Biocompatibility of Hydraulic Calcium Silicate-Based Cement MTA FlowTM on Human Dental Pulp Stem Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
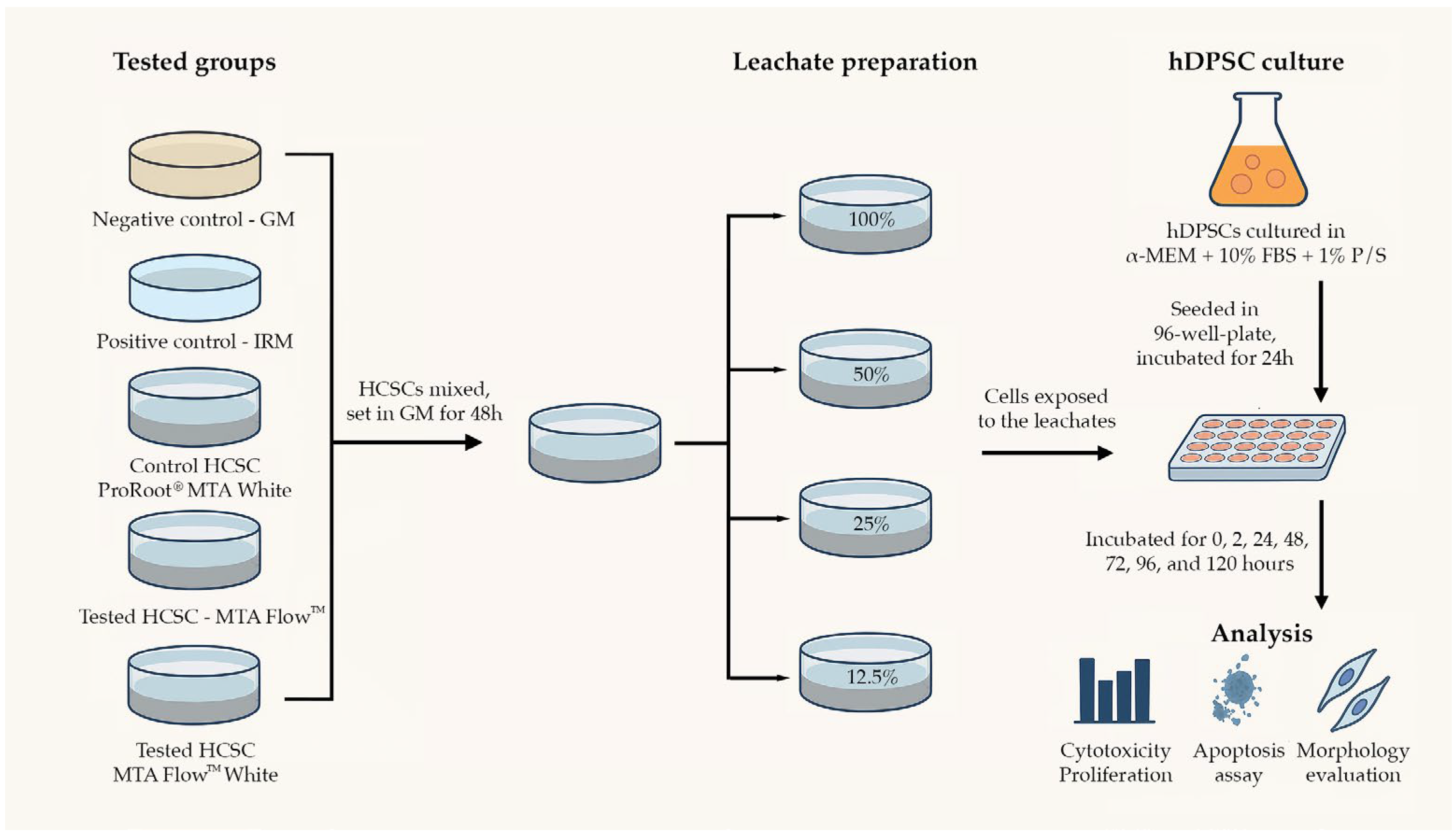
2.1. Groups and Tested Materials in the Study
- -
- Negative control group (NegativeCG)—growth medium. Serving as a reference control.
- -
- Positive control group (PositiveCG)—leachates extracted from intermediate restorative material (IRM, Dentsply Tulsa Dental, Tulsa, OK, USA). A control group to ensure induction of apoptosis or necrosis.
- -
- Control HCSC group (ProRootCG)—leachates extracted from gold-standard HCSC in research studies and dental clinical practice ProRoot® MTA (Dentsply Tulsa Dental, Tulsa, OK, USA).
