Synthesis of Ag2O/Ag Nanoparticles Using Puerarin: Characterization, Cytotoxicity, In Ovo Safety Profile, Antioxidant, and Antimicrobial Potential Against Nosocomial Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Microbial Strains
2.2. Synthesis Route of Ag2O/Ag NPs Using Isoflavone Puerarin
2.3. Characterization Techniques of PUE@Ag2O/Ag NPs
2.4. Antioxidant Activity Evaluation by DPPH Method
2.5. In Vitro Evaluation of the Antibacterial Activity
2.5.1. Disk Diffusion Method
2.5.2. Dilution Method for Quantifying Minimum Inhibitory Concentration
2.6. In Vitro Model–Cell Culturing Protocols
2.6.1. MTT Protocol—Mitochondrial Activity Assessment
2.6.2. Lactate Dehydrogenase (LDH) Released Method—Cytotoxicity Test
2.7. In Ovo Screening Profile Assessment Through HET-CAM Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. X-Ray Diffraction (XRD) and FTIR Characterization of PUE@Ag2O/Ag NPs
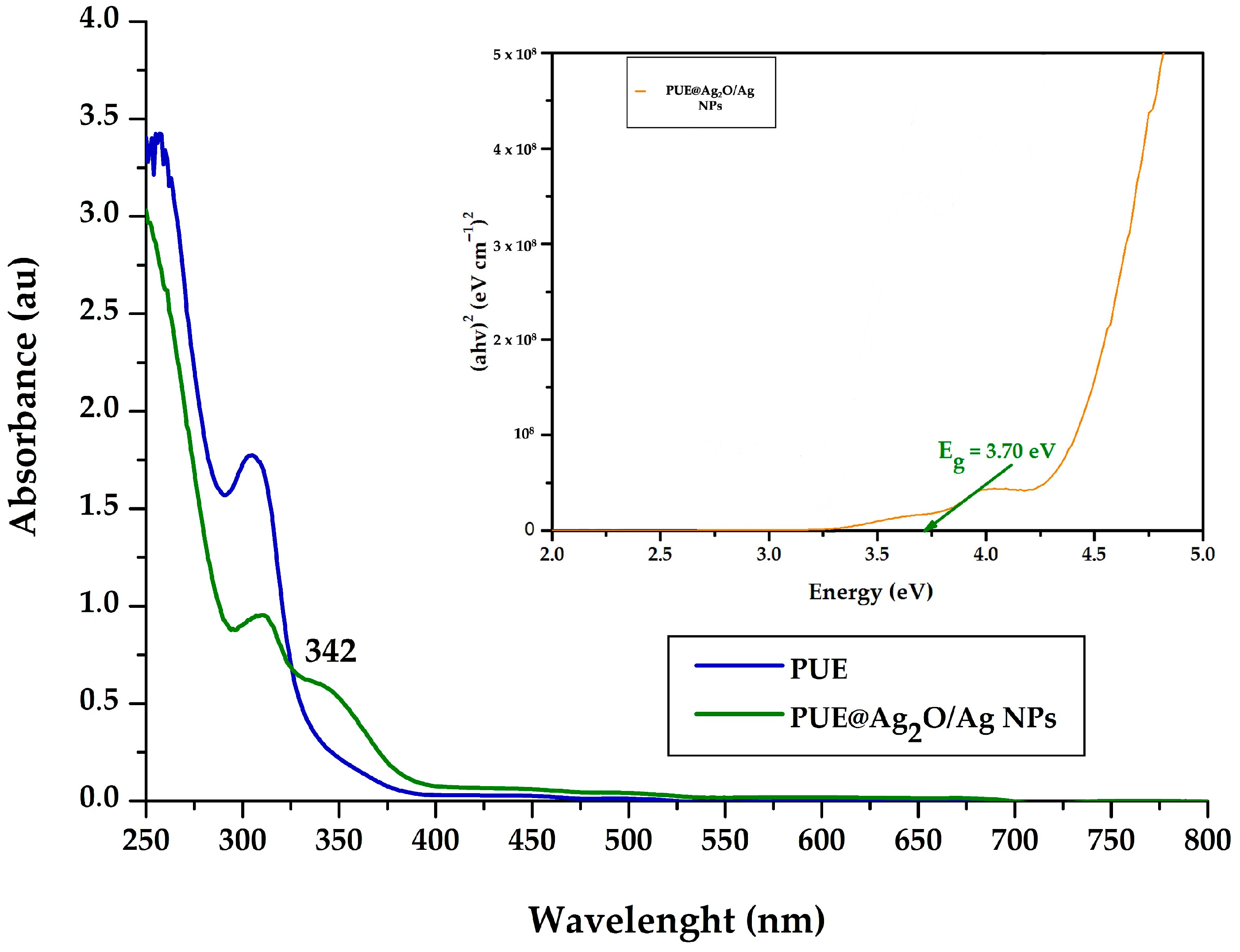
3.2. UV-Vis Spectroscopy
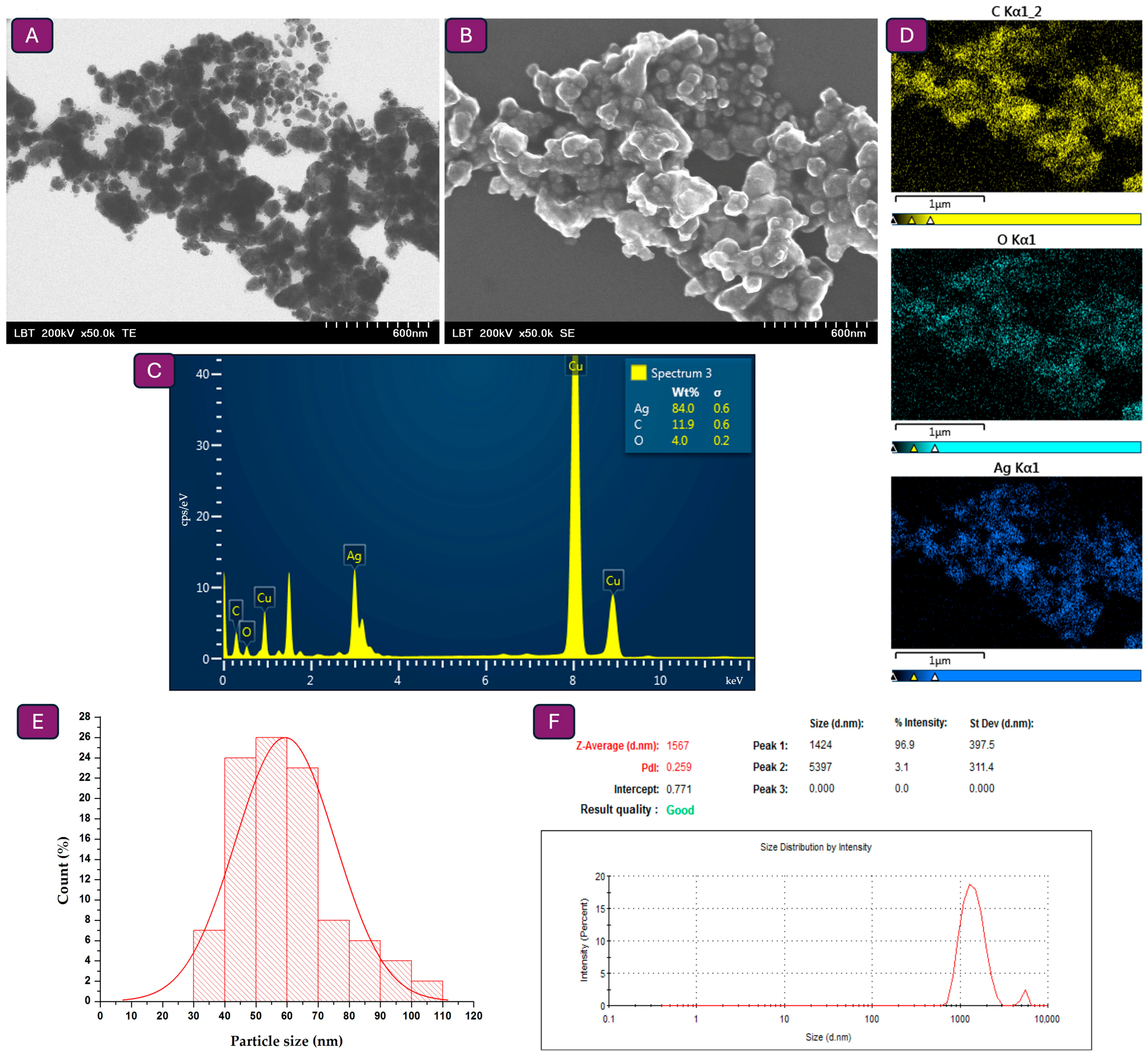
3.3. Particle Size Measurements Through Electron Microscopy and DLS Analysis
3.4. Biological Activities of PUE@Ag2O/Ag NPs
3.4.1. Antibacterial Activity
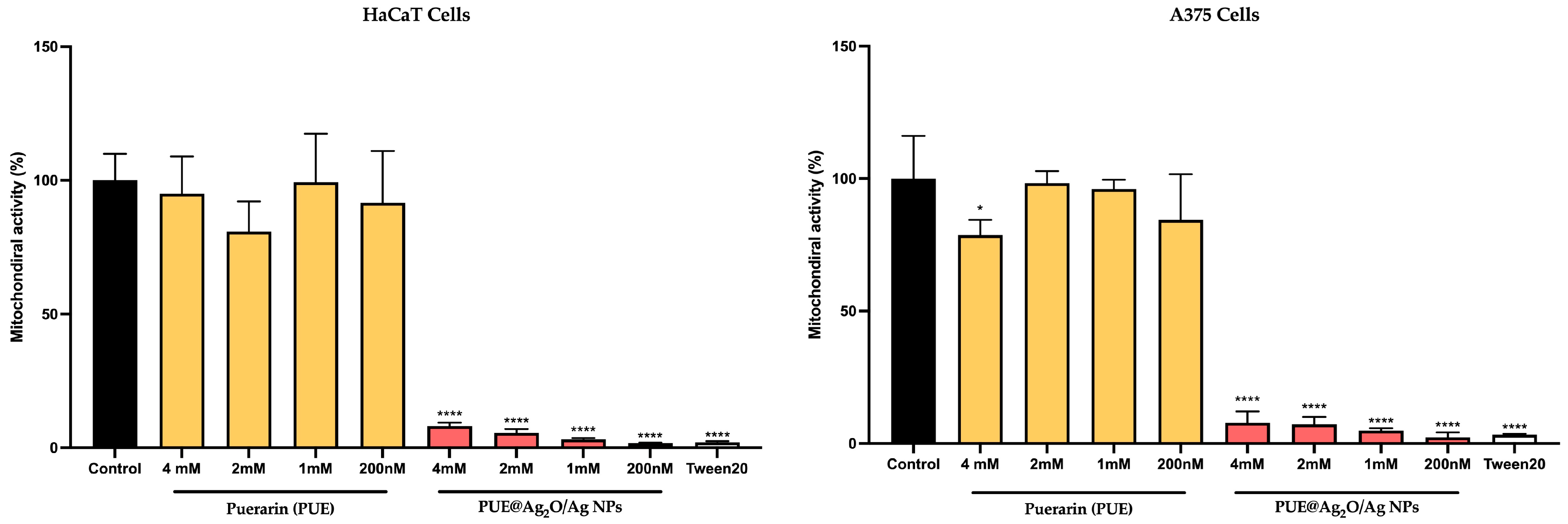
3.4.2. Impact on Mitochondrial Activity Using MTT Assay
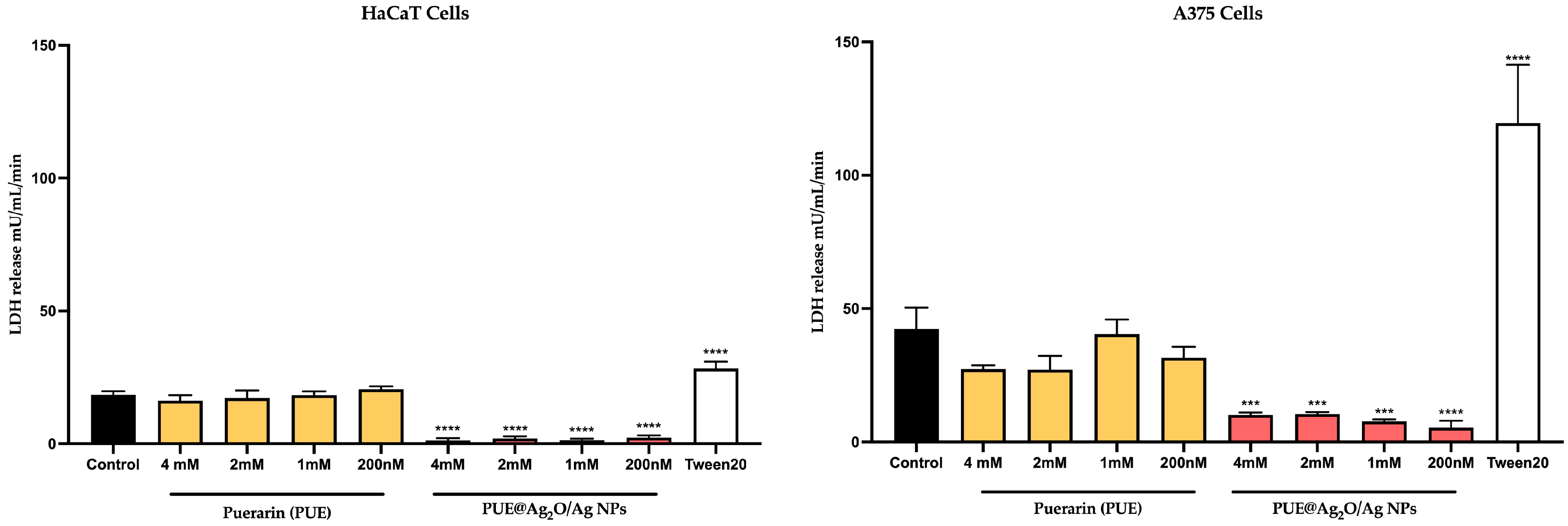
3.4.3. Cytotoxicity Evaluation via the LDH Release Method
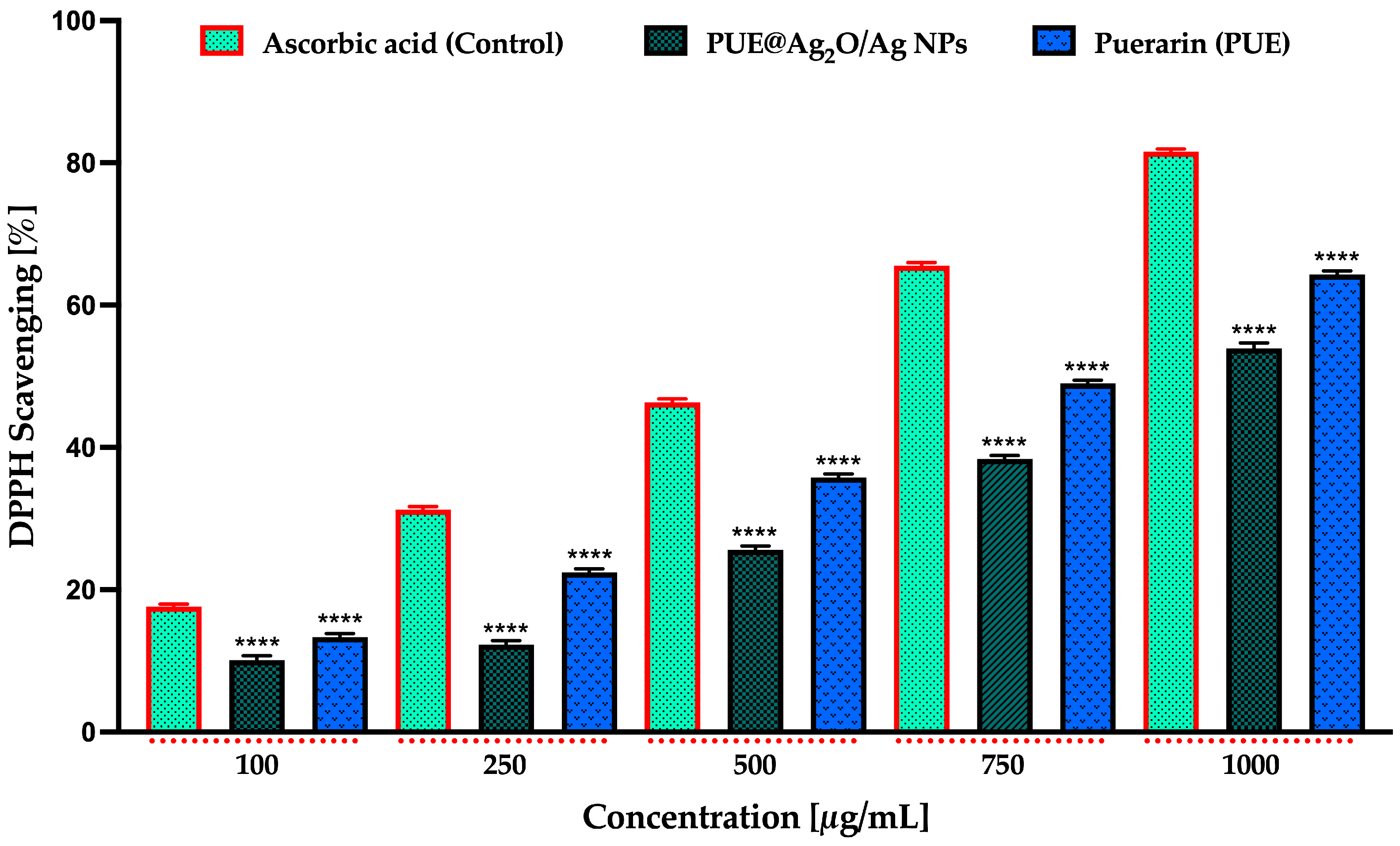
3.4.4. Antioxidant Activity Using DPPH Assay
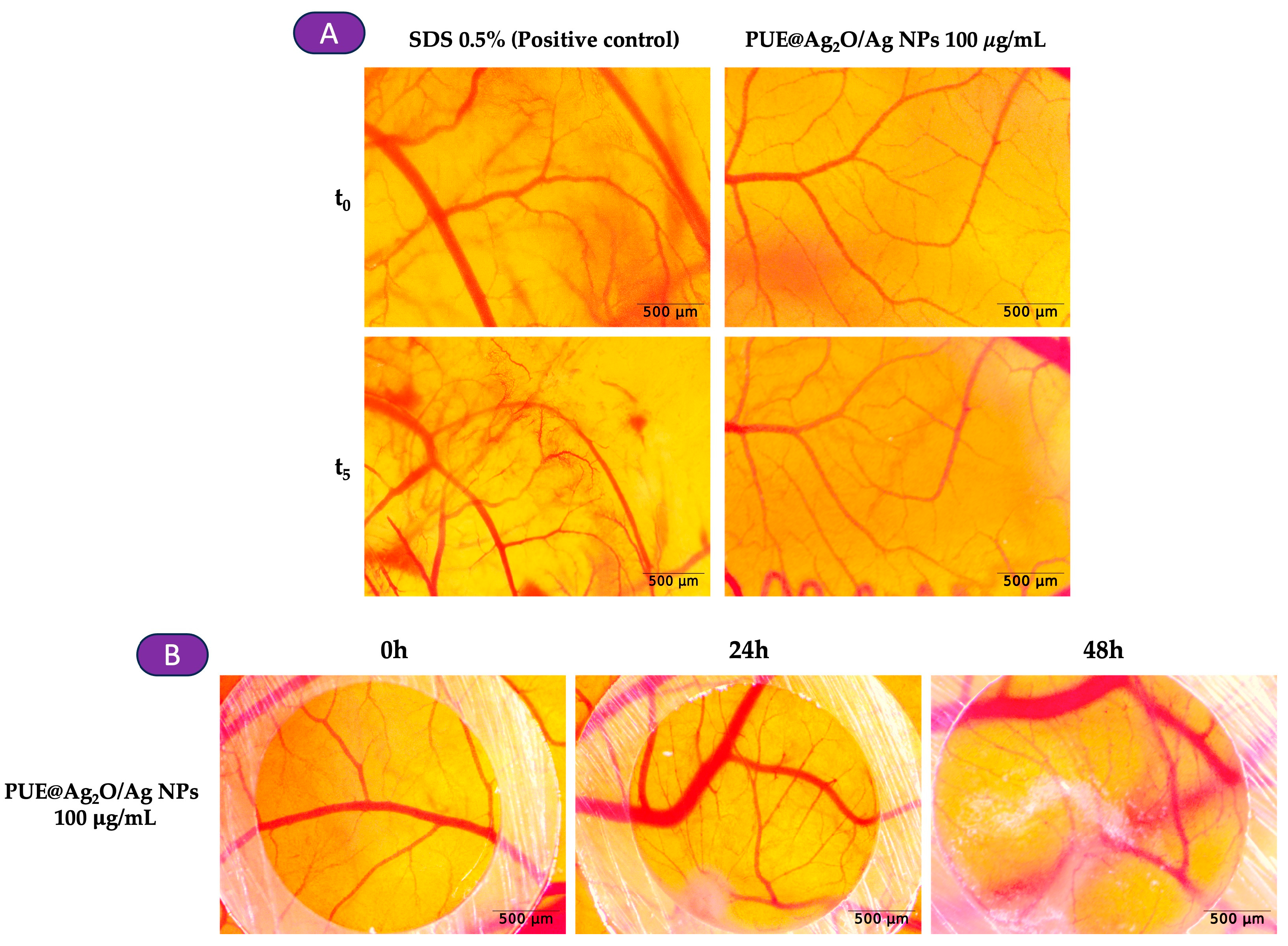
3.4.5. Irritant Potential and Influence of PUE@Ag2O/Ag NPs on In Ovo Angiogenesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szabó, S.; Kocsis, B.; Szabó, D. An Overview of Healthcare Associated Infections and Their Detection Methods. J. Clin. Med. 2022, 11, 3204. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial Infections: Epidemiology, Prevention, Control and Surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Sartelli, M.; Di Bella, S.; McFarland, L.V.; Ansaloni, L.; Coccolini, F.; Chichom-Mefire, A.; Hardcastle, T.C.; Abu-Zidan, F.M.; Catena, F. Preventing and Controlling Healthcare-Associated Infections: The First Principle of Every Antimicrobial Stewardship Program in Hospital Settings. Antibiotics 2024, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Nguyen, T.Y.N.; Le, T.H.N.; Le, T.N.T.; Chau, N.T.N.; Le, T.M.H.; Nguyen, B.Q.H. Medicinal Plants as a Potential Resource for the Discovery of Novel Structures Towards Cancer Drug Resistance Treatment. Heliyon 2024, 10, e39229. [Google Scholar] [CrossRef]
