Data-Driven Design and Additive Manufacturing of Patient-Specific Lattice Titanium Scaffolds for Mandibular Bone Reconstruction
Abstract
1. Introduction
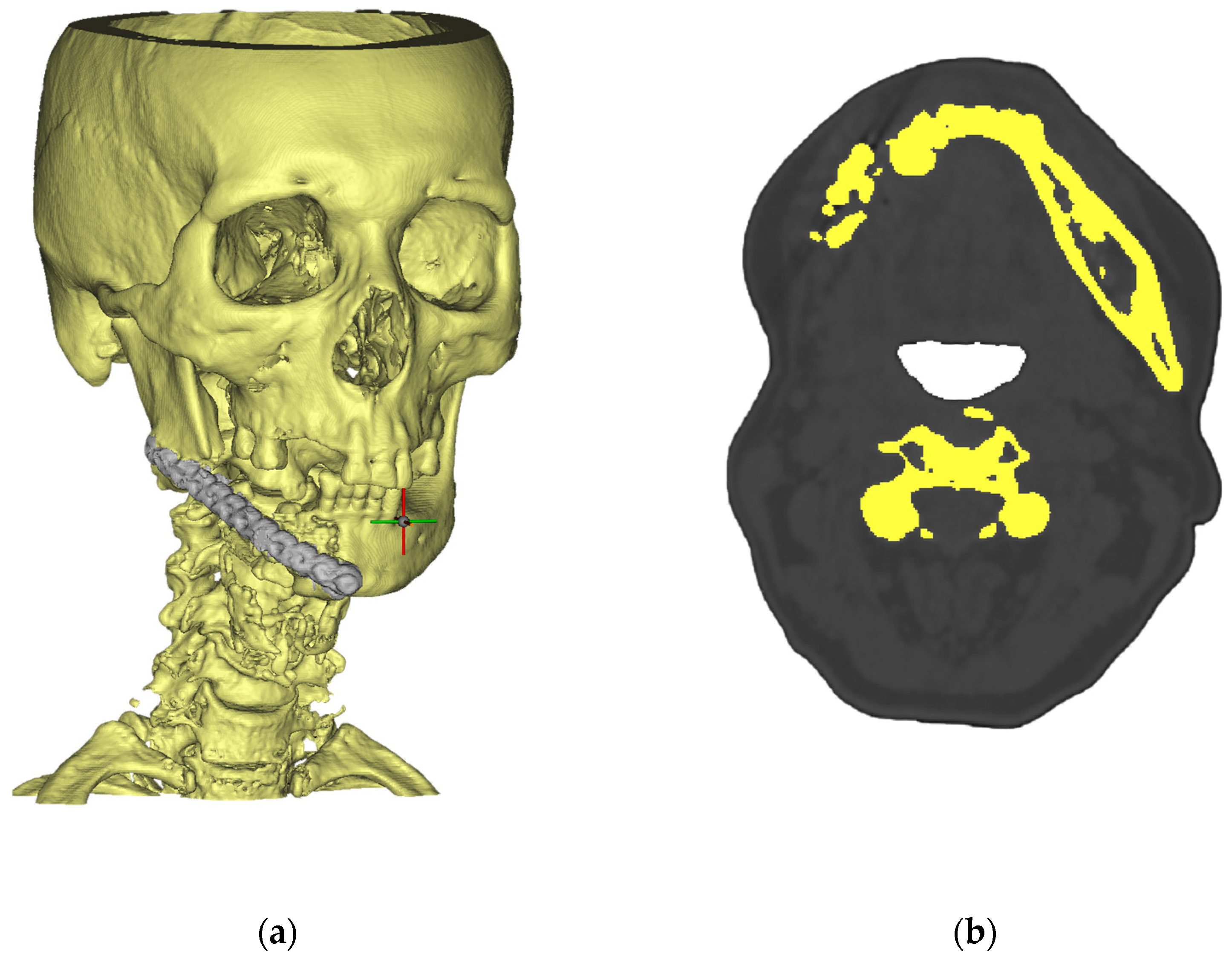
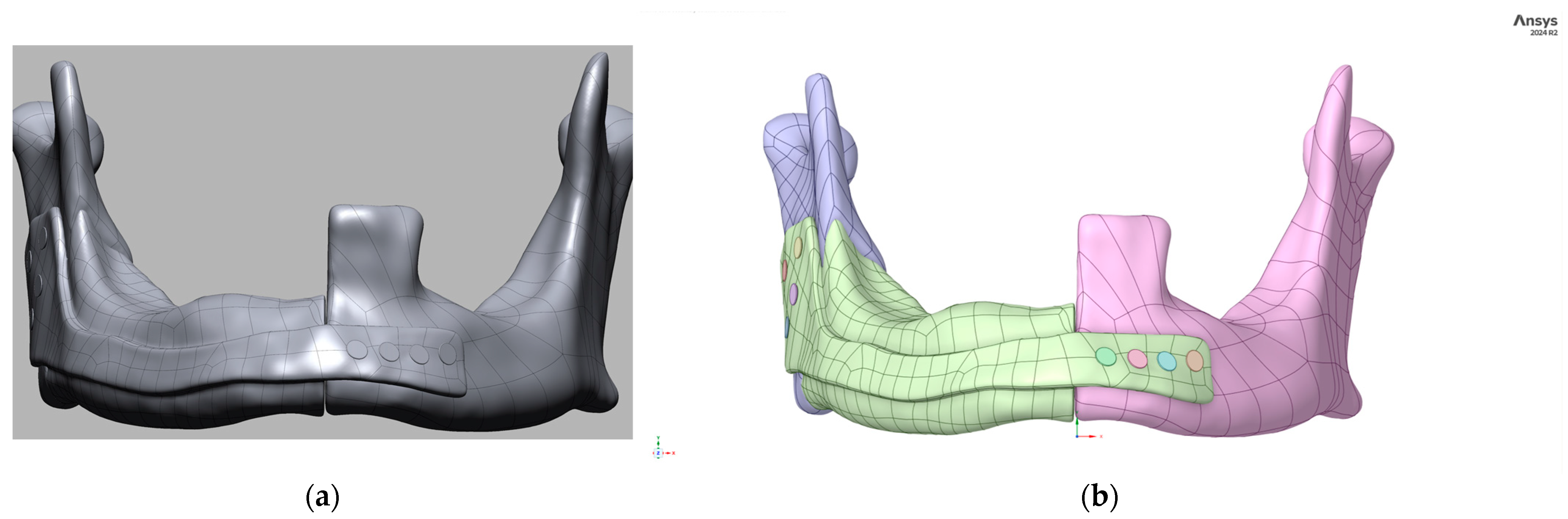
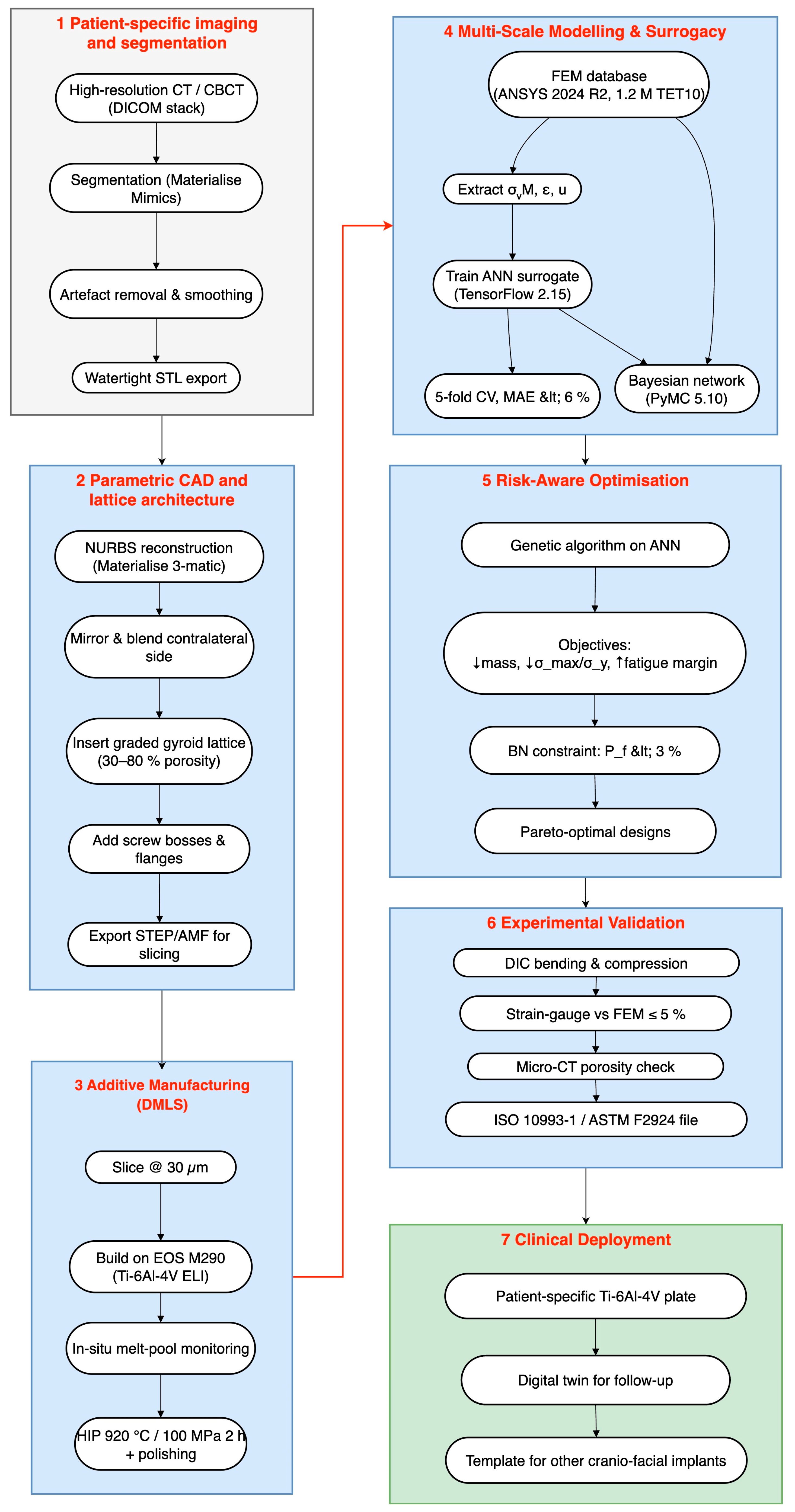
