Original Locking Rod System Designed for Diaphyseal Fractures of Long Bones
Abstract
1. Introduction
2. Materials and Methods
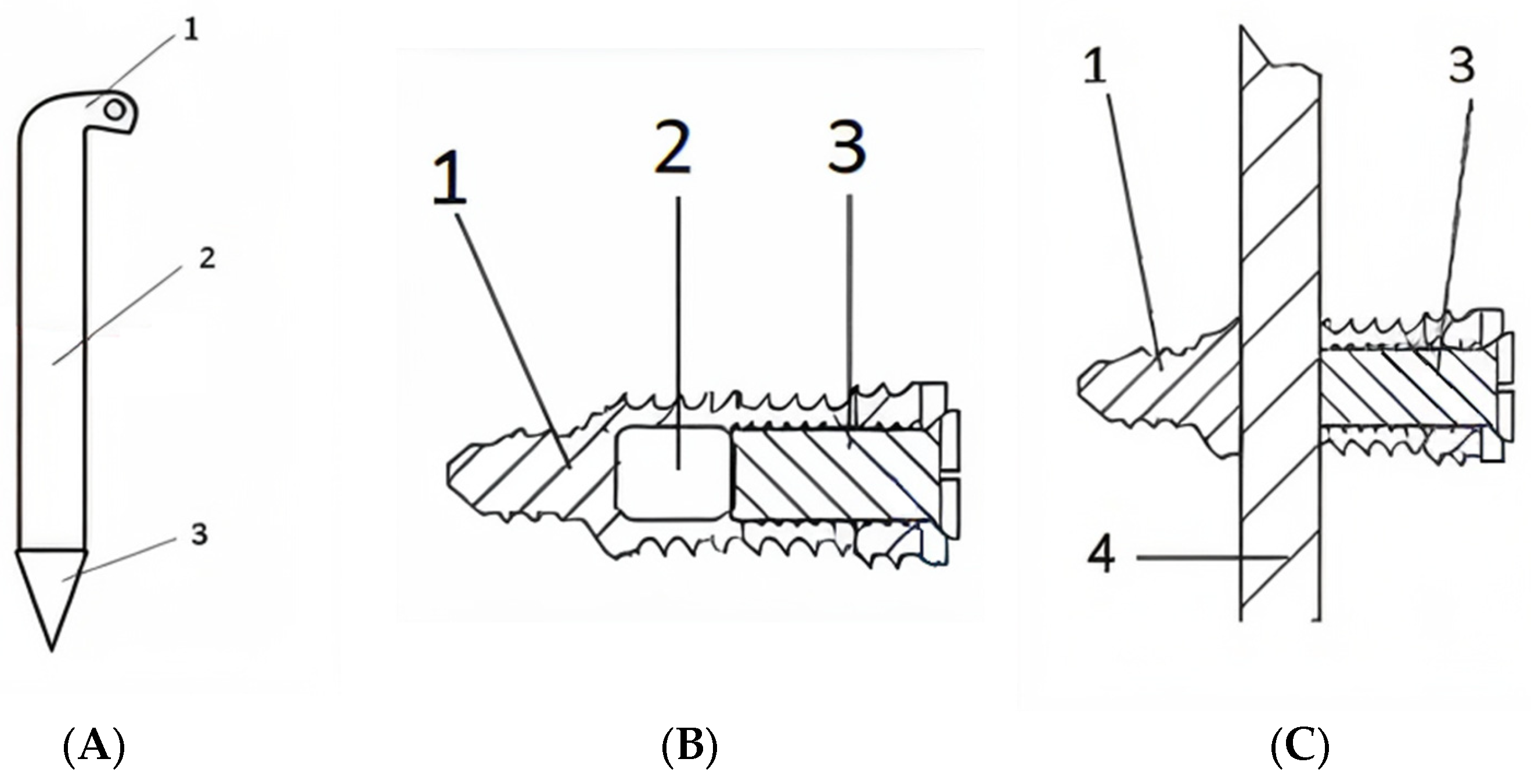
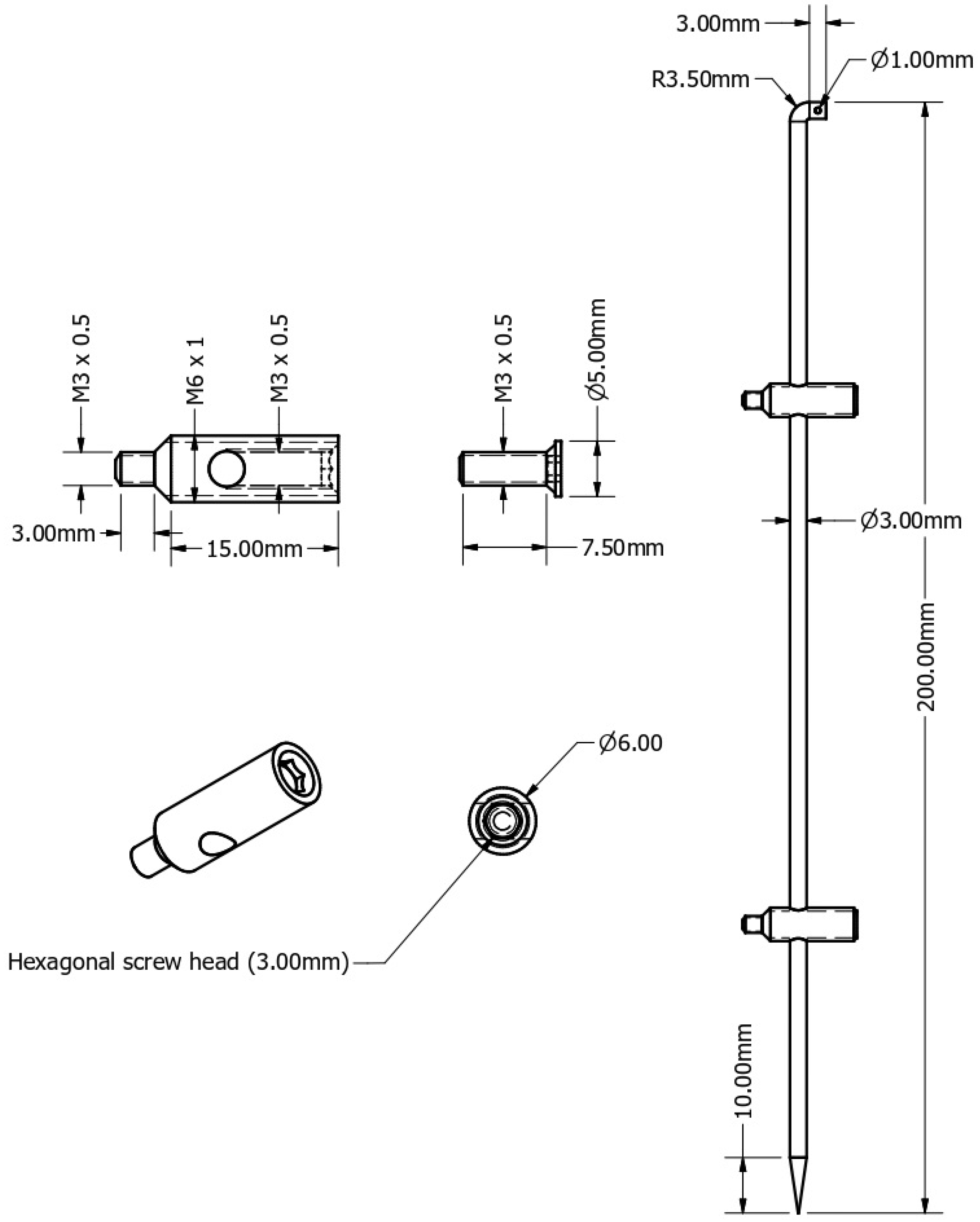
2.1. Hardware
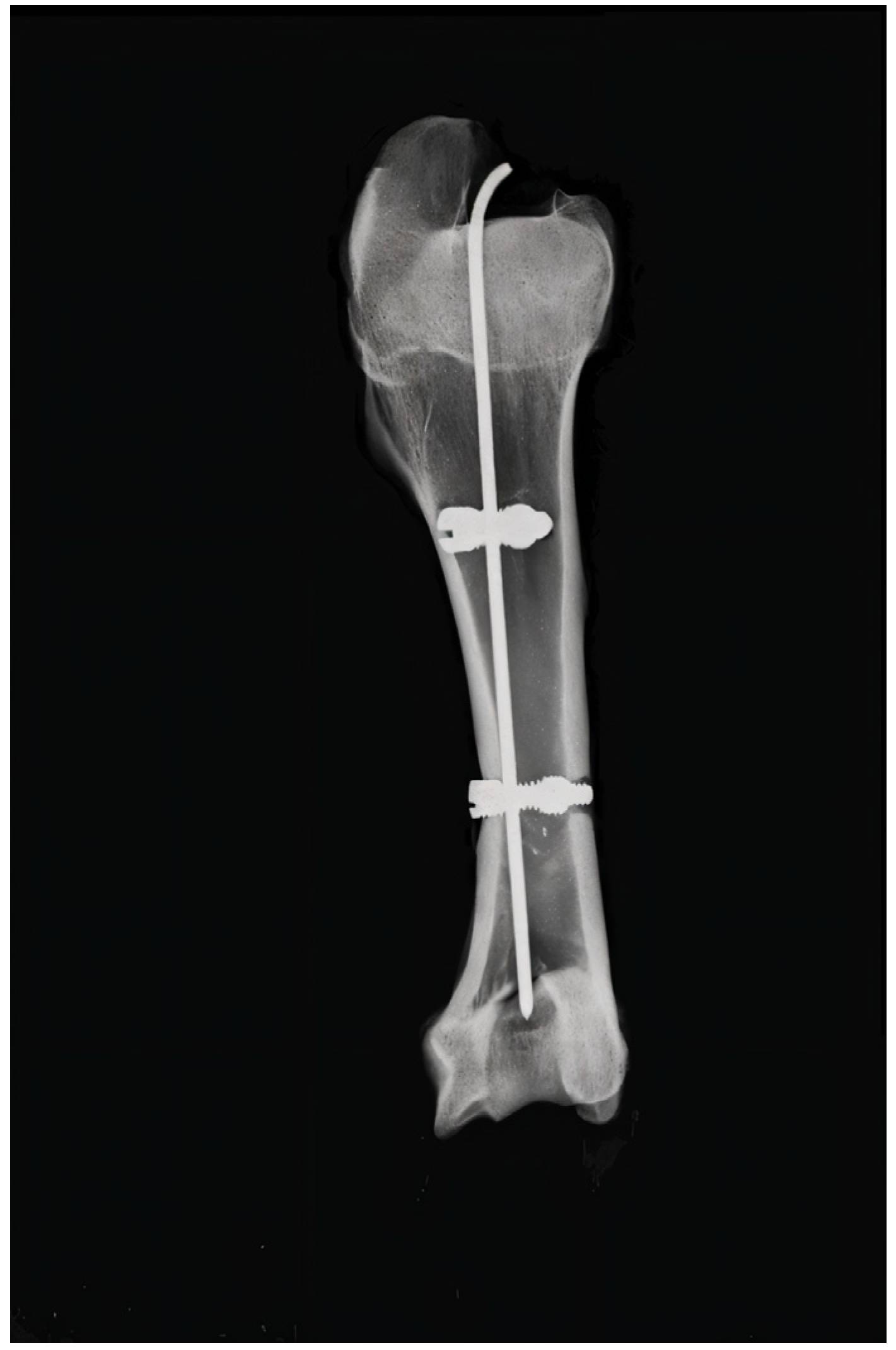
2.2. Surgical Technique