- Chandra, S.; Gahlot, M.; Choudhary, A.N.; Palai, S.; de Almeida, R.S.; de Vasconcelos, J.E.L.; dos Santos, F.A.V.; de Farias, P.A.M.; Coutinho, H.D.M. Scientific Evidences of Anticancer Potential of Medicinal Plants. Food Chem. Adv. 2023, 2, 100239. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent Advances in Green Synthesized Nanoparticles: From Production to Application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Serna-Gallén, P.; Mužina, K. Metallic Nanoparticles at the Forefront of Research: Novel Trends in Catalysis and Plasmonics. Nano Mater. Sci. 2024. [Google Scholar] [CrossRef]
- Shume, W.M.; Murthy, H.C.A.; Zereffa, E.A. A Review on Synthesis and Characterization of Ag2O Nanoparticles for Photocatalytic Applications. J. Chem. 2020, 2020, 5039479. [Google Scholar] [CrossRef]
- Dharmaraj, D.; Krishnamoorthy, M.; Rajendran, K.; Karuppiah, K.; Annamalai, J.; Durairaj, K.R.; Santhiyagu, P.; Ethiraj, K. Antibacterial and Cytotoxicity Activities of Biosynthesized Silver Oxide (Ag2O) Nanoparticles Using Bacillus paramycoides. J. Drug Deliv. Sci. Technol. 2021, 61, 102111. [Google Scholar] [CrossRef]
- Iwuji, C.; Saha, H.; Ghann, W.; Dotson, D.; Bhuiya, M.A.K.; Parvez, M.S.; Jahangir, Z.S.; Rahman, M.M.; Chowdhury, F.I.; Uddin, J. Synthesis and Characterization of Silver Nanoparticles and Their Promising Antimicrobial Effects. Chem. Phys. Impact 2024, 9, 100758. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Gusta, M.F.; Bastus, N.; Rello, J.; Puntes, V. Silver Nanoparticles and Antibiotics: A Promising Synergistic Approach to Multidrug-Resistant Infections. Microorganisms 2025, 13, 952. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, S.; Blanco-Agudín, N.; Rodríguez, D.; Fernández-Vega, I.; Merayo-Lloves, J.; Quirós, L.M. Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases. Antibiotics 2025, 14, 289. [Google Scholar] [CrossRef]
- Mahadevan, A.; Chinnadura, M.C.; Suresh, R.; Yogeshwaran, A.; Logababu, P. The Proliferation of Refined Optical and Emission Properties of Silver Oxide Nanoparticles Using Various Leaf Extracts. Front. Adv. Mater. Res. 2023, 1, 94–102. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella-Pajulas, L.L.; Alemaida, I.M.; Grilli, M.L.; Mikhaylov, A.; Senjyu, T. Green Synthesis of Silver Oxide Nanoparticles for Photocatalytic Environmental Remediation and Biomedical Applications. Metals 2022, 12, 769. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Terenteva, E.A.; Apyari, V.V.; Dmitrienko, S.G.; Zolotov, Y.A. Formation of Plasmonic Silver Nanoparticles by Flavonoid Reduction: A Comparative Study and Application for Determination of These Substances. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 89–95. