Clinical and Radiological Evaluation of Flap and Flapless Procedures with Biomaterials in Alveolar Ridge Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Groups
- -
- Necessity to remove a single tooth in the maxilla in the esthetic area.
- -
- Minimum age of 18 years.
- -
- Signing a written consent.
- -
- Systemic general diseases that affect surgical therapy (uncontrolled diabetes, uncontrolled hypertension, severe heart diseases).
- -
- Osteoporosis treated with bisphosphonates or other antiresorptive drugs.
- -
- Immunosuppression.
- -
- Pregnancy and breastfeeding.
- -
- Smoking.
- -
- Allergy to penicillin.
- -
- Knowledge of allergy to any of the biomaterials used in the study.
- -
- Acute local condition in the area of tooth qualified for removal that could prevent precise performance of the surgical procedure and preparation of temporary work.
2.2. Clinical and Radiographic Examination
- -
- Plaque index (FMPS—full mouth plaque score)—determined dichotomously as the presence or absence of dental plaque on the four tooth surfaces [18].
- -
- Bleeding on probing (FMBOP—full mouth bleeding on probing)—determined dichotomously as bleeding on probing or its absence on 4 tooth surfaces [19].
- -
- Width of keratinized gingiva (KT—keratinized tissue)—measured from the gingival margin to the mucogingival junction in the long axis of the tooth to be removed.
- -
- Papillae height (PHm-mesial, PHd-distal) and papillae width (PWm-mesial, PWd-distal) of the interdental papillae adjacent to the tooth qualified for removal.
- -
- Probing depth (PD), gingival recession (GR), clinical attachment level (CAL) of teeth adjacent to the tooth qualified for extraction.
- -
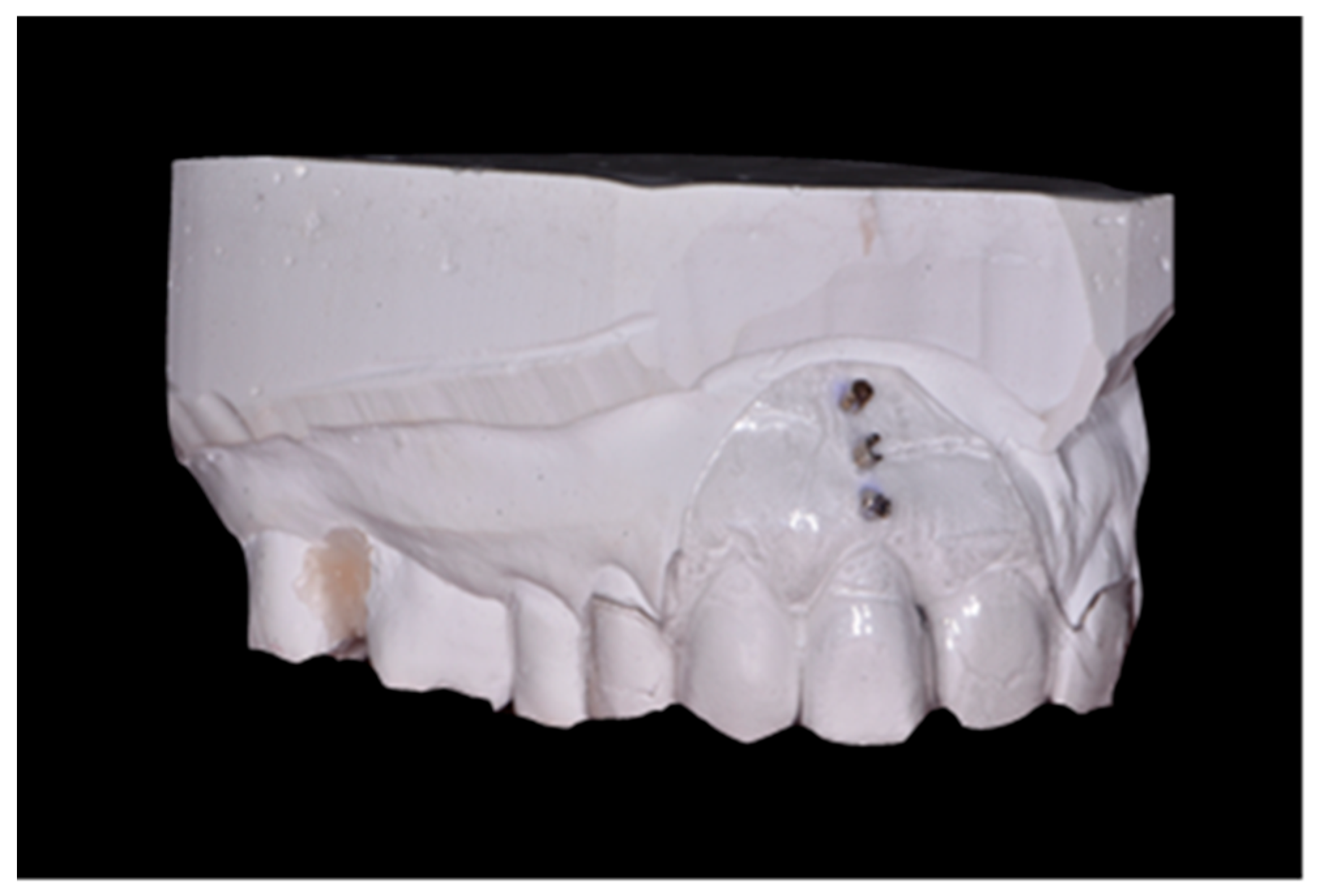
