 2018; pp. 3–35. [Google Scholar]
- Saxena, R.; Kotnala, S.; Bhatt, S.C.; Uniyal, M.; Rawat, B.S.; Negi, P.; Riyal, M.K. A review on green synthesis of nanoparticles toward sustainable environment. Sustain. Chem. Clim. Action 2025, 6, 100071. [Google Scholar] [CrossRef]
- Jackson, T.C.; Obiakor, N.M.; Iheanyichukwu, I.N.; Ita, O.O.; Ucheokoro, A.S. Biotechnology and Nanotechnology Drug Delivery: A Review. J. Pharm. Pharmacol. 2021, 9, 127–132. [Google Scholar] [CrossRef]
- Shelin, A.R.; Meenakshi, S. Bionanomaterials—An emerging field of nanotechnology. Arch. Mater. Sci. Eng. 2023, 121, 33–41. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Vadanasundari, V.; Pan, S.; Barhoum, A.; Danquah, M.K. Chapter 1—Bionanotechnology and Bionanomaterials: Emerging Applications, Market, and Commercialization. In Bionanotechnology: Emerging Applications of Bionanomaterials; Barhoum, A., Jeevanandam, J., Danquah, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Liua, C.; Yazdani, N.; Morana, C.S.; Salomonb, C.; Seneviratnea, C.J.; Ivanovski, S.; Han, P. Unveiling clinical applications of bacterial extracellular vesicles as natural nanomaterials in disease diagnosis and therapeutics. Acta Biomater. 2024, 180, 18–45. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M., Jr. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Nayak, V.; Singh, R.P. Chapter 1—Introduction to bionanomaterials: An overview. In Bionanomaterials—Fundamentals and Biomedical Applications; Singh, R.P., Singh, K.R.B., Eds.; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Mahmoodi, M.; Hashemi, P.M.; Imani, R. Characterization of a novel nanobiomaterial fabricated from HA, TiO2 and Al2O3 powders: An in vitro study. Prog. Biomater. 2014, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef]
- Heo, D.N.; Castro, N.J.; Lee, S.-J.; Noh, H.; Zhua, W.; Zhang, L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale 2017, 9, 5055. [Google Scholar] [CrossRef]
- Maji, S.K.; Mandal, A.K.; Nguyen, K.T.; Borah, P.; Zhao, Y. Cancer Cell Detection and Therapeutics Using Peroxidase-Active Nanohybrid of Gold Nanoparticle-Loaded Mesoporous Silica-Coated Graphene. ACS Appl. Mater. Interfaces 2015, 7, 9807–9816. [Google Scholar] [CrossRef]
- Beloqui, A.; Cortajarena, A.L. Protein-based functional hybrid bionanomaterials by bottom-up approaches. Curr. Opin. Struct. Biol. 2020, 63, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.P.; Bhuvaneshwari, V.; Ranjithkumar, R.; Sathiyavimal, S.; Malayamanc, V.; Chandarshekar, B. Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: As a bionanomaterials. Int. J. Biol. Macromol. 2017, 104, 1746–1752. [Google Scholar] [CrossRef]
- Nosrati, H.; Heydar, M. Titanium dioxide nanoparticles: A promising candidate for wound healing applications. Burn. Trauma 2025, 13, tkae069. [Google Scholar] [CrossRef]
- Poomrattanangoon, S.; Pissuwan, D. Gold nanoparticles coated with collagen-I and their wound healing activity in human skin fibroblast cells. Heliyon 2024, 10, e33302. [Google Scholar] [CrossRef]
- Almeida, F.A.; Srinivasan, R.; Vijayakumar, S. Editorial: Biosynthesis of bio-inspired nanoparticles/nanomaterials and evaluation of their therapeutic potential in the medical field. Front. Nanotechnol. 2023, 5, 1198994. [Google Scholar] [CrossRef]
- Dolci, M.; Wang, Y.; Nooteboom, S.W.; Rodriguez, P.E.D.S.; Sánchez, S.; Albertazzi, L.; Zijlstra, P. Real-Time Optical Tracking of Protein Corona Formation on Single Nanoparticles in Serum. ACS Nano 2023, 17, 20167–20178. [Google Scholar] [CrossRef] [PubMed]
