Biodegradable Metal-Based Stents: Advances, Challenges, and Prospects
Abstract
1. Introduction
2. Properties and Related Research Methods for Degradable Metal Stents
2.1. Mechanical Properties and Testing Parameters
- (1)
- Radial recoil performance, also known as intrinsic elastic recoil, refers to the change in the diameter of the vascular stent from balloon expansion to balloon deflation. It is usually represented by the radial recoil rate. Stents with a lower radial recoil rate can reduce vascular wall injury and the occurrence of restenosis.
- (2)
- Longitudinal shortage performance is usually represented by the longitudinal shortage rate, which refers to the change in length of the expansion of the vascular stent from the non-deployed state to the nominal diameter. In general, the clinically acceptable longitudinal shortage rate for vascular stents is 0~20%.
- (3)
- Radial support performance refers to the ability of vascular stents to resist vascular wall constriction. It is represented by the corresponding compression load when the compression reaches 50% of the unloaded stent’s outer diameter [16].
- (4)
- Flexibility refers to the longitudinal bending ability. Bending flexibility of a deployed stent may be one measure of its ability to flex with a vessel or to conform to the natural curvature of a vessel. The specimen is loaded onto a three-point bend fixture. The specimen is supported from below by two static supports separated by a known span and bent by a force applied at the top and midway between the lower supports, as shown in Figure 1. The bending flexibility of the stent is obtained from force-versus-deflection plots and/or midspan bending moment versus midspan curvature plots.
2.2. Methods and Parameters of the In Vitro Dynamic Immersion Test
2.2.1. Selection of Immersion Solution for the Corrosion Test
2.2.2. Ratio of Solution Volume to Sample Surface Area (V/S)
2.2.3. Hemodynamic Parameters
2.2.4. The Effect of pH, Dissolved Oxygen Concentration, and Temperature
The Effect of pH on In Vitro Experiments
The Effect of Dissolved Oxygen (DO) Concentration on In Vitro Experiments
The Effect of Temperature on In Vitro Experiments
| Normal Value | Normal Range | Approximate Short-Term Nonlethal Limit | Unit | |
|---|---|---|---|---|
| Acid–base | 7.4 | 7.3–7.5 | 6.9–8.0 | pH |
| Body temperature | 98.4 (37.0) | 98–98.8 (37.0) | 65–110 (18.3–43.3) | °F (°C) |
| Oxygen partial pressure | 40 | 35–45 | 10–1000 | mmHg |
3. Research Progress on Degradable Materials
3.1. Degradable Magnesium Alloys
3.1.1. Introduction to the Magnesium Alloys
3.1.2. Mechanism of Mg Corrosion In Vitro
3.1.3. Mechanism of Magnesium Metabolism in the Human Body
3.2. Degradable Iron Alloys
3.2.1. Introduction to the Iron Alloys
3.2.2. Mechanism of Fe Corrosion in Vitro
3.2.3. Mechanism of Iron Metabolism in the Human Body
3.3. Degradable Zinc Alloys
3.3.1. Introduction to the Zinc Alloys
3.3.2. Mechanism of Zn Corrosion in Vitro
3.3.3. Mechanism of Zinc Metabolism in the Human Body
4. Biosafety of Metal-Based Stents
4.1. Hemocompatibility
4.2. Cytocompatibility
4.3. In Vivo Studies
4.3.1. Progress in Clinical Trials of Mg-Based Metal Stents
4.3.2. In Vivo Experimental Progress of Fe-Based Metal Stents
4.3.3. In Vivo Experimental Progress on Zn-Based Metal Stents
5. Advances in Stent Structure Research
6. Summary and Outlook
6.1. Experimental Conditions for Dynamic Corrosion In Vitro
6.2. Optimization of Biodegradable Alloy Materials
- Magnesium alloy system: Relative to ideal stents, magnesium alloy stents exhibit weaker mechanical properties and a rapid degradation rate. Therefore, the development of biodegradable magnesium alloy stents should consider optimizing the stent size and controlling the degradation rate while maintaining their radial support. Currently, surface modification represents the primary strategy for enhancing the corrosion resistance of magnesium alloy stents, with coating application being the most prevalent method. However, given that the stent expands during implantation, the coating may fracture or detach due to disparities in mechanical properties between the coating and the base material, substantially increasing the risk during and post-surgery. To overcome this limitation, it is crucial to explore novel material systems, composite structures, and coating methods that can prevent the coated layer from separating from the metal base during stent implantation.
- Ferroalloy system: Iron-based stents primarily contend with a slow degradation rate, which can lead to late thrombosis and chronic inflammation, and may also interfere with MRI processes.
- Zinc alloy system: Zinc alloys degrade at a moderate rate, yet their overall strength and ductility remain inferior to the aforementioned materials. The research and development of biodegradable zinc alloy stents has received attention in recent years.
6.3. Biocompatibility of Degradable Alloy Materials
6.4. Stent Structure
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Material System | Stent Material | YS (Mpa) | UTS (Mpa) | EL (%) | Hardness | Degradation (mm·Year−1) | In Vitro Experiment Solution | Other Conditions | |
|---|---|---|---|---|---|---|---|---|---|
| Immersion Test | Potentiodynamic | ||||||||
| Mg Alloys | Pure Mg | 83 | 143 | 9.8 | - | - | - | - | - |
| AZ31 | 172 | - | 16 | - | 1.50–1.92 | - | Hanks’ | 5% CO2, 95% relative humidity, T = 37 °C | |
| AZ31B | 198 | 230 | 12 | - | - | - | - | - | |
| AZ91 | 98 | 172 | 4.3 | - | - | - | - | - | |
| JDBM | 123–220 | 267 | 26–48.8 | - | - | - | - | - | |
| WE43 | 113 | 340–440 | 10.0–21 | 102–114 | - | - | - | - | |
| ZE21B | 196 | 298 | 20 | - | - | 2.16 | - | - | |
| ZM21 | 340 | 353 | 11.5 | - | - | - | - | - | |
| Mg-4Zn-1Y | 240 | 330 | 20.4 | - | - | - | - | - | |
| Material System | Stent Material | YS (Mpa) | UTS (Mpa) | EL (%) | Hardness | Degradation (mm·Year−1) | In Vitro Experiment Solution | Other Conditions | |
|---|---|---|---|---|---|---|---|---|---|
| Immersion Test | Potentiodynamic | ||||||||
| Fe Alloys | Pure Fe | 298.72–387.08 | 200–250 | 20–30 | 149.68–160.16 | 0.44–0.70 (static, day 30) 0.63–1.59 (dynamic, day 30) | 0.043–0.071 | Hanks’ | 37 ± 0.5 °C, pH = 7.4, DO (2.8–3.2 mg−1), shear stress (0.68 Pa) |
| Nitrided Fe | 585.32–643.38 | 300–350 | 10.0–15 | 278.39–295.73 | - | 0.223–0.227 | - | - | |
| Fe-10Mn | 650–800 | 1300–1400 | 14 | 367–434 | 3.97 (day 1), 2.05 (day 2) | - | SBF | T = 37 ± 1 °C | |
| Fe-10Mn-1Pd | 850–950 | 1450–1550 | 2.0–11 | 370–440 | 6.68 (day 1), 9.05 (day 2) | - | |||
| Fe65Mn35 | - | - | - | - | 6.695 (day 30) | 0.51 | - | pH7.4 ± 0.2 | |
| Fe65Mn35Ag1 | - | - | - | - | 19.01 (day 30) | 0.96 | - | ||
| Polished Fe–Mn | - | - | - | - | - | 1.21 | - | pH7.8 | |
| Laser-textured Fe–Mn | - | - | - | - | - | 8.63 | - | - | |
| Fe–30Mn | - | - | - | - | 1.08 (day 30) | 0.127 | PBS | T = 37 °C, pH = 7.4 | |
| Fe–30Mn prepared by continuous laser | - | - | - | - | 1.01 (day 30) | 0.114 | |||
| Fe–30Mn prepared by nanosecond laser | - | - | - | - | 1.27 (day 30) | 0.136 | |||
| Fe–30Mn prepared by femtosecond laser | - | - | - | - | 1.51 (day 30) | 0.179 | |||
| Fe–30Mn–Ag | - | - | - | - | - | 0.012 | Hanks’ | - | |
| Fe-2.1Zn | 235–267 | 402–440 | 18.3–23.3 | 134–136 | 4.56 | 0.238 | SBF | T = 37 °C, pH = 7.4 | |
| Fe-4.6Zn | 208–284 | 437–489 | 12.6–20.8 | 146–150 | 5.77 | 0.323 | |||
| Fe-7.2Zn | 252–278 | 497–531 | 12.8–14.6 | 168–170 | 5.31 | 0.231 | |||
| Fe-2Ag | 210 | - | - | - | 0.72 (Static, day 30) | 0.12 | Hanks’ | T = 37 ± 0.5 °C, pH = 7.4, DO (2.8–3.2 mg−1), dissolved oxygen, shear stress (0.68 Pa) | |
| 1.74 (dynamic, day 30) | - | ||||||||
| Fe-5Ag | 380 | - | - | - | 0.90 (Static, day 30) | 0.14 | |||
| 2.06 (dynamic, day 30) | - | ||||||||
| Fe-10Ag | 200 | - | - | - | 0.78 (Static, day 30) | 0.175 | |||
| 1.87 (dynamic, day 30) | - | ||||||||
| Fe-2Au | 350 | - | - | - | 0.73 (Static, day 30) | 0.174 | |||
| 1.73 (dynamic, day 30) | - | ||||||||
| Fe-5Au | 250 | - | - | - | 0.98 (Static, day 30) | 0.131 | |||
| 2.31 (dynamic, day 30) | - | ||||||||
| Fe-10Au | 350 | - | - | - | 0.87 (Static, day 30) | 0.098 | |||
| 1.72 (dynamic, day 30) | - | ||||||||
| Fe-Pd | 445 | - | - | - | 0.74 (Static, day 30) | - | Hanks’ | T = 36.0–37.1 °C (immersion), T = 37 ± 0.5 °C (electrochemical), pH = 7.35 –7.45, DO (2.8–3.2 mg−1), shear stress (0.68 Pa) | |
| Fe-Pt | 503 | - | - | - | 1.63 (dynamic, day 30) | - | - | - | |
| M-Fe | - | - | - | - | 2.57 | 0.137 | SBF | T = 37 °C, pH = 7.4 | |
| E-Fe | 270–360 | 292–423 | - | - | 3.48 | 0.139 | |||
| Fe/0.3CNTs | - | - | - | - | 2.05 (day 28) | 0.090e | - | pH = 7.4–8, aerated | |
| Fe/0.6CNTs | - | - | - | - | 2.27 (day 28) | 0.160e | - | ||
| Fe/0.9CNTs | - | - | - | - | 3.20 (day 28) | 0.225e | - | ||
| Fe/1.2CNTs | - | - | - | - | 3.30 (day 28) | 0.241e | - | ||
| Material System | Stent Material | YS (Mpa) | UTS (Mpa) | EL (%) | Hardness | Degradation (mm·Year−1) | In Vitro Experiment Solution | Other Conditions | |
|---|---|---|---|---|---|---|---|---|---|
| Immersion Test | Potentiodynamic | ||||||||
| Zn Alloys | Pure Zn | 27.5–130 | 29.7–151 | 0.62–8.8 | - | 0.013–0.078 (static) | 0.03–0.19 | DPBS | pH = 7.4, agitation 80 rpm |
| Ringer’ | |||||||||
| Human plasma | |||||||||
| Whole blood | |||||||||
| Ultra-pure Zn plate | - | - | - | - | 0.013 (Static) | - | Hanks’ (30 days) | pH = 7.4, R = L125 | |
| Ultra-pure Zn: laser cut tube | - | - | - | - | 0.037 (Static) | - | |||
| Zn-0.08Mg | 221 | 339 | 40 | 103 | - | - | - | - | |
| Zn-0.15Mg | 114 | 250 | 22 | 52 | - | - | - | - | |
| Zn-0.5Mg | 159–192 | 102.02–297 | 2.97–28 | 60.32–73 | 0.071 (0.0134 mg·cm·day) (Static) | - | DMEM, MEM (1 days) | T = 37 °C agitation 125 rpm | |
| Zn-0.8Mg | 203 | 301 | 15 | 80–90 | - | - | - | - | |
| Zn-1Mg | 316–383 | 435–482 | 23–35 | - | 0.073–0.086 (Static) | 0.149–0.169 | aerated SBP (14 days) | T = 37 °C | |
| Zn-1.2Mg | 219.61 | 362.64 | 21.31 | 96.01 | - | - | |||
| Zn-1.5Mg | 112.29–250 | 250.55–300 | 1.25–17.5 | 70–150 | 0.069 (Static) | - | |||
| Zn-3Mg | 36–291 | 46–399 | 1–6.3 | 117–200 | 0.073 (Static) | - | |||
| Zn-4.2Mg | - | - | - | - | 0.07–0.09 (Static) | 0.12–0.19 | Hanks’ (90 days) | T = 37 °C | |
| Zn-5Mg | - | - | - | - | 0.101 (0.0 IP ms·cm·day) (Static) | - | DMEM, MEM (3 days) | T = 37 °C, pH = 7.4, 5% CO2: atmosphere | |
| Zn-5.0Mg-1.0Fe | - | - | - | - | 0.062 (Static) | - | SBF (20 days) | T = 37 °C | |
| Zn-1Mg-0.1Ca | 209.04 | 331.51 | 35.43 | 110–125 | - | - | - | - | |
| Zn-1Mg-1Ca | - | - | - | - | 0.090 (Static) | 0.169 | Hanks’ (30 days) | T = 37 °C | |
| Zn-1.0Mg-1.0Sr | - | - | - | - | 0.095 (Static) | 0.175 | Hanks’ (30 days) | T = 37 °C | |