- Soft tissue thickness in the root projection of the extracted tooth (3 mm, 6 mm and 9 mm from gingival margin of extracted tooth); to measure this parameter, an individual positioner with holes for the measuring tool was prepared before tooth extraction (Figure 2); a sharply pointed root canal tool No. 25 was used for the test; in order to perform measurements in the same point, through the embedded sleeves, a root canal tool No. 25 was passed through and the puncture was performed to determine the measurement point; after removing the stent in the marked areas, the tissue was punctured until palpable bone resistance was felt; measurements were made under local anesthesia.
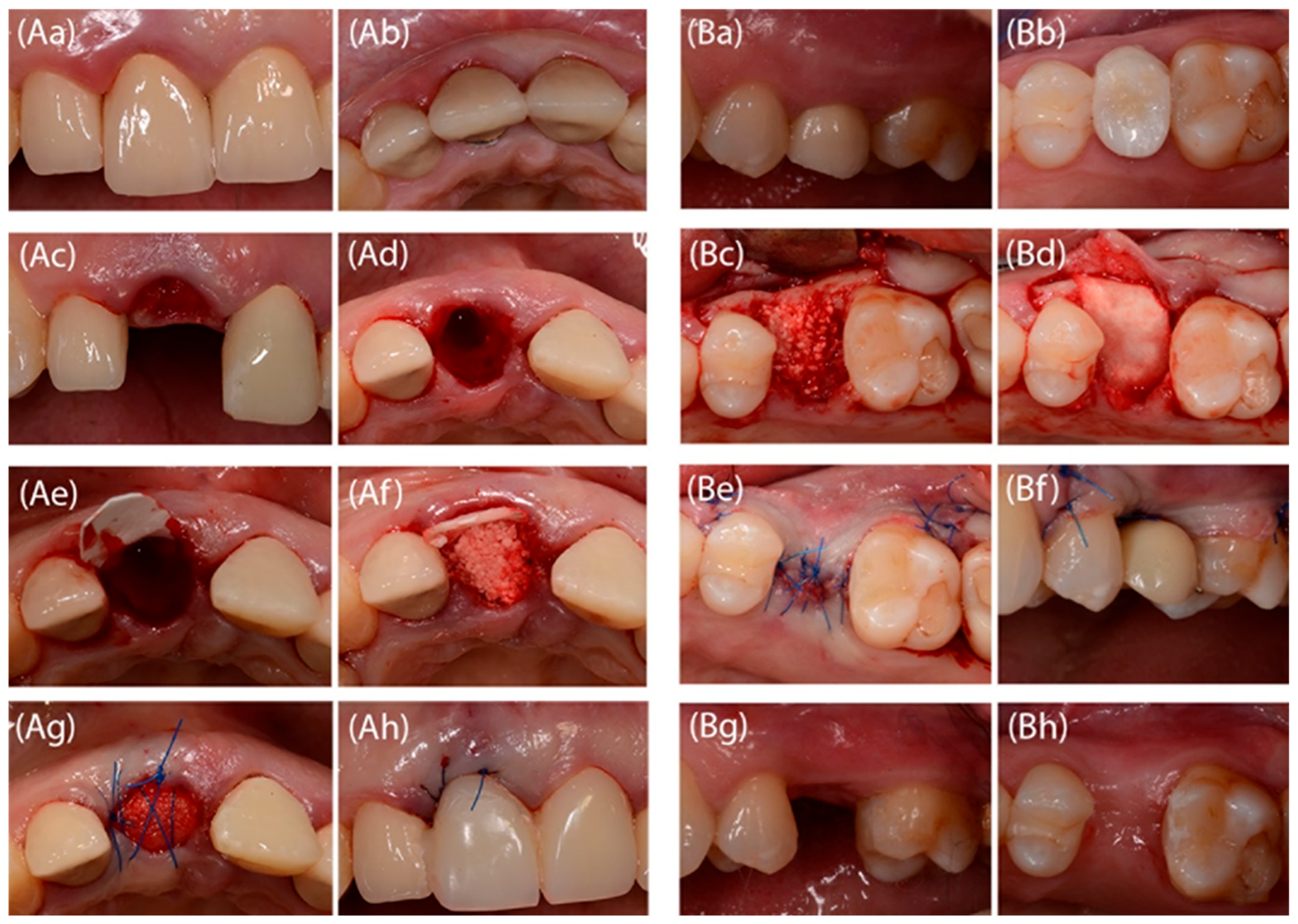
2.3. Surgical Procedure and Temporary Prosthetic Device
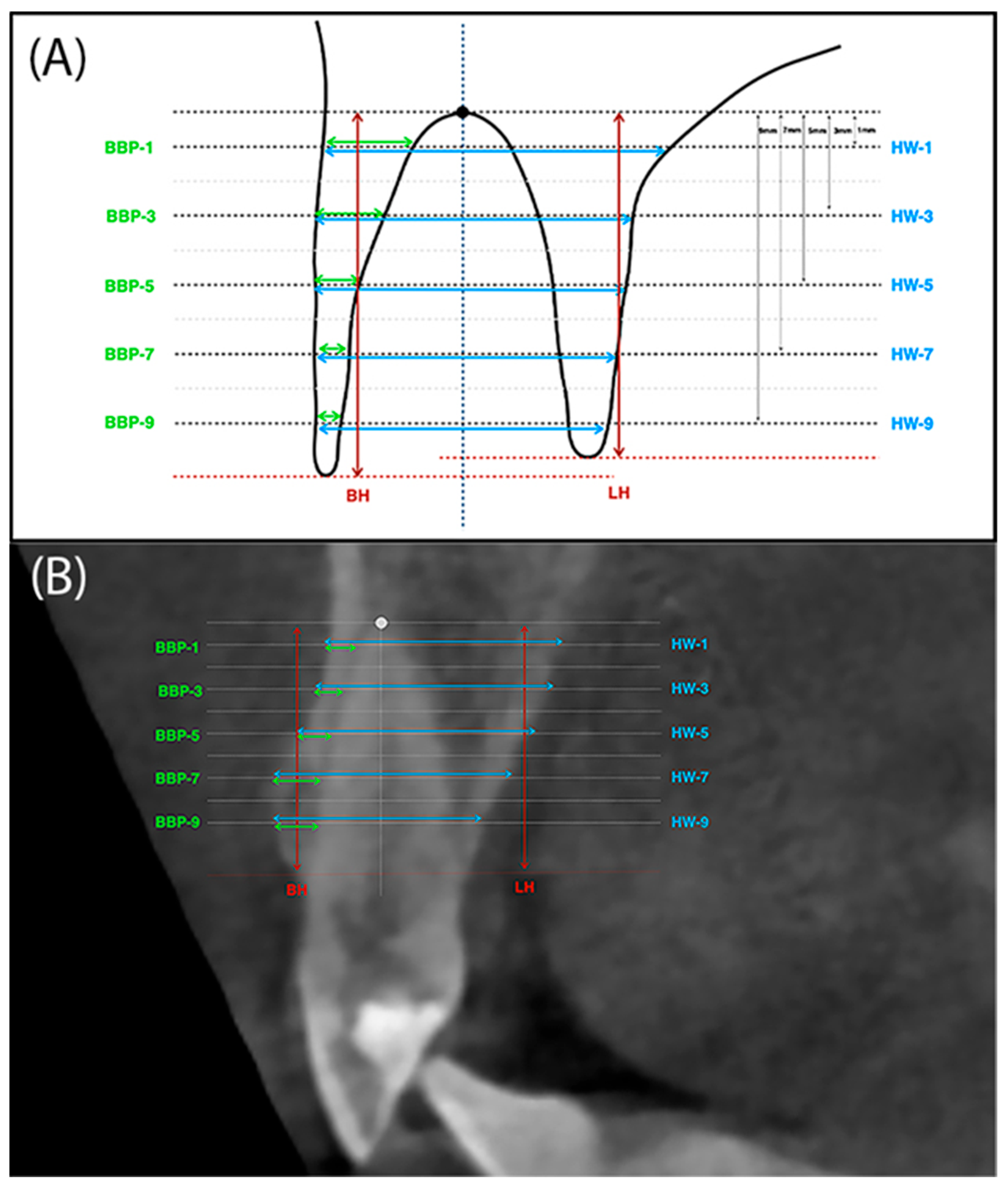
2.4. CBCT Analysis
- -
- BH (buccal height)—The height of the buccal plate measured from the edge of the bone margin on the buccal side to the perpendicular line running through the reference point at the alveolar floor.