- Tehseena, B.; Rehmana, A.; Rahmatc, M.; Bhattic, H.N.; Wud, A.; Butte, F.K.; Nazf, G.; Khana, W.S.; Bajwa, S.Z. Solution growth of 3D MnO2 mesh comprising 1D nanofibres as a novel sensor for selective and sensitive detection of biomolecules. Biosens. Bioelectron. 2018, 117, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Shad, N.A.; Bajwa, S.Z.; Amin, N.; Taj, A.; Hameed, S.; Khan, Y.; Dai, Z.; Cao, C.; Khan, W.S. Solution growth of 1D zinc tungstate (ZnWO4) nanowires; design, morphology, and electrochemical sensor fabrication for selective detection of chloramphenicol. J. Hazard. Mater. 2019, 367, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.; Maji, P.K. Synergistic effect of cellulose nanofibres and bio-extracts for fabricating high strength sodium alginate based composite bio-sponges with antibacterial properties. Carbohydr. Polym. 2019, 203, 396–408. [Google Scholar] [CrossRef]
- Morikawa, M.; Yoshihara, M.; Endo, T.; Kimizuka, N. ATP as Building Blocks for the Self-Assembly of Excitonic Nanowires. J. Am. Chem. Soc. 2005, 127, 1358–1359. [Google Scholar] [CrossRef]
- Choi, A.H.; Cazalbou, S.; Ben-Nissan, B. Nanobiomaterial Coatings in Dentistry. Front. Oral. Biol. 2015, 17, 49–61. [Google Scholar]
- Krishna, S.B.N.; Jakmunee, J.; Mishra, Y.K.; Prakash, J. ZnO based 0–3D diverse nano-architectures, films and coatings for biomedical applications. J. Mater. Chem. B 2024, 12, 2950. [Google Scholar] [CrossRef]
- Ma, L.; Zong, J.; Xun, X.; Hu, X.; Chen, Z.; Zhang, Q.; Peng, M.; Song, B.; Ao, H. Fabrication of gentamicin loaded Col-I/HA multilayers modified titanium coatings for prevention of implant infection. Front. Chem. 2022, 10, 1019332. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.R.S.; Eapen, A.S.; Zhang, W.; Roy, S. Polysaccharide Based Edible Biopolymer-Based Coatings for Fruit Preservation: A Review. Foods 2024, 13, 1529. [Google Scholar] [CrossRef] [PubMed]
- Zuo, R.; Gong, J.; Gao, X.; Huang, J.; Zhang, J.; Jiang, S.; Guo, D. Using halofuginone–silver thermosensitive nanohydrogels with antibacterial and anti-inflammatory properties for healing wounds infected with Staphylococcus aureus. Life Sci. 2024, 339, 122414. [Google Scholar] [CrossRef]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef]
- Sajjadi, M.; Nasrollahzadeh, M.; Ghafuri, H. Functionalized chitosan-inspired (nano)materials containing sulfonic acid groups: Synthesis and application. Carbohydr. Polym. 2024, 343, 122443. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef]
- Tyan, Y.-C.; Yang, M.-H.; Chang, C.-C.; Chung, T.-W. Biocompatibility of Materials for Biomedical Engineering. Adv. Exp. Med. Biol. 2020, 1250, 125–140. [Google Scholar] [PubMed]
- Guo, Y.; Chen, Y.; Han, P.; Liu, X.; Li, W.; Zhu, F.; Fu, K.; Chu, M. Biocompatible chitosan-carbon nanocage hybrids for sustained drug release and highly efficient laser and microwave co-irradiation induced cancer therapy. Acta Biomater. 2020, 103, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.R.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Srivastava, S.; Jan, S.Y.; Deka, P.; Sabahi, N. Recent advances in the production of bionanomaterials for development of sustainable food packaging: A comprehensive review. Environ. Res. 2023, 237, 116948. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, C.; Yang, Y.; Xu, S.; Huang, H.; Zhang, L.; Lei, B.; Zhao, F. A Highly Bioactive Organic-Inorganic Nanoparticle for Activating Wnt10b Mediated Osteogenesis by Specifically Anchor CCN3 Protein. Adv. Healthc. Mater. 2025, 14, e2404075. [Google Scholar] [CrossRef]