| Zn-1.0Mg-0.1Mn | 114.10–195.02 | 131.94–299.04 | 1.11–26.07 | 97.66–107.82 | 0.051–0.070 (Static) | 0.140–0.260 | Hanks’ (48 days) | T = 37 °C, pH = 7.4, R = 1/25 | |
| Zn-1.5Mg-0.1Mn | 114.71 | 121.72 | 0.77 | 148.69 | 0.061 (Static) | 0.25 | |||
| Zn-1.5Mg-0.1Ca | 173.81 | 241.72 | 1.72 | 150 | 0.115 (Static) | 0.28 | Hanks’ (20 days) | T = 37 °C, pH = 7.4, R = 1/25 | |
| Zn-1.5Mg-0.1Sr | 129.55 | 209.22 | 2.03 | 150 | 0.105 (Static) | 0.106 | |||
| Zn-5Mg-1Fe | 176–187 | 223–231 | 22–26 | - | 0.040 (Static) | - | SBF (20 days) | T = 37 °C | |
| Zn-0.5Al | 119 | 203 | 33 | 59 | 0.080 (Static) | - | SBF (30 days) | - | |
| Zn-0.5Al-0.1Mg | - | - | - | - | 0.110 (Static) | - | SBF (30 days) | - | |
| Zn-0.5Al-0.3Mg | - | - | - | - | 0.131 (Static) | - | - | - | |
| Zn-0.5Al-0.5Mg | - | 92 | 1.73 | 94 | 0.150 (Static) | - | SBF (30 days) | T = 37 °C, pH = 7.6 | |
| Zn-0.5Al-0.5Mg-0.1Bi | - | 102 | 2.38 | 102 | 0.170 (Static) | - | |||
| Zn-0.5Al-0.5Mg-0.3Bi | - | 108 | 2.74 | 109 | 0.210 (Static) | - | |||
| Zn-0.5Al-0.5Mg-0.5Bi | - | 98 | 1.97 | 99 | 0.280 (Static) | - | |||
| Zn-1Al | 134 | 223 | 24 | 73 | - | - | - | - | |
| Zn-2Al-0.4Li | 352.2 | 405.8 | 31.6 | - | - | - | - | - | |
| Zn-4Al-0.2Li | 362.7 | 382.2 | 33.3 | - | - | - | - | - | |
| Zn-4Al-0.4Li | 399.3 | 414.6 | 25.6 | - | - | - | - | - | |
| Zn-4Al-0.6Li | 429.6 | 451.4 | 46.3 | - | - | - | - | - | |
| Zn-6Al-0.4Li | 335.6 | 432.6 | 42.7 | - | - | - | - | - | |
| Zn-4Al-lCu | - | - | - | - | 0.075 (Static) | - | aerated SBP (14 days) | T = 37 °C, pH = 7 | |
| Zn-2Al-2Cu-0.6Li | 260 | 402 | 44 | - | - | - | - | - | |
| Zn-2Al-2Cu-0.8Li | 371 | 445 | 47 | - | - | - | - | - | |
| Zn-2Al-4Cu-0.6Li | 404 | 535 | 32 | - | - | - | - | - | |
| Zn-2Al-4Cu-0.8Li | 407 | 536 | 17 | - | - | - | - | - | |
| Zn-1Cu | 148.7 | 19.2–186.3 | 1.7–21 | 61.72 | 0.033 (Static) | – | SBF (20 days) | T = 37 °C, R = 1/25 | |
| Zn-2Cu | 199.7 | 68.5–240 | 2.77–46.8 | 68.78 | 0.026 (Static) | – | |||
| Zn-3Cu | 128–213.7 | 105.5–288 | 4.06–47.2 | 67.04–76.64 | 0.012–0.030 (Static) | 0.018 | |||
| Zn-4Cu | 227–250 | 270–270.7 | 50.6–51 | - | 0.025 (Static) | 0.006 | |||
| Zn-3Cu-0.1Mg | 325–350 | 350–375 | 5.0–10.0 | - | 0.022 (Static) | 0.024 | Hanks’ (14 days) | T = 37 °C, R = 1/25 | |
| Zn-3Cu-0.5Mg | 400–425 | 400–425 | 0–5 | - | 0.030 (Static) | 0.185 | |||
| Zn-3Cu-1Mg | 426.7 | 440.5 | 0.9 | - | 0.043 (Static) | 0.104 | |||
| Zn-3Cu-0.5Fe | 269 | 284–302 | 24.7–32.7 | 76.06 | 0.064 (Static) | 0.131 | SBF | T = 37 °C | |
| Zn-3Cu-1Fe | - | 272 | 19.6 | 82.24 | 0.069 (Static) | 0.133 | |||
| Zn-2.5Ag | 147 | 203 | 32–36 | - | 0.079 (Static) | 0.134 | Modified Hanks’ renewed every two days (14 days) | T = 37 °C, pH = 7.4 5%, CO2 atmosphere, 90% humidity | |
| Zn-7Ag | 236 | 287 | 32–36 | - | 0.084 (Static) | 0.147 | |||
| Zn-2Cu-0.3Li | 226.55 | 333.49 | 26 | 124.59 | - | - | - | - | |
| Zn-3Cu-0.5Fe | 269 | 302 | 24.7 | - | - | - | - | - | |
| Zn-3.5Cu-0.3Li | 231.63 | 382.8 | 18.33 | 128.79 | - | - | - | - | |
| Zn-4Cu-0.02Li | 256 | 342 | 39.8 | - | - | - | - | - | |
| Zn-5Cu-0.3Li | 272.87 | 427.92 | 19.3 | 133.85 | - | - | - | - | |
| Zn-0.3Li | 292 | 367.2 | 19.3 | - | - | - | - | - | |
| Zn-0.2Li | 87.1 | 88.7 | 0.5 | 100 | - | 0.06 | SBF | T = 37 °C, pH = 7.4, 5% CO2 atmosphere, R = 1/20 | |
| Zn-0.4Li | 352.2–363.7 | 398.5–406.5 | 27.4–27.8 | - | - | 0.05 | |||
| Zn-0.8Li | 261.5 | 401.4 | 80.8 | - | - | - | - | - | |
| Zn-0.8Li-0.4Mn | 449.7 | 505.1 | 40.5 | 137.6 | - | - | - | - | |
| Zn-3Ag-0.5Mg | 385 | 432 | 34 | - | - | - | - | - | |
| Zn-1.0Ca | - | - | - | - | 0.0591 (Static) | 0.16 | Hanks’ (14 days) | T = 37 °C | |
| Znl-1.0Sr | - | - | - | - | 0.098 (Static) | 0.176 | |||
| Zn-1.0Ca-1.0Sr | - | - | - | - | 0.095 (Static) | 0.175 | Hanks’ (30 days) | T = 37 °C | |
References
- Cardiovascular Diseases. World Health Organization. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 10 August 2025).
- Eshghi, N.; Hojjati, M.H.; Imani, M.; Goudarzi, A.M. Finite Element Analysis of Mechanical Behaviors of Coronary Stent. Procedia Eng. 2011, 10, 3056–3061. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Patel, V.G.; Banerjee, S. Medical Management After Coronary Stent Implantation: A Review. JAMA 2013, 310, 189–198. [Google Scholar] [CrossRef]
- When Do You Need a Heart Stent? Harvard Health Publications, Harvard Men’s Health Watch; Harvard Health Publications: Boston, MA, USA, 2020; Available online: https://www.health.harvard.edu/heart-health/when-do-you-need-a-heart-stent (accessed on 10 August 2025).
- Werkhoven, R.J.; Sillekens, W.H.; van Lieshout, J.B.J.M. Processing Aspects of Magnesium Alloy Stent Tube. In Magnesium Technology 2011; Springer International Publishing: Cham, Switzerland, 2011; pp. 419–424. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, H. Research Progress in Biodegradable Metals for Stent Application. Acta Metall. Sin. 2017, 53, 1227–1237. [Google Scholar] [CrossRef]
- Drelich, J.W.; Goldman, J. Bioresorbable vascular metallic scaffolds: Current status and research trends. Curr. Opin. Biomed. Eng. 2022, 24, 100411. [Google Scholar] [CrossRef]
- ISO 25539-2:2020; Cardiovascular Implants—Endovascular Devices Part2: Vascular Stents. ISO: Geneva, Switzerland, 2020.
- ISO/TS 17137; Cardiovascular Implants and Extracorporeal Systems-Cardiovascular Absorbable Implants. ISO: Geneva, Switzerland, 2021.
- ISO 10993-1:2018; Biological Evaluation of Medical Devices-Part 1: Evaluation and Testing Within a Risk Management Process. ISO: Geneva, Switzerland, 2018.
- ISO 14630:2012; Non-Active Surgical Implants—General Requirements. ISO: Geneva, Switzerland, 2012.
- ASTM F2606-08 (2014); Standard Guide for Three-Point Bending of Balloon Expandable Vascular Stent and Stent Systems. ASTM International Press: West Conshohocken, PA, USA, 2014; pp. 1–3.
- ASTM F3067-14; Guide for Radial Loading of Balloon Expandable and Self-Expanding Vascular Stents. ASTM International Press: West Conshohocken, PA, USA, 2014; pp. 1–19.
- ASTM F2477-19; Standard Test Methods For In Vitro Pulsatile Durability Testing of Vascular Stents. ASTM International Press: West Conshohocken, PA, USA, 2019; pp. 1–10.
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef]
- Pauck, R.G.; Reddy, B.D. Computational Analysis of the Radial Mechanical Performance of PLLA Coronary Artery Stents. Med. Eng. Phys. 2015, 37, 7–12. [Google Scholar] [CrossRef] [PubMed]
- De Beule, M.; Van Impe, R.; Verdonck, P. Finite Element Analysis and Stent Design: Reduction of Dogboning. Technol. Health Care 2006, 14, 506–519. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, Y.F. A Review on In Vitro Corrosion Performance Test of Biodegradable Metallic Materials. Trans. Nonferrous Met. Soc. China 2013, 23, 2283–2293. [Google Scholar] [CrossRef]
- Wang, J.; Smith, C.; Sankar, J.; Yun, Y.; Huang, N. Absorbable Magnesium-Based Stent: Physiological Factors to Consider for In Vitro Degradation Assessments. Regen. Biomater. 2015, 2, 59–69. [Google Scholar] [CrossRef]
- Muster, T.H.; Cole, I.S. The Protective Nature of Passivation Films on Zinc: Surface Charge. Corros. Sci. 2004, 46, 2319–2335. [Google Scholar] [CrossRef]
- Danaie, M.; Asmussen, R.M.; Jakupi, P.; Shoesmith, D.W.; Botton, G.A. The Role of Aluminum Distribution on the Local Corrosion Resistance of the Microstructure in a Sand-Cast AM50 Alloy. Corros. Sci. 2013, 77, 151–163. [Google Scholar] [CrossRef]
- Chu, P.-W.; Le Mire, E.; Marquis, E.A. Microstructure of Localized Corrosion Front on Mg Alloys and the Relationship with Hydrogen Evolution. Corros. Sci. 2017, 128, 253–264. [Google Scholar] [CrossRef]
- Gray-Munro, J.E.; Strong, M. A Study on the Interfacial Chemistry of Magnesium Hydroxide Surfaces in Aqueous Phosphate Solutions: Influence of Ca2+;, Cl− and Protein. J. Colloid Interface Sci. 2013, 393, 421–428. [Google Scholar] [CrossRef]
- Heakal, F.E.; Shehata, O.S.; Tantawy, N.S. Degradation Behaviour of AZ80E Magnesium Alloy Exposed to Phosphate 77Buffer Saline Medium. Corros. Sci. 2014, 86, 285–294. [Google Scholar] [CrossRef]
- Cao, X.; Jia, Q.G.; Xu, C.; Zhang, Z.; Ren, C.; Yang, W.; Zhang, J. Research on Dynamic Corrosion Behavior and the Microstructure of Biomedical Mg–Y–Zn–Zr–Sr in Simulated Body Fluid Solution after Processing by Solution Treatment. Adv. Eng. Mater. 2019, 22, 1901146. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. Guyton and Hall Textbook of Medical Physiology, 12th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2011. [Google Scholar]
- Cheng, J.; Huang, T.; Zheng, Y.F. Microstructure, Mechanical Property, Biodegradation Behavior, and Biocompatibility of Biodegradable Fe–Fe2O3 Composites. J. Biomed. Mater. Res. Part B 2014, 102, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Li, S.; Wang, G.; Yang, Y.; Peng, S.; Gao, C. Strong Corrosion Induced by Carbon Nanotubes to Accelerate Fe Biodegradation. Mater. Sci. Eng. C 2019, 104, 109935. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.B.; Singh Raman, R.K. In Vitro Degradation and Mechanical Integrity of Calcium-Containing Magnesium Alloys in Modified-Simulated Body Fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef]
- Alvarez-Lopez, M.; Pereda, M.D.; Del Valle, J.A.; Fernandez-Lorenzo, M.; Garcia-Alonso, M.C.; Ruano, O.A.; Escudero, M.L. Corrosion Behaviour of AZ31 Magnesium Alloy with Different Grain Sizes in Simulated Biological Fluids. Acta Biomater. 2010, 6, 1763–1771. [Google Scholar] [CrossRef]
- Frattolin, J.; Cattarinuzzi, L.; Roy, R.; Rajagopalan, S.; Walsh, M.; Yue, S.; Bertrand, O.F.; Mongrain, R. Development of a Micro-Scale Method to Assess the Effect of Corrosion on the Mechanical Properties of a Biodegradable Fe-316L Stent Material. J. Mech. Behav. Biomed. Mater. 2021, 113, 104173. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. A New In Vitro–In Vivo Correlation for Bioabsorbable Magnesium Stents from Mechanical Behavior. Mater. Sci. Eng. C 2013, 33, 5064–5070. [Google Scholar] [CrossRef]
- Zhang, Y.; Roux, C.; Rouchaud, A.; Meddahi-Pellé, A.; Guguen, V.; Mangeney, C.; Sun, F.; Pavon-Djavid, G.; Luo, Y. Recent Advances in Fe-Based Bioresorbable Stents: Materials Design and Biosafety. Bioact. Mater. 2023, 31, 333–354. [Google Scholar] [CrossRef]
- Yin, X.; Zhou, C.; Shi, P.; Shi, Z.; Lu, H.; Hao, Y.; Liu, H.; Wang, X.; Zhang, J.; Wang, N.; et al. Hemocompatibility of biodegradable Zn-0.8 wt% (Cu, Mn, Li) alloys. Mater. Sci. Eng. C 2019, 104, 109896. [Google Scholar] [CrossRef]
- Shearier, E.; Bowen, P.K.; Drelich, J.; Goldman, J. In Vitro Cytotoxicity, Adhesion, and Proliferation of Human Vascular Cells Exposed to Zinc. ACS Biomater. Sci. Eng. 2016, 2, 634–642. [Google Scholar] [CrossRef]
- ASTM G31-72(1999); Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 1999.
