Polydatin Modulates Inflammatory Cytokine Expression in Lipoteichoic Acid-Stimulated Human Dental-Pulp Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Methodology
2.2. Cells and Cell-Culture Conditions
Cell Expansion
2.3. Materials Preparation
2.3.1. Polydatin Preparation
2.3.2. Lipoteichoic Acid Stimulation of Dental-Pulp Stem Cells
2.4. Cellular Viability of hDPSCs Exposed to Polydatin
AlamarBlueTM Cell Viability Assay
2.5. Levels of Cytokines in LTA-Stimulated Dental-Pulp Stem Cells
2.5.1. Reverse Transcription—Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5.2. Enzyme-Linked Immunosorbent Assay
2.6. Statistical Analysis
3. Results
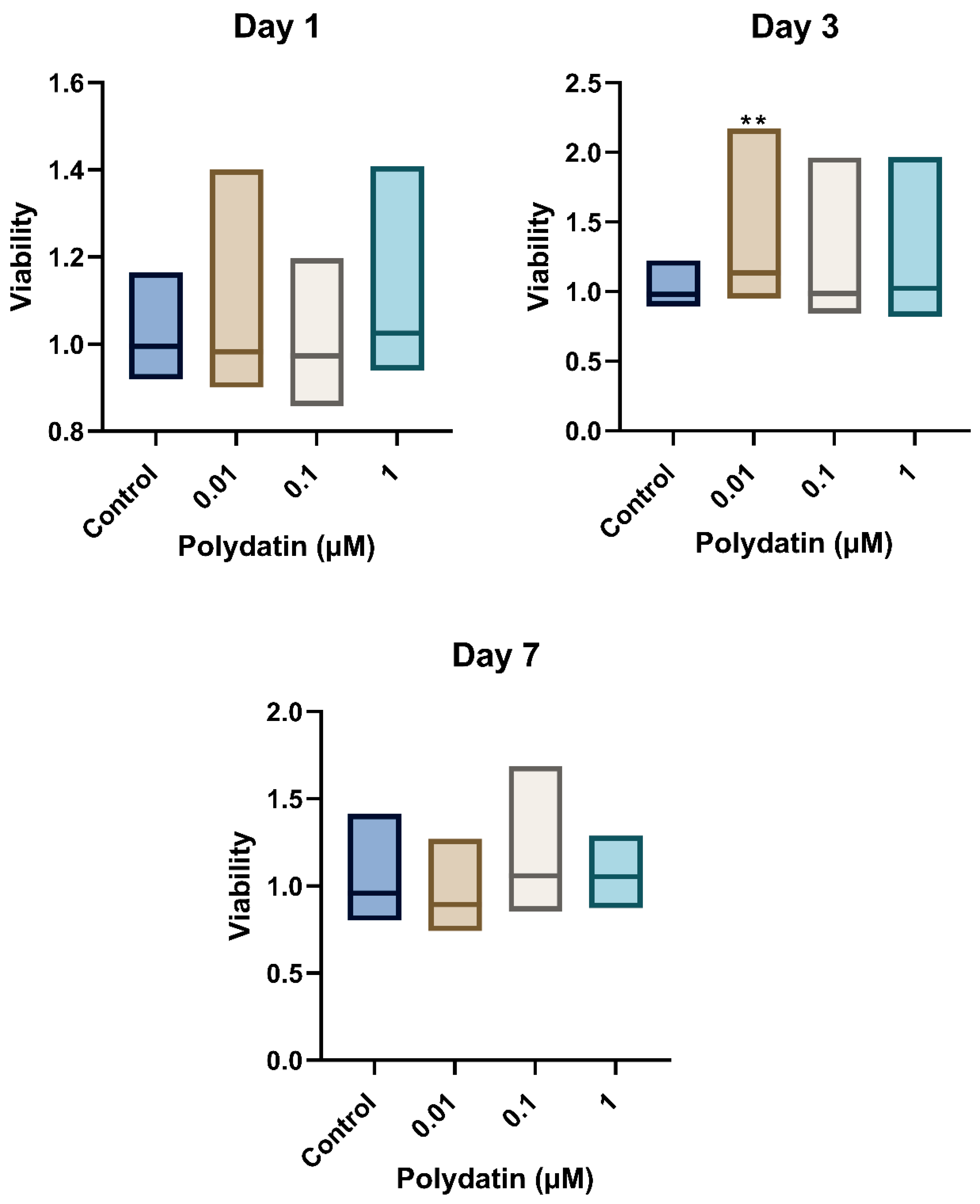
3.1. Viability of Stimulated Dental-Pulp Stem Cells
3.2. Effect of Polydatin Treatment on Messenger RNA Levels
3.3. Effect of Polydatin Treatment on Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LTA | Lipoteichoic acid |
| hDPSCs | Human dental-pulp stem cells |
| ELISA | Enzyme-linked immunosorbent assay |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| TNF-α | Tumor necrosis factor α |
| NF-ĸB | Nuclear factor kappa light chain enhancer of activated B cells |
| MTA® | Mineral trioxide aggregate® |
| α-MEM | Alpha-modified minimum essential medium |
| DMSO | Dimethyl sulfoxide |
| Staphylococcus aureus | S. aureus |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
References
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 4 June 2024).