- -
- LH (lingual height)—The height of the palatal plate measured from the edge of the alveolar bone margin on the buccal side to the perpendicular line running through the reference point at the alveolar floor.
- -
- BBP-1 (buccal bone plate 1)—Thickness of buccal plate measured parallel 1mm from the reference line running through the alveolar floor and analogously BBP-3, BBP-5, BBP-7 and BBP-9.
- -
- HW-1 (horizontal width 1)—The width of the alveolus measured parallel 1mm from the reference line running through the bottom of the alveolus. And analogously HW-3, HW-5, HW-7 and HW-9 (Figure 4A,B).
2.5. Statistical Analysis
3. Results
3.1. Clinical Examination
3.2. Radiological Examination-Evaluation of CBCT Scans
4. Discussion
5. Conclusions
- A reduction in interdental papillae height and keratinized tissue width.
- Increase in buccal soft tissues thickness at a height of 3 mm and 6 mm from the gingival margin.
- Decrease in radiological buccal bone plate width.
- Decrease in radiological buccal and lingual plate height (significantly for the group without flap preparation).
- Radiological alveolar process width reduction.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARP | Alveolar Preservation Procedure |
| BBP | buccal bone plate |
| BH | buccal height |
| CAL | clinical attachment level |
| CBCT | cone beam computed tomography |
| FMBOP | full mouth bleeding on probing |
| FMPS | full mouth plaque score |
| GR | gingival recession |
| HW | horizontal width |
| KT | keratinized tissue |
| LH | lingual height |
| PD | probing depth |
| PH | papilla height |
| PW | papilla width |
References
- Nanci, A. Ten Cate’s Oral Histology, 9th ed.; Elsevier: Amsterdam, Netherlands, 2013. [Google Scholar]
- Bamusa, B.; Alahmari, A.; Binjubair, M.; Badahdah, O.; Bakhadher, W. Buccal alveolar bone thickness: A review of studies. Med Med. Sci. 2019, 7, 018–023. [Google Scholar]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontol 2000 2015, 68, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Clozza, E.; Biasotto, M.; Cavalli, F.; Moimas, L.; Di Lenarda, R. Three-Dimensional Evaluation of Bone Changes Following Ridge Preservation Procedures. Int. J. Oral Maxillofac Impl. 2012, 27, 770–775. [Google Scholar]
- Pagni, G.; Pellegrini, G.; Giannobile, W.V.; Rasperini, G. Postextraction Alveolar Ridge Preservation: Biological Basis and Treatments. Int. J. Dent. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar] [PubMed]
- Shalu, C.; Kimpreet, K.; Navkiran, K.; Anish, M. Socket augmentation. J. Int. Clin. Dent. Res. Org. 2015, 7, 73–80. [Google Scholar]
- MacBeth, N.; Trullenque-Eriksson, A.; Donos, N.; Mardas, N. Hard and soft tissue changes following alveolar ridge preservation: A systematic review. Clin. Oral Implant. Res. 2016, 28, 982–1004. [Google Scholar] [CrossRef]
- Canullo, L.; Del Fabbro, M.; Khijmatgar, S.; Panda, S.; Ravidà, A.; Tommasato, G.; Sculean, A.; Pesce, P. Dimensional and histomorphometric evaluation of biomaterials used for alveolar ridge preservation: A systematic review and network meta-analysis. Clin. Oral Investig. 2021, 26, 141–158. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Trombelli, L.; Heitz, F.; Needleman, I.; Moles, D. A systematic review of the effect of surgical debridement vs. non-surgical debridement for the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29, 92–102. [Google Scholar] [CrossRef]
- Fickl, S.; Kebschull, M.; Schupbach, P.; Zuhr, O.; Schlagenhauf, U.; Hürzeler, M.B. Bone loss after full-thickness and partial-thickness flap elevation. J. Clin. Periodontol. 2011, 38, 157–162. [Google Scholar] [CrossRef]