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of Silver Nanoparticles Using Flavonoids: Hesperidin, Naringin and Diosmin, and Their Antibacterial Effects and Cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Kulkarni, D.; Sherkar, R.; Shirsathe, C.; Sonwane, R.; Varpe, N.; Shelke, S.; More, M.P.; Pardeshi, S.R.; Dhaneshwar, G.; Junnuthula, V.; et al. Biofabrication of Nanoparticles: Sources, Synthesis, and Biomedical Applications. Front. Bioeng. Biotechnol. 2023, 11, 1159193. [Google Scholar] [CrossRef] [PubMed]
- Zanbili, F.; Poursattar Marjani, A. Innovative Green and Bio-Based Approaches for Photosensitive Nanoparticle Synthesis: A Review on Methodologies, Characterization, and Applications. Micro Nano Syst. Lett. 2025, 13, 3. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Bodroth, R.P. Green Synthesis of Silver Nanoparticles Using Flavonoids and Assessment of Their Antimicrobial Properties. BioNanoSci 2023, 13, 186–193. [Google Scholar] [CrossRef]
- Sysak, S.; Czarczynska-Goslinska, B.; Szyk, P.; Koczorowski, T.; Mlynarczyk, D.T.; Szczolko, W.; Lesyk, R.; Goslinski, T. Metal Nanoparticle-Flavonoid Connections: Synthesis, Physicochemical and Biological Properties, as Well as Potential Applications in Medicine. Nanomaterials 2023, 13, 1531. [Google Scholar] [CrossRef]
- Gong, X.; Jadhav, N.D.; Lonikar, V.V.; Kulkarni, A.N.; Zhang, H.; Sankapal, B.R.; Ren, J.; Xu, B.B.; Pathan, H.M.; Ma, Y.; et al. An Overview of Green Synthesized Silver Nanoparticles towards Bioactive Antibacterial, Antimicrobial and Antifungal Applications. Adv. Colloid Interface Sci. 2024, 323, 103053. [Google Scholar] [CrossRef]
- Vidyasagar; Patel, R.R.; Singh, S.K.; Singh, M. Green Synthesis of Silver Nanoparticles: Methods, Biological Applications, Delivery and Toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar] [CrossRef]
- Dhaka, A.; Mali, S.C.; Sharma, S.; Trivedi, R. A Review on Biological Synthesis of Silver Nanoparticles and Their Potential Applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Ahmed, A.; Usman, M.; Ji, Z.; Rafiq, M.; Yu, B.; Shen, Y.; Cong, H. Nature-Inspired Biogenic Synthesis of Silver Nanoparticles for Antibacterial Applications. Mater. Today Chem. 2023, 27, 101339. [Google Scholar] [CrossRef]
- Gungure, A.S.; Jule, L.T.; Nagaprasad, N.; Dey, S.R.; Dhanabal, R.; Krishnaraj, R.; Mishra, N.; Saka, A. Studying the Properties of Green Synthesized Silver Oxide Nanoparticles in the Application of Organic Dye Degradation under Visible Light. Sci. Rep. 2024, 14, 26967. [Google Scholar] [CrossRef]