- The diameter of the bone canal to determine the optimal diameter of the central rod.
- The length of the bone and fracture site for the optimal selection of the central rod.
- The fracture pattern and the anatomical region to determine the final osteosynthesis.
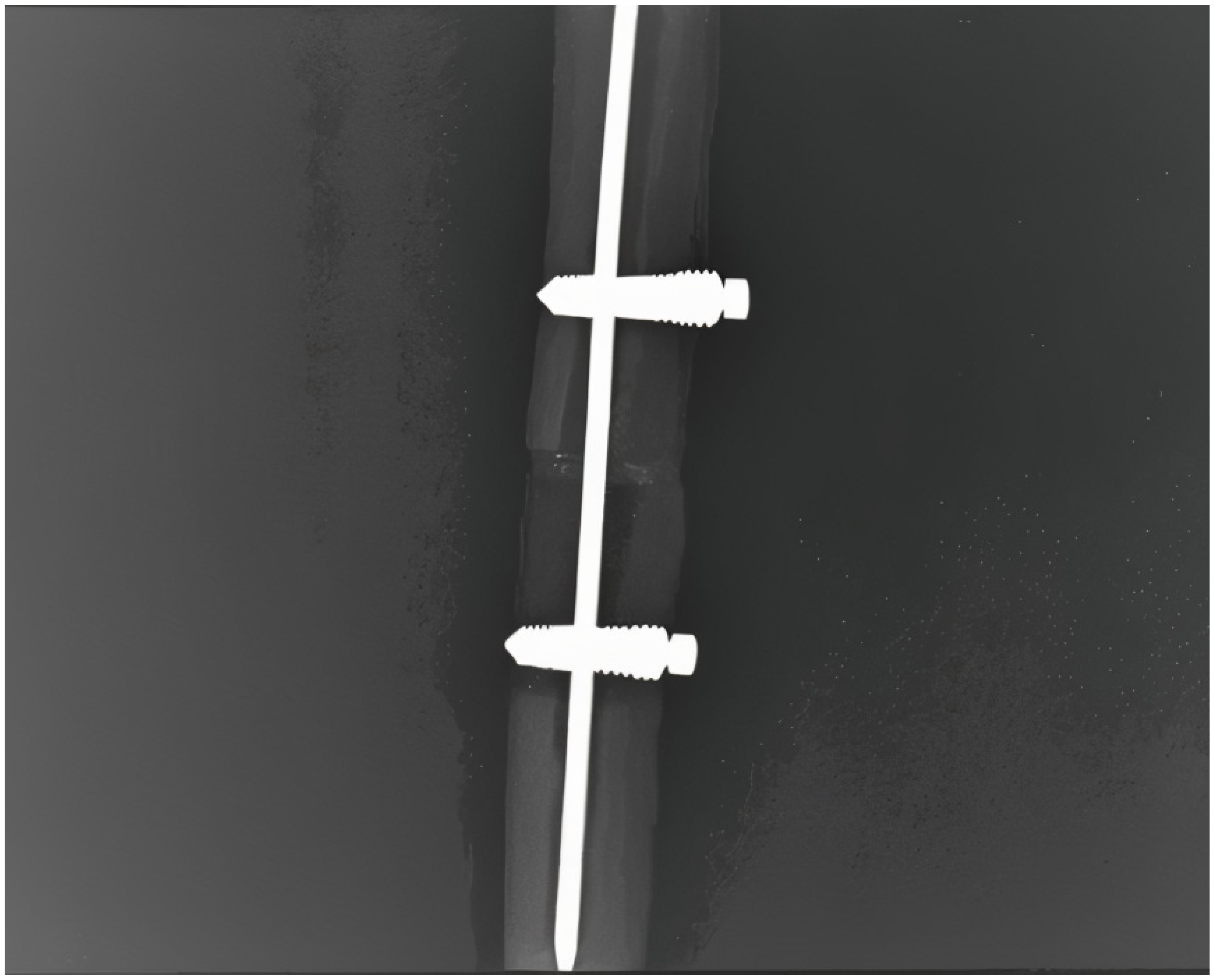
2.3. Surgical Procedure Steps