- Aladmawy, M.A.; Natto, Z.S.; Steffensen, B.; Levi, P.; Cheung, W.; Finkelman, M.; Ogata, Y.; Hur, Y. A Comparison between Primary and Secondary Flap Coverage in Ridge Preservation Procedures: A Pilot Randomized Controlled Clinical Trial. BioMed Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Saleh, M.H.; Couso-Queiruga, E.; Ravidà, A.; Dukka, H.; De Andrade, N.P.; Ou, A.; Ou, H.-L.; Wang, A. Impact of the periodontal phenotype in premolar and molar sites on bone loss following full-thickness mucoperiosteal flap: A 1-year prospective clinical trial. J. Periodontol. 2022, 93, 966–976. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Ridge alterations following tooth extraction with and without flap elevation: An experimental study in the dog. Clin. Oral Implant. Res. 2009, 20, 545–549. [Google Scholar] [CrossRef]
- Thoma, D.S.; Jung, R.E.; Schneider, D.; Cochran, D.L.; Ender, A.; Jones, A.A.; Görlach, C.; Uebersax, L.; Graf-Hausner, U.; Hämmerle, C.H.F. Soft tissue volume augmentation by the use of collagen-based matrices: A volumetric analysis. J. Clin. Periodontol. 2010, 37, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Schmidlin, P.R.; Philipp, A.; Annen, B.M.; Ronay, V.; Hämmerle, C.H.F.; Attin, T.; Jung, R.E. Labial soft tissue volume evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. J. Clin. Periodontol. 2014, 41, 612–617. [Google Scholar] [CrossRef] [PubMed]
- O’LEary, T.J.; Drake, R.B.; Naylor, J.E. The Plaque Control Record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Landsberg, C.J. Socket seal surgery combined with immediate implant placement: A novel approach for single-tooth replacement. Int. J. Periodontics Restor. Dent. 1997, 17, 140–149. [Google Scholar]
- Hong, H.R.; Chen, C.; Kim, D.M.; Machtei, E.E. Ridge preservation procedures revisited: A randomized controlled trial to evaluate dimensional changes with two different surgical protocols. J. Periodontol. 2018, 90, 331–338. [Google Scholar] [CrossRef]
- Barone, A.; Toti, P.; Piattelli, A.; Iezzi, G.; Derchi, G.; Covani, U. Extraction Socket Healing in Humans After Ridge Preservation Techniques: Comparison Between Flapless and Flapped Procedures in a Randomized Clinical Trial. J. Periodontol. 2014, 85, 14–23. [Google Scholar] [CrossRef]
- Engler-Hamm, D.; Cheung, W.S.; Yen, A.; Stark, P.C.; Griffin, T. Ridge Preservation Using a Composite Bone Graft and a Bioabsorbable Membrane With and Without Primary Wound Closure: A Comparative Clinical Trial. J. Periodontol. 2011, 82, 377–387. [Google Scholar] [CrossRef]
- Iasella, J.M.; Greenwell, H.; Miller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge Preservation with Freeze-Dried Bone Allograft and a Collagen Membrane Compared to Extraction Alone for Implant Site Development: A Clinical and Histologic Study in Humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef]
- Jung, R.E.; Philipp, A.; Annen, B.M.; Signorelli, L.; Thoma, D.S.; Hämmerle, C.H.; Attin, T.; Schmidlin, P. Radiographic evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. J. Clin. Periodontol. 2012, 40, 90–98. [Google Scholar] [CrossRef]
- Manavella, V.; Romano, F.; Corano, L.; Bignardi, C.; Aimetti, M. Three-Dimensional Volumetric Changes in Severely Resorbed Alveolar Sockets After Ridge Augmentation with Bovine-Derived Xenograft and Resorbable Barrier: A Preliminary Study on CBCT Imaging. Int. J. Oral Maxillofac. Implant. 2018, 33, 373–382. [Google Scholar] [CrossRef]