- Saka, A.; Dey, S.R.; Jule, L.T.; Krishnaraj, R.; Dhanabal, R.; Mishra, N.; Nagaprasad, N. Investigating Antibacterial Activity of Biosynthesized Silver Oxide Nanoparticles Using Phragmanthera macrosolen L. Leaf Extract. Sci. Rep. 2024, 14, 26850. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.Z.R.; Falak, U.; Naz, T.; Baig, M.M.; Ahmad, R.T.M.; Rasheed, A.; Dastgeer, G. Synthesis of Silver/Silver Oxide Heterostructures via Partial Reduction of AgNO3 Using a Novel Green Reducing Agent. Ceram. Int. 2022, 48, 37194–37202. [Google Scholar] [CrossRef]
- Belaiche, Y.; Khelef, A.; Laouini, S.E.; Bouafia, A.; Tedjani, M.L.; Barhoum, A. Green Synthesis and Characterization of Silver/Silver Oxide Nanoparticles Using Aqueous Leaves Extract of Artemisia Herba-Alba as Reducing and Capping Agents. Rev. Rom. Mater. 2021, 51, 342–352. [Google Scholar]
- Gallardo, O.A.D.; Moiraghi, R.; Macchione, M.A.; Godoy, J.A.; Pérez, M.A.; Coronado, E.A.; Macagno, V.A. Silver Oxide Particles/Silver Nanoparticles Interconversion: Susceptibility of Forward/Backward Reactions to the Chemical Environment at Room Temperature. RSC Adv. 2012, 2, 2923–2929. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plant Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Velidandi, A.; Dahariya, S.; Pabbathi, N.P.P.; Kalivarathan, D.; Baadhe, R.R. A Review on Synthesis, Applications, Toxicity, Risk Assessment and Limitations of Plant Extracts Synthesized Silver Nanoparticles. NanoWorld J. 2020, 6, 35–60. [Google Scholar] [CrossRef]
- Mohammadi, E.; Amini, S.M. Green Synthesis of Stable and Biocompatible Silver Nanoparticles with Natural Flavonoid Apigenin. Nano-Struct. Nano-Obj. 2024, 38, 101175. [Google Scholar] [CrossRef]
- Li, Z.; Ma, W.; Ali, I.; Zhao, H.; Wang, D.; Qiu, J. Green Synthesis of Silver Nanoparticles Using Plant Extracts and Their Antibacterial Activity. ACS Omega 2020, 5, 32632–32640. [Google Scholar] [CrossRef]
- Wang, D.; Bu, T.; Li, Y.; He, Y.; Yang, F.; Zou, L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants 2022, 11, 2121. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C. Puerarin—A Promising Flavonoid: Biosynthesis, Extraction Methods, Analytical Techniques, and Biological Effects. Int. J. Mol. Sci. 2024, 25, 5222. [Google Scholar] [CrossRef]
- Li, H.; Dong, L.; Liu, Y.; Wang, G.; Wang, G.; Qiao, Y. Biopharmaceutics Classification of Puerarin and Comparison of Perfusion Approaches in Rats. Int. J. Pharm. 2014, 466, 133–138. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Zhang, H.; Peng, C. Effects of Puerarin on the Prevention and Treatment of Cardiovascular Diseases. Front. Pharmacol. 2021, 12, 771793. [Google Scholar] [CrossRef] [PubMed]