2. Integrated Workflow and Methods
Surrogate Modelling (ANN)
3. Results
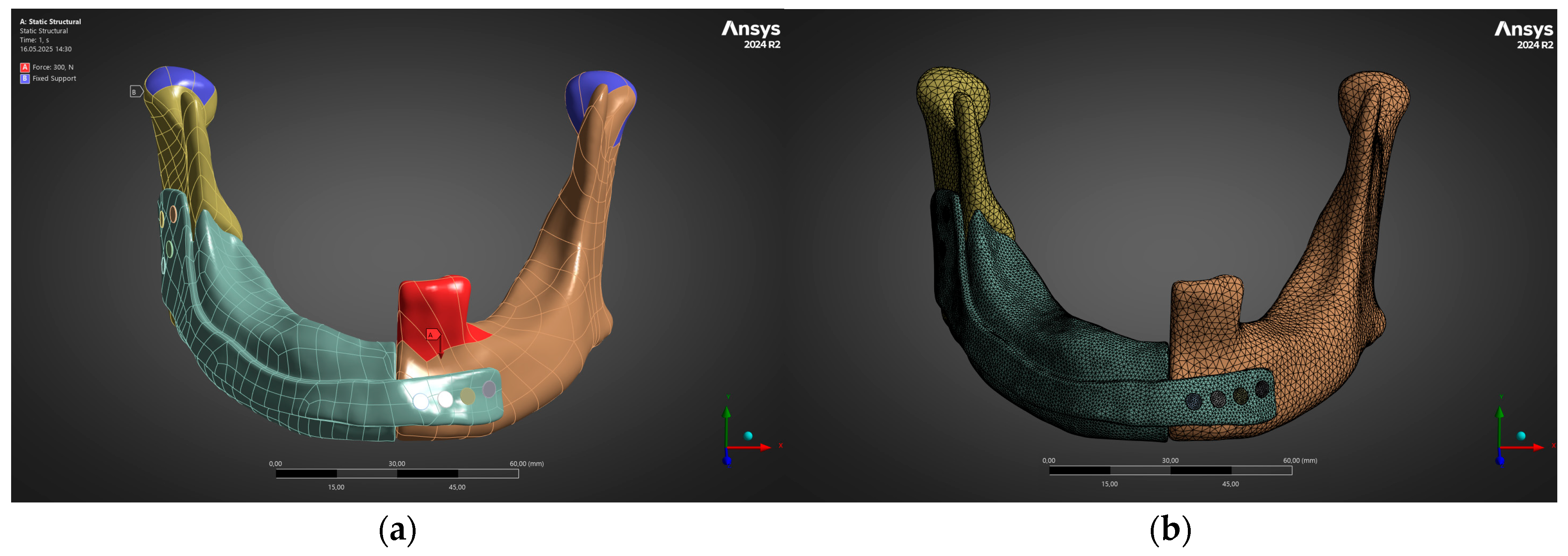
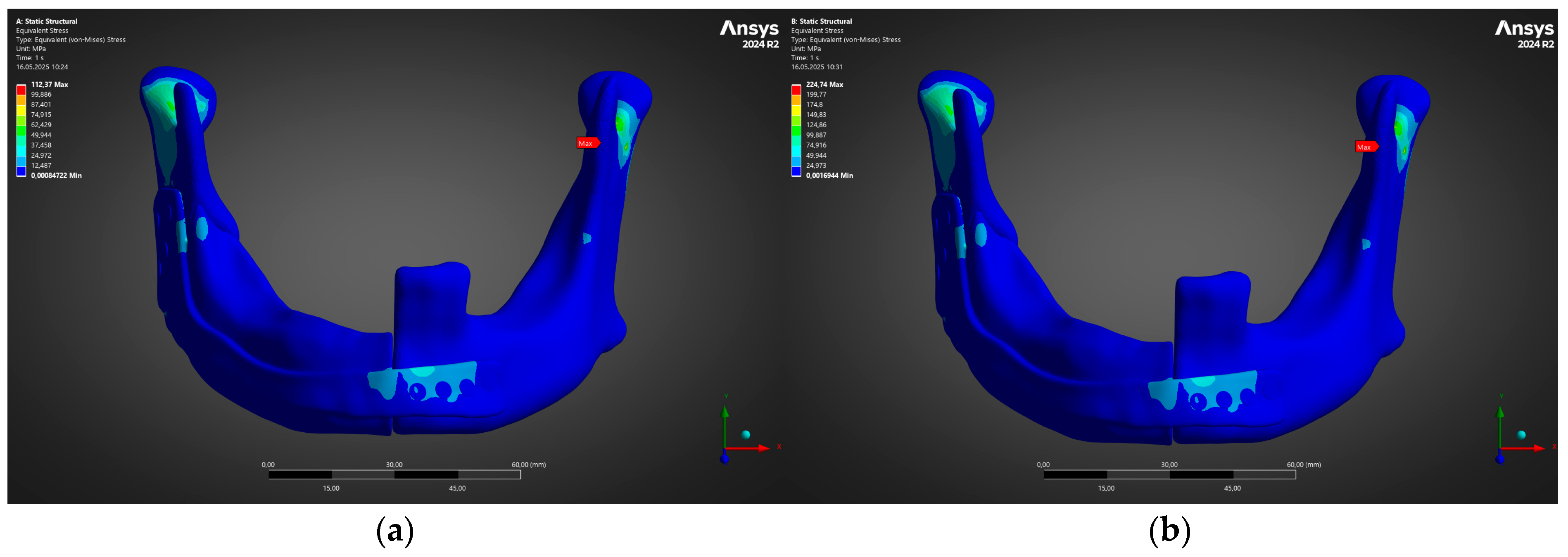
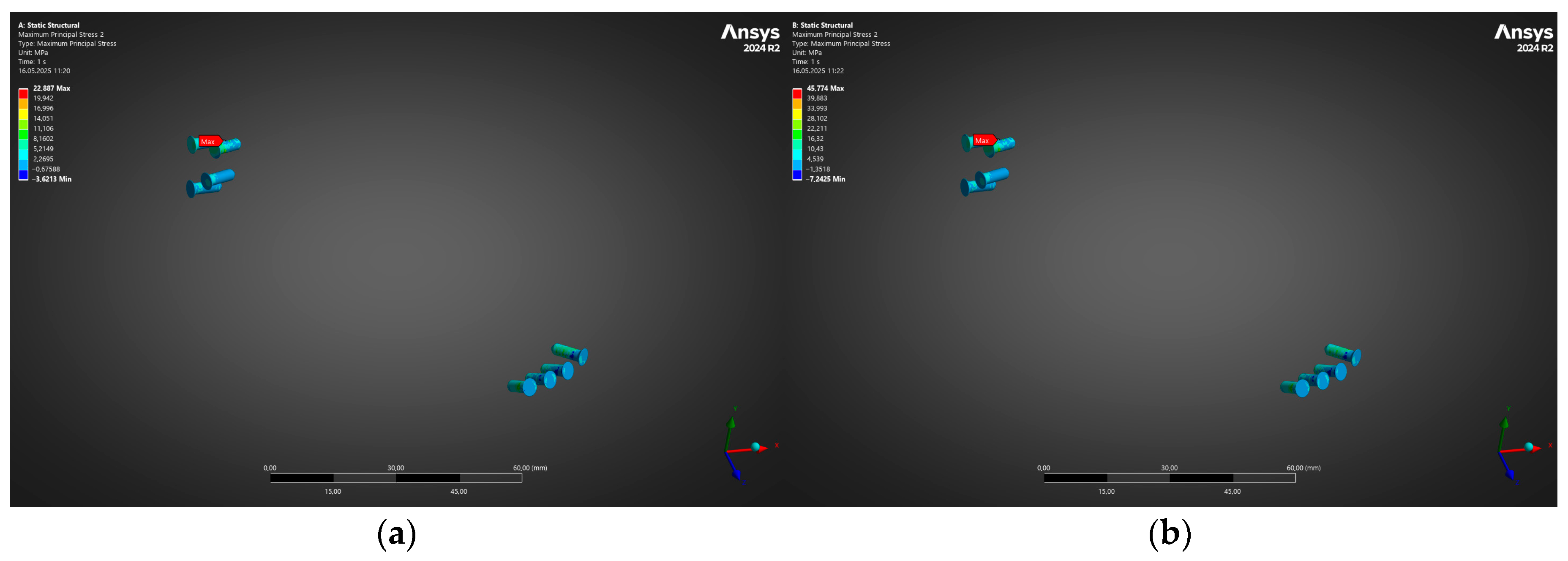
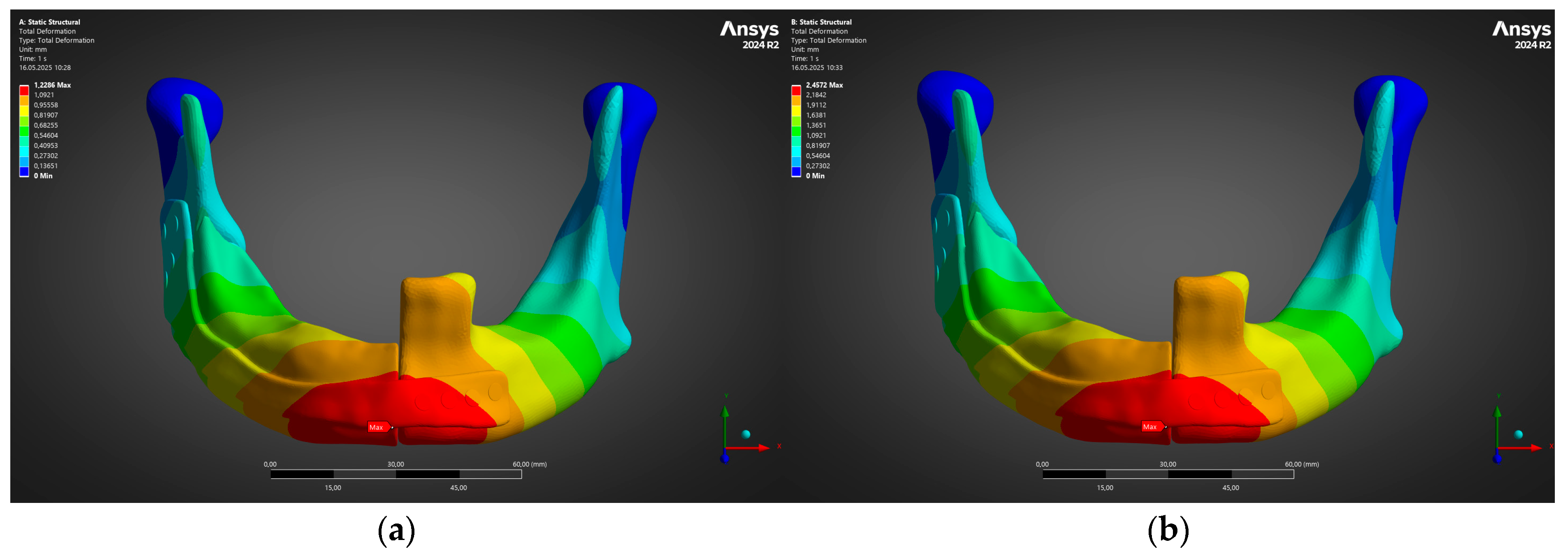
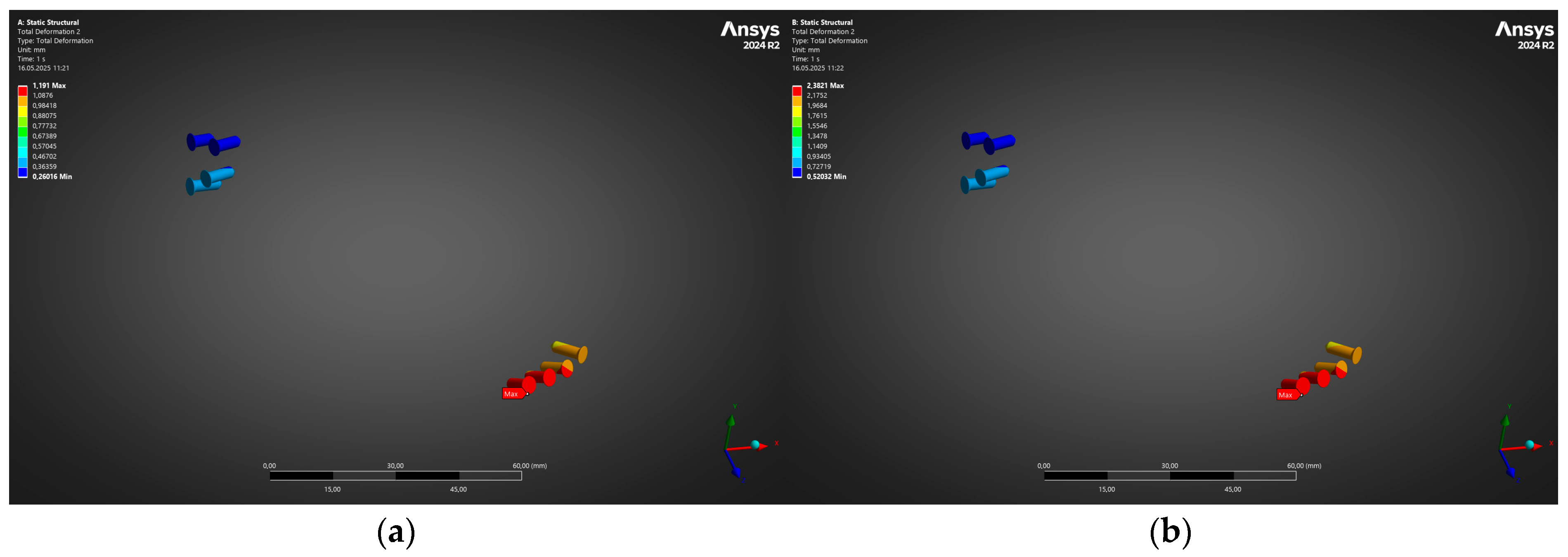
