Ultrasound-Responsive Drug Delivery System Based on Piezoelectric Catalytic Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Materials
2.1.2. Characterization of BTO
2.2. Synthesis of BTO Nanoparticles
2.3. COMSOL Physical Field Simulation
2.4. Investigation of the Piezocatalysis Performance and Mechanism of BTO
2.4.1. Radical Scavenging Experiment
2.4.2. Radical Specificity Test
2.5. Synthesis of ROS-Responsive Hydrogel
2.6. Release Experiment of Hydrogel Composite
2.6.1. Drug Release of Composite Hydrogel Under ROS-Responsive Conditions
2.6.2. Drug Release of Composite Hydrogel Under Ultrasound Conditions
2.7. Cell Safety Experiment
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of BTO Nanoparticles
3.2. COMSOL Physical Field Simulation Results
3.3. BTO NP Performance and Mechanism Investigation
3.3.1. Identification of the Dominant ROS via Radical Scavenging Assays
3.3.2. Validation of the Dominant ROS via Specific Fluorescent Probes
3.4. Verification and Analysis of the Release Performance of Composite Hydrogels
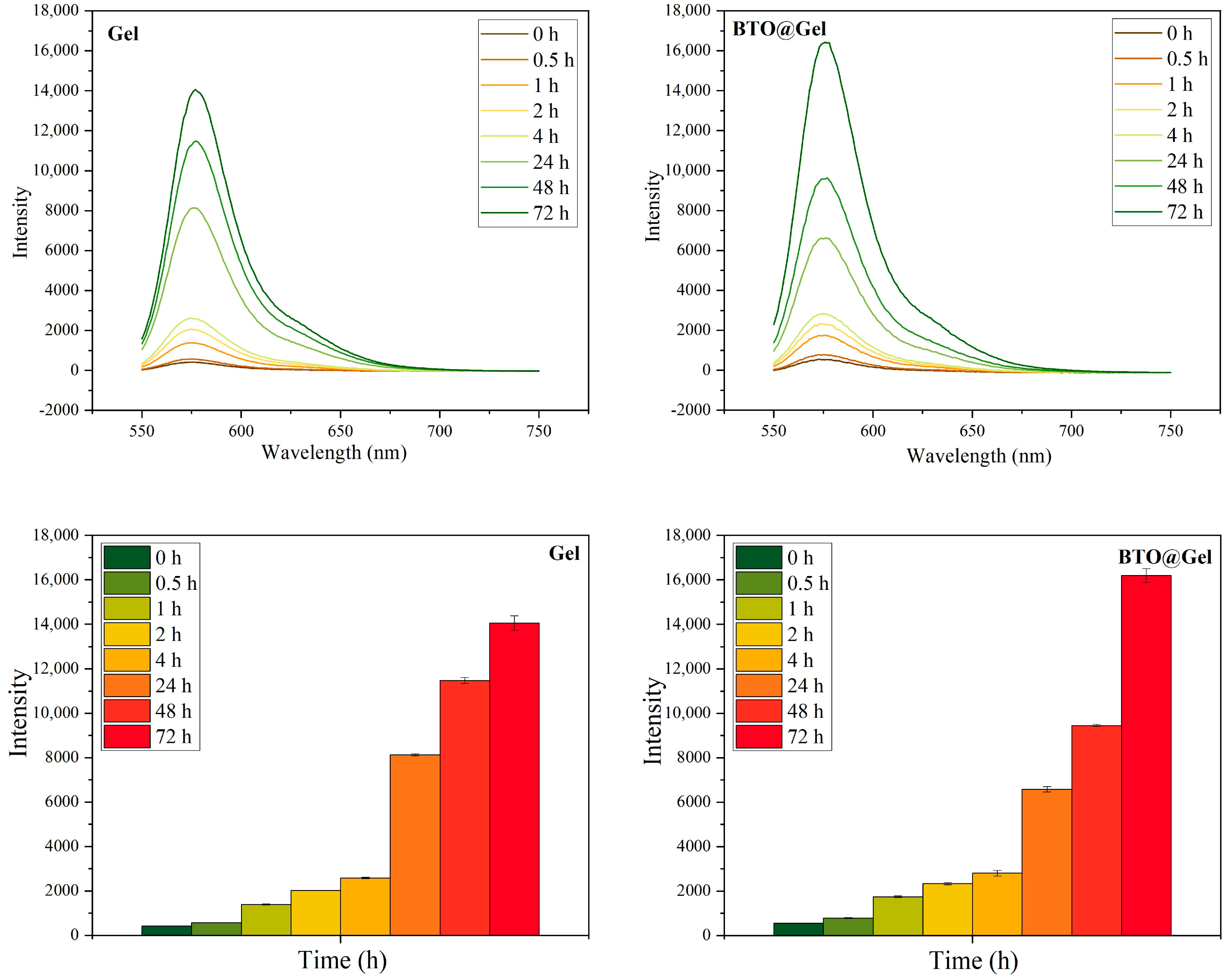
3.4.1. Drug Release Behavior of the Composite Hydrogel Under H2O2 Conditions
3.4.2. Drug Release Properties of Composite Hydrogels Under Ultrasound Stimulation
3.5. In Vitro Biological Safety Evaluation of BTO and Its Hydrogel Composites
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTO | Barium Titanate |
| CCK-8 | Cell Counting Kit-8 |
| COMSOL | COMSOL Multiphysics |
| DOX | Doxorubicin |
| FFA | Furfuryl Alcohol |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| MB | Methylene Blue |
| NBT | Nitroblue Tetrazolium |
| NPs | Nanoparticles |
| OD | Optical Density |
| PBS | Phosphate-Buffered Saline |
| pBQ | p-Benzoquinone |
| ROS | Reactive Oxygen Species |
| •OH | Hydroxyl Radical |
| 1O2 | Singlet Oxygen |
| O2− | Superoxide Anion |
| SOSG | Singlet Oxygen Sensor Green |
| TA | Terephthalic Acid |
| TBA | Tert-Butanol |
| TEM | Transmission Electron Microscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
References
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Zhang, H.; Li, C.; Zhang, T.; Liu, H.; Zhu, S.; Chen, J. Tumor microenvironment and immunotherapy of oral cancer. Eur. J. Med. Res. 2022, 27, 198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Ultrasound responsive mesoporous silica nanoparticles for biomedical applications. Chem. Commun. 2019, 55, 2731–2740. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Jakhmola, A.; Hornsby, T.K.; Tavakkoli, J.; Kolios, M.C. Ultrasound-mediated nano drug delivery for treating cancer: Fundamental physics to future directions. J. Controll. Release. 