- Zehra, T.; Ahsan, F.; Versiani, M.A.; Wahid, S.; Jahangir, S.; Shah, M.R. Puerarin-Coated Gold Nanoparticles (PUE-AuNPs) Synthesized via Green Synthetic Route: A New Colorimetric Probe for the Detection of Ciprofloxacin. Turk. J. Chem. 2021, 45, 1814–1827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Xiong, J.; Su, S.; Hao, M.; Wei, S. The Fabrication of Silver Nanoparticles Using Puerarin and the Photothermal Sterilization and Diabetic Wound Healing Behavior. Acta Chim. Sin. 2024, 82, 1150–1161. [Google Scholar] [CrossRef]
- Liga, S.; Vodă, R.; Lupa, L.; Paul, C.; Nemeş, N.S.; Muntean, D.; Avram, Ș.; Gherban, M.; Péter, F. Green Synthesis of Zinc Oxide Nanoparticles Using Puerarin: Characterization, Antimicrobial Potential, Angiogenesis, and In Ovo Safety Profile Assessment. Pharmaceutics 2024, 16, 1464. [Google Scholar] [CrossRef]
- Santos, U.P.; Campos, J.F.; Torquato, H.F.V.; Paredes-Gamero, E.J.; Carollo, C.A.; Estevinho, L.M.; De Picoli Souza, K.; Dos Santos, E.L. Antioxidant, Antimicrobial and Cytotoxic Properties as Well as the Phenolic Content of the Extract from Hancornia speciosa Gomes. PLoS ONE 2016, 11, e0167531. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane in the Study of Tumor Angiogenesis. Rom. J. Morphol. Embryol. 2008, 49, 131–135. [Google Scholar]
- Luepke, N.P. Hen’s Egg Chorioallantoic Membrane Test for Irritation Potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.K.; Han, J.W.; Zhang, X.F.; Park, J.H.; Kim, J.H. Multidimensional Effects of Biologically Synthesized Silver Nanoparticles in Helicobacter pylori, Helicobacter felis, and Human Lung (L132) and Lung Carcinoma A549 Cells. Nanoscale Res. Lett. 2015, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J. Comparative Assessment of the Apoptotic Potential of Silver Nanoparticles Synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 Human Breast Cancer Cells: Targeting p53 for Anticancer Therapy. Int. J. Nanomed. 2015, 10, 4203–4223. [Google Scholar] [CrossRef]
- Fowsiya, J.; Madhumitha, G. Biomolecules Derived from Carissa edulis for the Microwave Assisted Synthesis of Ag2O Nanoparticles: A Study Against S. incertulas, C. medinalis and S. mauritia. J. Clust. Sci. 2019, 30, 1243–1252. [Google Scholar] [CrossRef]
- Aiswariya, K.S.; Jose, V. Bioactive Molecules Coated Silver Oxide Nanoparticle Synthesis from Curcuma zanthorrhiza and HR-LCMS Monitored Validation of Its Photocatalytic Potency Towards Malachite Green Degradation. J. Clust. Sci. 2022, 33, 1685–1696. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Jamal, A.; Faisal, M.; Asiri, A.M. Fabrication of Highly Sensitive Acetone Sensor Based on Sonochemically Prepared As-Grown Ag2O Nanostructures. Chem. Eng. J. 2012, 192, 122–128. [Google Scholar] [CrossRef]