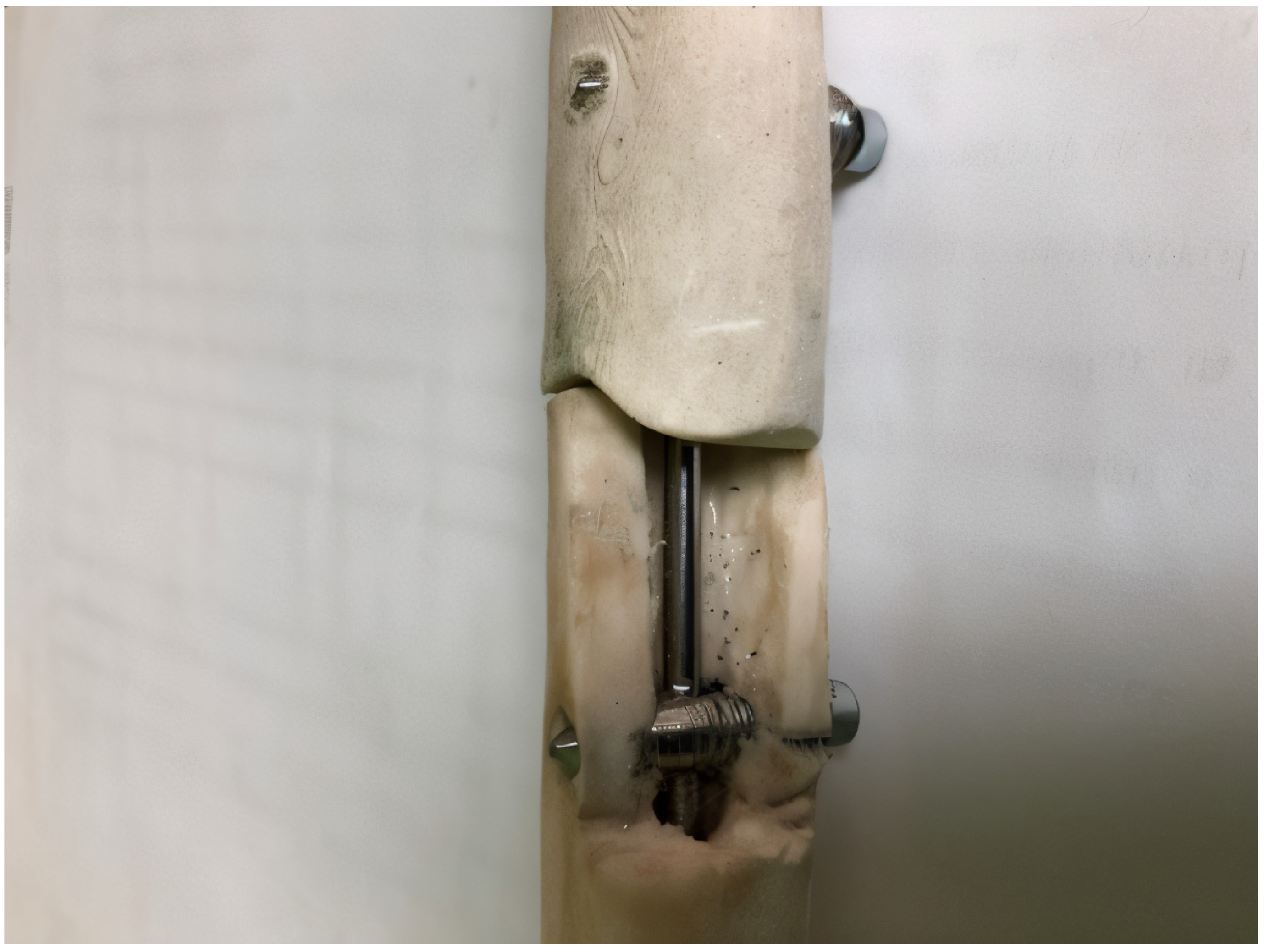
2.4. Prototype and Mechanical Stability
- Tensile–compression test: This type of testing simulates axial loading, which is experienced in a clinical setting during weight-bearing activities, walking, or running. Tensile–compression testing determines the implant stiffness, elastic modulus, and load-bearing capacity of an implant. A well-fixed fracture site using an intramedullary rod should exhibit minimal axial displacement and high stiffness; the excessive deformation of the implant or fracture fragments would suggest implant instability or material fatigue [19].