- Fung, Y.-C. Biomechanics: Circulation; Springer: New York, NY, USA, 1997. [Google Scholar]
- Sen, S.; Petraco, R.; Mayet, J.; Davies, J. Wave Intensity Analysis in the Human Coronary Circulation in Health and Disease. Curr. Cardiol. Rev. 2014, 10, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.T., Jr.; Brown, B.G.; Bolson, E.L.; Dodge, H.T. Lumen Diameter of Normal Human Coronary Arteries: Influence of Age, Sex, Anatomic Variation, and Left Ventricular Hypertrophy or Dilation. Circulation 1992, 86, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Maurel, B.; Sarraf, C.; Bakir, F.; Dufranc, A.; Alric, P.; Branchereau, A.; Ducasse, E. A New Hemodynamic Ex Vivo Model for Medical Devices Assessment. Ann. Vasc. Surg. 2015, 29, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable Biomaterials Based on Magnesium Corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdul-Kadir, M.R.; Idris, M.H.; Farahany, S. Relationship between the Corrosion Behavior and the Thermal Characteristics and Microstructure of Mg–0.5Ca–xZn Alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar] [CrossRef]
- Thomas, S.; Birbilis, N.; Venkatraman, M.S.; Cole, I.S. Corrosion of Zinc as a Function of pH. Corrosion 2012, 68, 015009-1–015009-9. [Google Scholar] [CrossRef]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Plenum Press: New York, NY, USA, 1996; p. 142. [Google Scholar]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed Pure Iron as a New Biomaterial for Degradable Stents: In Vitro Degradation and Preliminary Cell Viability Studies. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B.; Tissot, J.-D. Physiology of Iron Metabolism. Transfus. Med. Hemother. 2014, 41, 213–221. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the Corrosion of Biodegradable Magnesium Implants: A Critical Review of Current Methodologies and Their Limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Willumeit, R.; Feyerabend, F.; Huber, N. Magnesium Degradation as Determined by Artificial Neural Networks. Acta Biomater. 2013, 9, 8722–8729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, N.; Shu, H.; Wu, Y.; Xu, L. Corrosion Resistance and Blood Compatibility of Lanthanum Ion Implanted Pure Iron by MEVVA. Appl. Surf. Sci. 2009, 256, 99–104. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F. Effects of Alloying Elements (Mn, Co, Al, W, Sn, B, C and S) on Biodegradability and In Vitro Biocompatibility of Pure Iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W. Effect of Grain Size and Twins on Corrosion Behaviour of AZ31B Magnesium Alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- Palmaz, J.C.; Richter, G.M.; Noeldge, G.; Schatz, R.A.; Robison, P.D.; Gardiner, G.A.; Becker, G.J.; McLean, G.K.; Denny, D.F.; Lammer, J. Intraluminal Stents in Atherosclerotic Iliac Artery Stenosis: Preliminary Report of a Multicenter Study. Radiology 1988, 168, 727–731. [Google Scholar] [CrossRef]
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Cobalt–Chromium Dental Alloys: Metal Exposures, Toxicological Risks, CMR Classification, and EU Regulatory Framework. Crystals 2020, 10, 1151. [Google Scholar] [CrossRef]
- McMahon, S.; Bertollo, N.; O’ Cearbhaill, E.D.; Salber, J.; Pierucci, L.; Duffy, P.; Dürrig, T.; Bi, V.; Wang, W. Bio-Resorbable Polymer Stents: A Review of Material Progress and Prospects. Prog. Polym. Sci. 2018, 87, 146–176. [Google Scholar] [CrossRef]
- Liu, D.; Yang, K.; Chen, S. Development and Future Trends of Protective Strategies for Magnesium Alloy Vascular Stents. Materials 2024, 17, 68. [Google Scholar] [CrossRef]
- Slottow, T.L.P.; Pakala, R.; Okabe, T.; Hellinga, D.; Lovec, R.J.; Tio, F.O.; Bui, A.B.; Waksman, R. Optical Coherence Tomography and Intravascular Ultrasound Imaging of Bioabsorbable Magnesium Stent Degradation in Porcine Coronary Arteries. Cardiovasc. Revasc. Med. 2008, 9, 248–254. [Google Scholar] [CrossRef]
- Kannan, M.B. Influence of microstructure on the in-vitro degradation behaviour of magnesium alloys. Mater. Lett. 2010, 64, 739–742. [Google Scholar] [CrossRef]
- Yuen, C.K.; Ip, W.Y. Theoretical Risk Assessment of Magnesium Alloys as Degradable Biomedical Implants. Acta Biomater. 2010, 6, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Rapetto, C.; Leoncini, M. Magmaris: A New Generation Metallic Sirolimus-Eluting Fully Bioresorbable Scaffold: Present Status and Future Perspectives. J. Thorac. Dis. 2017, 9, S903–S913. [Google Scholar] [CrossRef] [PubMed]
- Haude, M.; Wlodarczak, A.; van der Schaaf, R.J.; Torzewski, J.; Ferdinande, B.; Escaned, J.; Iglesias, J.F.; Bennett, J.; Toth, G.; Joner, M.; et al. Safety and Performance of the Third-Generation Drug-Eluting Resorbable Coronary Magnesium Scaffold System in the Treatment of Subjects with De Novo Coronary Artery Lesions: 6-Month Results of the Prospective, Multicenter BIOMAG-I First-in-Human Study. Eclinicalmedicine 2023, 59, 101944. [Google Scholar] [CrossRef]
- Serruys, P.W.; Chevalier, B.; Sotomi, Y.; Cequier, A.; Carrié, D.; Piek, J.J.; Van Boven, A.J.; Dominici, M.; Dudek, D.; McClean, D.; et al. Comparison of an Everolimus-Eluting Bioresorbable Scaffold with an Everolimus-Eluting Metallic Stent for the Treatment of Coronary Artery Stenosis (ABSORB II): A 3 Year, Randomised, Controlled, Single-Blind, Multicentre Clinical Trial. Lancet 2016, 388, 2479–2491. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.-J.; Kaiser, C.; et al. Safety and Performance of the Second-Generation Drug-Eluting Absorbable Metal Scaffold in Patients with De-Novo Coronary Artery Lesions (BIOSOLVE-II): 6-Month Results of a Prospective, Multicentre, Non-Randomised, First-in-Man Trial. Lancet 2015, 387, 31–39. [Google Scholar] [CrossRef]
- Serruys, P.W.; Chevalier, B.; Dudek, D.; Cequier, A.; Carrié, D.; Iniguez, A.; Dominici, M.; van der Schaaf, R.J.; Haude, M.; Wasungu, L.; et al. A Bioresorbable Everolimus-Eluting Scaffold versus a Metallic Everolimus-Eluting Stent for Ischaemic Heart Disease Caused by De-Novo Native Coronary Artery Lesions (ABSORB II): An Interim 1-Year Analysis of Clinical and Procedural Secondary Outcomes from a Randomised Controlled Trial. Lancet 2014, 385, 43–54. [Google Scholar] [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Böse, D.; Vermeersch, P.; Wijnbergen, I.; Weissman, N.; Prati, F.; et al. Safety and Performance of the Drug-Eluting Absorbable Metal Scaffold (DREAMS) in Patients with De-Novo Coronary Lesions: 12-Month Results of the Prospective, Multicentre, First-in-Man BIOSOLVE-I Trial. Lancet 2013, 381, 836–844. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Guan, S.; Zhu, S.; Ren, C.; Hou, S. Microstructure and Corrosion Properties of as Sub-Rapid Solidification Mg–Zn–Y–Nd Alloy in Dynamic Simulated Body Fluid for Vascular Stent Application. J. Mater. Sci. Mater. Med. 2010, 21, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Yuan, G.; Zhang, X.; Mao, L.; Niu, J.; Ding, W. Comparison of Biodegradable Behaviors of AZ31 and Mg–Nd–Zn–Zr Alloys in Hank’s Physiological Solution. Mater. Sci. Eng. B 2012, 177, 395–401. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, S.; Wang, L.; Liu, Q.; Yue, G.; Wang, J.; Guan, S. The Microstructure and Properties of Cyclic Extrusion Compression Treated Mg–Zn–Y–Nd Alloy for Vascular Stent Application. J. Mech. Behav. Biomed. Mater. 2012, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Yang, Z.; Zhu, S.; Wang, L.; Guan, S. Processing and Properties of Magnesium Alloy Micro-Tubes for Biodegradable Vascular Stents. Mater. Sci. Eng. C 2018, 90, 504–513. [Google Scholar] [CrossRef]
- Du, P.; Mei, D.; Furushima, T.; Zhu, S.; Wang, L.; Zhou, Y.; Guan, S. In Vitro Corrosion Properties of HTHEed Mg–Zn–Y–Nd Alloy Microtubes for Stent Applications: Influence of Second Phase Particles and Crystal Orientation. J. Magnes. Alloy. 2022, 10, 1286–1295. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Bian, D.; Gao, S.; Leeflang, S.; Guo, H.; Zheng, Y.; Zhou, J. Study on the Mg–Li–Zn Ternary Alloy System with Improved Mechanical Properties, Good Degradation Performance and Different Responses to Cells. Acta Biomater. 2017, 62, 418–433. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, D.; Lee, B.; Roy, A.; Yao, R.; Chen, S.; Dong, Z.; Heineman, W.R.; Kumta, P.N. Effect of Lithium and Aluminum on the Mechanical Properties, In Vivo and In Vitro Degradation, and Toxicity of Multiphase Ultrahigh Ductility Mg–Li–Al–Zn Quaternary Alloys for Vascular Stent Application. ACS Biomater. Sci. Eng. 2020, 6, 1950–1964. [Google Scholar] [CrossRef]
- Ormiston, J.A.; Serruys, P.W.; Evelyn, R.; Dariusz, D.; Leif, T.; Webster, M.W.I.; Yoshinobu, O.; Garcia-Garcia, H.M.; Robert, M.G.; Susan, V. A Bioabsorbable Everolimus-Eluting Coronary Stent System for Patients with Single De-Novo Coronary Artery Lesions (ABSORB): A Prospective Open-Label Trial. Lancet 2008, 371, 899–907. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Duque, V.; Quintero, D.; Harmsen, M.C.; Echeverria, F. Novel Coatings Obtained by Plasma Electrolytic Oxidation to Improve the Corrosion Resistance of Magnesium-Based Biodegradable Implants. Surf. Coat. Technol. 2018, 354, 28–37. [Google Scholar] [CrossRef]
- Rahim, M.I.; Tavares, A.; Evertz, F.; Kieke, M.; Seitz, J.-M.; Eifler, R.; Weizbauer, A.; Willbold, E.; Maier, H.J.; Glasmacher, B.; et al. Phosphate Conversion Coating Reduces the Degradation Rate and Suppresses Side Effects of Metallic Magnesium Implants in an Animal Model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1622–1635. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, G.; Zhang, Y.-H.; Zhao, Q. Growth and Characterization of Mg(OH)2 Film on Magnesium Alloy AZ31. Appl. Surf. Sci. 2011, 257, 6129–6137. [Google Scholar] [CrossRef]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg–Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Yuan, G.; Niu, J.; Zong, Y.; Ding, W. In Vitro Degradation Behavior and Biocompatibility of Mg–Nd–Zn–Zr Alloy by Hydrofluoric Acid Treatment. Mater. Sci. Eng. C 2013, 33, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, X.; Niu, J.; Shi, Y.; Zhu, Z.; Dai, D.; Chen, C.; Pei, J.; Yuan, G.; Zhang, R. Biosafety and Efficacy Evaluation of a Biodegradable Magnesium-Based Drug-Eluting Stent in Porcine Coronary Artery. Sci. Rep. 2021, 11, 7330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Cao, P.; Zhang, X.N.; Zhang, S.X.; He, Y.H. In Vitro Degradation and Cell Attachment of a PLGA Coated Biodegradable Mg–6Zn Based Alloy. J. Mater. Sci. 2010, 45, 6038–6045. [Google Scholar] [CrossRef]
- Li, L.-Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Chen, X.-B.; Zheng, Y.; Kannan, M.B. Advances in Functionalized Polymer Coatings on Biodegradable Magnesium Alloys—A Review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Maitz, M.F.; Collins, B.; Xiong, K.; Guo, L.; Yun, Y.; Wan, G.; Huang, N. A Surface-Eroding Poly(1,3-Trimethylene Carbonate) Coating for Fully Biodegradable Magnesium-Based Stent Applications: Toward Better Biofunction, Biodegradation and Biocompatibility. Acta Biomater. 2013, 9, 8678–8689. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Chu, C.-C.; Xi, T. A Novel Biodegradable and Biologically Functional Arginine-Based Poly(Ester Urea Urethane) Coating for Mg–Zn–Y–Nd Alloy: Enhancement in Corrosion Resistance and Biocompatibility. J. Mater. Chem. B 2017, 5, 1787–1802. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Chu, C.-C.; Xi, T. Arginine-Leucine Based Poly(Ester Urea Urethane) Coating for Mg–Zn–Y–Nd Alloy in Cardiovascular Stent Applications. Colloids Surf. B Biointerfaces 2017, 159, 78–88. [Google Scholar] [CrossRef]
- Shen, D.; Qi, H.; Lin, W.; Zhang, W.; Bian, D.; Shi, X.; Qin, L.; Zhang, G.; Fu, W.; Dou, K.; et al. PDLLA-Zn-Nitrided Fe Bioresorbable Scaffold with 53-μm-Thick Metallic Struts and Tunable Multistage Biodegradation Function. Sci. Adv. 2021, 7, eabf0614. [Google Scholar] [CrossRef]
- Jablonská, E.; Vojtěch, D.; Fousová, M.; Kubásek, J.; Lipov, J.; Fojt, J.; Ruml, T. Influence of Surface Pre-Treatment on the Cytocompatibility of a Novel Biodegradable ZnMg Alloy. Mater. Sci. Eng. C 2016, 68, 198–204. [Google Scholar] [CrossRef]
- Swaminathan, R. Magnesium Metabolism and Its Disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed] [PubMed Central]
- Seo, J.W.; Park, T.J. Magnesium Metabolism. Electrolyte Blood Press. 2008, 6, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Chen, Y.; Biao, M.; Zhang, X.; Yang, B. Bio-Functionalization of Biomedical Metals. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Noviana, D.; Paramitha, D.; Ulum, M.F.; Hermawan, H. The Effect of Hydrogen Gas Evolution of Magnesium Implant on the Post-Implantation Mortality of Rats. J. Orthop. Transl. 2016, 5, 9–15. [Google Scholar] [CrossRef]
- Seetharaman, S.; Sankaranarayanan, D.; Gupta, M. Magnesium-Based Temporary Implants: Potential, Current Status, Applications, and Challenges. J. Funct. Biomater. 2023, 14, 23. [Google Scholar] [CrossRef]
- Lewis, J.L. Hypermagnesemia. In Merck Manual Professional Version; Brookwood Baptist Health and Saint Vincent’s Ascension Health: Birmingham, UK, 2023. [Google Scholar]
- Ma, J.; Zhao, N.; Betts, L.; Zhu, D. Bio-Adaption between Magnesium Alloy Stent and the Blood Vessel: A Review. J. Mater. Sci. Technol. 2015, 31, 815–826. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Wang, W. The Degradation and Transport Mechanism of a Mg–Nd–Zn–Zr Stent in Rabbit Common Carotid Artery: A 20-Month Study. Acta Biomater. 2018, 69, 372–384. [Google Scholar] [CrossRef]
- Liu, C.; Hou, Y.; Li, J. A Mini Review on Biodegradable Magnesium Alloy Vascular Stent. Adv. Mater. Lett. 2020, 11, 1–5. [Google Scholar] [CrossRef]
- Wei, L.; Gao, Z. Recent Research Advances on Corrosion Mechanism and Protection, and Novel Coating Materials of Magnesium Alloys: A Review. RSC Adv. 2023, 13, 8427–8463. [Google Scholar] [CrossRef]