- Maureira, M.; Cuadra, F.; Cádiz, M.; Torres, M.; Marttens, A.V.; Covarrubias, C. Preparation and osteogenic properties of nanocomposite hydrogel beads loaded with nanometric bioactive glass particles. Biomed. Mater. 2021, 16, 045043. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, Y.; Zhong, C.; Ma, Z.; Wang, H.; Dong, X.; Yu, F.; Li, J.; Chen, Q.; Lin, C.; et al. Advanced bioactive nanomaterials for diagnosis and treatment of major chronic diseases. Front. Mol. Biosci. 2023, 10, 1121429. [Google Scholar] [CrossRef]
- Khan, F.; Ahmad, S.R. Bioactive Polymers and Nanobiomaterials Composites for Bone Tissue Engineering. In Biomimetics; Ramalingam, M., Wang, X., Eds.; Scrivener Publishing: Beverly, MA, USA, 2013; pp. 91–118. [Google Scholar]
- Sharma, P.; Mittal, M.; Yadav, A.; Aggarwal, N.K. Bacterial cellulose: Nano-biomaterial for biodegradable face masks—A greener approach towards environment. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100759. [Google Scholar] [CrossRef]
- Bakola, V.; Karagkiozaki, V.; Tsiapla, A.R.; Pappa, F.; Moutsios, I.; Pavlidou, E.; Logothetidis, S. Dipyridamole-loaded biodegradable PLA nanoplatforms as coatings for cardiovascular stents. Nanotechnology 2018, 29, 275101. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; Wang, W.; Wang, S.; Pan, X.; Zhang, F.; Li, S.; Liu, S.; Wang, H.; Gao, G.; et al. Biodegradable Poly(amino acid)-Gold-Magnetic Complex with Efficient Endocytosis for Multimodal Imaging-Guided Chemo-photothermal Therapy. ACS Nano 2018, 12, 9022–9032. [Google Scholar] [CrossRef]
- Leiro, V.; Garcia, J.P.; Moreno, P.M.D.; Spencer, A.P.; Fernandez-Villamarin, M.; Riguera, R.; Fernandez-Megia, E.; Pêgo, A.P. Biodegradable PEG-dendritic block copolymers: Synthesis and biofunctionality assessment as vectors of siRNA. J. Mater. Chem. B 2017, 5, 4901–4917. [Google Scholar] [CrossRef]
- Subhan, M.A.; Torchilin, V.P. Advances in siRNA Drug Delivery Strategies for Targeted TNBC Therapy. Bioengineering 2024, 11, 830. [Google Scholar] [CrossRef]
- Lara, P.; Chan, A.B.; Cruz, L.J.; Quest, A.F.G.; Kogan, M.J. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery towards Specific Cells and Tissues. Pharmaceutics 2020, 12, 1022. [Google Scholar] [CrossRef]
- Chen, X.; Lei, L.; Yan, J.; Wang, X.; Li, L.; Liu, Q.; Wang, Y.; Chen, T.; Shao, J.; Yu, L.; et al. Bifunctional Phage Particles Augment CD40 Activation and Enhance Lymph Node-Targeted Delivery of Personalized Neoantigen Vaccines. ACS Nano 2025, 19, 6955–6976. [Google Scholar] [CrossRef]
- Cho, C.F.; Shukla, S.; Simpson, E.J.; Steinmetz, N.F.; Luyt, L.G.; Lewis, J.D. Molecular targeted viral nanoparticles as tools for imaging cancer. Methods Mol. Biol. 2014, 1108, 211–230. [Google Scholar]
- Laskar, P.; Varghese, O.P.; Shastri, V.P. Advances in Intracellular and On-Surface Polymerization in Living Cells: Implications for Nanobiomedicines. Adv. NanoBiomed. Res. 2023, 3, 2200174. [Google Scholar] [CrossRef]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.-A.; Sudesh, K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar] [CrossRef]