2023, 355, 522–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, W.; Wang, Z. Designing next-generation immune cell therapies with nanomaterials. BMEMat 2025, 3, 70003. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, K.; Zhang, R.; She, Z.; Tan, R.; Fan, Y.; Li, X. Magnetic nanoparticles applied in targeted therapy and magnetic resonance imaging: Crucial preparation parameters, indispensable pre-treatments, updated research advancements and future perspectives. J. Mater. Chem. B. 2020, 8, 5973–5991. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Eshaghi, M.M.; Ostovar, S.; Mohammadi, Z.; Sharma, R.K.; Paiva-Santos, A.C.; Rahmani, E.; Rahdar, A.; Pandey, S. Innovative nanomaterials for cancer diagnosis, imaging, and therapy: Drug delivery applications. J. Drug Deliv. Sci. Technol. 2023, 82, 104357. [Google Scholar] [CrossRef]
- Wei, J.; Xia, J.; Liu, X.; Ran, P.; Zhang, G.; Wang, C.; Li, X. Hollow-structured BaTiO3 nanoparticles with cerium-regulated defect engineering to promote piezocatalytic antibacterial treatment. Appl. Catal. B Environ. 2023, 328, 122520. [Google Scholar] [CrossRef]
- Wang, P.; Tang, Q.; Zhang, L.; Xu, M.; Sun, L.; Sun, S.; Zhang, J.; Wang, S.; Liang, X. Ultrasmall Barium Titanate Nanoparticles for Highly Efficient Hypoxic Tumor Therapy via Ultrasound Triggered Piezocatalysis and Water Splitting. ACS Nano. 2021, 15, 11326–11340. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, X.; Zhou, X.; Li, J. Nanosonosensitizers With Ultrasound-Induced Reactive Oxygen Species Generation for Cancer Sonodynamic Immunotherapy. Front. Bioeng. Biotechnol. 2021, 9, 761218. [Google Scholar] [CrossRef]
- Yu, J.; Hu, J.; Tian, Y.; Lei, Y.; Hu, H.; Lei, B.; Zhang, G.; Sun, Y.; Ye, H. Nanosensitizer-assisted sonodynamic therapy for breast cancer. J. Nanobiotechnol. 2025, 23, 281. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Kiritoshi, Y.; Watanabe, J.; Ishihara, K. Degradation of phospholipid polymer hydrogel by hydrogen peroxide aiming at insulin release device. Biomaterials 2003, 24, 5183–5190. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, B.; Chen, X.; Zhu, G.; Du, W.; Qing, L.; Wu, P.; Wang, Z.; Tang, J.; Xie, H. Enhanced piezocatalytic therapy of MRSA-infected osteomyelitis using ultrasound-triggered copper nanocrystals-doped barium titanate. Bioact. Mater. 2025, 51, 450–468. [Google Scholar] [CrossRef]