- Peuster, M.; Wohlsein, P.; Brügmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A Novel Approach to Temporary Stenting: Degradable Cardiovascular Stents Produced from Corrodible Metal—Results 6–18 Months after Implantation into New Zealand White Rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-Term Biocompatibility of a Corrodible Peripheral Iron Stent in the Porcine Descending Aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Pakala, R.; Baffour, R.; Seabron, R.; Hellinga, D.; Tio, F.O. Short-Term Effects of Biocorrodible Iron Stents in Porcine Coronary Arteries. J. Interv. Cardiol. 2008, 21, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.P.; Arnold, S.; Badar, M.; Bormann, D.; Bach, F.W.; Drynda, A.; Meyer-Lindenberg, A.; Hauser, H.; Peuster, M. Histological and Molecular Evaluation of Iron as Degradable Medical Implant Material in a Murine Animal Model. J. Biomed. Mater. Res. Part A 2012, 100, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhang, D.; Xin, C.; Liu, X.; Lin, W.; Zhang, W.; Chen, S.; Sun, K. Characterization and In Vivo Evaluation of a Bio-Corrodible Nitrided Iron Stent. J. Mater. Sci. Mater. Med. 2013, 24, 713–724. [Google Scholar] [CrossRef]
- Priyadarshini, S.B.P. A Comprehensive Study on Architecture of Neural Networks and Its Prospects in Cognitive Computing. Int. J. Synth. Emot. 2020, 11, 37–55. [Google Scholar] [CrossRef]
- Indolfi, C.; Torella, D.; Cavuto, L. Mechanisms of In-Stent Restenosis and Remodeling: Implications for Design of Drug-Eluting Stents. Nat. Clin. Pract. Cardiovasc. Med. 2004, 1, 44–49. [Google Scholar]
- Nakazawa, G.; Finn, A.V.; Vorpahl, M.; Ladich, E.R.; Kolodgie, F.D.; Virmani, R. Coronary Responses and Differential Mechanisms of Late Stent Thrombosis Attributed to First-Generation Sirolimus- and Paclitaxel-Eluting Stents. J. Am. Coll. Cardiol. 2011, 57, 390–398. [Google Scholar] [CrossRef]
- Niccoli, G.; Montone, R.A.; Ferrante, G.; Crea, F. The Evolving Role of Inflammatory Biomarkers in Risk Assessment after Stent Implantation. J. Am. Coll. Cardiol. 2010, 56, 1783–1793. [Google Scholar] [CrossRef]
- Ielasi, A.; Al-Lamee, R.; Colombo, A. Stent Thrombosis and Duration of Dual Antiplatelet Therapy. Curr. Pharm. Des. 2010, 16, 4052–4063. [Google Scholar] [CrossRef]
- Lin, W.; Qin, L.; Qi, H.; Zhang, D.; Zhang, G.; Gao, R.; Qiu, H.; Xia, Y.; Cao, P.; Wang, X. Long-Term In Vivo Corrosion Behavior, Biocompatibility and Bioresorption Mechanism of a Bioresorbable Nitrided Iron Scaffold. Acta Biomater. 2017, 54, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Zhang, D.Y.; Zhang, G.; Sun, H.T.; Qi, H.P.; Chen, L.P.; Liu, Z.Q.; Gao, R.L.; Zheng, W. Design and Characterization of a Novel Biocorrodible Iron-Based Drug-Eluting Coronary Scaffold. Mater. Des. 2016, 91, 72–79. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F.; Ruan, L. In Vitro Investigation of Fe–30Mn–6Si Shape Memory Alloy as Potential Bioresorbable Metallic Material. Mater. Lett. 2011, 65, 540–543. [Google Scholar] [CrossRef]
- Schinhammer, M.; Pecnik, C.M.; Rechberger, F.; Hanzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Recrystallization Behavior, Microstructure Evolution and Mechanical Properties of Bioresorbable Fe–Mn–C(–Pd) TWIP Alloys. Acta Mater. 2012, 60, 2756–2764. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation Performance of Bioresorbable Fe–Mn–C(–Pd) Alloys. Mater. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design Strategy for Biodegradable Fe-Based Alloys for Medical Applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef]
- Schinhammer, M.; Gerber, I.; Hanzi, A.C.; Uggowitzer, P.J. On the Cytocompatibility of Bioresorbable Fe-Based Alloys. Mater. Sci. Eng. C 2013, 33, 782–789. [Google Scholar] [CrossRef]
- Purnama, A.; Hermawan, H.; Champetier, S.; Mantovani, D.; Couet, J. Gene Expression Profile of Mouse Fibroblasts Exposed to a Biodegradable Iron Alloy for Stents. Acta Biomater. 2013, 9, 8746–8753. [Google Scholar] [CrossRef]
- Drynda, A.; Hassel, T.; Bach, F.W.; Peuster, M. In Vitro and In Vivo Corrosion Properties of New Iron-Manganese Alloys Designed for Cardiovascular Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 649–660. [Google Scholar] [CrossRef]
- Scarcello, E.; Lobysheva, I.; Bouzin, C.; Jacques, P.J.; Lison, D.; Dessy, C. Endothelial Dysfunction Induced by Hydroxyl Radicals—The Hidden Face of Biodegradable Fe-Based Materials for Coronary Stents. Mater. Sci. Eng. C 2020, 112, 110938. [Google Scholar] [CrossRef]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current Use of Fenton Reaction in Drugs and Food Molecules. Molecules 2022, 27, 5451. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Guzman, M.L.; Rivella, S. Iron and Reactive Oxygen Species: Friends or Foes of Cancer Cells? Antioxid. Redox Signal 2014, 20, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, R.S.; Martenson, M.S.; Tamargo, J.A.; McLaren, C.; Ezzati, A.; Lin, Y.; Yang, J.J.; Yoon, H.-S.; McElroy, T.; Collins, J.F.; et al. Iron Homeostasis in Older Adults: Balancing Nutritional Requirements and Health Risks. J. Nutr. Health Aging 2024, 28, 100212. [Google Scholar] [CrossRef] [PubMed]
- Penkova, M.; Ivanova, N. Serum Iron Metabolism Variables in Clinically Healthy Persons. Open Access Maced. J. Med. Sci. 2019, 7, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, M.; Conboy, H.L.; Hojyo, S.; Fukada, T.; Budnik, B.; Bartnikas, T.B. Biliary Excretion of Excess Iron in Mice Requires Hepatocyte Iron Import by Slc39a14. J. Biol. Chem. 2021, 297, 100812. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Tapiero, H.; Tew, K.D. Trace Elements in Human Physiology and Pathology: Zinc and Metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Vojtech, D.; Kubasek, J.; Serak, J.; Novak, P. Mechanical and Corrosion Properties of Newly Developed Biodegradable Zn-Based Alloys for Bone Fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J., II; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stent Application: Interests and New Opportunities. J. Biomed. Mater. Res. Part A 2015, 103, 1289–1300. [Google Scholar] [CrossRef]
- Chen, S.; Du, T.; Zhang, H.; Qi, J.; Zhang, Y.; Mu, Y.; Qiao, A. Methods for Improving the Properties of Zinc for the Application of Biodegradable Vascular Stents. Biomater. Adv. 2024, 156, 213693. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, Mechanical Characteristics and In Vitro Degradation, Cytotoxicity, Genotoxicity and Mutagenicity of Novel Biodegradable Zn–Mg Alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef]
- Jarzębska, A.; Bieda, M.; Kawałko, J.; Rogal, A.; Koprowski, P.; Sztwiertnia, K.; Pachla, W.; Kulczyk, M. A New Approach to Plastic Deformation of Biodegradable Zinc Alloy with Magnesium and Its Effect on Microstructure and Mechanical Properties. Mater. Lett. 2018, 211, 58–61. [Google Scholar] [CrossRef]
- Jarzębska, A.; Bieda, M.; Maj, A.; Chulist, R.; Wojtas, D.; Strąg, M.; Sułkowski, B.; Przybysz, S.; Pachla, W.; Sztwiertnia, K. Controlled Grain Refinement of Biodegradable Zn–Mg Alloy: The Effect of Magnesium Alloying and Multi-Pass Hydrostatic Extrusion Preceded by Hot Extrusion. Metall. Mater. Trans. A 2020, 51, 6784–6796. [Google Scholar] [CrossRef]
- Shen, C.; Liu, X.; Fan, B.; Lan, P.; Zhou, F.; Li, X.; Wang, H.; Xiao, X.; Li, L.; Zhao, S.; et al. Mechanical Properties, In Vitro Degradation Behavior, Hemocompatibility and Cytotoxicity Evaluation of Zn–1.2Mg Alloy for Biodegradable Implants. RSC Adv. 2016, 6, 86410–86419. [Google Scholar] [CrossRef]
- Li, H.; Shen, C.; Ruan, D.; Liu, X.; Li, X.; Guo, S.; Guo, Z. Microstructure, Mechanical Properties, and In Vitro Behavior of Biodegradable Zn–1Mg–0.1Ca and Zn–1Mg–0.5Ca. J. Mater. Sci. Mater. Med. 2020, 31, 88. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wang, X.; Xu, F.; Dai, T.; Zhou, J.G.; Liu, J.; Song, K.; Tian, L.; Liu, B.; Liu, Y. In Vivo Biocompatibility and Degradability of a Zn–Mg–Fe Alloy Osteosynthesis System. Bioact. Mater. 2022, 7, 122–139. [Google Scholar] [CrossRef]
- Farabi, E.; Sharp, J.A.; Vahid, A.; Fabijanic, D.M.; Barnett, M.R.; Gallo, S.C. Development of High Strength and Ductile Zn–Al–Li Alloys for Potential Use in Bioresorbable Medical Devices. Mater. Sci. Eng. C 2021, 122, 111897. [Google Scholar] [CrossRef]
- Farabi, E.; Sharp, J.; Vahid, A.; Wang, J.; Fabijanic, D.M.; Barnett, M.R.; Corujeira Gallo, S. Novel Biodegradable Zn Alloy with Exceptional Mechanical and In Vitro Corrosion Properties for Biomedical Applications. ACS Biomater. Sci. Eng. 2021, 7, 5555–5572. [Google Scholar] [CrossRef]
- Young, J.; Reddy, R.G. Synthesis, Mechanical Properties, and In Vitro Corrosion Behavior of Biodegradable Zn–Li–Cu Alloys. J. Alloys Compd. 2020, 844, 156257. [Google Scholar] [CrossRef]
- Yue, R.; Zhang, J.; Ke, G.; Jia, G.; Huang, H.; Pei, J.; Kang, B.; Zeng, H.; Yuan, G. Effects of Extrusion Temperature on Microstructure, Mechanical Properties and In Vitro Degradation Behavior of Biodegradable Zn–3Cu–0.5Fe Alloy. Mater. Sci. Eng. C 2019, 105, 110106. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Zheng, Y.; Qiao, L.; Yan, Y. In Vitro Degradation Behavior of Novel Zn–Cu–Li Alloys: Roles of Alloy Composition and Rolling Processing. Mater. Des. 2021, 212, 110288. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, C.; Li, G.; Zheng, Y.; Nie, J. Microstructure, Mechanical Properties and Creep Behaviour of Extruded Zn–xLi (x = 0.1, 0.3 and 0.4) Alloys for Biodegradable Vascular Stent Applications. Mater. Sci. Eng. A 2020, 777, 139082. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Z.; Hao, Y.; Li, H.; Liu, X.; Volinsky, A.A.; Zhang, H.; Wang, L. High-Performance Hot-Warm Rolled Zn-0.8Li Alloy with Nano-Sized Metastable Precipitates and Sub-Micron Grains for Biodegradable Stents. J. Mater. Sci. Technol. 2019, 35, 2618–2624. [Google Scholar] [CrossRef]
- Watroba, M.; Bednarczyk, W.; Kawalko, J.; Bala, P. Fine-Tuning of Mechanical Properties in a Zn–Ag–Mg Alloy via Cold Plastic Deformation Process and Post-Deformation Annealing. Bioact. Mater. 2021, 6, 3424–3436. [Google Scholar] [CrossRef]
- Ren, H.; Pan, C.; Liu, Y.; Liu, D.; He, X.; Li, X.; Sun, X. Fabrication, In Vitro and In Vivo Properties of Porous Zn–Cu Alloy Scaffolds for Bone Tissue Engineering. Mater. Chem. Phys. 2022, 289, 126458. [Google Scholar] [CrossRef]
- Akinwekomi, A.D.; Akhtar, F. Tunability of Mechanical and Biodegradation Properties of Zinc-Based Biomaterial with Calcium Micronutrient Alloying. J. Mech. Behav. Biomed. Mater. 2023, 140, 105724. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, K.; Sun, T.; Jing, X.; Wan, Y.; Chen, K.; Gao, H.; Wang, Y.; Chen, L.; Guo, X.; et al. Material-Structure-Function Integrated Additive Manufacturing of Degradable Metallic Bone Implants for Load-Bearing Applications. Adv. Funct. Mater. 2023, 33, 2212715. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, A.; Fan, J.; Wang, M.; Dai, J.; Jin, X.; Deng, H.; Wang, X.; Liang, Y.; Li, H.; et al. A Drug-Loaded Composite Coating to Improve Osteogenic and Antibacterial Properties of Zn–1Mg Porous Scaffolds as Biodegradable Bone Implants. Bioact. Mater. 2023, 27, 488–504. [Google Scholar] [CrossRef]
- Waqas, M.; He, D.; Tan, Z.; Yang, P.; Gao, M.; Guo, X. A Study of Selective Laser Melting Process for Pure Zinc and Zn–10Mg Alloy on Process Parameters and Mechanical Properties. Rapid Prototyp. J. 2023; in press. [Google Scholar] [CrossRef]
- Kulczyk, M.; Skorupska, M.; Skiba, J.; Przybysz, S.; Smalc-Koziorowska, J. Effects of HE and ECAP Processes on Changes in Microstructure and Mechanical Properties in Copper, Iron and Zinc. Bull. Pol. Acad. Sci. Tech. Sci. 2023, 71, e145563. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Meng, B.; Wan, M. Effect of Equal-Channel Angular Pressing on Microstructural Evolution, Mechanical Property and Biodegradability of an Ultrafine-Grained Zinc Alloy. Mater. Sci. Eng. A 2021, 824, 141857. [Google Scholar] [CrossRef]
- Bednarczyk, W.; Kawalko, J.; Rutkowski, B.; Watroba, M.; Gao, N.; Starink, M.J.; Bala, P.; Langdon, T.G. Abnormal Grain Growth in a Zn–0.8Ag Alloy after Processing by High-Pressure Torsion. Acta Mater. 2021, 207, 116667. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-Based Alloys for Degradable Vascular Stent Applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Falk, T.; Svensson, J.-E.; Johansson, L.-G. The Influence of CO2 and NaCl on the Atmospheric Corrosion of Zinc: A Laboratory Study. J. Electrochem. Soc. 1998, 145, 2993–2999. [Google Scholar] [CrossRef]
- Lévesque, J.; Hermawan, H.; Dubé, D.; Mantovani, D. Design of a Pseudo-Physiological Test Bench Specific to the Development of Biodegradable Metallic Biomaterials. Acta Biomater. 2008, 4, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and Its Importance for Human Health: An Integrative Review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed] [PubMed Central]
- Drelich, A.; Zhao, S.; Guillory, R.J., II; Drelich, J.W. Long-Term Surveillance of Zinc Implant in Murine Artery: Surprisingly Steady Biocorrosion Rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, S.; Guillory, R.; Bowen, P.K.; Yin, Z.; Griebel, A.; Schaffer, J.; Earley, E.J.; Goldman, J.; Drelich, J.W. Novel High-Strength, low-alloy Zn-Mg (<0.1 wt% Mg) and Their Arterial Biodegradation. Mater. Sci. Eng. C 2018, 84, 67–79. [Google Scholar] [CrossRef]
- Williams, D.F. Definitions in Biomaterials: Proceedings of a Consensus Conference of the EUROPEAN Society for Biomaterials, Chester, UK, 3–5 March 1986; Elsevier: New York, NY, USA, 1987; Volume 4. [Google Scholar]
- Ahmed, M.H.; Byrne, J.A.; Keyes, T.E.; Ahmed, W.; Elhissi, A.; Jackson, M.J.; Ahmed, E. 1—Characteristics and applications of titanium oxide as a biomaterial for medical implants. In The Design and Manufacture of Medical Devices; Woodhead Publishing: Cambridge, UK, 2012; pp. 1–57. [Google Scholar] [CrossRef]
- ISO 10993-4: 2017; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. ISO: Geneva, Switzerland, 2017.