- Elgersma, R.C.; van Dijk, M.; Dechesne, A.C.; van Nostrum, C.F.; Hennink, W.E.; Rijkers, D.T.; Liskamp, R.M. Microwave-assisted click polymerization for the synthesis of Abeta(16–22) cyclic oligomers and their self-assembly into polymorphous aggregates. Org. Biomol. Chem. 2009, 7, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Ramasamy, K.; Leena, M.; Jiménez, C.; Campos, J.; Ibarra, P.; Haidar, Z.S.; Ramalingam, M. Surface functionalization of nanobiomaterials for application in stem cell culture, tissue engineering, and regenerative medicine. Biotechnol. Prog. 2016, 32, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Theus, A.S.; Ning, L.; Jin, L.; Roeder, R.K.; Zhang, J.; Serpooshan, V. Nanomaterials for bioprinting: Functionalization of tissue-specific bioinks. Essays Biochem. 2021, 65, 429–439. [Google Scholar] [CrossRef]
- Mukhtar, M.; Zeeshan, M.; Saba, M.; Saghir, A.; Ayub, R. Chapter 9—Functionalization of green synthesized bionanomaterials. In Micro and Nano Technologies, Synthesis of Bionanomaterials for Biomedical Applications; Ozturk, M., Roy, A., Bhat, R.A., Vardar-Sukan, F., Tonelli, F.M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 187–207. [Google Scholar]
- Durmaz, E.; Sertkaya, S.; Yilmaz, H.; Olgun, C.; Ozcelik, O.; Tozluoglu, A.; Candan, Z. Lignocellulosic Bionanomaterials for Biosensor Applications. Micromachines 2023, 14, 1450. [Google Scholar] [CrossRef]
- Olguín, Y.; Acuna-Mendoza, S.; Otero, C.; Acevedo, C.A.; Covarrubias, C. Osteoconductive Effect of a Nanocomposite Membrane Treated with UV Radiation. Polymers 2022, 14, 289. [Google Scholar] [CrossRef]
- Civantos, A.; Barnwell, A.; Shetty, A.R.; Pavón, J.J.; El-Atwani, O.; Arias, S.L.; Lang, E.; Reece, L.M.; Chen, M.; Allain, J.P. Designing Nanostructured Ti6Al4V Bioactive Interfaces with Directed Irradiation Synthesis toward Cell Stimulation to Promote Host-Tissue-Implant Integration. ACS Biomater. Sci. Eng. 2019, 5, 3325–3339. [Google Scholar] [CrossRef]
- Zaharescu, T.; Mari¸s, M. Irradiation Effects in Polymer Composites for Their Conversion into Hybrids. J. Compos. Sci. 2022, 6, 109. [Google Scholar] [CrossRef]
- Ul Ahad, I.; Bartnik, A.; Fiedorowicz, H.; Kostecki, J.; Korczyc, B.; Ciach, T.; Brabazon, D. Surface modification of polymers for biocompatibility via exposure to extreme ultraviolet radiation. J. Biomed. Mater. Res. Part A 2014, 102, 3298. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.J.; Zhang, J.X.; Wen, M.L.; Wei, Y.; Tang, T.T.; Yang, T.T.; Bai, H.T.; Guo, C.Q.; Gao, X.; Wang, Z.C.; et al. Preparation of polyvinyl alcohol/chitosan nanofibrous films incorporating graphene oxide and lanthanum chloride by electrospinning method for potential photothermal and chemical synergistic antibacterial applications in wound dressings. J. Mech. Behav. Biomed. Mater. 2023, 148, 106162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ji, P.; Bu, T.; Mao, Y.; He, H.; Ge, N. Recent Progress in the Application of Electrospinning Technology in the Biomedical Field. J. Funct. Biomater. 2025, 16, 266. [Google Scholar] [CrossRef] [PubMed]
- Shabani, A.; Al, G.A.; Berri, N.; Castro-Dominguez, B.; Leese, H.S.; Martinez-Hernande, U. Electrospinning Technology, Machine Learning, and Control Approaches: A Review. Adv. Eng. Mater. 2025, 27, 2401353. [Google Scholar] [CrossRef]
- Ortega-Nieto, C.; Losada-Garcia, N.; Pessela, B.C.; Domingo-Calap, P.; Palomo, J.M. Design and Synthesis of Copper Nanobiomaterials with Antimicrobial Properties. ACS Bio Med. Chem. Au 2023, 3, 349–358. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, S.; Peng, M.; Gama, M.; Yang, Z.; Deng, X.; Zhou, J.; Ouyang, C.; Luo, H. Controllable synthesis of biomimetic nano/submicro-fibrous tubes for potential small-diameter vascular grafts. J. Mater. Chem. B 2020, 8, 5694–5706. [Google Scholar] [CrossRef]