- Ortiz-Blanco, B.; Sanz, J.L.; Llena, C.; Lozano, A.; Forner, L. Dentin Sealing of Calcium Silicate-Based Sealers in Root Canal Retreatment: A Confocal Laser Microscopy Study. J. Funct. Biomater. 2022, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Adolpho, L.F.; Ribeiro, L.M.S.; Freitas, G.P.; Lopes, H.B.; Gomes, M.P.O.; Ferraz, E.P.; Gimenes, R.; Beloti, M.M.; Rosa, A.L. Mesenchymal Stem Cells Combined with a P(VDF-TrFE)/BaTiO3 Scaffold and Photobiomodulation Therapy Enhance Bone Repair in Rat Calvarial Defects. J. Funct. Biomater. 2023, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Zhang, Q.; Wang, H.; Wu, Z.; Guo, Y.; Li, Y.; Xiong, X.; Liu, K.; Fu, W.; Ma, Y.; et al. Synthesis of BaTiO3 nanoparticles by sol-gel assisted solid phase method and its formation mechanism and photocatalytic activity. Ceram. Int. 2020, 46, 10619–10633. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhong, Y.; He, X.; Mai, S. A Collagen Membrane Pretreated with Citrate Promotes Collagen Mineralization and Bone Regeneration. J. Funct. Biomater. 2025, 16, 261. [Google Scholar] [CrossRef]
- Liga, S.; Vodă, R.; Lupa, L.; Moacă, E.-A.; Muntean, D.; Barbu-Tudoran, L.; Suciu, M.; Socoliuc, V.; Péter, F. Synthesis of Ag2O/Ag Nanoparticles Using Puerarin: Characterization, Cytotoxicity, In Ovo Safety Profile, Antioxidant, and Antimicrobial Potential Against Nosocomial Pathogens. J. Funct. Biomater. 2025, 16, 258. [Google Scholar] [CrossRef]
- Alawak, M.; Mahmoud, G.; Dayyih, A.A.; Duse, L.; Pinnapireddy, S.R.; Engelhardt, K.; Awak, I.; Wölk, C.; König, A.M.; Brüßler, J.; et al. Magnetic resonance activatable thermosensitive liposomes for controlled doxorubicin delivery. Mater. Sci. Eng. C. 2020, 115, 111116. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Guo, Z.; Wei, J.; Liu, L.; Song, Y. Performance and mechanism of rhodamine B degradation by Fe-modified magnetic biochar via PMS activation. J. Environ. Eng. 2025, 15, 924–934. [Google Scholar]
- Tungjitkusolmun, S.; Staelin, S.T.; Haemmerich, D.; Tsai, J.Z.; Cao, H.; Webster, J.G.; Lee, F.T.; Mahvi, D.M.; Vorperian, V.R. Three-dimensional finite-element analyses for radio-frequency hepatic tumor ablation. IEEE Trans. Biomed. Eng. 2002, 49, 3–9. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Bueno, L.S.R.; Bertemes-Filho, P. Evaluation of electric field in polymeric electrodes geometries for liquid biosensing applications using COMSOL multiphysics. Sens. Bio-Sens. Res. 2024, 44, 100663. [Google Scholar] [CrossRef]
- Gollmer, A.; Arnbjerg, J.; Blaikie, F.H.; Pedersen, B.W.; Breitenbach, T.; Daasbjerg, K.; Glasius, M.; Ogilby, P.R. Singlet Oxygen Sensor Green®: Photochemical behavior in solution and in a mammalian cell. Redox Rep. 2011, 87, 671–679. [Google Scholar] [CrossRef]