- Liu, H.; Pan, C.; Zhou, S.; Li, J.; Huang, N.; Dong, L. Improving hemocompatibility and accelerating endothelialization of vascular stents by a copper-titanium film. Mater. Sci. Eng. C 2016, 69, 1175–1182. [Google Scholar] [CrossRef]
- ISO-10993-5; Biological Evaluation of Medical Devices-Part 5: Tests for Cytotoxicity: In Vitro Methods. ANSI/AAMI: Arlington, VA, USA, 1999.
- Meng, L.; Liu, X.; Liu, L.; Hong, Q.; Cheng, Y.; Gao, F.; Chen, J.; Zhang, Q.; Pan, C. Comparative Investigation of the Corrosion Behavior and Biocompatibility of the Different Chemical Conversion Coatings on the Magnesium Alloy Surfaces. Metals 2022, 12, 1644. [Google Scholar] [CrossRef]
- F04 Committee. Practice for Assessment of Hemolytic Properties of Materials; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Verhaegen, C.; Lepropre, S.; Octave, M.; Brusa, D.; Bertrand, L.; Beauloye, C.; Jacques, P.J.; Kefer, J.; Horman, S. Bioreactivity of Stent Material: In Vitro Impact of New Twinning—Induced Plasticity Steel on Platelet Activation. J. Biomater. Nanobiotechnol. 2019, 10, 175–189. [Google Scholar] [CrossRef]
- Gasior, G.; Szczepa’nski, J.; Radtke, A. Biodegradable Iron—Based Materials—What Was Done and What More Can Be Done? Materials 2021, 14, 3381. [Google Scholar] [CrossRef]
- Zhang, E.; Chen, H.; Shen, F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010, 21, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cheng, J.; Zheng, Y.F. In vitro degradation and biocompatibility of Fe-Pd and Fe-Pt composites fabricated by spark plasma sintering. Mater. Sci. Eng. C 2014, 35, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Niu, J.; Li, Y.; Ke, G.; Huang, H.; Pei, J.; Ding, W.; Yuan, G. In vitro cytocompatibility, hemocompatibility and antibacterial properties of biodegradable Zn—Cu—Fe alloys for cardiovascular stents applications. Mater. Sci. Eng. C 2020, 113, 11100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Sun, J.K.; Yang, Y.H.; Zhou, F.; Pu, Z.; Li, L.; Zheng, Y. Microstructure, mechanical properties, in vitro degradation behavior and hemocompatibility of novel Zn—Mg—Sr alloys as biode-gradable metals. Mater. Lett. 2016, 162, 242–245. [Google Scholar] [CrossRef]
- Liu, X.W.; Sun, J.K.; Zhou, F.Y.; Yang, Y.; Chang, R.; Qiu, K.; Pu, Z.; Li, L.; Zheng, Y. Micro-alloying with Mn in Zn-Mg alloy for future biodegradable metals application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]
- Zhen, Z.; Liu, X.; Huang, T.; Xi, T.; Zheng, Y. Hemolysis and Cytotoxicity Mechanisms of Biodegradable Magnesium and Its Alloys. Mater. Sci. Eng. C 2015, 46, 202–206. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Wang, L.; Zeng, M.Q.; Zeng, R.C.; Kannan, M.B.; Lin, C.G.; Zheng, Y.F. Biodegradation Behavior of Micro-Arc Oxidation Coating on Magnesium Alloy-from a Protein Perspective. Bioact. Mater. 2020, 5, 398–409. [Google Scholar] [CrossRef]
- Zheng, Q.; Sun, Z.; Wang, Z.; Duan, T.; Xu, K.; Cai, M.; Wang, B. Corrosion and Biocompatibility Behaviours of Microarc Oxidation/Phytic Acid Coated Magnesium Alloy Clips for Use in Cholecystectomy in a Rabbit Model. RSC Adv. 2021, 11, 20730–20736. [Google Scholar] [CrossRef]
- Zhou, W.R.; Zheng, Y.F.; Leeflang, M.A.; Zhou, J. Mechanical Property, Biocorrosion and in Vitro Biocompatibility Evaluations of Mg–Li–(Al)–(RE) Alloys for Future Cardiovascular Stent Application. Acta Biomater. 2013, 9, 8488–8498. [Google Scholar] [CrossRef] [PubMed]
- ASTM F756-08; Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM International: West Conshohocken, PA, USA, 2008. [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H.; Huang, L.; Tian, L.; et al. Corrigendum: Development of biodegradable Zn—1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yin, D.S.; Xu, L.P.; Yang, L.; Yang, K. Microstructure, mechanical and corrosion properties and biocompatibility of Mg–Zn–Mn alloys for biomedical application. Mater. Sci. Eng. C 2009, 29, 987–993. [Google Scholar] [CrossRef]
- Erikson, K.M.; Syversen, T.; Aschner, J.L.; Aschner, M. Interactions between excessive manganese exposures and dietary iron—Deficiency in neurodegeneration. Environ. Toxicol. Pharmacol. 2005, 19, 415–421. [Google Scholar] [CrossRef]
- Sun, S.; Ren, Y.; Wang, L.; Yang, B.; Li, H.; Qin, G. Abnormal effect of Mn addition on the mechanical properties of as—Extruded Zn alloys. Mater. Sci. Eng. A 2017, 701, 129–133. [Google Scholar] [CrossRef]
- Boutrand, J.-P. (Ed.) Biocompatibility and Performance of Medical Devices; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Philadelphia, PA, USA, 2012. [Google Scholar]
- Loffredo, S.; Paternoster, C.; Mantovani, D. Iron—Based Degradable Implants. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 374–385. [Google Scholar]
- Lin, W.; Zhang, G.; Cao, P.; Zhang, D.; Zheng, Y.; Wu, R.; Qin, L.; Wang, G.; Wen, T. Cytotoxicity and Its Test Methodology for a Bioabsorbable Nitrided Iron Stent. J. Biomed. Mater. Res. Part. B 2015, 103, 764–776. [Google Scholar] [CrossRef]
- Nie, F.L.; Zheng, Y.F.; Wei, S.C.; Hu, C.; Yang, G. In Vitro Corrosion, Cytotoxicity and Hemocompatibility of Bulk Nanocrystalline Pure Iron. Biomed. Mater. 2010, 5, 065015. [Google Scholar] [CrossRef]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.J.; Lison, D. Hydroxyl Radicals and Oxidative Stress: The Dark Side of Fe Corrosion. Colloids Surf. B Biointerfaces 2020, 185, 110542. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, B.; Wu, Y.; Zheng, Y. Comparative in Vitro Study on Pure Metals (Fe, Mn, Mg, Zn and W) as Biodegradable Metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Chen, J.; Zhang, X.; Kwak, M.; Wu, Y.; Fan, R.; Zhang, L.; Pei, J.; Yuan, G.; et al. A Promising Biodegradable Magnesium Alloy Suitable for Clinical Vascular Stent Application. Sci. Rep. 2017, 7, 46343. [Google Scholar] [CrossRef]
- Liu, J.; Xi, T. Enhanced Anti-Corrosion Ability and Biocompatibility of PLGA Coatings on MgZnYNd Alloy by BTSE-APTES Pre-Treatment for Cardiovascular Stent. J. Mater. Sci. Technol. 2016, 32, 845–857. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, N.; Shi, Y.; Niu, J.; Mao, L.; Li, H.; Xiong, M.; Yuan, G. Influence of Proteins and Cells on in Vitro Corrosion of Mg–Nd–Zn–Zr Alloy. Corros. Sci. 2014, 85, 477–481. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential Biodegradable Zn-Cu Binary Alloys Developed for Cardiovascular Implant Applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef] [PubMed]
- García-Mintegui, C.; Córdoba, L.C.; Buxadera-Palomero, J.; Marquina, A.; Jiménez-Piqué, E.; Ginebra, M.-P.; Cortina, J.L.; Pegueroles, M. Zn-Mg and Zn-Cu Alloys for Stenting Applications: From Nanoscale Mechanical Characterization to in Vitro Degradation and Biocompatibility. Bioact. Mater. 2021, 6, 4430–4446. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Low, H.T.; Kasiri-Asgarani, M.; Farahany, S.; Akbari, E.; Cho, M.H. Fabrication of Biodegradable Zn-Al-Mg Alloy: Mechanical Properties, Corrosion Behavior, Cytotoxicity and Antibacterial Activities. Mater. Sci. Eng. C 2017, 73, 215–219. [Google Scholar] [CrossRef]
- Niu, J.; Huang, H.; Pei, J.; Jin, Z.; Guan, S.; Yuan, G. Research and Development Strategy for Biodegradable Magnesium-based Vascular Stents: A Review. Biomater. Transl. 2021, 2, 236–247. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D. Endothelial Cellular Responses to Biodegradable Metal Zinc. ACS Biomater. Sci. Eng. 2015, 1, 1174–1182. [Google Scholar] [CrossRef]
- Meir, K.S.; Leitersdorf, E. Atherosclerosis in the Apolipoprotein-E-deficient Mouse: A Decade of Progress. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1006–1014. [Google Scholar] [CrossRef]
- Erbel, R.; Di Mario, C.; Bartunek, J.; Bonnier, H.; Legrand, V.; Kalesan, B.; Neumann, F.J.; Schömig, A.; Serruys, P.W. Temporary stenting of coronary arteries with bioabsorbable magnesium stents: A prospective, non-randomised multicentre trial. Lancet 2007, 369, 1869–1875. [Google Scholar] [CrossRef]
- Waksman, R.; Erbel, R.; Di Mario, C.; Bonnier, H.; Legrand, V.; Kalesan, B.; Neumann, F.J.; Schömig, A.; Serruys, P.W. Early-and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc. Interv. 2009, 2, 312–320. [Google Scholar] [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Gloekler, S.; Legrand, V.; Bonnier, H.; Kalesan, B.; Neumann, F.J.; Schömig, A.; Serruys, P.W. TCT-38 two-year clinical data of cohort 1 and multi-modality imaging resuts up to 1-year follow-up of the BIOSOLVE-I study with the paclitaxel-eluting bioabsorbable magnesium stent (DREAMS). J. Am. Coll. Cardiol. 2012, 60, B11. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Toelg, R.; Abizaid, A.; Colombo, A.; van der Giessen, W.; Di Mario, C.; Erbel, R.; Erne, P.; Gloekler, S.; et al. Safety and performance of the second-generation drug-eluting absorbable metal stent (DREAMS2G) in patients with de novo coronary lesions: Three-year clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. EuroIntervention 2020, 15, e1375–e1382. [Google Scholar] [CrossRef] [PubMed]
- Verheye, S.; Wlodarczak, A.; Montorsi, P.; Morice, M.C.; Wijns, W.; Colombo, A.; van der Giessen, W.; Di Mario, C.; Erbel, R.; Erne, P.; et al. BIOSOLVE-IV–registry: Safety and performance of the Magmaris stent: 12-month outcomes of the first cohort of 1,075 patients. Catheter. Cardiovasc. Interv. 2021, 98, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.; Testa, L.; Montorsi, P.; Verheye, S.; Morice, M.C.; Wijns, W.; Colombo, A.; van der Giessen, W.; Di Mario, C.; Erbel, R.; et al. SICI-GISE Position Document on the Use of the Magmaris Resorbable Magnesium Stent in Clinical Practice. Cardiovasc. Revasc Med. 2022, 34, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.L.; Xu, B.; Sun, Z.W.; Guan, C.D.; Song, L.; Gao, L.J.; Li, C.J.; Cui, J.G.; Zhang, Y.; Dou, K.F.; et al. First in human elution of a novel ultrathin sirolimus-eluting iron bioresorbable stent: 3-year outcomes of Ibs-FIM trial. EuroIntervention 2023, 19, e137–e146. [Google Scholar] [CrossRef]
- Saliba, L.; Vashaee, D.; Shadman, K.; Kiani, A.; Sahraei, A.; Alavi, M.; Pourdeyhimi, B.; Shalek, R.; Boccaccini, A.R. FeMn and FeMnAg biodegradable alloys: An in vitro and in vivo investigation. Heliyon 2023, 9, e15671. [Google Scholar] [CrossRef]
- Bowen, P.K.; Guillory, R.J., II; Shearier, E.R.; Wang, X.; Liu, C.; Xu, X.; Wang, J.; Yang, H.T. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C 2015, 56, 467–474. [Google Scholar] [CrossRef]
- Zhu, D.H.; Cockerill, I.; Su, Y.C.; Zhang, Z.X.; Fu, J.Y.; Lee, K.W.; Ma, J.; Okpokwasili, C.; Tang, L.P.; Zheng, Y.F.; et al. Mechanical Strength, Biodegradation, and in Vitro and in Vivo Biocompatibility of Zn Biomaterials. ACS Appl. Mater. Interfaces 2019, 11, 6809–6819. [Google Scholar] [CrossRef]
- Yang, H.T.; Wang, C.; Liu, C.Q.; Li, J.; Zhang, Y.; Zhao, C.; Wang, J. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef]
- Zhao, S.; Seitz, J.M.; Eifler, R.; Zettl, H.; Niendorf, T.; Witte, F. Zn—Li alloy after extrusion and drawing: Structural, mechanical characterization, and biodegradation in abdominal aorta of rat. Mater. Sci. Eng. C 2017, 76, 301–310. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.F.; Yin, Y.X.; Li, C.; Huang, N.; Wang, Y.; Zheng, Y.F. Long-term in-vivo study of biodegradable Zn-Cu stent: A 2-year implantation evaluation in porcine coronary artery. Acta Biomater. 2019, 97, 657–666. [Google Scholar] [CrossRef]