- Torsion testing: This test evaluates the rotational stability of the implant and the efficiency of the locking mechanism. Torsion testing simulates the twisting forces encountered during gait, turning, accidental falls or rotational movements. High torsional stiffness indicates good rotational fixation, while excessive rotational displacement might suggest loosening of the locking mechanism or implant failure [20].
- 3.
- Combined torsion–tensile–compression testing: This specific test mimics realistic in vivo conditions, where multiple forces act simultaneously, this type of testing helps assess the complex stress interactions in fracture fixation systems. Combined torsion–tensile–compression testing can identify the stress concentration points where implant failure is most likely to occur and also provides insight into implant fatigue under complex load cycles [21].
- 4.
- Three-point bending testing: This type of test was conducted in order to evaluate flexural strength and fatigue resistance. The three-point bending test simulates direct impact forces from falls or external trauma. High bending stiffness without secondary displacement of the fracture indicates a mechanically stable implant, while low resistance during bending testing suggests implant fragility or material deficiencies [22].
3. Results
- Tensile compression testing was performed in two scenarios:
- Bone in physical contact with a mechanical load of 200 N (with a preloading force of 50 N). The number of cycles in this setting was 200,000.
- Bone with a 1 mm gap at the level of the fracture site with a mechanical load of 200 N (with a preloading force of 50 N). The number of cycles in this setting was 100,000.
- Torsion testing was performed with the fracture fragments in physical contact with a torque force of 1.0 Nm. Torsion testing was repeated for 50,000 cycles.
- Combined tensile–compression–torsion testing was performed with the fracture fragments in physical contact with a torque force of 0.8 Nm and a tensile force of 200 N. The number of cycles for this test was 150,000.
- Three-point bend testing was performed with the fracture fragments in physical contact with a mechanical load of 70 N. The number of cycles for this test was 30,000.
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IM | Intramedullary |
| Fig | Figure |
| mm | Millimetres |
| N | Newton |
| Nm | Newton-metre |
| E | Young’s modulus |
| G | Shear modulus |
| I | Moment of inertia |
| D | Diameter |
| π | ≈3.14 |
Appendix A
| Property | Titanium (Ti-6Al-4V) | Stainless Steel (316 L) |
|---|---|---|
| Young’s Modulus (E) | ~110 GPa | ~200 GPa |
| Shear Modulus (G) | ~44 GPa | ~77 GPa |
| Density | ~4.4 g/cm3 | ~8.0 g/cm3 |
| Tensile Strength | ~1000 MPa | ~570 MPa |
| Fatigue Strength | Higher | Lower |
| Corrosion Resistance | Excellent | Moderate |
| Stress Shielding Risk | Lower (better load sharing) | Higher (stiffer implant) |
- = 3 mm (rod diameter tested biomechanically);
- = average bending moment in femoral shaft fractures (which is described in the literature to be approximately 10 to 12 times greater in fractures of the femoral shaft compared to metacarpal fractures);
- = bending moment of the tested 3 mm central rod.