- Fickl, S.; Zuhr, O.; Wachtel, H.; Bolz, W.; Huerzeler, M. Tissue alterations after tooth extraction with and without surgical trauma: A volumetric study in the beagle dog. J. Clin. Periodontol. 2008, 35, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Nuñez, V.; Aracil, L.; Muñoz, F.; Ramos, I. Ridge alterations following immediate implant placement in the dog: Flap versus flapless surgery. J. Clin. Periodontol. 2008, 35, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Mareque, S.; Liñares, A.; Muñoz, F. Vertical and horizontal ridge alterations after tooth extraction in the dog: Flap vs. flapless surgery. Clin. Oral Implant. Res. 2011, 22, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Borgia, V.; Covani, U.; Ricci, M.; Piattelli, A.; Iezzi, G. Flap versus flapless procedure for ridge preservation in alveolar extraction sockets: A histological evaluation in a randomized clinical trial. Clin. Oral Implant. Res. 2014, 26, 806–813. [Google Scholar] [CrossRef]
- Siu, T.L.; Dukka, H.; Saleh, M.H.A.; Tattan, M.; Dib, Z.; Ravidà, A.; Greenwell, H.; Wang, H.; Araujo, M.G. Flap versus flapless alveolar ridge preservation: A clinical and histological single-blinded, randomized controlled trial. J. Periodontol. 2022, 94, 184–192. [Google Scholar] [CrossRef]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implant. Res. 2012, 23, 22–38. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.-B.; Koo, K.-T.; Seol, Y.-J.; Lee, Y.-M. Flap Management in Alveolar Ridge Preservation: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.; Alfardan, L.; Alsabeeha, N. Flapped versus flapless alveolar ridge preservation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2022, 51, 133–142. [Google Scholar] [CrossRef]
- Sela, M.N.; Babitski, E.; Steinberg, D.; Kohavi, D.; Rosen, G. Degradation of collagen-guided tissue regeneration membranes by proteolytic enzymes of Porphyromonas gingivalis and its inhibition by antibacterial agents. Clin. Oral Implant. Res. 2009, 20, 496–502. [Google Scholar] [CrossRef]
- Orgeas, V.G.; Clementini, M.; De Risi, V.; de Sanctis, M. Surgical techniques for alveolar socket preservation: A systematic review. Int. J. Oral Maxillofac Implant. 2013, 28, 1049–1061. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 195–223. [Google Scholar] [CrossRef]
- MacBeth, N.D.; Donos, N.; Mardas, N. Alveolar ridge preservation with guided bone regeneration or socket seal technique. A randomised, single-blind controlled clinical trial. Clin. Oral Implant. Res. 2022, 33, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Laino, L.; Cicciù, M.; Lo Russo, L. Combination of Bone Graft and Resorbable Membrane for Alveolar Ridge Preservation: A Systematic Review, Meta-analysis and Trial Sequential Analysis. J. Periodontol. 2018, 89, 46–57. [Google Scholar] [CrossRef]
- Barootchi, S.; Tavelli, L.; Majzoub, J.; Stefanini, M.; Wang, H.; Avila-Ortiz, G. Alveolar ridge preservation: Complications and cost-effectiveness. Periodontology 2000 2022, 92, 235–262. [Google Scholar] [CrossRef]
- Couso-Queiruga, E.; Weber, H.A.; Garaicoa-Pazmino, C.; Barwacz, C.; Kalleme, M.; Galindo-Moreno, P.; Avila-Ortiz, G. Influence of healing time on the outcomes of alveolar ridge preservation using a collagenated bovine bone xenograft: A randomized clinical trial. J. Clin. Periodontol. 2022, 50, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E. The Effect of Membrane Exposure on the Outcome of Regenerative Procedures in Humans: A Meta-Analysis. J. Periodontol. 2001, 72, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.; Meraw, S.J.; Lee, E.; Giannobile, W.V.; Wang, H. Comparative analysis of collagen membranes for the treatment of implant dehiscence defects. Clin. Oral Implant. Res. 2003, 14, 80–90. [Google Scholar] [CrossRef]