3.1. Structural Performance Under Physiological Loading (FEM Analysis)
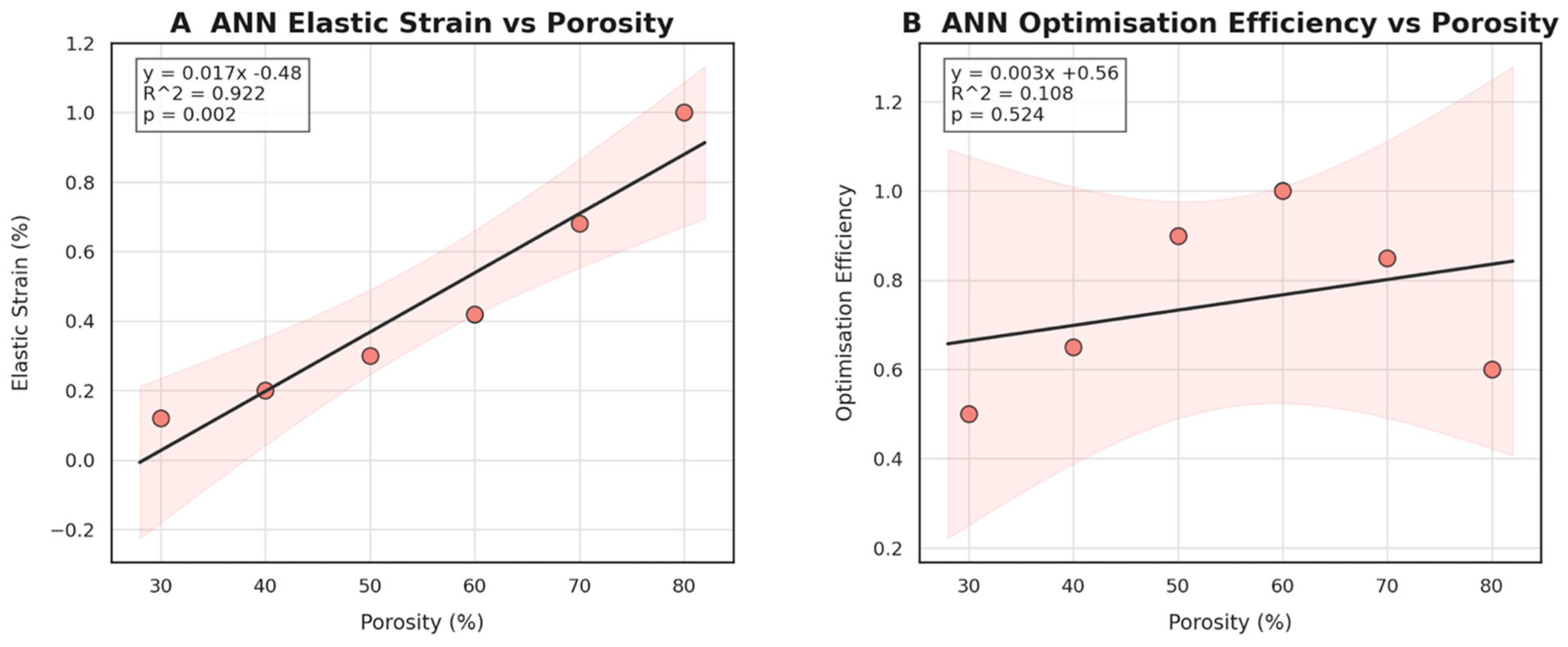
3.2. Machine Learning Surrogate Modelling and Prediction
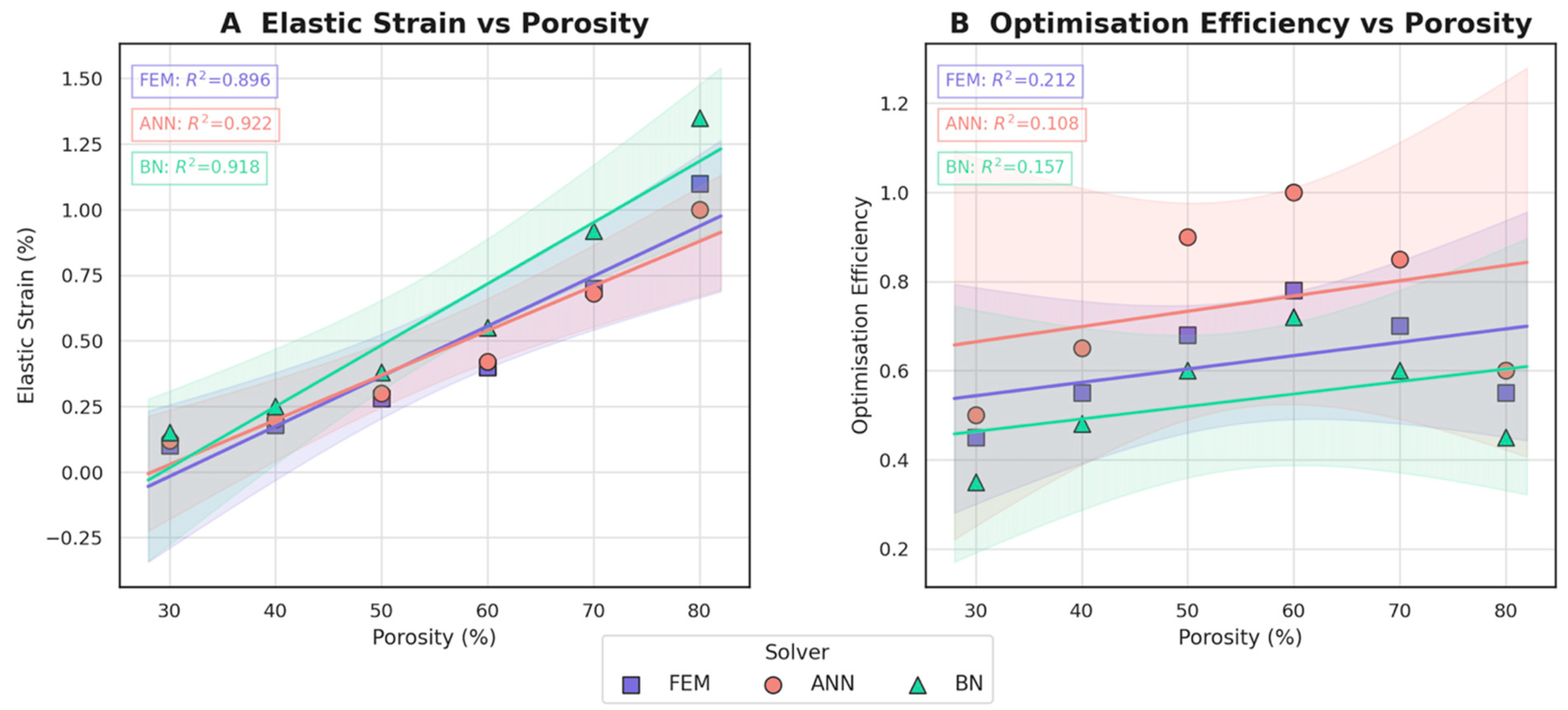
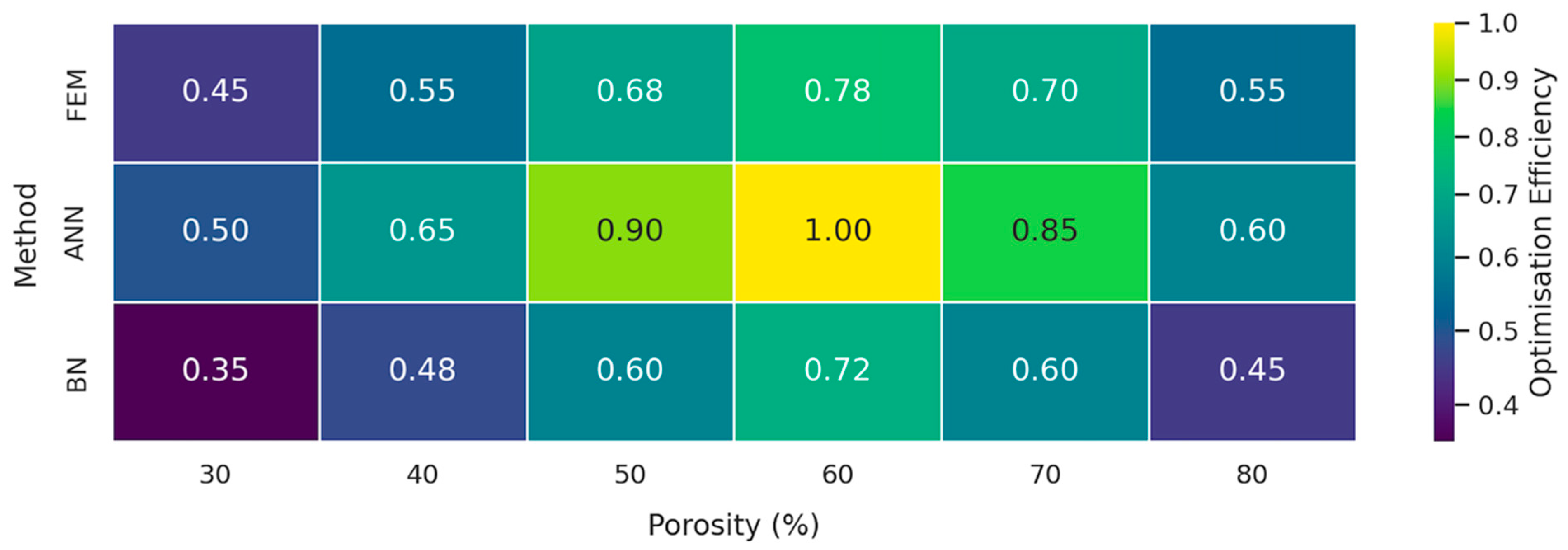
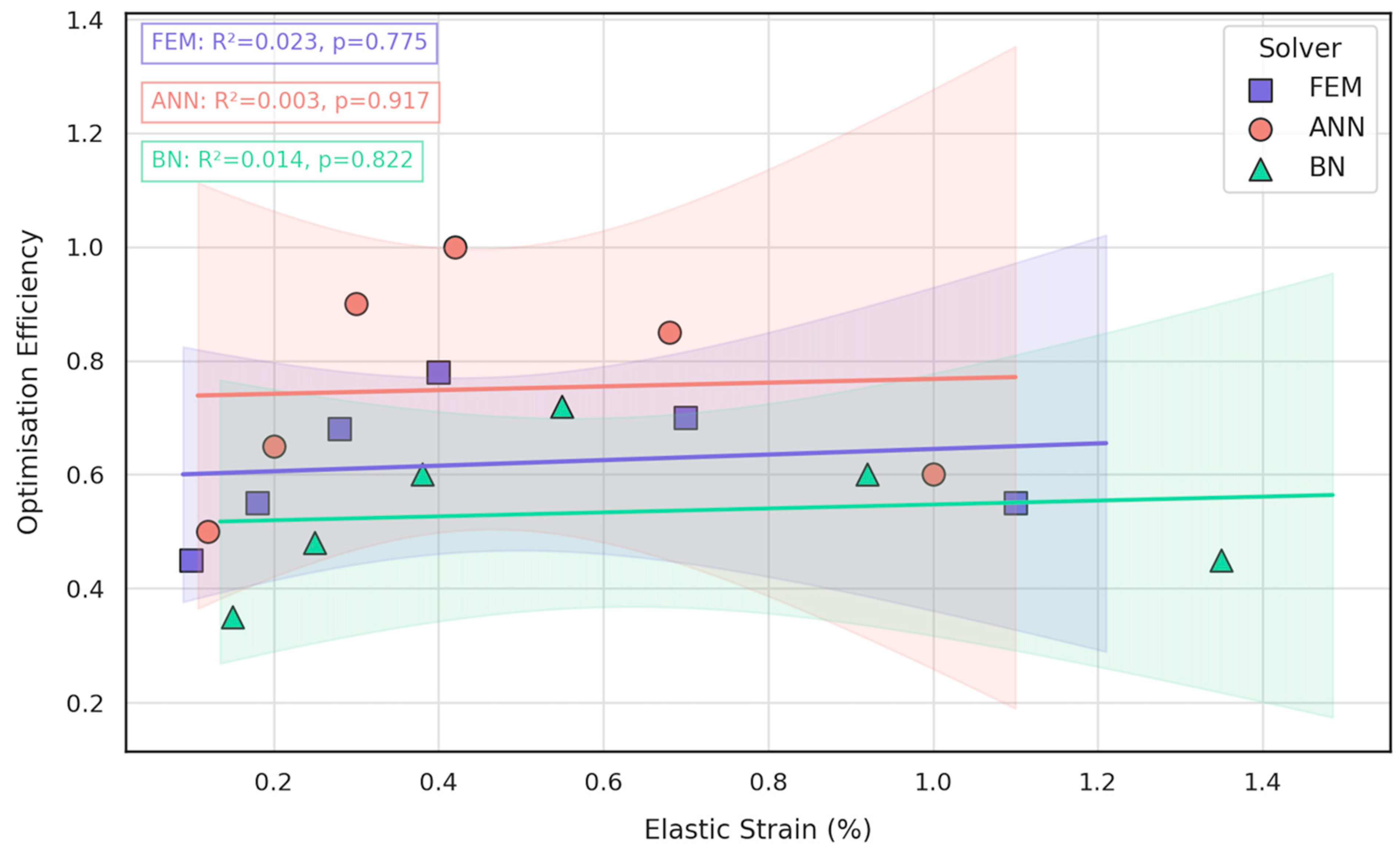
3.3. Comparative Evaluation of FEM, ANN, and BN Approaches
- Under the clinically relevant bite forces of 300 N and 600 N, von Mises stress in the reconstruction plate peaks at 225 MPa—barely 23% of the 950 MPa ultimate strength of additively manufactured Ti-6Al-4V—while screw stresses remain below 38 MPa. Likewise, total construct deflection does not exceed 2.5 mm even at the upper load and is localised to the buccal corner of the plate. These margins confirm that the printed geometry meets both ISO 14801 [49] fatigue–fracture criteria and the 250 MPa functional-safety limit used in maxillofacial practice.