- Contreras, D.C.; Cisternas, M.A.; Congreve, R.C.; Varaprasad, K.; Chandrasekaran, K.; Sadiku, E.R. Antimicrobial hydrogel scaffolds from Barba de Viejo microfibers, alginate and Ago nanoparticles via green synthesis. Int. J. Biol. Macromol. 2025, 284, 138048. [Google Scholar] [CrossRef]
- Dugganaboyana, G.K.; Mukunda, C.K.; Jain, A.; Kantharaju, R.M.; Nithya, R.R.; Ninganna, D.; Ahalliya, R.M.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; et al. Environmentally benign silver bio-nanomaterials as potent antioxidant, antibacterial, and antidiabetic agents: Green synthesis using Salacia oblonga root extract. Front. Chem. 2023, 11, 1114109. [Google Scholar] [CrossRef]
- Elgersma, R.C.; Posthuma, G.; Rijkers, D.T.; Liskamp, R.M. Backbone-modified amylin derivatives: Implications for amyloid inhibitor design and as template for self-assembling bionanomaterials. J. Pept. Sci. 2007, 13, 709–716. [Google Scholar] [CrossRef]
- Srinivasarao, D.A.; Shah, S.; Famta, P.; Vambhurkar, G.; Jain, N.; Pindiprolu, S.K.S.S.; Sharma, A.; Kumar, R.; Padhy, H.P.; Kumari, M.; et al. Unravelling the role of tumor microenvironment responsive nanobiomaterials in spatiotemporal controlled drug delivery for lung cancer therapy. Drug Deliv. Transl. Res. 2025, 15, 407–435. [Google Scholar] [CrossRef] [PubMed]
- Shoja, Z.; Jalilvand, S.; Latifi, T.; Roohvand, F. Rotavirus VP6: Involvement in immunogenicity, adjuvant activity, and use as a vector for heterologous peptides, drug delivery, and production of nano-biomaterials. Arch. Virol. 2022, 167, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Ogunnaike, E.A.; Pitz, M.; Elbaz, N.M.; Panda, D.K.; Alexander-Bryant, A.; Saha, S.; Whitehead, D.C.; Kabanov, A. Enhancing drug delivery with supramolecular amphiphilic macrocycle nanoparticles: Selective targeting of CDK4/6 inhibitor palbociclib to melanoma. Biomater. Sci. 2024, 12, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, E.; Dehshahri, A.; Izadpanah, K.; Ahmadi, F. Plant virus nanoparticles: Novel and robust nanocarriers for drug delivery and imaging. Colloids Surf. B Biointerfaces 2018, 167, 20–27. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, C.; Sun, F.; Chen, Z.; Zou, J.; Chen, X.; Yang, Z. J-Aggregation Strategy toward Potentiated NIR-II Fluorescence Bioimaging of Molecular Fluorophores. Adv. Mater. 2024, 36, 2304848. [Google Scholar] [CrossRef]
- Mehta, N.; Sahu, S.P.; Shaik, S.; Devireddy, R.; Gartia, M.R. Dark-field hyperspectral imaging for label free detection of nano-bio-materials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1661. [Google Scholar] [CrossRef]
- Park, S.; Mondal, S. Editorial: Bio-nanomaterials and systems for enhanced bioimaging in biomedical applications. Front. Mol. Biosci. 2024, 11, 1491376. [Google Scholar] [CrossRef]
- Wen, A.M.; Lee, K.L.; Yildiz, I.; Bruckman, M.A.; Shukla, S.; Steinmetz, N.F. Viral nanoparticles for in vivo tumor imaging. J. Vis. Exp. 2012, 69, e4352. [Google Scholar]
- Zhang, S.; Chen, X.; Shan, M.; Hao, Z.; Zhang, X.; Meng, L.; Zhai, Z.; Zhang, L.; Liu, X.; Wang, X. Convergence of 3D Bioprinting and Nanotechnology in Tissue Engineering Scaffolds. Biomimetics 2023, 8, 94. [Google Scholar] [CrossRef]
- Hung, H.S.; Hsu, S.H. Surface Modification by Nanobiomaterials for Vascular Tissue Engineering Applications. Curr. Med. Chem. 2020, 27, 1634–1646. [Google Scholar] [CrossRef]