References
- Bartoníček, J. Early history of operative treatment of fractures. Arch. Orthop. Trauma Surg. 2010, 130, 1385–1396. [Google Scholar] [CrossRef]
- Dan, P.; Petreus, T.; Asaftei, R.; Berea, G.; Botez, P. Minimally Invasive Plate Osteosynthesis (MIPO) in Long Bone Fractures—Biomechanics—Design—Clinical Results. In Biomechanics in Applications; IntechOpen Limited: London, UK, 2011. [Google Scholar] [CrossRef]
- Bekos, A.; Sioutis, S.; Kostroglou, A.; Saranteas, T.; Mavrogenis, A.F. The history of intramedullary nailing. Int. Orthop. 2021, 45, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Pogorelic, Z.; Vodopić, T.; Jukić, M.; Furlan, D. Elastic Stable Intramedullary Nailing for Treatment of Pediatric Femoral Fractures; A 15-Year Single Centre Experience. Bull. Emerg. Trauma 2019, 7, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aznar, C.; Mateo, J.; Ibarz, E.; Gracia, L.; Rosell, J.; Puértolas, S. Biomechanical Behavior of Dynamic vs. Static Distal Locking Intramedullary Nails Subtrochanteric Femur Fractures. Bioengineering 2023, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, M.; Demir, T. Evaluation of the mechanical behaviour of the expandable wedge locked nail fixation in retrograde use: A finite element study. Comput. Biol. Med. 2024, 174, 108419. [Google Scholar] [CrossRef]
- Özkaya, M.; Tunalı, S.; Köksal, İ.; Demir, T. Mechanical comparison of standard interlocking, clawed, and expandable wedge locked nail fixations: An experimental and numerical study. Injury 2023, 54, 379–394. [Google Scholar] [CrossRef]
- Putame, G.; Pascoletti, G.; Terzini, M.; Zanetti, E.M.; Audenino, A.L. Mechanical Behavior of Elastic Self-Locking Nails for Intramedullary Fracture Fixation: A Numerical Analysis of Innovative Nail Designs. Front. Bioeng. Biotechnol. 2020, 8, 557. [Google Scholar] [CrossRef]
- Ruffilli, A.; Traina, F.; Pilla, F.; Fenga, D.; Faldini, C. Marchetti Vicenzi elastic retrograde nail in the treatment of humeral shaft fractures: Review of the current literature. Musculoskelet. Surg. 2015, 99, 201–209. [Google Scholar] [CrossRef]
- Lepore, L.; Lepore, S.; Maffulli, N. Intramedullary nailing of the femur with an inflatable self-locking nail: Comparison with locked nailing. J. Orthop. Sci. 2003, 8, 796–801. [Google Scholar] [CrossRef]
- Liu, B.; Xiong, Y.; Deng, H.; Gu, S.; Jia, F.; Li, Q.; Wang, D.; Gan, X.; Liu, W. Comparison of our self-designed rotary self-locking intramedullary nail and interlocking intramedullary nail in the treatment of long bone fractures. J. Orthop. Surg. Res. 2014, 9, 47. [Google Scholar] [CrossRef]
- Fang, Y.; Fu, X.; Chi, L.; Wang, G.; Yang, T. The biomechanical study of rotating-arm self-locking intramedullary nails in comminuted femoral shaft fractures. J. Biomed. Eng./Shengwu Yixue Gongchengxue Zazhi 2006, 23, 1041–1044. Available online: https://pubmed.ncbi.nlm.nih.gov/17121350/ (accessed on 14 January 2025).
- Kim, D.H.; Jang, H.S.; Kwak, S.H.; Jung, S.Y.; Jeon, J.M.; Ahn, T.Y.; Lee, S.H. Surgical outcomes of segmental diaphyseal forearm fractures in adults: A small case series on plate osteosynthesis, intramedullary nailing, and other surgical methods. BMC Musculoskelet. Disord. 2023, 24, 731. [Google Scholar] [CrossRef]
- Saul, D.; Menger, M.M.; Ehnert, S.; Nüssler, A.K.; Histing, T.; Laschke, M.W. Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions—Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors. Bioengineering 2023, 10, 85. [Google Scholar] [CrossRef]