- Hämmerle, C.H.F.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontology 2000 2003, 33, 36–53. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Cardaropoli, G. Preservation of the postextraction alveolar ridge: A clinical and histologic study. Int. J. Periodont. Rest. Dent. 2008, 28, 469–477. [Google Scholar]



| Flapless (Group A) | With a Flap (Group B) | |
|---|---|---|
| n | 15 | 14 |
| Female/Male | 10F, 5M | 9F, 5M |
| Mean age ± SD | 43 ± 10 years | 43 ± 10 years |
| Age range | 27–62 years | 24–59 years |
| Tooth Position: | ||
| incisor | 12 | 5 |
| canine | 0 | 0 |
| premolar | 3 | 9 |
| Reasons for Tooth Loss: | ||
| fracture/rupture of a tooth | 9 | 4 |
| endodontic treatment failure | 5 | 7 |
| root resorption | 1 | 2 |
| periodontitis | 0 | 1 |
| other | 0 | 0 |
| Baseline (0) | 3 Months | 4 Months | 6 Months | p-Time Changes | ||
|---|---|---|---|---|---|---|
| PHm | Flapless group (Group A) Flap group (Group B) p* between groups | 3.37 ± 1.04 ^ 2.75 ± 1.05 p* = ns | 2.50 ± 0.94 ^ 2.11 ± 0.76 p* = ns | 2.80 ± 0.96 2.14 ± 0.86 p* = ns | 3.10 ± 1.23 2.21 ± 0.73 p* = 0.028 | p = 0.0011 p = 0.0068 |
| PWm | Flapless group (Group A) Flap group (Group B) p* between groups | 4.93 ± 0.80 4.79 ± 0.80 p* = ns | 4.80 ± 0.77 4.50 ± 0.94 p* = ns | 4.73 ± 0.80 4.50 ± 0.85 p* = ns | 4.73 ± 0.59 4.21 ± 0.97 p* = ns | p = ns p = ns |
| PHd | Flapless group (Group A) Flap group (Group B) p* between groups | 2.97 ± 0.97 ^ 3.07 ± 1.22 ^ p* = ns | 2.17 ± 0.92 ^ 2.18 ± 1.27 p* = ns | 2.47 ± 1.20 2.04 ± 1.06 p* = ns | 2.67 ± 1.36 1.86 ± 1.08 ^ p* = ns | p = 0.0043 p = 0.0022 |
| PWd | Flapless group (Group A) Flap group (Group B) p* between groups | 5.37 ± 0.90 5.46 ± 1.69 p* = ns | 4.67 ± 0.72 4.43 ± 1.22 p* = ns | 4.40 ± 0.91 4.36 ± 1.08 p* = ns | 4.53 ± 0.74 4.43 ± 1.09 p* = ns | p < 0,0001 p = 0.0122 |
| Baseline (0) | 3 Months | 4 Months | 6 Months | p | Diff ± SD (0–3m) | Diff ± SD (0–4m) | Diff ± SD (0–6m) | ||
|---|---|---|---|---|---|---|---|---|---|
| KT | Flapless group Flap group p* between groups | 6.5 ± 2.54 4.79 ± 2.01 p* = 0.021 | 5.53 ± 2.36 3.61 ± 1.47 p* = 0.027 | 5.50 ± 2.29 3.82 ± 1.64 p* = 0.032 | 5.40 ± 2.10 3.82 ± 1.96 p* = 0.046 | p = 0.015 p = 0.04 | 0.97 ± 1.3 1.18 ± 1.54 | 1.0 ± 1.3 0.96 ± 1.55 | 1.1 ± 1.17 0.96 ± 1.97 |
| Baseline (0) | 3 Months | 4 Months | 6 Months | p | ||
|---|---|---|---|---|---|---|
| Measurement on 3 mm | Flapless group (Group A) Flap group (Group B) p* between groups | 2.07 ± 0.86 2.79 ± 2.46 p* = ns | 7.03 ± 2.58 6.43 ± 3.16 p* = ns | 7.00 ± 2.33 6.25 ± 3.07 p* = ns | 6.60 ± 2.51 6.43 ± 3.01 p* = ns | p < 0.0001 p = 0.0002 |
| Measurement on 6 mm | Flapless group (Group A) Flap group (Group B) p* between groups | 2.97 ± 1.38 2.64 ± 1.98 p = ns | 4.73 ± 2.16 5.36 ± 2.82 p = ns | 4.27 ± 2.15 4.75 ± 1.90 p = ns | 3.77 ± 1.66 4.54 ± 1.89 p = ns | p = 0.0488 p = 0.0008 |
| Measurement on 9 mm | Flapless group (Group A) Flap group (Group B) p* between groups | 3.23 ± 2.51 3.11 ± 2.14 p* = ns | 3.37 ± 1.54 5.46 ± 3.75 p* = 0.046 | 2.87 ± 1.20 4.11 ± 1.30 p* = 0.013 | 2.67 ± 0.98 3.50 ± 1.51 p* = ns | p = ns p = 0.007 |
| Baseline (0) | 6 Months | p | Diff ± SD | ||
|---|---|---|---|---|---|
| BH | Flapless group (Group A) Flap group (Group B) p* between groups | 8.19 ± 3.74 7.99 ± 3.93 p* = ns | 7.11 ± 2.85 7.50 ± 2.40 p* = ns | p = 0.0353 p = ns | −1.08 ± 1.90 −0.49 ± 3.45 |