- Hehrlein, C.; Schorch, B.; Kress, N.; Arab, A.; von zur Muhlen, C.; Bode, C.; Epting, T.; Haberstroh, J.; Mey, L.; Schwarzbach, H. Zn-alloy provides a novel platform for mechanically stable bioresorbable vascular stents. PLoS ONE 2019, 14, e0209111. [Google Scholar] [CrossRef]
- Lin, S.; Ran, X.; Yan, X.; Wang, Q.; Zhou, J.G.; Hu, T.; Wang, G. Systematical evolution on a Zn-Mg alloy potentially developed for biodegradable cardiovascular stents. Journal of materials science. Mater. Med. 2019, 30, 122. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Qiao, A. Structural design and mechanical analysis of a novel biodegradable zinc alloy stent. Comput. Model. Eng. Sci. 2018, 117, 17–28. [Google Scholar]
- Peng, K.; Li, J.; Wang, S.R.; Xia, J.; Qiao, A. Research Progress on the Structure Design and Optimization of Biodegradable Stents. Chin. J. Biomed. Eng. 2019, 38, 367–374. [Google Scholar] [CrossRef]
- LaDisa, J.F. Stent design properties and deployment ratio influence indexes of wall shear stress: A three-dimensional computational fluid dynamics investigation within a normal artery. J. Appl. Physiol. 2004, 97, 424–430. [Google Scholar] [CrossRef]
- Li, H.; Gu, J.; Wang, M.; Zhao, D.; Bao, Z. Multi-objective optimization of coronary stent using Kriging surrogate model. Biomed. Eng. OnLine 2016, 15, 275–291. [Google Scholar] [CrossRef]
- Li, N.; Zhang, H.W. Optimization model of longitudinal flexibility of a coronary stent. Chin. J. Comput. Mech. 2011, 28, 315–319. [Google Scholar] [CrossRef]
- Heo, D.N.; Lee, J.B.; Bae, M.S.; Hwang, Y.S.; Kwon, K.H.; Kwon, K. Development of nanofiber coated indomethacin-eluting stent for tracheal regeneration. J. Nanosci. Nanotechnol. 2011, 11, 5711. [Google Scholar] [CrossRef]
- Prithipaul, P.K.M.; Kokkolaras, M.; Pasini, D. Assessment of structural and hemodynamic performance of vascular stents modelled as periodic lattices. Med. Eng. Phys. 2018, 57, 11–18. [Google Scholar] [CrossRef]
- Pan, C.; Han, Y.; Lu, J. Structural Design of Vascular Stents: A Review. Micromachines 2021, 12, 770. [Google Scholar] [CrossRef]
- Petrini, L.; Migliavacca, F.; Auricchio, F.; Dubini, G. Numerical investigation of the intravascular coronary stent flexibility. J. Biomech. 2004, 37, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Azaouzi, M.; Makradi, A.; Belouettar, S. Numerical investigations of the structural behavior of a balloon expandable stent design using finite element method. Comput. Mater. Sci. 2013, 72, 54–61. [Google Scholar] [CrossRef]
- Bedoya, J.; Meyer, C.A.; Timmins, L.H.; Moreno, M.R.; Moore, J.E. Effects of Stent Design Parameters on Normal Artery Wall Mechanics. J. Biomech. Eng. 2006, 128, 757–765. [Google Scholar] [CrossRef]
- Hsiao, H.M.; Lin, C.H.; Liao, Y.C.; Chen, H.Y.; Wang, T.W. Hemodynamic Behavior of Coronary Stents in Straight and Curved Arteries. Curr. Nanosci. 2014, 10, 205–211. [Google Scholar] [CrossRef]
- Rieu, R.; Barragan, P.; Garitey, V.; Roquebert, P.O.; Fuseri, J.; Commeau, P.; Sainsous, J. Assessment of the trackability, flexibility, and conformability of coronary stents: A comparative analysis. Catheter. Cardiovasc. Interv. 2003, 59, 496–503. [Google Scholar] [CrossRef]
- Behrend, D.; Behrens, P.; Schmidt, W. Comparative Studies of Different Stent Designs. Prog. Biomed. Res. 1999, 4, 52–58. [Google Scholar]
- Ormiston, J.A.; Dixon, S.R.; Webster, M.W.I.; Ruygrok, P.N.; Stewart, J.T.; Minchington, I.; West, T. Stent longitudinal flexibility: A comparison of 13 stent designs before and after balloon expansion. Catheter. Cardiovasc. Interv. 2000, 50, 120–124. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Q.; Li, Z. Study on the Impact of Straight Stents on with Different Curvatures. J. Mech. Med. Biol. 2016, 16, 1650093. [Google Scholar] [CrossRef]
- Wei, Y.B.; Wang, M.J.; Zhao, D.Y.; Li, H.X. In vitro experimental study on the mechanical properties of biodegradable polymer stents. J. Biomed. Eng. 2019, 36, 604–612. [Google Scholar] [CrossRef]
- Wei, Y.B.; Zhao, D.Y.; Wang, M.J.; Li, H.X. Design and Mechanics Analysis of Biodegradable Polymer Vascular Stents with High Radial Supporting Property. Chin. J. Mech. Eng. 2020, 31, 1098–1107. [Google Scholar] [CrossRef]
- Mori, K.; Saito, T. Effects of Stent Structure on Stent Flexibility Measurements. Ann. Biomed. Eng. 2005, 33, 733–742. [Google Scholar] [CrossRef]
- Xia, Z.; Ju, F.; Sasaki, K. A general finite element analysis method for balloon expandable stents based on repeated unit cell (RUC) model. Finite Elem. Anal. Des. 2007, 43, 649–658. [Google Scholar] [CrossRef]
- Abad, E.M.K.; Pasini, D.; Cecere, R. Shape optimization of stress concentration-free lattice for self-expandable Nitinol stent-grafts. J. Biomech. 2012, 45, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Lith, R.V.; Baker, E.; Ware, H.; Jian, Y.; Farsheed, A.C.; Sun, C.; Ameer, G. 3D—Printing Strong High—Resolution Antioxidant Bioresorbable Vascular Stents. Adv. Mater. Technol. 2016, 1, 1600138. [Google Scholar] [CrossRef]
- Ware, H.O.T.; Farsheed, A.C.; Akar, B.; Duan, C.; Chen, X.F.; Ameer, G.; Sun, C. High-speed on-demand 3D printed bioresorbable vascular scaffolds. Mater. Today Chem. 2018, 7, 25–34. [Google Scholar] [CrossRef]
- Ware, H.O.T.; Farsheed, A.C.; Baker, E.; Ameer, G.; Sun, C. Fabrication Speed Optimization for High-resolution 3D-printing of Bioresorbable Vascular Scaffolds. Procedia CIRP 2017, 65, 131–138. [Google Scholar] [CrossRef]
- Douglas, G.R.; Phani, A.S.; Gagnon, J. Analyses and design of expansion mechanisms of balloon expandable vascular stents. J. Biomech. 2014, 47, 1438–1446. [Google Scholar] [CrossRef]
- Dolla, W.J.S.; Fricke, B.A.; Becker, B.R. Structural and Drug Diffusion Models of Conventional and Auxetic Drug-Eluting Stents. J. Med. Devices 2007, 1, 47. [Google Scholar] [CrossRef]
- Tan, T.W.; Douglas, G.R.; Bond, T.; Phani, A.S. Compliance and Longitudinal Strain of Cardiovascular Stents: Influence of Cell Geometry. J. Med. Devices 2011, 5, 041002. [Google Scholar] [CrossRef]
- Liu, R.; Xu, S.; Luo, X.; Liu, Z.S. Theoretical and Numerical Analysis of Mechanical Behaviors of a Metamaterial-Based Shape Memory Polymer Stent. Polymers 2020, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.L.; Li, J.J.; Song, X.K.; Zhou, H.J.; Yuan, W.; Wu, W.W.; Xia, R. Mechanical Design of Antichiral-Reentrant Hybrid Intravascular Stent. Int. J. Appl. Mech. 2019, 10, 1850105. [Google Scholar] [CrossRef]
- Pant, S.; Limbert, G.; Curzen, N.P.; Bressloff, N.W. Multiobjective design optimisation of coronary stents. Biomaterials 2011, 32, 7755–7773. [Google Scholar] [CrossRef] [PubMed]
- Torki, M.M.; Hassanajili, S.; Jalisi, M.M. Design optimizations of PLA stent structure by FEM and investigating its function in a simulated plaque artery. Math. Comput. Simul. 2020, 169, 103–116. [Google Scholar] [CrossRef]
- Hsiao, H.M.; Chiu, Y.H. Assessment of mechanical integrity for drug-eluting renal stent with micro-sized drug reservoirs. Comput. Methods Biomech. Biomed. Eng. 2012, 16, 1307–1318. [Google Scholar] [CrossRef]
- Grogan, J.A.; O’Brien, B.J.; Leen, S.B.; McHugh, P.E. A corrosion model for bioabsorbable metallic stents. Acta Biomater. 2011, 7, 3523–3533. [Google Scholar] [CrossRef]
- Wu, W.; Chen, S.; Gastaldi, D.; Petrini, L.; Mantovani, D.; Yang, K.; Tan, L.; Migliavacca, F. Experimental data confirm numerical modeling of the degradation process of magnesium alloys stents. Acta Biomater. 2013, 9, 8730–8739. [Google Scholar] [CrossRef]
- Wu, W.; Gastaldi, D.; Yang, K.; Tan, L.; Petrini, L.; Migliavacca, F. Finite element analyses for design evaluation of biodegradable magnesium alloy stents in arterial vessels. Mater. Sci. Eng. B. 2011, 176, 1733–1740. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Wu, W.; Shi, Y.; Jin, L.; Petrini, L.; Shen, L.; Yuan, G.; Ding, W.; Ge, J.; et al. In vivo and in vitro evaluation of a biodegradable magnesium vascular stent designed by shape optimization strategy. Biomaterials 2019, 221, 119414. [Google Scholar] [CrossRef]
- Yuanxin Technology (Shenzhen) Co., Ltd. Absorbable Stent. Patent Application CN202210308364.0, 2 August 2022. [Google Scholar]
- Yuanxin Technology (Shenzhen) Co., Ltd. Absorbable Metal Device. Patent Application CN201911412829.1, 16 July 2021. [Google Scholar]
- Xianjian Technology (Shenzhen) Co., Ltd. Absorbable Metal Stent. Patent Application CN201811557509.0, 26 June 2020. [Google Scholar]
- Van der Heiden, K.; Gijsen, F.J.H.; Narracott, A.; Hsiao, S.; Halliday, I.; Gunn, J.; Wentzel, J.J.; Evans, P.C. The effects of stenting on shear stress: Relevance to endothelial injury and repair. Cardiovasc. Res. 2013, 99, 269–275. [Google Scholar] [CrossRef]
- Lagache, M.; Coppel, R.; Finet, G.; Derimay, F.; Pettigrew, R.I.; Ohayon, J.; Malvè, M. Impact of Malapposed and Overlapping Stents on Hemodynamics: A 2D Parametric Computational Fluid Dynamics Study. Mathematics 2021, 9, 795. [Google Scholar] [CrossRef]
- Schueler, B.A.; Sen, A.; Hsiung, H.-H.; Latchaw, R.E.; Hu, X.P. Three-Dimensional Vascular Reconstruction with a Clinical X-ray Angiography System. Acad. Radiol. 1997, 4, 693–699. [Google Scholar] [CrossRef]
- Hua, W.; Shi, W.; Mitchell, K.; Raymond, L.; Coulter, R.; Zhao, D.; Jin, Y. 3D Printing of Biodegradable Polymer Vascular Stents: A Review. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100020. [Google Scholar] [CrossRef]
- Han, Y.F.; Lu, W.F. Optimizing the deformation behavior of stent with nonuniform Poisson’s ratio distribution for curved artery. J. Mech. Behav. Biomed. 2018, 88, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Song, J.; Peng, Y.P. New insights and perspectives into biodegradable metals in cardiovascular stents: A mini review. J. Alloys Compd. 2024, 1002, 175313. [Google Scholar] [CrossRef]
- Morlacchi, S.; Migliavacca, F.; Petrini, L.; Dubini, G.; Auricchio, F.; Totaro, P.; Chiesa, R. Simulation of stent deployment in a realistic bifurcated coronary artery. J. Biomech. 2007, 40, 2557–2564. [Google Scholar] [CrossRef]
- Bütev Öcal, E.; Esen, Z.; Aydınol, K.; Dericioğlu, A.F. Comparison of the short and long-term degradation behaviors of as-cast pure Mg, AZ91 and WE43 alloys. Mater. Chem. Phys. 2020, 241, 122350. [Google Scholar] [CrossRef]
- Savaedi, Z.; Mirzadeh, H.; Aghdam, R.M.; Mahmudi, R. Effect of grain size on the mechanical properties and bio-corrosion resistance of pure magnesium. J. Mater. Res. Technol. 2022, 19, 3100–3109. [Google Scholar] [CrossRef]
- Liu, F.; Chen, C.; Niu, J.; Pei, J.; Zhang, H.; Huang, H.; Yuan, G. The processing of Mg alloy micro-tubes for biodegradable vascular stents. Mater. Sci. Eng. C 2015, 48, 400–407. [Google Scholar] [CrossRef]
- Furushima, T.; Manabe, K.-i. Large reduction die-less mandrel drawing of magnesium alloy micro-tubes. CIRP Ann. 2018, 67, 309–312. [Google Scholar] [CrossRef]
- Pan, C.-J.; Pang, L.-Q.; Hou, Y.; Lin, Y.-B.; Gong, T.; Liu, T.; Ye, W.; Ding, H.-Y. Improving Corrosion Resistance and Biocompatibility of Magnesium Alloy by Sodium Hydroxide and Hydrofluoric Acid Treatments. Appl. Sci. 2016, 7, 33. [Google Scholar] [CrossRef]