- Mundi, R.; Chaudhry, H.; Niroopan, G.; Petrisor, B.; Bhandari, M. Open Tibial Fractures. JBJS Rev. 2015, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhaya, J.; Bhadani, J.S. Fixation Failure in Osteoporotic Bone: A Review of Complications and Outcomes. Indian J. Orthop. 2024, 59, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Hackett, D.J.; Rothenberg, A.C.; Chen, A.F.; Gutowski, C.; Jaekel, D.; Tomek, I.M.; Parsley, B.S.; Ducheyne, P.; Manner, P.A. The Economic Significance of Orthopaedic Infections. J. Am. Acad. Orthop. Surg. 2015, 23, S1–S7. Available online: https://www.scirp.org/reference/referencespapers?referenceid=3885469 (accessed on 13 July 2025). [CrossRef] [PubMed]
- Grant, C.A.; Schuetz, M.; Epari, D. Mechanical testing of internal fixation devices: A theoretical and practical examination of current methods. J. Biomech. 2015, 48, 3989–3994. [Google Scholar] [CrossRef]
- Havaldar, R.; Pilli, S.C.; Putti, B.B. Insights into the effects of tensile and compressive loadings on human femur bone. Adv. Biomed. Res. 2014, 3, 101. [Google Scholar] [CrossRef]
- Samiezadeh, S.; Tavakkoli Avval, P.; Fawaz, Z.; Bougherara, H. Biomechanical assessment of composite versus metallic intramedullary nailing system in femoral shaft fractures: A finite element study. Clin. Biomech. 2014, 29, 803–810. [Google Scholar] [CrossRef]
- Zderic, I.; Kraus, M.; Rossenberg, L.V.; Puls, L.; Pastor, T.; Gueorguiev, B.; Richards, G.; Pape, H.C. Evaluating the Biomechanical Efficacy of 2.5-mm and 2.0-mm Double Plating Against 3.5-mm Single Plating in Ulnar Shaft Fracture Fixation: A Cadaveric Study. Orthop. Proc. 2024, 106-B, 10. [Google Scholar] [CrossRef]
- Beaumont, C.M.; Beason, D.P.; McKeon, K.E. Fracture Fixation Strength in Metacarpal Plating Versus Intramedullary Nailing Using a 3-Point Bending Model: A Cadaveri c, Biomechanical Study. J. Hand Surg. 2024, 49, 57.E1–57.E6. [Google Scholar] [CrossRef]
- Barber, C.C.; Burnham, M.; Ojameruaye, O.; McKee, M.D. A systematic review of the use of titanium versus stainless steel implants for fracture fixation. OTA Int. 2021, 4, e138. [Google Scholar] [CrossRef]
- ISO 5832-1:2024; Implants for Surgery—Metallic Materials. Part 1: Wrought Stainless Steel. ISO: Geneva, Switzerland, 2024.
- ISO 6475:1989; Implants for Surgery—Metal Bone Screws with Asymmetrical Thread and Spherical Under-Surface—Mechanical Requirements and Test Methods. ISO: Geneva, Switzerland, 1989.
- ASTM F543; Axial and Torsion Testing of Metallic Medical Bone Screws. ASTM: West Conshohocken, PA, USA, 2017.
- Fernandes, H.J.A.; Saad, E.A.; dos Reis, F.B. Osteosynthesis with Intramedullary Nails in Children. Rev. Bras. Ortop. Engl. Ed. 2009, 44, 380–385. [Google Scholar] [CrossRef]
- Mahar, A.; Sink, E.; Faro, F.; Oka, R.; Newton, P.O. Differences in biomechanical stability of femur fracture fixation when using titanium nails of increasing diameter. J. Child. Orthop. 2007, 1, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Klausmeyer, M.; Mudgal, C.; Tobert, D. Intramedullary Fixation of Metacarpal Fractures Using Headless Compression Screws. J. Hand Microsurg. 2016, 8, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Padegimas, E.M.; Weikert, N.; Greulich, S.; Ilyas, A.M.; Siegler, S. Headless Screw Fixation of Metacarpal Neck Fractures: A Mechanical Comparative Analysis. HAND 2017, 14, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Anand, S.; Bhardwaj, J.; Sachdeva, V.; Sapru, B. Bone Response To Stainless Steel And Titanium Bone Plates. Med. J. Armed Forces India 1994, 50, 10–14. [Google Scholar] [CrossRef][Green Version]
- Ladani, L.; Palmieri, M. Review of the Use of Metals in Biomedical Applications: Biocompatibility, Additive Manufacturing Technologies, and Standards and Regulations. Metals 2024, 14, 1039. [Google Scholar] [CrossRef]
- Holt, J.M.; Mindlin, H.; Ho, C.Y. Structural Alloys Handbook; CINDAS/Purdue University: West Lafayette, IN, USA, 1997; pp. 17–104. [Google Scholar]
- Daood, K. Mechanics of Materials by B. J. 7th. Available online: https://www.academia.edu/35205827/Mechanics_of_Materials_By_B_J_7th (accessed on 2 February 2025).