- Yu, C.; Abbott, P. An Overview of the Dental Pulp: Its Functions and Responses to Injury. Aust. Dent. J. 2007, 52, S4–S6. [Google Scholar] [CrossRef]
- Bowen, W.H.; Koo, H. Biology of Streptococcus Mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- da Costa Rosa, T.; de Almeida Neves, A.; Azcarate-Peril, M.A.; Divaris, K.; Wu, D.; Cho, H.; Moss, K.; Paster, B.J.; Chen, T.; Freitas-Fernandes, L.B.; et al. The Bacterial Microbiome and Metabolome in Caries Progression and Arrest. J. Oral Microbiol. 2021, 13, 1886748. [Google Scholar] [CrossRef] [PubMed]
- Morath, S.; Stadelmaier, A.; Geyer, A.; Schmidt, R.R.; Hartung, T. Synthetic Lipoteichoic Acid from Staphylococcus Aureus Is a Potent Stimulus of Cytokine Release. J. Exp. Med. 2002, 195, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, A.; Zucchi, A.; De Swert, K.; Balau, B.; Truyens, C.; Nicaise, C. Lipoteichoic Acid Stimulates the Proliferation, Migration and Cytokine Production of Adult Dental Pulp Stem Cells without Affecting Osteogenic Differentiation. Int. Endod. J. 2021, 54, 585–600. [Google Scholar] [CrossRef]
- Goldberg, M.; Farges, J.C.; Lacerda-Pinheiro, S.; Six, N.; Jegat, N.; Decup, F.; Septier, D.; Carrouel, F.; Durand, S.; Chaussain-Miller, C.; et al. Inflammatory and Immunological Aspects of Dental Pulp Repair. Pharmacol. Res. 2008, 58, 137–147. [Google Scholar] [CrossRef]
- Farges, J.C.; Carrouel, F.; Keller, J.F.; Baudouin, C.; Msika, P.; Bleicher, F.; Staquet, M.J. Cytokine Production by Human Odontoblast-like Cells upon Toll-like Receptor-2 Engagement. Immunobiology 2011, 216, 513–517. [Google Scholar] [CrossRef]
- Xu, G.; Kuang, G.; Jiang, W.; Jiang, R.; Jiang, D. Polydatin Promotes Apoptosis through Upregulation the Ratio of Bax/Bcl-2 and Inhibits Proliferation by Attenuating the β-Catenin Signaling in Human Osteosarcoma Cells. Am. J. Transl. Res. 2016, 8, 922. [Google Scholar]
- Hirsch, V.; Wolgin, M.; Mitronin, A.V.; Kielbassa, A.M. Inflammatory Cytokines in Normal and Irreversibly Inflamed Pulps: A Systematic Review. Arch. Oral Biol. 2017, 82, 38–46. [Google Scholar] [CrossRef]
- Copeland, S.; Shaw Warren, H.; Lowry, S.F.; Galvano, S.E.; Remick, D. Acute Inflammatory Response to Endotoxin in Mice and Humans. Clin. Diagn. Lab. Immunol. 2005, 12, 60–67. [Google Scholar] [CrossRef]
- Hosoya, S.; Ohbayashi, E.; Matsushima, K.; Takeuchi, H.; Yamazaki, M.; Shibata, Y.; Abiko, Y. Stimulatory Effect of Interleukin-6 on Plasminogen Activator Activity from Human Dental Pulp Cells. J. Endod. 1998, 24, 331–334. [Google Scholar] [CrossRef]
- Stashenko, P.; Dewhirst, F.E.; Peros, W.J.; Kent, R.L.; Ago, J.M. Synergistic Interactions between Interleukin 1, Tumor Necrosis Factor, and Lymphotoxin in Bone Resorption. J. Immunol. 1987, 138, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, M.; Nagaoka, S.; Torii, M. Interleukin-10 Inhibits Expression of Interleukin-6 and -8 MRNA in Human Dental Pulp Cell Cultures via Nuclear Factor-ΚB Deactivation. J. Endod. 2002, 28, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Paula-Silva, F.W.G.; Ghosh, A.; Silva, L.A.B.; Kapila, Y.L. TNF-Alpha Promotes an Odontoblastic Phenotype in Dental Pulp Cells. J. Dent. Res. 2009, 88, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sonmez Kaplan, S.; Sazak Ovecoglu, H.; Genc, D.; Akkoc, T. TNF-α, IL-1B and IL-6 Affect the Differentiation Ability of Dental Pulp Stem Cells. BMC Oral Health 2023, 23, 555. [Google Scholar] [CrossRef]