- Zhou, P.; Beeh, E.; Friedrich, H.E.; Grünheid, T. Mechanical Behavior and Microstructural Analysis of Extruded AZ31B Magnesium Alloy Processed by Backward Extrusion. J. Mater. Eng. Perform. 2016, 25, 2866–2877. [Google Scholar] [CrossRef]
- Pan, C.-J.; Hou, Y.; Wang, Y.-N.; Gao, F.; Liu, T.; Hou, Y.-H.; Zhu, Y.-F.; Ye, W.; Wang, L.-R. Effects of self-assembly of 3-phosphonopropionic acid, 3-aminopropyltrimethoxysilane and dopamine on the corrosion behaviors and biocompatibility of a magnesium alloy. Mater. Sci. Eng. C 2016, 67, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yue, R.; Miao, H.; Pei, J.; Huang, H.; Yuan, G. Enhanced plasticity of magnesium alloy micro-tubes for vascular stents by double extrusion with large plastic deformation. Mater. Lett. 2019, 245, 155–157. [Google Scholar] [CrossRef]
- Amani, S.; Faraji, G.; Kazemi Mehrabadi, H.; Abrinia, K.; Ghanbari, H. A combined method for producing high strength and ductility magnesium microtubes for biodegradable vascular stents application. J. Alloys Compd. 2017, 723, 467–476. [Google Scholar] [CrossRef]
- Amani, S.; Faraji, G. Recrystallization and mechanical properties of WE43 magnesium alloy processed via cyclic expansion extrusion. Int. J. Miner. Met. Mater. 2018, 25, 672–681. [Google Scholar] [CrossRef]
- Amani, S.; Faraji, G.; Mehrabadi, H.K.; Baghani, M. Manufacturing and mechanical characterization of Mg-4Y-2Nd-0.4Zr-0.25La magnesium microtubes by combined severe plastic deformation process for biodegradable vascular stents. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2018, 233, 1196–1205. [Google Scholar] [CrossRef]
- Wang, L.; Fang, G.; Qian, L.; Leeflang, S.; Duszczyk, J.; Zhou, J. Forming of magnesium alloy microtubes in the fabrication of biodegradable stents. Prog. Nat. Sci. 2014, 24, 500–506. [Google Scholar] [CrossRef]
- Fang, G.; Ai, W.-j.; Leeflang, S.; Duszczyk, J.; Zhou, J. Multipass cold drawing of magnesium alloy minitubes for biodegradable vascular stents. Mater. Sci. Eng. C 2013, 33, 3481–3488. [Google Scholar] [CrossRef]
- Ge, Q.; Dellasega, D.; Demir, A.G.; Vedani, M. The processing of ultrafine-grained Mg tubes for biodegradable stents. Acta Biomater. 2013, 9, 8604–8610. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, B.; Cai, Z. Recent Progress on Mg- and Zn-Based Alloys for Biodegradable Vascular Stent Applications. J. Nanomater. 2019, 2019, 1310792. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Sun, D.; Xue, H.; Guan, R.-g.; Liu, H. Microstructure of Biodegradable Zn-Fe Alloys and Mechanical and Corrosion Properties. JOM 2020, 72, 3661–3671. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, W.; Yu, F.; Wang, Y.; Qi, M.; Zhao, Y.; Wang, Y. Effects of pulse frequency and current density on microstructure and properties of biodegradable Fe-Zn alloy. J. Mater. Res. Technol. 2022, 18, 44–58. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Zhou, F.; Ma, E. High tensile ductility in a nanostructured metal. Nature 2002, 419, 912–915. [Google Scholar] [CrossRef]
- Huang, T.; Cheng, J.; Bian, D.; Zheng, Y. Fe–Au and Fe–Ag composites as candidates for biodegradable stent materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Van Swygenhoven, H.; Derlet, P.M.; Frøseth, A.G. Nucleation and propagation of dislocations in nanocrystalline fcc metals. Acta Mater. 2006, 54, 1975–1983. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.G.; Previtali, B.; Mantovani, D.; Beanland, R.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, L.; Cui, L.; Chen, L.; Lai, Y.; Guo, H.; Liu, Y. Study on Properties of Zn-x Mg (x = 0.5, 0.8, 1) Alloys for Potential Stent Material. J. Mater. Eng. Perform. 2023, 32, 7468–7479. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L.; Zheng, Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1.5Mg alloy. J. Alloys Compd. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Kurniawan, D.; Hermawan, H. Processing of Zn-3Mg alloy by equal channel angular pressing for biodegradable metal implants. J. King Saud Univ.-Sci. 2017, 29, 455–461. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Z.; Cui, Y.; Zhang, Y.; Yu, S.; Qu, G.; Gong, H. Processing of a Novel Zn Alloy Micro-Tube for Biodegradable Vascular Stent Application. J. Mater. Sci. Technol. 2016, 32, 925–929. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Low, H.T.; Cho, M.H.; Kasiri-Asgarani, M.; Farahany, S.; Mostafa, A.; Medraj, M. Thermal Characteristics, Mechanical Properties, In Vitro Degradation and Cytotoxicity of Novel Biodegradable Zn–Al–Mg and Zn–Al–Mg–xBi Alloys. Acta Met. Sin. Engl. Lett. 2017, 30, 201–211. [Google Scholar] [CrossRef]
- Huang, J.; Lai, Y.; Jin, H.; Guo, H.; Ai, F.; Xing, Q.; Yang, X.; Ross, D.J. Preparation and Properties of Zn-Cu Alloy for Potential Stent Material. J. Mater. Eng. Perform. 2020, 29, 6484–6493. [Google Scholar] [CrossRef]
- Yue, R.; Huang, H.; Ke, G.; Zhang, H.; Pei, J.; Xue, G.; Yuan, G. Microstructure, mechanical properties and in vitro degradation behavior of novel Zn-Cu-Fe alloys. Mater. Charact. 2017, 134, 114–122. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, H.; Niu, J.; Zhang, L.; Zhang, H.; Pei, J.; Tan, J.; Yuan, G. Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater. Des. 2017, 117, 84–94. [Google Scholar] [CrossRef]
- Niu, J.; Tang, Z.; Huang, H.; Pei, J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater. Sci. Eng. C 2016, 69, 407–413. [Google Scholar] [CrossRef]
- Grabow, N.; Bünger, C.M.; Schultze, C.; Schmohl, K.; Martin, D.P.; Williams, S.F.; Sternberg, K.; Schmitz, K.-P. A biodegradable slotted tube stent based on poly(L-lactide) and poly(4-hydroxybutyrate) for rapid balloon-expansion. Ann. Biomed. Eng. 2007, 35, 2031–2038. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed ZnAg alloys for degradable implant applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Z.-Z.; Hao, Y.; Li, H.-F.; Zhang, H.-J.; Liu, X.-F.; Wang, L.-N. Insight into role and mechanism of Li on the key aspects of biodegradable ZnLi alloys: Microstructure evolution, mechanical properties, corrosion behavior and cytotoxicity. Mater. Sci. Eng. C 2020, 114, 111049. [Google Scholar] [CrossRef]
- Zhao, S.; McNamara, C.T.; Bowen, P.K.; Verhun, N.; Braykovich, J.P.; Goldman, J.; Drelich, J.W. Structural Characteristics and In Vitro Biodegradation of a Novel Zn-Li Alloy Prepared by Induction Melting and Hot Rolling. Met. Mater. Trans. A 2017, 48, 1204–1215. [Google Scholar] [CrossRef]
- Yang, X.; Du, P.; Li, K.; Bao, W.; Xiang, T.; Chen, J.; Liu, X.; Xie, G. Optimization of mechanical properties and corrosion resistance of Zn-0.4Mn-0.8Li alloy using the hot rolling process. J. Mater. Sci. Technol. 2022, 145, 136–147. [Google Scholar] [CrossRef]
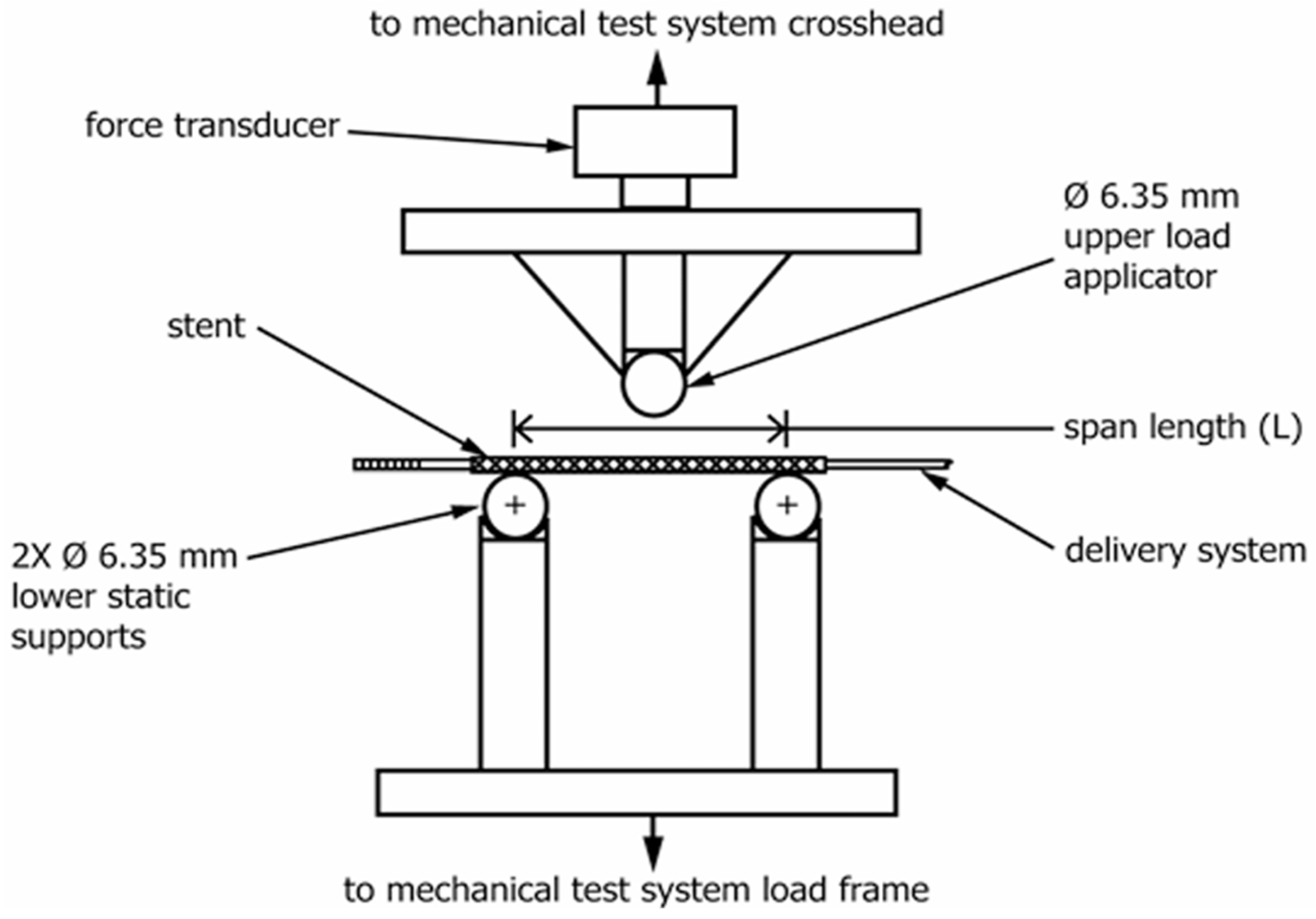













| Criterion | Constraint |
|---|---|
| Biodegradation | Mechanical integrity for 3~6 months; Full absorption in 12~24 months |
| Biocompatibility | Non-toxic and non-inflammatory; No allergenic potential; No harmful release or retention of particles |
| Mechanical properties | Yield strength > 200 MPa, Ultimate tensile strength > 300 MPa; Yield strength/elastic modulus ratio > 0.16; Elongation to failure > 15~18%; Elastic recoil on expansion < 4% |
| Microstructure | Homogeneous and approximately isotropic |
| Small grain size | <30 um |
| Corrosion rate | Penetration rate < 0.02 mm∙a−1 |
| Normal Value | Normal Range | Approximate Short-Term Nonlethal Limit | Unit | |
|---|---|---|---|---|
| Sodium ion | 142 | 138–146 | 115–175 | mmol/L |
| Potassium ion | 4.2 | 3.8–5.0 | 1.5–9.0 | mmol/L |
| Calcium ion | 1.2 | 1.0–1.4 | 0.5–2.0 | mmol/L |
| Chloride ion | 108 | 103–112 | 70–130 | mmol/L |
| Bicarbonate ion | 28 | 24–32 | 8–45 | mmol/L |
| Solution | Test | Composition | Ref. |
|---|---|---|---|
| Hank’s | Immersion Electrochemical | 8.0 g/L NaCl, | [27] |
| 0.4 g/L KCl, | |||
| 0.14 g/L CaCl2, | |||
| 0.35 g/L NaHCO3, | |||
| 1.0 g/L C6H6O6 (glucose), | |||
| 0.2 g/L MgSO4·7H2O, | |||
| 0.1 g/L KH2PO4·H2O, | |||
| 0.06 g/L Na2HPO4·7H2O | |||
| Simulated Body Fluid (SBF) | Immersion Electrochemical | 142.0 (mmol/L) Na+ | [28] |
| 5.0 (mmol/L) K+ | |||
| 2.5 (mmol/L) Ca2+ | |||
| 1.5 (mmol/L) Mg2+ | |||
| 147.8 (mmol/L) Cl− | |||
| 4.2 (mmol/L) HCO3− | |||
| 0.5 (mmol/L) SO42− | |||
| 1.0 (mmol/L) HPO42− | |||
| Modified Simulated Body Fluid (m-SBF) | Immersion Electrochemical | 5.403 g/L NaCl, | [29] |
| 0.504 g/L NaHCO3, | |||
| 0.426 g/L Na2CO3, | |||
| 0.225 g/L KCl, | |||
| 0.230 g/L K2HPO4·3H2O, | |||
| 0.311 g/L MgCl·6H2O, | |||
| 100 mL 0.2 mol/L NaOH, | |||
| 17.892 g/L HEPES, | |||
| 0.293 g/L CaCl2, | |||
| 0.072 g/L Na2SO4 | |||
| PBS | Immersion Electrochemical | 0.20 g/L KCl, | [30] |
| 0.20 g/L KH2PO4, | |||
| 8.00 g/L NaCl, | |||
| 1.15 g/L Na2HPO4 | |||
| Modified HBSS | Immersion Electrochemical | 8.0 g/L NaCl, | [31] |
| 0.4 g/L KCl, | |||
| 0.14 g/L CaCl2, | |||
| 0.35 g/L NaHCO3, | |||
| 1.0 g/L C6H6O6 (glucose), | |||
| 0.2 g/L MgSO4·7H 2O, | |||
| 0.1 g/L KH2PO4·H2O, | |||
| 0.06 g/L Na2HPO4·7H2O | |||
| 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid | |||
| DMEM | Immersion | Dulbecco’s modified Eagle’s medium | [32] |
| Culture medium | Immersion | Contains inorganic ions, organic compounds, and proteins | [33] |
| Human plasma | Electrochemical | Contains inorganic ions, organic compounds, and proteins | [34] |
| Whole blood | Electrochemical | Contains inorganic ions, organic compounds, and proteins | [35] |
| Parameter | Value | Unit | Ref. |
|---|---|---|---|
| Blood flow range | 200–250 (About 4 to 5 percent of the total cardiac output.) | mL/min | [38] |
| Pressure | 100–180 | mmHg | [38] |
| Viscosity | 3.5 | N·s/m2 | [39] |
| Diameter (left main artery) | 4.5 ± 0.5 | mm | [39] |
| Diameter (right coronary artery) | 3.9 ± 0.6 and 2.8 ± 0.5 | mm | [38] |
| Material | YS (Mpa) | UTS (Mpa) | EL (%) | HV |
|---|---|---|---|---|
| Mg-3Al-1Zn (AZ31) [57,58] | 172 | - | 16 | - |
| Mg-2-1Zn-0.2Zr (JDBM) [59,60] | 123–220 | 267 | 26–48.8 | - |
| Mg-4Y-3RE (WE43) [61,62,63] | 113 | 340–440 | 10–21 | 102–114 |
| Mg-2Zn-1RE-B (ZE21B) [64] | 196 | 298 | 20 | - |
| Mg-2Zn-1Mn (ZM21) [65,66,67] | 340 | 353 | 11.5 | - |
| Mg-4Zn-1Y [51] | 240 | 330 | 20.4 | - |
| σ0.2 (MPa) | Tensile Strength (MPa) | Radial Strength (kPa) | Stent Stiffness (kN/m) | Microhardness (HV) | |
|---|---|---|---|---|---|
| Pure iron stents | 236.27 ± 22.40 | 342.90 ± 44.18 | 59.07 ± 3.69 | 37.87 ± 2.93 | 154.92 ± 5.24 |
| Nitrided iron stents | 561.41 ± 30.99 | 614.35 ± 29.03 | 92.61 ± 5.26 | 52.95 ± 3.96 | 287.06 ± 8.67 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| (µA cm−2) | (mm year−1) | ||
|---|---|---|---|
| Pure iron stents | −439 ± 10 | 10.887 ± 0.715 | 0.127 ± 0.008 |
| Nitrided iron stents | −405 ± 39 | 19.365 ± 1.731 | 0.225 ± 0.020 |
| P | >0.05 | <0.01 | <0.01 |
| Designation | YS(MPa) | UTS (MPa) | εu (%) | εf (%) | H (HV10) |
|---|---|---|---|---|---|
| Fe (Armco) | 250 | 300 | 19.5 | 37.5 | 85 ± 1 |
| Fe (sht) | 700 | 900 | 3.5 | 9.5 | 403 ± 2 |
| Fe-10Mn (sht) | NA * | NA * | NA * | NA * | 428 ± 6 |
| Fe-10Mn (ht 1) | 800 | 1400 | 9.5 | 14.0 | 384 ± 5 |
| Fe-10Mn (ht 2) | 650 | 1300 | 9.0 | 14.0 | 374 ± 7 |
| Fe-10Mn-1Pd (sht) | 950 | 1500 | 2.0 | 2.0 | 432 ± 8 |
| Fe-10Mn-1Pd (ht 1) | 900 | 1550 | 6.5 | 7.0 | 437 ± 3 |