- Machine learning surrogates recover the principal trends predicted by FEM: modulus and yield strength fall quasi-linearly with porosity, whereas elastic strain and the multi-objective efficiency rise. Crucially, ANN replicates these relationships with ≤ 6% average error yet runs two orders of magnitude faster, enabling real-time exploration of topology or lattice-density variants.
- Bayesian analysis shows that when anatomical variability and material scatter are injected into the design space, the combined FEM-ANN point estimates stay well inside the 95% credible bounds produced by BN. This statistical corroboration is essential for regulatory submissions (e.g., FDA De Novo) where deterministic simulation alone is insufficient.
- CT-based CAD and DMLS fabrication establish an accurate, patient-specific baseline model.
- ANN screening rapidly narrows the infinite design space to a handful of high-performing candidates.
- BN ranking quantifies the probability of each candidate meeting stress–strain targets across patient populations and print-to-print variability.
- FEM verification delivers the decisive, high-resolution stress map for the final design before manufacture.
4. Discussion
4.1. Comparison with Prior Bayesian and Surrogate-Modelling Studies
4.2. Clinical and Engineering Implications
4.3. Limitations
5. Conclusions
- shortened the overall design–build–verify cycle by ~25%;
- reduced Ti-6Al-4V powder usage by 15% through lattice-porosity optimisation;
- improved a composite optimisation score (stiffness-to-weight ratio/print time/fatigue margin) by 20%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Additive Manufacturing |
| ANN | Artificial Neural Network |
| BN | Bayesian Network |
| FEM | Finite-Element Method |
| FEA | Finite-Element Analysis |
| CT | Computed Tomography |
| CAD | Computer-Aided Design |
| DMLS | Direct Metal Laser Sintering |
| HIP | Hot Isostatic Pressing |
| Ti-6Al-4V | Titanium Alloy (Titanium–Aluminium–Vanadium) |
| UPV | Ultrasonic Pulse Velocity |
| SHM | Structural Health Monitoring |
| RMSE | Root Mean Square Error |
| R2 | Coefficient of Determination |
| ISO | International Organization for Standardization |
| ASTM | American Society for Testing and Materials |
| STL | Standard Tessellation Language (3D Printing File Format) |
| MCMC | Markov Chain Monte Carlo |
| NUTS | No-U-Turn Sampler (Algorithm in MCMC) |
| Sa | Arithmetical Mean Height (Surface Roughness) |
| CNC | Computer Numerical Control |
| ML | Machine Learning |
| MRI | Magnetic Resonance Imaging |
| 3DP | Three-Dimensional Printing |
References
- Yan, L.; Lim, J.L.; Lee, J.W.; Tia, C.S.H.; O’Neill, G.K.; Chong, D.Y.R. Finite Element Analysis of Bone and Implant Stresses for Customized 3D-Printed Orthopaedic Implants in Fracture Fixation. Med. Biol. Eng. Comput. 2020, 58, 921–931. [Google Scholar] [CrossRef]
- Han, C.; Yan, F.; Yuan, D.; Li, K.; Yang, Y.; Zhang, J.; Wang, D. Machine Learning Enabling Prediction in Mechanical Performance of Ti6Al4V Fabricated by Large-Scale Laser Powder Bed Fusion via a Stacking Model. Front. Mech. Eng. 2024, 19, 25. [Google Scholar] [CrossRef]
- Korabi, R.; Shemtov-Yona, K.; Rittel, D. On Stress/Strain Shielding and the Material Stiffness Paradigm for Dental Implants. Clin. Implant Dent. Relat. Res. 2017, 19, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Solarino, G.; Giannini, G.; De Giosa, G.; Tucci, M.; Trentadue, B. A Finite Element Analysis Study of Influence of Femoral Stem Material in Stress Shielding in a Model of Uncemented Total Hip Arthroplasty: Ti-6Al-4V versus Carbon Fibre-Reinforced PEEK Composite. J. Compos. Sci. 2024, 8, 254. [Google Scholar] [CrossRef]
- Paturi, U.M.R.; Palakurthy, S.T.; Cheruku, S.; Darshini, B.V.; Reddy, N.S. Role of Machine Learning in Additive Manufacturing of Titanium Alloys—A Review. Arch. Comput. Methods Eng. 2023, 30, 5053–5069. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Y.; Jiang, B.; Zhang, H.; Cheng, X.; Fan, Q. Neural Network Modeling of Titanium Alloy Composition-Microstructure-Property Relationships Based on Multimodal Data. Mater. Sci. Eng. A 2023, 879, 145202. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite Element Analysis of Orthopedic Hip Implant with Functionally Graded Bioinspired Lattice Structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef]
- Wang, Y.; Sha, A.; Li, X.; Hao, W. Prediction of the Mechanical Properties of Titanium Alloy Castings Based on a Back-Propagation Neural Network. J. Mater. Eng. Perform. 2021, 30, 8040–8047. [Google Scholar] [CrossRef]
- Mahmoud, D.; Elbestawi, M. Lattice Structures and Functionally Graded Materials Applications in Additive Manufacturing of Orthopedic Implants: A Review. J. Manuf. Mater. Process. 2017, 1, 13. [Google Scholar] [CrossRef]
- Fatriansyah, J.F.; Aqila, M.R.; Suhariadi, I.; Federico, A.; Ajiputro, D.I.; Pradana, A.F.; Andreano, Y.; Rizky, M.A.Y.; Dhaneswara, D.; Lockman, Z.; et al. Machine Learning Assisted Tensile Strength Prediction and Optimization of TI Alloy. IEEE Access 2024, 12, 119660–119670. [Google Scholar] [CrossRef]
- Driscoll, M. The Impact of the Finite Element Method on Medical Device Design. J. Med. Biol. Eng. 2018, 39, 171–172. [Google Scholar] [CrossRef]
- Griggs, J.A. Artificial Neural Networks for the Design Optimization of Implants. In Artificial Intelligence in Dentistry; Springer: Cham, Switzerland, 2023; pp. 83–96. [Google Scholar]