- Jeanneau, C.; Laurent, P.; Rombouts, C.; Giraud, T.; About, I. Light-Cured Tricalcium Silicate Toxicity to the Dental Pulp. J. Endod. 2017, 43, 2074–2080. [Google Scholar] [CrossRef]
- Iwamoto, C.E.; Adachi, E.; Pameijer, C.H.; Barnes, D.; Romberg, E.E.; Jefferies, S. Clinical and Histological Evaluation of White ProRoot MTA in Direct Pulp Capping. Am. J. Dent. 2006, 19, 85–90. [Google Scholar]
- Yuan, Z.; Zhu, X.; Li, Y.; Yan, P.; Jiang, H. Influence of iRoot SP and Mineral Trioxide Aggregate on the Activation and Polarization of Macrophages Induced by Lipopolysaccharide. BMC Oral Health 2018, 18, 56. [Google Scholar] [CrossRef]
- Çelik, N.; Işcan Yapar, M.; Taghizadehghalehjoughi, A.; Nalcı, K.A. Influence of Resveratrol Application with Pulp-capping Materials on the Genetic Expression Levels of Stem Cells. Int. Endod. J. 2020, 53, 1253–1263. [Google Scholar] [CrossRef]
- Giraud, T.; Jeanneau, C.; Bergmann, M.; Laurent, P.; About, I. Tricalcium Silicate Capping Materials Modulate Pulp Healing and Inflammatory Activity In Vitro. J. Endod. 2018, 44, 1686–1691. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Posa, F.; De Maria, S.; Ravagnan, G.; Ballini, A.; Porro, C.; Trotta, T.; Grano, M.; Muzio, L.L.; Mori, G. Polydatin, Natural Precursor of Resveratrol, Promotes Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Al-Ateeq, R.; Elsafadi, M.; Al-Hadlaq, S. Effect of Polydatin on the Viability and Odontogenic Differentiation of Human Dental Pulp Stem Cells: An In-Vitro Study. J. Dent. Sci. 2024, 19, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, T.; Lei, X.; Li, Y.; Dai, X.; Cao, Y.; Ding, Q.; Lei, X.; Li, T.; Lin, X. Neuroprotective Effects of Polydatin against Mitochondrial-Dependent Apoptosis in the Rat Cerebral Cortex Following Ischemia/Reperfusion Injury. Mol. Med. Rep. 2016, 14, 5481–5488. [Google Scholar] [CrossRef]
- Khalil, A.A.K.; Park, W.S.; Kim, H.J.; Akter, K.M.; Ahn, M.J. Anti-Helicobacter Pylori Compounds from Polygonum Cuspidatum. Nat. Prod. Sci. 2016, 22, 220–224. [Google Scholar] [CrossRef]
- Lanzilli, G.; Cottarelli, A.; Nicotera, G.; Guida, S.; Ravagnan, G.; Fuggetta, M.P. Anti-Inflammatory Effect of Resveratrol and Polydatin by In Vitro IL-17 Modulation. Inflammation 2012, 35, 240–248. [Google Scholar] [CrossRef]
- Xu, L.Q.; Xie, Y.L.; Gui, S.H.; Zhang, X.; Mo, Z.Z.; Sun, C.Y.; Li, C.L.; Luo, D.D.; Zhang, Z.B.; Su, Z.R.; et al. Polydatin Attenuates D-Galactose-Induced Liver and Brain Damage through Its Anti-Oxidative, Anti-Inflammatory and Anti-Apoptotic Effects in Mice. Food Funct. 2016, 7, 4545–4555. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Wen, D.; Guo, J.; Xiong, Q.; Li, P.; Zhao, L.; Wang, J.; Wu, C.; Dong, L. Polydatin Has Anti-Inflammatory and Antioxidant Effects in LPS-Induced Macrophages and Improves DSS-Induced Mice Colitis. Immun. Inflamm. Dis. 2021, 9, 959–970. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, S.H.; Lin, T.; Liao, Y.W.; Chang, Y.C.; Chen, C.C.; Yu, C.C.; Chen, C.J. Resveratrol Attenuates Advanced Glycation End Product-Induced Senescence and Inflammation in Human Gingival Fibroblasts. J. Dent. Sci. 2024, 19, 580–586. [Google Scholar] [CrossRef]