- Wahbeh, J.M.; Kelley, B.V.; Shokoohi, C.; Park, S.-H.; Devana, S.K.; Ebramzadeh, E.; Sangiorgio, S.N.; Jeffcoat, D.M. Comparison of a 2.7-mm and 3.5-mm locking compression plate for ulnar fractures: A biomechanical evaluation. OTA Int. 2023, 6, e278. [Google Scholar] [CrossRef]
- Penzkofer, R.; Maier, M.; Nolte, A.; von Oldenburg, G.; Püschel, K.; Bühren, V.; Augat, P. Influence of intramedullary nail diameter and locking mode on the stability of tibial shaft fracture fixation. Arch. Orthop. Trauma Surg. 2008, 129, 525–531. [Google Scholar] [CrossRef]
- Antekeier, S.B.; Burden, R.L.; Voor, M.J.; Roberts, C.S. Mechanical study of the safe distance between distal femoral fracture site and distal locking screws in antegrade intramedullary nailing. J. Orthop. Trauma 2005, 19, 693–697. [Google Scholar] [CrossRef]
- Guo, J.; Zha, J.; Di, J.; Yin, Y.; Hou, Z.; Zhang, Y. Outcome Analysis of Intramedullary Nailing Augmented with Poller Screws for Treating Difficult Reduction Fractures of Femur and Tibia: A Retrospective Cohort Study. BioMed Res. Int. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Shanto, R.A.; Khalil, M.; Sultana, S.Z.; Epsi, E.Z.; Bose, S.K.; Latif, M.S.; Siddiquee, T.; Russel, M.T.H.; Sumi, S.A. Variation of Mid shaft Antero-posterior and Transverse Diameter of Femur in Bangladeshi People. Mymensingh Med. J. MMJ 2024, 33, 234–238. Available online: https://pubmed.ncbi.nlm.nih.gov/38163798/ (accessed on 13 July 2025).
- Singh, A.; Nagar, M.; Kumar, A. An Anthropometric Study of the Humerus in Adults. Res. Rev. J. Ofâ Med. Health Sci. 2025, 3, 77–82. Available online: https://www.rroij.com/open-access/an-anthropometric-study-of-the-humerus-in-adults.php?aid=34958 (accessed on 13 July 2025).
- Hu, H.; Chen, G.; Wu, X.; Lin, M.; Lin, H. Reduction with Pre-Drilling Combined with a Finger Reduction Tool in Difficult-to-Reduce Intertrochanteric Fracture. Orthop. Surg. 2022, 14, 2750–2756. [Google Scholar] [CrossRef]
- ASTM F1264-16e1; Standard Specification and Test Methods for Intramedullary Fixation Devices. ASTM: West Conshohocken, PA, USA, 2016.
| Type of Mechanical Testing | Bone Contact | Frequency [Hz] | Mechanical Load | Imposed Number of Cycles (Not to Failure) | Displacement | Stiffness |
|---|---|---|---|---|---|---|
| Tensile–compression | In physical contact | 10 | 200 N (−50 N preloading) | 200,000 | <0.01 mm | ≈20,000 N/mm |
| 1 mm gap between bones | 5 | 200 N (−50 N preloading) | 100,000 | <0.01 mm | ≈20,000 N/mm | |
| Torsion test | In physical contact | 5 | 1.0 Nm | 50,000 | <0.1° rotation | ≈573 Nm/° |
| Combined testing (tensile–compression–torsion) | In physical contact | 5 | Torsion: 0.8 Nm Tensile: 200 N | 150,000 | <0.01 mm and <0.1° rotation | ≈20,000 N/mm and ≈573 Nm/° |
| Three-point bend | In physical contact | 0.5 | 70 N | 30,000 | <0.02 mm deflection | Flexural stiffness ≈3500 Nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misca, L.-C.; Croicu, C.C.; Lazarescu, A.E.; Sandesc, M.-A.; Patrascu, J.M., Jr.; Florescu, S.; Patrascu, J.M., Sr. Original Locking Rod System Designed for Diaphyseal Fractures of Long Bones. J. Funct. Biomater. 2025, 16, 348. https://doi.org/10.3390/jfb16090348
Misca L-C, Croicu CC, Lazarescu AE, Sandesc M-A, Patrascu JM Jr., Florescu S, Patrascu JM Sr. Original Locking Rod System Designed for Diaphyseal Fractures of Long Bones. Journal of Functional Biomaterials. 2025; 16(9):348. https://doi.org/10.3390/jfb16090348
Chicago/Turabian StyleMisca, Liviu-Coriolan, Cristian Constantin Croicu, Adrian Emil Lazarescu, Mihai-Alexandru Sandesc, Jenel Marian Patrascu, Jr., Sorin Florescu, and Jenel Marian Patrascu, Sr. 2025. "Original Locking Rod System Designed for Diaphyseal Fractures of Long Bones" Journal of Functional Biomaterials 16, no. 9: 348. https://doi.org/10.3390/jfb16090348
APA StyleMisca, L.-C., Croicu, C. C., Lazarescu, A. E., Sandesc, M.-A., Patrascu, J. M., Jr., Florescu, S., & Patrascu, J. M., Sr. (2025). Original Locking Rod System Designed for Diaphyseal Fractures of Long Bones. Journal of Functional Biomaterials, 16(9), 348. https://doi.org/10.3390/jfb16090348