- Borges, J.S.; Campos, G.C.M.; Ferreira, J.A.; Romanazzi, G. Drug Release from Polymeric Platforms for Non Smooth Solutions. Appl. Numer. Math. 2025, 213, 12–37. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Kong, L.; Long, H.; Teng, J.; Zhen, H.; Ding, Q.; Pan, R.; Tian, X. Black Phosphorus Nanosheet Hydrogels Elicit a Thermogenic Effect and Enhance Diabetic Wound Healing through Controlled Drug Release. Mater. Des. 2025, 254, 113967. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, H.; Li, Z.; Xu, X.; Xing, H.; Wang, M.; Jia, H.; Liang, L.; Li, C.; Sun, L.; et al. Responsive Hydrogels Based on Triggered Click Reactions for Liver Cancer. Adv. Mater. 2022, 34, 2201651. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Feng, N.; Zhao, S.; Shi, J.; Yang, G.; Guo, W.; Liu, Y.; Fan, K. Microenvironment-Responsive Injectable Thermosensitive Hydrogel Incorporating Nanozymes for Synergistic Breast Cancer Therapy and Postsurgical Adjuvant Treatment. Adv. Funct. Mater. 2025, 35, 2421176. [Google Scholar] [CrossRef]
- Qiu, L.; Kumpf, S.W.; Oziolor, E.M.; Sheehan, M.; Finley, J.E.; Rubitski, D.M.; Qian, J.; Gosink, M.M.; Kopec, A.K.; Lanz, T.A.; et al. In vitro NIH3T3 mouse embryonic fibroblast cell model does not predict AAV2 or AAVdj-mediated cell transformation. Toxicol. Appl. Pharmacol. 2025, 495, 117229. [Google Scholar] [CrossRef]
- Srikanth, M.; Khan, W.S.; Asmatulu, R.; Misak, H.E.; Yang, S.Y.; Asmatulu, E. In vitro Cytotoxicity Studies of Industrially Used Common Nanomaterials on L929 and 3T3 Fibroblast Cells. J. Biomed. Res. Environ. Sci. 2020, 1, 192–200. [Google Scholar] [CrossRef]








| Sample Name | Zeta Potential (mV) | Mobility (μ/s)/(V/cm) |
|---|---|---|
| BTO | −11.18 | −0.87 |
| −12.20 | −0.95 | |
| −15.95 | −1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, K.; Li, T.; Ma, Y.; Zhang, C.; Zhang, K.; Qi, C.; Cai, K. Ultrasound-Responsive Drug Delivery System Based on Piezoelectric Catalytic Mechanisms. J. Funct. Biomater. 2025, 16, 304. https://doi.org/10.3390/jfb16080304
Cui K, Li T, Ma Y, Zhang C, Zhang K, Qi C, Cai K. Ultrasound-Responsive Drug Delivery System Based on Piezoelectric Catalytic Mechanisms. Journal of Functional Biomaterials. 2025; 16(8):304. https://doi.org/10.3390/jfb16080304
Chicago/Turabian StyleCui, Kaixi, Tianzheng Li, Yifei Ma, Chuanjin Zhang, Ke Zhang, Chao Qi, and Kaiyong Cai. 2025. "Ultrasound-Responsive Drug Delivery System Based on Piezoelectric Catalytic Mechanisms" Journal of Functional Biomaterials 16, no. 8: 304. https://doi.org/10.3390/jfb16080304
APA StyleCui, K., Li, T., Ma, Y., Zhang, C., Zhang, K., Qi, C., & Cai, K. (2025). Ultrasound-Responsive Drug Delivery System Based on Piezoelectric Catalytic Mechanisms. Journal of Functional Biomaterials, 16(8), 304. https://doi.org/10.3390/jfb16080304