- Ravagnan, G.; De Filippis, A.; Cartenì, M.; De Maria, S.; Cozza, V.; Petrazzuolo, M.; Tufano, M.A.; Donnarumma, G. Polydatin, a Natural Precursor of Resveratrol, Induces β-Defensin Production and Reduces Inflammatory Response. Inflammation 2013, 36, 26–34. [Google Scholar] [CrossRef]
- Wang, F.M.; Hu, Z.; Liu, X.; Feng, J.Q.; Augsburger, R.A.; Gutmann, J.L.; Glickman, G.N. Resveratrol Represses Tumor Necrosis Factor α/c-Jun N-Terminal Kinase Signaling via Autophagy in Human Dental Pulp Stem Cells. Arch. Oral Biol. 2019, 97, 116–121. [Google Scholar] [CrossRef]
- Ye, J.; Piao, H.; Jiang, J.; Jin, G.; Zheng, M.; Yang, J.; Jin, X.; Sun, T.; Choi, Y.H.; Li, L.; et al. Polydatin Inhibits Mast Cell-Mediated Allergic Inflammation by Targeting PI3K/Akt, MAPK, NF-ΚB and Nrf2/HO-1 Pathways. Sci. Rep. 2017, 7, 11895. [Google Scholar] [CrossRef]
- Berghe, W.V.; De Bosscher, K.; Boone, E.; Plaisance, S.; Haegeman, G. The Nuclear Factor-KappaB Engages CBP/P300 and Histone Acetyltransferase Activity for Transcriptional Activation of the Interleukin-6 Gene Promoter. J. Biol. Chem. 1999, 274, 32091–32098. [Google Scholar] [CrossRef]
- Jiang, K.F.; Zhao, G.; Deng, G.Z.; Wu, H.C.; Yin, N.N.; Chen, X.Y.; Qiu, C.W.; Peng, X.L. Polydatin Ameliorates Staphylococcus Aureus-Induced Mastitis in Mice via Inhibiting TLR2-Mediated Activation of the P38 MAPK/NF-ΚB Pathway. Acta Pharmacol. Sin. 2016, 38, 211–222. [Google Scholar] [CrossRef]
- Roebuck, K.A. Regulation of Interleukin-8 Gene Expression. J. Interferon Cytokine Res. 1999, 19, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Lettieri, T. Comparison of Four Different Colorimetric and Fluorometric Cytotoxicity Assays in a Zebrafish Liver Cell Line. BMC Pharmacol. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Alrshedan, A.; Elsafadi, M.; Muthurangan, M.; Al-Hadlaq, S. Tumor Necrosis Factor Superfamily 14 Regulates the Inflammatory Response of Human Dental Pulp Stem Cells. Curr. Issues Mol. Biol. 2024, 46, 13979–13990. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, Z.; Niu, K.; Xie, Y.; Hu, X.; Fu, J.; Tian, D.; Fu, K.; Zhao, B.; Kong, W.; et al. Microbiome of Deep Dentinal Caries from Reversible Pulpitis to Irreversible Pulpitis. J. Endod. 2019, 45, 302–309.e1. [Google Scholar] [CrossRef]
- Zhao, G.; Jiang, K.; Wu, H.; Qiu, C.; Deng, G.; Peng, X. Polydatin Reduces Staphylococcus Aureus Lipoteichoic Acid-Induced Injury by Attenuating Reactive Oxygen Species Generation and TLR2-NFκB Signalling. J. Cell. Mol. Med. 2017, 21, 2796–2808. [Google Scholar] [CrossRef]
- Hamada, S.; Slade, H.D. Biology, Immunology, and Cariogenicity of Streptococcus Mutans. Microbiol. Mol. Biol. Rev. 1980, 44, 331–384. [Google Scholar] [CrossRef]
- Hong, S.W.; Seo, D.G.; Baik, J.E.; Cho, K.; Yun, C.H.; Han, S.H. Differential Profiles of Salivary Proteins with Affinity to Streptococcus Mutans Lipoteichoic Acid in Caries-Free and Caries-Positive Human Subjects. Mol. Oral Microbiol. 2014, 29, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; About, I.; Cooper, L.F.; Chung, S.H. C5L2 Silencing in Human Pulp Fibroblasts Enhances Nerve Outgrowth Under Lipoteichoic Acid Stimulation. J. Endod. 2018, 44, 1396–1401. [Google Scholar] [CrossRef]