| LH | Flapless group (Group A) Flap group (Group B) p* between groups | 8.69 ± 2.98 8.91 ± 2.63 p* = ns | 7.40 ± 2.32 8.46 ± 2.09 p* = ns | p = 0.0046 p = ns | −1.29 ± 1.49 −0.46 ± 1.38 |
| Baseline (0) | 6 Months | p in Time | Diff ± SD | ||
|---|---|---|---|---|---|
| BBP-1 | Flapless group (Group A) Flap group (Group B) p* between groups | 0.68 ± 0.51 1.03 ± 1.10 p* = ns | 0.71 ± 0.47 0.87 ± 1.07 p* = ns | p = ns p = ns | 0.03 ± 0.15 −0.16 ± 0.42 |
| BBP-3 | Flapless group (Group A) Flap group (Group B) p* between groups | 0.51 ± 0.35 0.81 ± 0.86 p* = ns | 0.47 ± 0.34 0.54 ± 0.77 p* = ns | p = ns p = 0.0342 | −0.04 ± 0.11 −0.27 ± 0.43 |
| BBP-5 | Flapless group (Group A) Flap group (Group B) p* between groups | 0.57 ± 0.45 0.61 ± 0.63 p* = ns | 0.33 ± 0.41 0.26 ± 0.31 p* = ns | p = 0.0156 p = 0.0161 | −0.24 ± 0.33 −0.36 ± 0.55 |
| BBP-7 | Flapless group (Gorup A) Flap group (Group B) p* between groups | 0.63 ± 0.74 0.50 ± 0.63 p* = ns | 0.25 ± 0.37 0.04 ± 0.12 p* = ns | p = 0.0312 p = 0.0039 | −0.37 ± 0.62 −0.46 ± 0.62 |
| BBP-9 | Flapless group (Group A) Flap group (Group B) p* between groups | 0.67 ± 0.74 0.19 ± 0.27 p* = 0.0312 | 0.24 ± 0.38 0.01 ± 0.05 p* = ns | p = 0.0078 p = ns | −0.43 ± 0.64 −0.17 ± 0.25 |
| Baseline (0) | 6 Miesięcy | p Time Changes | Diff ± SD | ||
|---|---|---|---|---|---|
| HW-1 | Flaplesss group (GroupA) Flap group (Group B) p* between groups | 7.87 ± 2.37 10.19 ± 2.99 p* = 0.0280 | 7.29 ± 2.04 9.79 ± 2.29 p* = 0.0016 | p = ns p = ns | −0.57 ± 1.22 −0.40 ± 2.20 |
| HW-3 | Flapless group (Group A) Flap group (Group B) p* between groups | 7.15 ± 2.80 9.36 ± 2.95 p* = 0.0490 | 6.64 ± 1.69 8.94 ± 2.05 p* = 0.0026 | p = ns p = ns | −0.51 ± 2.05 −0.41 ± 2.72 |
| HW-5 | Flapless group (Group A) Flap group (Group B) p* between group | 6.23 ± 3.93 7.86 ± 3.99 p* = ns | 5.44 ± 2.40 7.73 ± 2.79 p* = 0.0251 | p = 0.0156 p = ns | −0.79 ± 2.31 −0.13 ± 3.69 |
| HW-7 | Flapless group (Group A) Flap group (Group B) p* between groups | 5.43 ± 4.44 6.24 ± 4.83 p* = ns | 4.31 ± 3.25 5.15 ± 3.14 p* = ns | p = 0.0186 p = ns | −1.12 ± 1.56 −1.09 ± 3.67 |
| HW-9 | Flapless group (Group A) Flap group (Group B) p* between groups | 3.72 ± 4.44 3.15 ± 4.61 p* = ns | 2.25 ± 3.15 2.44 ± 3.05 p* = ns | p = ns p = ns | −1.47 ± 5.31 −0.71 ± 2.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolińska, E.; Duraj, E.; Bernaczyk, M.; Sulewska, M.; Pietruska, M. Clinical and Radiological Evaluation of Flap and Flapless Procedures with Biomaterials in Alveolar Ridge Preservation. J. Funct. Biomater. 2025, 16, 345. https://doi.org/10.3390/jfb16090345
Dolińska E, Duraj E, Bernaczyk M, Sulewska M, Pietruska M. Clinical and Radiological Evaluation of Flap and Flapless Procedures with Biomaterials in Alveolar Ridge Preservation. Journal of Functional Biomaterials. 2025; 16(9):345. https://doi.org/10.3390/jfb16090345
Chicago/Turabian StyleDolińska, Ewa, Ewa Duraj, Marcin Bernaczyk, Magdalena Sulewska, and Małgorzata Pietruska. 2025. "Clinical and Radiological Evaluation of Flap and Flapless Procedures with Biomaterials in Alveolar Ridge Preservation" Journal of Functional Biomaterials 16, no. 9: 345. https://doi.org/10.3390/jfb16090345
APA StyleDolińska, E., Duraj, E., Bernaczyk, M., Sulewska, M., & Pietruska, M. (2025). Clinical and Radiological Evaluation of Flap and Flapless Procedures with Biomaterials in Alveolar Ridge Preservation. Journal of Functional Biomaterials, 16(9), 345. https://doi.org/10.3390/jfb16090345