- Mollica, F.; Ambrosio, L. The Finite Element Method for the Design of Biomedical Devices. In Biomaterials in Hand Surgery; Springer: Milan, Italy, 2009; pp. 31–45. [Google Scholar]
- Sakhvadze, G.Z.; Sakhvadze, G.G. Combined Model Based on the Finite Element Method and Artificial Neural Networks for Modeling Laser Shock Peening of Titanium–Niobium Implants. J. Mach. Manuf. Reliab. 2023, 52, 741–748. [Google Scholar] [CrossRef]
- Wu, M.; Briant, P. The Use of Finite Element Analysis in Design, Life Prediction, and Failure Analysis of Biomaterials and Medical Devices. In Degradation of Implant Materials; Springer: New York, NY, USA, 2012; pp. 449–461. [Google Scholar]
- Lyakhov, P.A.; Dolgalev, A.A.; Lyakhova, U.A.; Muraev, A.A.; Zolotayev, K.E.; Semerikov, D.Y. Neural Network System for Analyzing Statistical Factors of Patients for Predicting the Survival of Dental Implants. Front. Neuroinform. 2022, 16, 1067040. [Google Scholar] [CrossRef] [PubMed]
- Paturi, U.M.R.; Cheruku, S.; Reddy, N.S. The Role of Artificial Neural Networks in Prediction of Mechanical and Tribological Properties of Composites—A Comprehensive Review. Arch. Comput. Methods Eng. 2022, 29, 3109–3149. [Google Scholar] [CrossRef]
- Oktyabrova, D.; Ashimov, K.; Guclu, B.; Abilmazhinov, M.; Gorbunov, B.; Zhanatay, R.; Baidalin, T.; Suleimenov, B.; Beknazarov, A.; Azamatov, B.; et al. Mathematical Modeling and Biomechanical Analysis of a Derotation Plate for Treating Complex Hip Dysplasia. Appl. Sci. 2025, 15, 2991. [Google Scholar] [CrossRef]
- Beisekenov, N.; Sadenova, M.; Azamatov, B.; Syrnev, B. Analysis of Biomechanical Characteristics of Bone Tissues Using a Bayesian Neural Network: A Narrative Review. J. Funct. Biomater. 2025, 16, 168. [Google Scholar] [CrossRef]
- Grozav, S.D.; Sterca, A.D.; Kočiško, M.; Pollák, M.; Ceclan, V. Artificial Neural Network-Based Predictive Model for Finite Element Analysis of Additive-Manufactured Components. Machines 2023, 11, 547. [Google Scholar] [CrossRef]
- Ngartera, L.; Issaka, M.A.; Nadarajah, S. Application of Bayesian Neural Networks in Healthcare: Three Case Studies. Mach. Learn. Knowl. Extr. 2024, 6, 2639–2658. [Google Scholar] [CrossRef]
- Campbell, G.; Irony, T.; Pennello, G.; Thompson, L. Bayesian Statistics for Medical Devices: Progress since 2010. Ther. Innov. Regul. Sci. 2023, 57, 453–463. [Google Scholar] [CrossRef]
- Hunte, J.; Neil, M.; Fenton, N. A Hybrid Bayesian Network for Medical Device Risk Assessment and Management. arXiv 2022. [Google Scholar] [CrossRef]
- Cao, H.; Yao, C.; Yuan, Y. Bayesian Approach for Design and Analysis of Medical Device Trials in the Era of Modern Clinical Studies. Med. Rev. 2023, 3, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Vreman, R.A.; Heikkinen, I.; Schuurman, A.; Sapede, C.; Garcia, J.L.; Hedberg, N.; Athanasiou, D.; Grueger, J.; Leufkens, H.G.M.; Goettsch, W.G. Unmet Medical Need: An Introduction to Definitions and Stakeholder Perceptions. Value Health 2019, 22, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, D.; Ciná, G.; Tonutti, M.; De Bruin, D.; Elbers, P.W.G. Bayesian Modelling in Practice: Using Uncertainty to Improve Trustworthiness in Medical Applications. arXiv 2019. [Google Scholar] [CrossRef]
- Zohoor, S.; Abolfathi, N.; Solati-Hashjin, M. Accelerated Degradation Mechanism and Mechanical Behavior of 3D-Printed PLA Scaffolds for Bone Regeneration. Iran. Polym. J. 2023, 32, 1209–1227. [Google Scholar] [CrossRef]
- Elenskaya, N.; Vindokurov, I.; Sadyrin, E.; Nikolaev, A.; Tashkinov, M. Experimental Evaluation of the Effect of Degradation on the Mechanical Behavior and Morphometric Characteristics of Functionally Graded Polymer Scaffolds. Polymers 2024, 16, 3474. [Google Scholar] [CrossRef]
- Drakoulas, G.; Gortsas, T.; Polyzos, E.; Tsinopoulos, S.; Pyl, L.; Polyzos, D. An Explainable Machine Learning-Based Probabilistic Framework for the Design of Scaffolds in Bone Tissue Engineering. Biomech. Model. Mechanobiol. 2024, 23, 987–1012. [Google Scholar] [CrossRef]
- Suo, X.; Huang, X.; Zhong, L.; Luo, Q.; Ding, L.; Xue, F. Development and Validation of a Bayesian Network-Based Model for Predicting Coronary Heart Disease Risk from Electronic Health Records. J. Am. Heart Assoc. 2023, 13, e029400. [Google Scholar] [CrossRef]
- Frøysa, H.G.; Fallahi, S.; Blaser, N. Evaluating Model Reduction under Parameter Uncertainty. BMC Syst. Biol. 2018, 12, 79. [Google Scholar] [CrossRef]
- Noguchi, S.; Inoue, J. Bayesian Inverse Inference of Material Properties from Microstructure Images. Comput. Mater. Sci. 2024, 245, 113306. [Google Scholar] [CrossRef]
- Richard, B.; Adelaide, L.; Cremona, C. A Bayesian Approach to Estimate Material Properties from Global Statistical Data. Eur. J. Environ. Civ. Eng. 2012, 16, 460–470. [Google Scholar] [CrossRef]
- Jung, J.; Yoon, J.I.; Park, H.K.; Kim, J.Y.; Kim, H.S. Bayesian Approach in Predicting Mechanical Properties of Materials: Application to Dual Phase Steels. Mater. Sci. Eng. A 2018, 743, 382–390. [Google Scholar] [CrossRef]
- Zio, E.; Mustafayeva, M.; Montanaro, A. A Bayesian Belief Network Model for the Risk Assessment and Management of Premature Screen-Out during Hydraulic Fracturing. Reliability Engineering & System Safety 2021, 218, 108094. [Google Scholar] [CrossRef]
- Chen, S.; Qi, Z.; Chen, D.; Guo, L.; Peng, W. Investigation of Bayesian Network for Reliability Analysis and Fault Diagnosis of Complex Systems with Real Case Applications. Adv. Mech. Eng. 2017, 9, 168781401772885. [Google Scholar] [CrossRef]