- Hermann, C.; Spreitzer, I.; Schröder, N.W.J.; Morath, S.; Lehner, M.D.; Fischer, W.; Schütt, C.; Schumann, R.R.; Hartung, T. Cytokine Induction by Purified Lipoteichoic Acids from Various Bacterial Species--Role of LBP, SCD14, CD14 and Failure to Induce IL-12 and Subsequent IFN-Gamma Release. Eur. J. Immunol. 2002, 32, 541–551. [Google Scholar] [CrossRef]
- Hong, S.W.; Baik, J.E.; Kang, S.S.; Yun, C.H.; Seo, D.G.; Han, S.H. Lipoteichoic Acid of Streptococcus Mutans Interacts with Toll-like Receptor 2 through the Lipid Moiety for Induction of Inflammatory Mediators in Murine Macrophages. Mol. Immunol. 2014, 57, 284–291. [Google Scholar] [CrossRef]
- Keller, J.F.; Carrouel, F.; Colomb, E.; Durand, S.H.; Baudouin, C.; Msika, P.; Bleicher, F.; Vincent, C.; Staquet, M.J.; Farges, J.C. Toll-like Receptor 2 Activation by Lipoteichoic Acid Induces Differential Production of pro-Inflammatory Cytokines in Human Odontoblasts, Dental Pulp Fibroblasts and Immature Dendritic Cells. Immunobiology 2010, 215, 53–59. [Google Scholar] [CrossRef]
- Kuru, S.; Sepet, E.; İrez, T.; Aktaş, E.; Yazır, Y.; Duruksu, G.; Osmanoglu Akyol, E.; Ergüven, M. Effects of Different Pulp-Capping Materials on Cell Death Signaling Pathways of Lipoteichoic Acid-Stimulated Human Dental Pulp Stem Cells. Odontology 2021, 109, 547–559. [Google Scholar] [CrossRef]


| Gene | Sequence (5′-3′) |
|---|---|
| GAPDH | Sense (forward primer) 5′-CTGGTAAAGTGGATATTGTTGCCAT-3′ Antisense (reverse primer) 5′-TGGAATCATATTGGAACATGTAAACC-3′ |
| IL-6 | Sense (forward primer) 5′-GCCCAGCTATGAACTCCTTCT-3′ Antisense (reverse primer) 5′-GAAGGCAGCAGGCAACAC-3′ |
| IL-8 | Sense (forward primer) 5′-GGCACAAACTTTCAGAGA CAG-3′ Antisense (reverse primer) 5′-ACACAGAGCTGCAGAAATCAGG-3′ |
| IL-10 | Sense (forward primer) 5′-TGAGCTTCTCTGTGAACGATTTA-3′ Antisense (reverse primer) 5′-GTCACCCTATGGAAACAGCTTA-3′ |
| TNF-α | Sense (forward primer) 5′-GAGGCCAAGCCCTGGTATG-3′ Antisense (reverse primer) 5′-CGGGCCGATTGATCTCAGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ateeq, R.; Elsafadi, M.; Muthurangan, M.; Al-Hadlaq, S. Polydatin Modulates Inflammatory Cytokine Expression in Lipoteichoic Acid-Stimulated Human Dental-Pulp Stem Cells. J. Funct. Biomater. 2025, 16, 331. https://doi.org/10.3390/jfb16090331
Al-Ateeq R, Elsafadi M, Muthurangan M, Al-Hadlaq S. Polydatin Modulates Inflammatory Cytokine Expression in Lipoteichoic Acid-Stimulated Human Dental-Pulp Stem Cells. Journal of Functional Biomaterials. 2025; 16(9):331. https://doi.org/10.3390/jfb16090331
Chicago/Turabian StyleAl-Ateeq, Rawan, Mona Elsafadi, Manikandan Muthurangan, and Solaiman Al-Hadlaq. 2025. "Polydatin Modulates Inflammatory Cytokine Expression in Lipoteichoic Acid-Stimulated Human Dental-Pulp Stem Cells" Journal of Functional Biomaterials 16, no. 9: 331. https://doi.org/10.3390/jfb16090331
APA StyleAl-Ateeq, R., Elsafadi, M., Muthurangan, M., & Al-Hadlaq, S. (2025). Polydatin Modulates Inflammatory Cytokine Expression in Lipoteichoic Acid-Stimulated Human Dental-Pulp Stem Cells. Journal of Functional Biomaterials, 16(9), 331. https://doi.org/10.3390/jfb16090331