- Balan, L.; Malval, J.-P.; Schneider, R.; Burget, D. Silver Nanoparticles: New Synthesis, Characterization and Photophysical Properties. Mater. Chem. Phys. 2007, 104, 417–421. [Google Scholar] [CrossRef]
- Švecová, M.; Ulbrich, P.; Dendisová, M.; Matějka, P. SERS Study of Riboflavin on Green-Synthesized Silver Nanoparticles Prepared by Reduction Using Different Flavonoids: What Is the Role of Flavonoid Used? Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 195, 236–245. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the Gap. J. Non Cryst. Solids 1972, 8–10, 569–585. [Google Scholar] [CrossRef]
- Wagner, E.P.I.I. Investigating Bandgap Energies, Materials, and Design of Light-Emitting Diodes. J. Chem. Educ. 2016, 93, 1289–1298. [Google Scholar] [CrossRef]
- Lakshmi, P.; Kumar, G.A. Nanosuspension Technology: A Review. Int. J. Pharm. Pharm. Sci. 2010, 2, 35–40. [Google Scholar]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Falke, S.; Betzel, C. Dynamic Light Scattering (DLS). In Radiation in Bioanalysis; Pereira, A.S., Tavares, P., Limão-Vieira, P., Eds.; Bioanalysis; Springer: Cham, Switzerland, 2019; Volume 8, pp. 173–193. [Google Scholar] [CrossRef]
- Gangal, A.; Bachhar, V.; Joshi, V.; Akhtar, N.; Duseja, M.; Sethiya, N.K.; Shukla, R.K. Green Synthesis of Silver Nanoparticles from the Essential Oil of Curcuma amada and Their Antihyperglycemic Effect in STZ-Induced Diabetic Rats. Inorg. Chem. Commun. 2024, 168, 112873. [Google Scholar] [CrossRef]
- Azzi, M.; Laib, I.; Bouafia, A.; Medila, I.; Tliba, A.; Laouini, S.E.; Alsaeedi, H.; Cornu, D.; Bechelany, M.; Barhoum, A. Antimutagenic and Anticoagulant Therapeutic Effects of Ag/Ag2O Nanoparticles from Olea europaea Leaf Extract: Mitigating Metribuzin-Induced Hepato- and Nephrotoxicity. Front. Pharmacol. 2024, 15, 1485525. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, T.; Gupta, J. A Review on the Toxicity of Silver Nanoparticles on Human Health. Mater. Today Proc. 2023, 81, 859–863. [Google Scholar] [CrossRef]
- Kubavat, K.; Trivedi, P.; Ansari, H.; Kongor, A.; Panchal, M.; Jain, V.; Sindhav, G. Green Synthesis of Silver Nanoparticles Using Dietary Antioxidant Rutin and Its Biological Contour. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 115. [Google Scholar] [CrossRef]
- Tasca, F.; Antiochia, R. Biocide Activity of Green Quercetin-Mediated Synthesized Silver Nanoparticles. Nanomaterials 2020, 10, 909. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical Devices—ISO 10993, Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 1999.