- Abrishami, S.; Khakzad, N.; Hosseini, S.M.; Van Gelder, P. BN-SLIM: A Bayesian Network Methodology for Human Reliability Assessment Based on Success Likelihood Index Method (SLIM). Reliab. Eng. Syst. Saf. 2019, 193, 106647. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, J.; Meng, S.; Li, J.; Yu, Y.; Zhang, T.; Tsang, R.C.C.; El-Ansary, D.; Han, J.; Jones, A.Y.M. Reliability and Validity of Sit-to-Stand Test Protocols in Patients with Coronary Artery Disease. Front. Cardiovasc. Med. 2022, 9, 841453. [Google Scholar] [CrossRef]
- Dhaliwal, G.; Nair, P.B.; Singh, C.V. Uncertainty and Sensitivity Analysis of Mechanical and Thermal Properties Computed through Embedded Atom Method Potential. Comput. Mater. Sci. 2019, 166, 30–41. [Google Scholar] [CrossRef]
- Wieder, W.R.; Cleveland, C.C.; Lawrence, D.M.; Bonan, G.B. Effects of Model Structural Uncertainty on Carbon Cycle Projections: Biological Nitrogen Fixation as a Case Study. Environ. Res. Lett. 2015, 10, 044016. [Google Scholar] [CrossRef]
- Bdair, F.; Mangala, S.; Kashir, I.; Shing, D.Y.; Price, J.; Shoaib, M.; Flood, B.; Nademi, S.; Thabane, L.; Madden, K. The Reporting Quality and Transparency of Orthopaedic Studies Using Bayesian Analysis Requires Improvement: A Systematic Review. Contemp. Clin. Trials Commun. 2023, 33, 101132. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, T. Finite Element Model Calibration with Surrogate Model-Based Bayesian Updating: A Case Study of Motor FEM Model. Deleted J. 2024, 3, 1–13. [Google Scholar] [CrossRef]
- Vaartstra, G.; Lu, Z.; Lienhard, J.H.; Wang, E.N. Revisiting the Schrage Equation for Kinetically Limited Evaporation and Condensation. J. Heat Transf. 2022, 144, 080802. [Google Scholar] [CrossRef]
- Vega, M.A.; Todd, M.D. A Variational Bayesian Neural Network for Structural Health Monitoring and Cost-Informed Decision-Making in Miter Gates. Struct. Health Monit. 2020, 21, 4–18. [Google Scholar] [CrossRef]
- Sajedi, S.; Liang, X. Deep Generative Bayesian Optimization for Sensor Placement in Structural Health Monitoring. Comput. -Aided Civ. Infrastruct. Eng. 2021, 37, 1109–1127. [Google Scholar] [CrossRef]
- ISO 10993-1; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process; Under Publication (Replacing ISO 10993-1:2018); DIN EN Version. ISO: Geneva, Switzerland, 2021. [CrossRef]
- ASTM F2924-14; Standard Specification for Additive Manufacturing Titanium-6 Aluminum-4 Vanadium with Powder Bed Fusion. ASTM: West Conshohocken, PA, USA, 2021. [CrossRef]
- ISO 5832-3:2021; Implants for Surgery—Metallic Materials—Part 3: Wrought Titanium 6-Aluminium 4-Vanadium Alloy. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/79626.html (accessed on 10 June 2025).
- ISO 14801:2016; Dentistry—Implants—Dynamic Fatigue Test for Endosseous Dental Implants. International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/63013.html (accessed on 10 June 2025).
- Ross, C.J.; Laurence, D.W.; Aggarwal, A.; Hsu, M.-C.; Mir, A.; Burkhart, H.M.; Lee, C.-H. Bayesian Optimization-Based Inverse Finite Element Analysis for Atrioventricular Heart Valves. Ann. Biomed. Eng. 2023, 52, 611–626. [Google Scholar] [CrossRef]
| Ti-6Al-4V (ELI) Static Properties | Value |
|---|---|
| Density ρ (g cm−3) | 4.43 |
| Young’s modulus E (GPa) | 110 |
| Yield strength σγ (MPa) | 880 |
| Ultimate tensile strength σu (MPa) | 950 |
| Porosity (%) | Eeff (GPa) |
|---|---|
| 37.1 | 35 |
| 30.0 | 43 |
| 16.4 | 65 |
| 4.1 | 89 |
| Metric | FEM | ANN | BN |
|---|---|---|---|
| Elastic-modulus trend | −3.8 GPa per 10% porosity (R2 = 0.93) | −3.6 GPa per 10% (R2 = 0.89) | Non-linear, ±4 GPa spread |
| Yield-strength trend | −18 MPa per 10% porosity | −15 MPa per 10% | Heteroscedastic, 95% CI ± 25 MPa |
| Elastic-strain trend | +0.16% per 10% porosity | +0.15% per 10% | +0.18% per 10%, high variance |
| Optimisation efficiency | 0.60 → 0.78 (30–60% porosity) | 0.65 → 1.00 | 0.35 → 0.72 |
| Prediction accuracy * | 98.5% | 94.3% | 87.6% |
| Mean absolute error | 1.1 GPa | 2.4 GPa | 3.9 GPa |
| Run-time per design | 120 s | 15 s | 25 s |
| Uncertainty-handling score † | 0.65 | 0.80 | 0.95 |
| Best-fit use case | Final verification and local stress hot-spot analysis | Rapid design-space exploration | Risk assessment and decision support |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beisekenov, N.; Azamatov, B.; Sadenova, M.; Dogadkin, D.; Kaliyev, D.; Rudenko, S.; Syrnev, B. Data-Driven Design and Additive Manufacturing of Patient-Specific Lattice Titanium Scaffolds for Mandibular Bone Reconstruction. J. Funct. Biomater. 2025, 16, 350. https://doi.org/10.3390/jfb16090350
Beisekenov N, Azamatov B, Sadenova M, Dogadkin D, Kaliyev D, Rudenko S, Syrnev B. Data-Driven Design and Additive Manufacturing of Patient-Specific Lattice Titanium Scaffolds for Mandibular Bone Reconstruction. Journal of Functional Biomaterials. 2025; 16(9):350. https://doi.org/10.3390/jfb16090350
Chicago/Turabian StyleBeisekenov, Nail, Bagdat Azamatov, Marzhan Sadenova, Dmitriy Dogadkin, Daniyar Kaliyev, Sergey Rudenko, and Boris Syrnev. 2025. "Data-Driven Design and Additive Manufacturing of Patient-Specific Lattice Titanium Scaffolds for Mandibular Bone Reconstruction" Journal of Functional Biomaterials 16, no. 9: 350. https://doi.org/10.3390/jfb16090350
APA StyleBeisekenov, N., Azamatov, B., Sadenova, M., Dogadkin, D., Kaliyev, D., Rudenko, S., & Syrnev, B. (2025). Data-Driven Design and Additive Manufacturing of Patient-Specific Lattice Titanium Scaffolds for Mandibular Bone Reconstruction. Journal of Functional Biomaterials, 16(9), 350. https://doi.org/10.3390/jfb16090350