- Urquhart, T.; Daub, E.; Honek, J.F. Bioorthogonal Modification of the Major Sheath Protein of Bacteriophage M13: Extending the Versatility of Bionanomaterial Scaffolds. Bioconjugate Chem. 2016, 27, 2276–2280. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Roberts, S.J.; Meade, S.J.; Gerrard, J.A. Amyloid fibrils as a nanoscaffold for enzyme immobilization. Biotechnol. Prog. 2010, 26, 93–100. [Google Scholar] [CrossRef]
- Singh, A.V.; Gemmati, D.; Kanase, A.; Pandey, I.; Misra, V.; Kishore, V.; Jahnke, T.; Bill, J. Nanobiomaterials for vascular biology and wound management: A review. Veins Lymphat. 2018, 7, 7196, 34–47. [Google Scholar] [CrossRef]
- Leonida, M.D.; Kumar, I. Bionanomaterials for Skin Regeneration; Springer Briefs in Bioengineering; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Mittal, P.; Rana, P.; Goyal, C.; Kapoor, R.; Soni, M.; Gautam, R. Bionanomaterials in Wound Dressings. In Bionanomaterials for Industrial Applications; Shakeel, A., Ed.; CRC Press: Boca Raton, FL, USA, 2024; pp. 301–309. [Google Scholar]
- Ezoji, H.; Rahimnejad, M. Nanobiomaterials for Point-of-Care Diagnostics. In Handbook of Nanobioelectrochemistry; Azad, U.P., Chandra, P., Eds.; Springer: Singapore, 2023. [Google Scholar]
- Karmakar, S.; Saxena, V.; Chandra, P.; Pandey, L.M. Novel Therapeutics and Diagnostics Strategies Based on Engineered Nanobiomaterials. In Nanotechnology in Modern Animal Biotechnology, Recent Trends and Future Perspectives; Singh, S., Maurya, P.K., Eds.; Springer Nature: Singapore, 2019. [Google Scholar]
- Park, S.M.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014. [Google Scholar] [CrossRef]
- Palchoudhury, S.; Palchaudhury, S. Chapter 14—Bionanomaterials for diagnosis and therapy of SARS-CoV-2. In Micro and Nano Technologies, Bionanotechnology: Emerging Applications of Bionanomaterials; Barhoum, A., Jeevanandam, J., Danquah, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 469–489. [Google Scholar]
- Khan, T.; Umer, R. Self-sensing piezoresistive aerospace composites based on CNTs and 2D material coated fabric sensors. Electron 2024, 2, e61. [Google Scholar] [CrossRef]
- Jiang, S.; Newton, E.; Yuen, C.-W.M.; Kan, C.-W. Application of Chemical Silver Plating on Polyester and Cotton Blended Fabric. Text. Res. J. 2007, 77, 85–91. [Google Scholar] [CrossRef]
- Das, S.; Shanmugam, N.; Kumar, A.; Jose, S. Review: Potential of biomimicry in the field of textile technology. Bioinspired Biomim. Nanobiomater. 2017, 6, 1–42. [Google Scholar] [CrossRef]
- Park, Y.; Shin, S.; Shukla, N.; Kim, K.; Park, M.H. Effects of Nanobubbles in Dermal Delivery of Drugs and Cosmetics. Nanomaterials 2022, 12, 3286. [Google Scholar] [CrossRef]
- Singh, T.G.; Sharma, N. Chapter 7—Nanobiomaterials in cosmetics: Current status and future prospects. In Nanobiomaterials in Galenic Formulations and Cosmetics; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 149–174. [Google Scholar]
- Rehman, N.; Pandey, A. Green Nanomaterials Revolution in Cosmetic Products and Skin Treatment. In Green Nanoarchitectonics; Pal, K., Ed.; Jenny Stanford Publishing: Singapore, 2023; pp. 271–288. ISBN 978-1-003-31860-6. [Google Scholar]