- Cataldi, S.; Ceccarini, M.R.; Patria, F.; Beccari, T.; Mandarano, M.; Ferri, I.; Lazzarini, A.; Curcio, F.; Albi, E. The Effect of Vitamin D3 and Silver Nanoparticles on HaCaT Cell Viability. Int. J. Mol. Sci. 2022, 23, 1410. [Google Scholar] [CrossRef]
- Franková, J.; Juráňová, J.; Kamarád, V.; Zálešák, B.; Ulrichová, J. Effect of AgNPs on the Human Reconstructed Epidermis. Interdiscip. Toxicol. 2018, 11, 289–293. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Correa, R.; Coronado, L.; Caballero, Z.; Faral-Tello, P.; Robello, C.; Spadafora, C. Extracellular Vesicles Carrying Lactate Dehydrogenase Induce Suicide in Increased Population Density of Plasmodium falciparum In Vitro. Sci. Rep. 2019, 9, 5042. [Google Scholar] [CrossRef]
- Chahardoli, A.; Hajmomeni, P.; Ghowsi, M.; Qalekhani, F.; Shokoohinia, Y.; Fattahi, A. Optimization of Quercetin-Assisted Silver Nanoparticles Synthesis and Evaluation of Their Hemocompatibility, Antioxidant, Anti-Inflammatory, and Antibacterial Effects. Glob. Chall. 2021, 5, 2100075. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Basist, P.; Alhalmi, A.; Khan, R.; Noman, O.M.; Alahdab, A. Synthesis of Quercetin-Loaded Silver Nanoparticles and Assessing Their Anti-Bacterial Potential. Micromachines 2023, 14, 2154. [Google Scholar] [CrossRef] [PubMed]
- Pandian, S.R.K.; Kunjiappan, S.; Ravishankar, V.; Sundarapandian, V. Synthesis of Quercetin-Functionalized Silver Nanoparticles by Rapid One-Pot Approach. Biotechnologia 2021, 102, 75–84. [Google Scholar] [CrossRef]
- Batish, S.; Rajput, J.K. Quercetin Capped Silver Nanoparticles as an Electrochemical Sensor for Ultrasensitive Detection of Chloramphenicol in Food and Water Samples. J. Food Compos. Anal. 2023, 122, 105421. [Google Scholar] [CrossRef]
- Lafta, M.Z.; Al-Samarrai, R.R.H.; Bouaziz, M. Green Synthesis of Silver and Gold Nanoparticles Using Quercetin Extracted from Arctium lappa by HPLC, Characterization and Estimation of Antioxidant Activity. Results Chem. 2025, 13, 102028. [Google Scholar] [CrossRef]
- Ramírez-Rosas, S.L.; Delgado-Alvarado, E.; Sanchez-Vargas, L.O.; Herrera-May, A.L.; Peña-Juarez, M.G.; Gonzalez-Calderon, J.A. Green Route to Produce Silver Nanoparticles Using the Bioactive Flavonoid Quercetin as a Reducing Agent and Food Anti-Caking Agents as Stabilizers. Nanomaterials 2022, 12, 3545. [Google Scholar] [CrossRef]
- Wu, H.; Su, M.; Jin, H.; Li, X.; Wang, P.; Chen, J.; Chen, J. Rutin-Loaded Silver Nanoparticles with Antithrombotic Function. Front. Bioeng. Biotechnol. 2020, 8, 598977. [Google Scholar] [CrossRef]
- Nemčeková, K.; Svitková, V.; Sochr, J.; Gemeiner, P.; Labuda, J. Gallic Acid-Coated Silver Nanoparticles as Perspective Drug Nanocarriers: Bioanalytical Study. Anal. Bioanal. Chem. 2022, 414, 5493–5505. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Yuan, Y.; Liu, Y.; Niu, F. Green Synthesis of Gallic Acid-Coated Silver Nanoparticles with High Antimicrobial Activity and Low Cytotoxicity to Normal Cells. Process Biochem. 2015, 50, 357–366. [Google Scholar] [CrossRef]
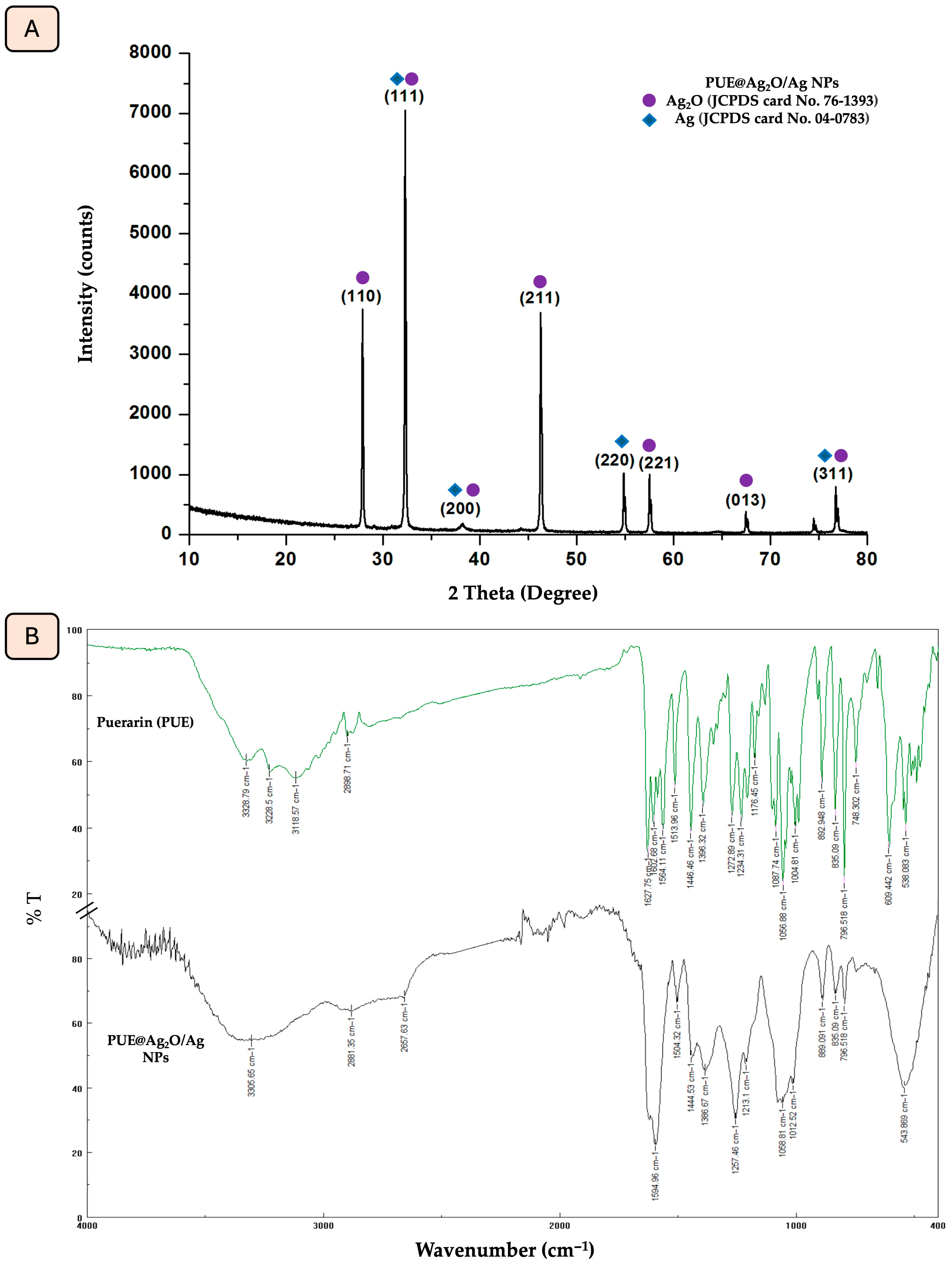