| Fe-10Mn-1Pd (ht 2) | 850 | 1450 | 8.0 | 11.0 | 376 ± 6 |
| Alloys | YS (Mpa) | UTS (Mpa) | EL (%) | Hardness |
|---|---|---|---|---|
| Zn-0.08Mg [129] | 221 | 339 | 40 | 103 |
| Zn-0.8Mg [130] | 203 | 301 | 15 | 80–90 |
| Zn-1Mg [131,132] | 316–383 | 435–482 | 23–35 | - |
| Zn-1.2Mg [133] | 219.61 | 362.64 | 21.31 | 96.01 |
| Zn-1Mg-0.1Ca [134] | 209.04 | 331.51 | 35.43 | 110–125 |
| Zn-2Al-0.4Li [135] | 352.2 | 405.8 | 31.6 | - |
| Zn-4Al-0.2Li [135] | 362.7 | 382.2 | 33.3 | - |
| Zn-4Al-0.4Li [135] | 399.3 | 414.6 | 25.6 | - |
| Zn-4Al-0.6Li [135] | 429.6 | 451.4 | 46.3 | - |
| Zn-6Al-0.4Li [135] | 335.6 | 432.6 | 42.7 | - |
| Zn-2Al-2Cu-0.6Li [136] | 260 | 402 | 44 | - |
| Zn-2Al-2Cu-0.8Li [136] | 371 | 445 | 47 | - |
| Zn-2Al-4Cu-0.6Li [136] | 404 | 535 | 32 | - |
| Zn-2Al-4Cu-0.8Li [136] | 407 | 536 | 17 | - |
| Zn-2Cu-0.3Li [137] | 226.55 | 333.49 | 26 | 124.59 |
| Zn-3Cu-0.5Fe [138] | 269 | 302 | 24.7 | - |
| Zn-3.5Cu-0.3Li [137] | 231.63 | 382.8 | 18.33 | 128.79 |
| Zn-4Cu-0.02Li [139] | 256 | 342 | 39.8 | - |
| Zn-5Cu-0.3Li [137] | 272.87 | 427.92 | 19.30 | 133.85 |
| Zn-0.3Li [140] | 292 | 367.2 | 19.3 | - |
| Zn-0.4Li [135,140] | 352.2–363.7 | 398.5–406.5 | 27.4–27.8 | - |
| Zn-0.8Li [141] | 261.5 | 401.4 | 80.8 | - |
| Zn-0.8Li-0.4Mn [142] | 449.7 | 505.1 | 40.5 | 137.6 |
| Zn-3Ag-0.5Mg [142] | 385 | 432 | 34 | - |
| Materials | Hemocompatibility | Reference | |
|---|---|---|---|
| Fe | Pure Fe | ~2.44% | [166] |
| Fe-5wt.%Pd | Slightly higher than that of pure iron, but still less than 5% | [167] | |
| Fe-5wt.%Pt | Slightly higher than that of pure iron, but still less than 5% | [167] | |
| Fe30Mn | Below 2% | [110] | |
| Fe30Mn6Si | Below 2% | [110] | |
| Zn | Pure Zn | 1.04% | [34] |
| Zn-0.8Cu | 0.47% | [34] | |
| Zn-0.8Mn | 0.57% | [34] | |
| Zn-0.8Li | 0.52% | [34] | |
| Zn-3Cu | ~1% | [168] | |
| Zn-Cu-Fe | ~1% | [168] | |
| Zn-1.2Mg | 1.62% | [132] | |
| Zn-1Mg-0.1Sr | 1.10% | [169] | |
| Zn-1Mg-0.1Mn | 1.10% | [170] | |
| Mg | Pure Mg | Up to 37% | [171] |
| Mg-OH | Below 5% | [162] | |
| Mg-HF | Below 5% | [162] | |
| Mg-P | Below 5% | [172] | |
| MG-PA | Below 5% | [173] | |
| Mg-1Zn-1Mn | 65.75% | [173] | |
| Mg–Nd–Zn–Zr | 52% | [162] | |
| Mg-Li | 3~3.8% | [79,166] | |
| Mg8.5Li1Al | ~4% | [174] | |
| Mg-Li-Al-RE | ~4.2–7% | [174] | |
| Device | Backbone Material | Trial | Late Lumen Loss (mm) | Target Lesion Failure (%) | Reference |
|---|---|---|---|---|---|
| AMS | Magmaris | Follow up 63 patients for 12 months | 1.08 ± 0.49 mm at 4 months | 23.8% after 4 months, 45% after 1 year | [195] |
| DREAMS (Biotronik) | Magmaris, PLGA, Paclitaxe | Follow up 46 patients for 12 months | 0.65 ± 0.50 mm at 4 months, 0.52 ± 0.39 mm at 12 months | 4.3% at 12 months | [196] |
| 3-year follow-up | 0.51 ± 0.46 mm at 12 months, 0.32 ± 0.32 mm at 28 ± 4 months | 4.3% at 3 years | [197] | ||
| DREAMS 2G (Biotronik) | Magmaris, PLGA, Sirolimaus | Follow up 123 patients for 6 months | 0.44 ± 0.36 mm at 6 months | 1.7% at 6 months | [64] |
| 12-month follow-up | 0.37 ± 0.25 mm at 6 months, 0.39 ± 0.27 mm at 12 months | 1.7% at 12 months | [64] | ||
| 3-year follow-up | 0.39 ± 0.27 mm at 12 months, 0.54 ± 0.38 mm at 36 months | 4.3% at 36 months | [198] | ||
| Magmaris (Biotronik) | Magmaris, PLGA, Sirolimaus | Follow up 61 patients for 6 months | 0.39 ± 0.39 mm | 1.7% at 6 months | [198] |
| Follow up 61 patients for 12 months | NA | 1.6% at 12 months | [198] | ||
| Follow up 1075 patients for 12 months | NA | 3.9% at 12 months | [199] | ||
| Follow up 2066 patients for 2 years | NA | 6% at 24 months | [200] |
| Device | Backbone Materials | Trial | Late Lumen Loss (mm) | Target Lesion Failure (%) | Reference |
|---|---|---|---|---|---|
| IBS (Lifetech) | Nitrided iron, Zn, PLA, Sirolimaus | Follow up 45 patients for 6 months | 0.33 ± 0.27 mm | 2.2% at 6 months | [66] |
| Follow up 45 patients for 12 months | 0.39 ± 0.50 mm | 6.7% at 1 year | |||
| Follow up 45 patients for 24 months | 0.40 ± 0.31 mm | 6.7% at 2 years | |||
| Follow up 45 patients for 36 months | 0.37 ± 0.57 mm | 6.7% at 3 years | |||
| NOR-I | >99.8% Pure Fe | Follow up 16 minipigs for 18 months | The loss of luminal area was less than 10% | - | [98] |
| Peripheral iron stent | >99.5% Fe | Follow up 29 patients for 360 days | There is no difference with the 316L stent. The loss of luminal area was less than 50% | - | [99] |
| Animal Model | Material | Experiment Period | Biocompatibility | Degradation | Reference |
|---|---|---|---|---|---|
| Rat abdominal aorta | Pure zinc wire | 6 months | None of the major contributors to restenosis observed | Retained about 70% area after 4 months, then degradation increased rapidly | [203] |
| Pure zinc wire, Zn-1.5Mg wire, Zn-1.5Sr wire | 1 month | No extensive inflammation was observed | Corrosion rate of Zn-based materials was 0.4 mm/year, significantly slower than that of AZ31 | [204] | |
| Pure zinc stent | 12 months | None of the major contributors to restenosis observed | Maintained mechanical integrity for 6 months and degraded 41.75 ± 29.72% of volume after 12 months | [205] | |
| Zn-5.5Al strip | 6 months | Chronic and acute inflammatory indications were present | Cross-sectional reduction of pure Zn and Zn-Al alloys was 30–40% and 40–50% after 4.5 and 6 months | [176] | |
| Zn-0.1Li wire | 12 months | Indicated moderate inflammation with a nonobstructive neointima | Indicated a moderate to low degradation rate of ~0.02 mm/year at 6.5 months, which increased to -0.05 mm/year at 12 months | [206] | |
| Porcine coronary artery | Zn-0.8Cu stent | 24 months | Endothelialization process could be completed in the first month. No inflammation responses or thrombosis formation were observed within 24 months | The implanted stent maintained its structural integrity after 6 months. After 24 months, approximately 28 ± 13 vol.% of the stent remained | [207] |
| Porcine iliofemoral artery | Zn-3Ag stent | 6 months | Stent struts were completely covered by neointima at the 4-week follow-up; no stent thrombosis or vascular occlusion at 6 months | Maintained the stent for a minimum of 6 months | [208] |
| Rabbit carotid artery | Zn-0.02Mg-0.02Cu stent | 12 months | Rapid endothelialization on the Zn-based alloy stent at 1 week, suggesting low cytotoxicity and thrombosis risk | Almost intact 6 months after implantation. Became incomplete, presented severe localized corrosion after 12 months | [209] |
| Reference | Objects | Method | Result |
|---|---|---|---|
| Behrend [223] | L-shaped, V-shaped, and S-shaped | Cantilever method | The L-shaped bridge had the smallest axial stiffness. |
| Ormiston et al. [224] | L-shaped, V-shaped, and S-shaped bridges | Three-point support method | The performance of the S-shaped vascular stent was better than that of the L-shaped and V-shaped stents, and the axial flexibility of the L-shaped and V-shaped stents was almost the same. |
| Wei et al. [225] | I-shaped, C-shaped, S-shaped, U-shaped, N-shaped, and W-shaped bridges | Finite element simulation of the ideal model analysis | When the vascular curvature was 0° or 15°, the stent with an S-shaped bridge structure was the most flexible. When the vascular curvature was 30°, 45°, or 60°, the U-shaped stent had the best flexibility. |
| Azaouzi et al. [219] | V-shaped, N-shaped, unsymmetrical V-shaped, and unsymmetrical N-shaped bridges | Finite element analysis | In terms of bending performance, the symmetrical N-shaped bridge and unsymmetrical V-shaped bridge had better flexibility; in terms of torsional performance, the symmetrical V-shaped bridge stent had the worst flexibility, and the unsymmetrical N-shaped stent had the best flexibility; since the radial force and stress of the symmetrical N-shaped bridge structure are small, it is the structure with the best radial support performance among the stents. |
| Wei et al. [226,227] | JS-shaped, OCS-shaped, and CCS-shaped bridges | Plane compression method, V-groove compression method, and three-point bending method | The radial strengths of the JS-shaped, open OCS-shaped, and closed CCS-shaped stents were 14%, 34%, and 42% higher than that of the ordinary biodegradable stent, respectively. The bending stiffness of the JS-shaped and OCS-shaped stents was similar to that of the ordinary stent, while the CCS-shaped stent had about 73% lower stiffness. All stents showed no axial foreshortening. |
| Mori and Saito [228] | W-shaped, S-shaped, WD-shaped, and N-shaped bridges | Four-point bending test method | The bending stiffness of the S-shaped stent was 85.28 N , that of the N-shaped stent was 41.67 N , that of the improved WD-shaped stent was 78.79 N , and that of the W-shaped stent was 188.67 N . |
| Reference | Objects | Result |
|---|---|---|
| Prithipaul [216] and Douglas et al. [234] | Diamond, Re-entrant Auxetic, Hybrid A, Hybrid C, Chevron B stent | Diamond structure exhibited poor mechanical properties; Re-entrant Auxetic, Hybrid A, Hybrid C, and Chevron B exhibited better radial stiffness and foreshortening. |
| Dolla et al. [235] and Tan et al. [236] | Diamond, Auxetic stent | Re-entrant Auxetic stent had good mechanical properties. |
| Liu et al. [237] | Re-entrant shape memory polymer vascular stent | A re-entrant stent with a smaller radius has a higher critical buckling load and smaller buckling displacement. Compared to traditional stents, it has a smaller contact area with the vessel and lower stress after implantation. |
| Ruan [238] | Antichiral Reentrant vascular stent | Antichiral re-entrant stent had good mechanical properties after being implanted in the blocked lesion by designing stents of different sizes. |
| Alloy | Advantage | Concern |
|---|---|---|
| Mg-based | Excellent biocompatibility | High corrosion or biodegradation rate |
| Fe-based | Excellent mechanical properties; biological benefits: decreasing the proliferation of smooth muscle cells and inhibition of neointimal hyperplasia. | Low corrosion or biodegradation rate |
| Zn-based | Good biocompatibility; optimal degradation rate: 10 and 20 mm/year, similar to the ideal bioabsorbable material [155]. | Cytotoxicity: excessive concentration of Zn ions can inhibit cell activity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Zeng, Y.; Shen, Z.; Yue, C.; Yang, Y.; Gao, J.; Zhang, J.; Yuan, Q.; Cha, L. Biodegradable Metal-Based Stents: Advances, Challenges, and Prospects. J. Funct. Biomater. 2025, 16, 315. https://doi.org/10.3390/jfb16090315
Sun L, Zeng Y, Shen Z, Yue C, Yang Y, Gao J, Zhang J, Yuan Q, Cha L. Biodegradable Metal-Based Stents: Advances, Challenges, and Prospects. Journal of Functional Biomaterials. 2025; 16(9):315. https://doi.org/10.3390/jfb16090315
Chicago/Turabian StyleSun, Lifeng, Yuanyuan Zeng, Zhengyu Shen, Chongsheng Yue, Yahan Yang, Jia Gao, Junhao Zhang, Qi Yuan, and Limei Cha. 2025. "Biodegradable Metal-Based Stents: Advances, Challenges, and Prospects" Journal of Functional Biomaterials 16, no. 9: 315. https://doi.org/10.3390/jfb16090315
APA StyleSun, L., Zeng, Y., Shen, Z., Yue, C., Yang, Y., Gao, J., Zhang, J., Yuan, Q., & Cha, L. (2025). Biodegradable Metal-Based Stents: Advances, Challenges, and Prospects. Journal of Functional Biomaterials, 16(9), 315. https://doi.org/10.3390/jfb16090315