- Ashtiani, H.R.A.; Bishe, P.; Lashgari, N.-A.; Nilforoushzadeh, M.A.; Zare, S. Liposomes in Cosmetics. J. Skin Stem Cell 2016, 3, e65815. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Nayak, V.; Singh, J.; Adetunji, C.O.; Singh, R.P. Chapter 1—Introduction: Potentialities of bionanomaterials towards the environmental and agricultural domain. In Bionanomaterials for Environmental and Agricultural Applications; Singh, R.P., Singh, K.R.B., Eds.; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Saha, A. Chapter 3—Bionanotechnology-Based Nanopesticide Application in Crop Protection Systems. In Functionalized Nanomaterials for Catalytic Application; Hussain, C.M., Shukla, S.K., Mangla, B., Eds.; Scrivener Publishing LLC: Austin, TX, USA, 2021. [Google Scholar]
- Boregowda, N.; Jogigowda, S.C.; Bhavya, G.; Sunilkumar, C.R.; Geetha, N.; Udikeri, S.S.; Chowdappa, S.; Govarthanan, M.; Jogaiah, S. Recent advances in nanoremediation: Carving sustainable solution to clean-up polluted agriculture soils. Environ. Pollut. 2022, 297, 118728. [Google Scholar] [CrossRef]
- Chellasamy, G.; Kiriyanthan, R.M.; Maharajan, T.; Radha, A.; Yun, K. Remediation of microplastics using bionanomaterials: A review. Environ. Res. 2022, 208, 112724. [Google Scholar] [CrossRef]
- Xu, M.; Liu, S.; Wen, J.; Wang, B.; Wang, H.; Lian, X.; Gao, X.; Niu, B.; Li, W. Preparation of sodium alginate modified silver-metal organic framework and application in citric acid/PVA antimicrobial packaging. Food Chem. 2024, 451, 139464. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Ogundolie, F.A.; Olaniyan, O.T.; Mathew, J.T.; Inobeme, A.; Titilayo, O.; Ghazanfar, S.; Ijabadeniyi, O.A.; Ajiboye, M.D.; Ajayi, O.O.; et al. Chapter 8—Nanobiomaterials for Food Packaging Sensor Applications. In Bio- and Nano-Sensing Technologies for Food Processing and Packaging; Shukla, A.K., Ed.; The Royal Society of Chemistry: London, UK, 2022; Volume 8, pp. 167–180. [Google Scholar]
- Primozic, M.; Knez, Z.; Leitgeb, M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef]
- Puglia, M.K.; Malhotra, M.; Kumar, C.V. Engineering functional inorganic nanobiomaterials: Controlling interactions between 2D-nanosheets and enzymes. Dalton Trans. 2020, 49, 3917. [Google Scholar] [CrossRef]
- Reardon, P.J.T.; Huang, J.; Tang, J. Mesoporous calcium phosphate bionanomaterials withcontrolled morphology by an energy-efficient microwave method. J. Biomed. Mater. Res. Part A 2015, 103A, 3781–3789. [Google Scholar] [CrossRef]
- de Souza Silva, K.F.; Costa-Orlandi, C.B.; Fernandes, M.A.; Brasil, G.S.P.; Mussagy, C.U.; Scontri, M.; da Silva Sasaki, J.C.; de Sousa Abreu, A.P.; Guerra, N.B.; Floriano, J.F.; et al. Biocompatible anti-aging face mask prepared with curcumin and natural rubber with antioxidant properties. Int. J. Biol. Macromol. 2023, 242, 124778. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkowiak, B.; Siatkowska, M.; Komorowski, P. Bionanomaterials or Nanobiomaterials: Differences in Definitions and Applications. J. Funct. Biomater. 2025, 16, 351. https://doi.org/10.3390/jfb16090351
Walkowiak B, Siatkowska M, Komorowski P. Bionanomaterials or Nanobiomaterials: Differences in Definitions and Applications. Journal of Functional Biomaterials. 2025; 16(9):351. https://doi.org/10.3390/jfb16090351
Chicago/Turabian StyleWalkowiak, Bogdan, Małgorzata Siatkowska, and Piotr Komorowski. 2025. "Bionanomaterials or Nanobiomaterials: Differences in Definitions and Applications" Journal of Functional Biomaterials 16, no. 9: 351. https://doi.org/10.3390/jfb16090351
APA StyleWalkowiak, B., Siatkowska, M., & Komorowski, P. (2025). Bionanomaterials or Nanobiomaterials: Differences in Definitions and Applications. Journal of Functional Biomaterials, 16(9), 351. https://doi.org/10.3390/jfb16090351