| Possible Assignment for Compound Class | Absorption Peaks of Puerarin (cm−1) | Absorption Peaks of PUE@Ag2O/Ag NPs (cm−1) |
|---|---|---|
| O-H stretching vibrations | 3328.79 3228.5 | 3305.65 |
| C-H aromatic vibration | 3118.57 2898.71 | 2881.35 |
| C=O stretching vibrations of the aryl ketone group | 1627.75 | 1594.66 |
| -C-O vibrational stretching | 1564.11 1513.96 1446.46 1056.88 | 1504.32 1444.53 1058.81 |
| Ag-O stretching vibration | - | 543.86 |
| Pathogenic Strains | Test Sample | Disk Diffusion Method (Inhibition Zones in mm) | Minimum Inhibitory Concentration (mg/mL) |
|---|---|---|---|
| Streptococcus pyogenes ATCC 19615 | PUE@Ag2O/Ag NPs Levofloxacin 5 µg | 20 24 | 125 * NA |
| Staphylococcus aureus ATCC 25923 | PUE@Ag2O/Ag NPs Gentamicin 10 µg | 21 26 | 125 * NA |
| Escherichia coli ATCC 25922 | PUE@Ag2O/Ag NPs Gentamicin 10 µg | 20 26 | 125 * NA |
| Pseudomonas aeruginosa ATCC 27853 | PUE@Ag2O/Ag NPs Gentamicin 10 µg | 22 23 | 125 * NA |
| Polyphenolic Compound | Physico-Chemical Parameters, Antioxidant and Antimicrobial Activities, Cytotoxicity Results | References |
|---|---|---|
| Quercetin |
| [75] |
| [76] | |
| [77] | |
| [78] | |
| [79] | |
| Apigenin |
| [37] |
| Rutin |
| [80] |
| [67] | |
| Myricetin |
| [38] |
| Gallic acid |
| [81] |
| [82] | |
| Puerarin |
| Our study |
| Sample | Irritation Score (IS) * | Irritation Category/Type of Effect |
|---|---|---|
| Positive control—SDS 0.5% | 15.58 ± 0.24 | Strongly irritant |
| PUE@Ag2O/Ag NPs, 100 μg/mL | 0 ± 0 | Non-irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liga, S.; Vodă, R.; Lupa, L.; Moacă, E.-A.; Muntean, D.; Barbu-Tudoran, L.; Suciu, M.; Socoliuc, V.; Péter, F. Synthesis of Ag2O/Ag Nanoparticles Using Puerarin: Characterization, Cytotoxicity, In Ovo Safety Profile, Antioxidant, and Antimicrobial Potential Against Nosocomial Pathogens. J. Funct. Biomater. 2025, 16, 258. https://doi.org/10.3390/jfb16070258
Liga S, Vodă R, Lupa L, Moacă E-A, Muntean D, Barbu-Tudoran L, Suciu M, Socoliuc V, Péter F. Synthesis of Ag2O/Ag Nanoparticles Using Puerarin: Characterization, Cytotoxicity, In Ovo Safety Profile, Antioxidant, and Antimicrobial Potential Against Nosocomial Pathogens. Journal of Functional Biomaterials. 2025; 16(7):258. https://doi.org/10.3390/jfb16070258
Chicago/Turabian StyleLiga, Sergio, Raluca Vodă, Lavinia Lupa, Elena-Alina Moacă, Delia Muntean, Lucian Barbu-Tudoran, Maria Suciu, Vlad Socoliuc, and Francisc Péter. 2025. "Synthesis of Ag2O/Ag Nanoparticles Using Puerarin: Characterization, Cytotoxicity, In Ovo Safety Profile, Antioxidant, and Antimicrobial Potential Against Nosocomial Pathogens" Journal of Functional Biomaterials 16, no. 7: 258. https://doi.org/10.3390/jfb16070258
APA StyleLiga, S., Vodă, R., Lupa, L., Moacă, E.-A., Muntean, D., Barbu-Tudoran, L., Suciu, M., Socoliuc, V., & Péter, F. (2025). Synthesis of Ag2O/Ag Nanoparticles Using Puerarin: Characterization, Cytotoxicity, In Ovo Safety Profile, Antioxidant, and Antimicrobial Potential Against Nosocomial Pathogens. Journal of Functional Biomaterials, 16(7), 258. https://doi.org/10.3390/jfb16070258