- -
- Tested HCSC groups:
- o
- MTA FlowTM group (MF)—leachates extracted from MTA FlowTM 3:2 liquid-to-powder ratio consistency (Ultradent Products, Inc., South Jordan, UT, USA).
- o
- MTA FlowTM White group (MFWhite)—leachates extracted from MTA FlowTM White 3:2 liquid-to-powder ratio consistency (Ultradent Products, Inc., South Jordan, UT, USA).
2.2. Cell Culture
2.3. Material (Eluate/Leachate) Preparation
2.4. pH Measurement
2.5. Cell Cytotoxicity and Proliferation
2.6. Annexin V Apoptosis Assay
2.7. Cell Morphology Assessment
2.8. Statistical Analysis
3. Results
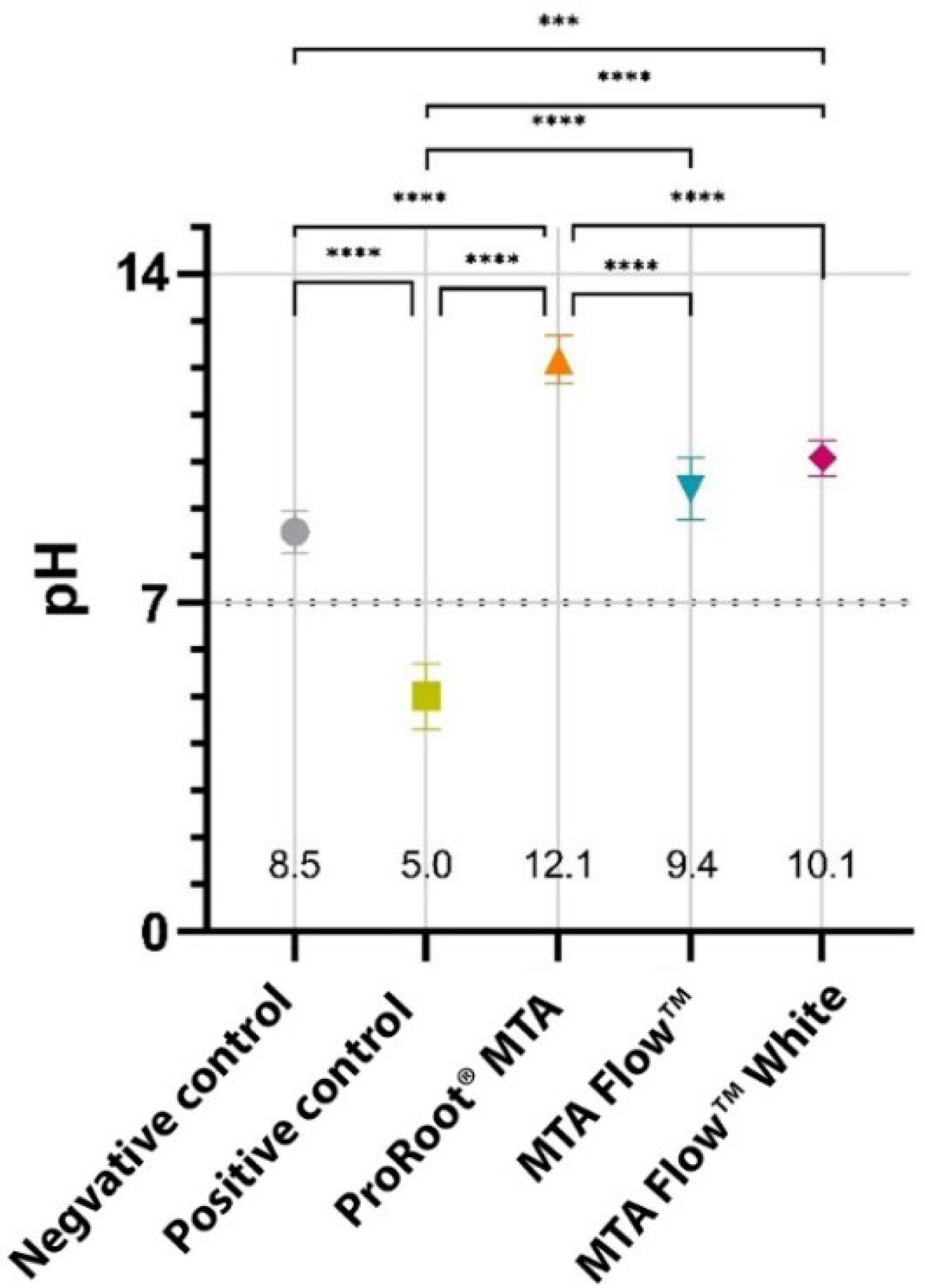
3.1. Characteristics of the Leachates
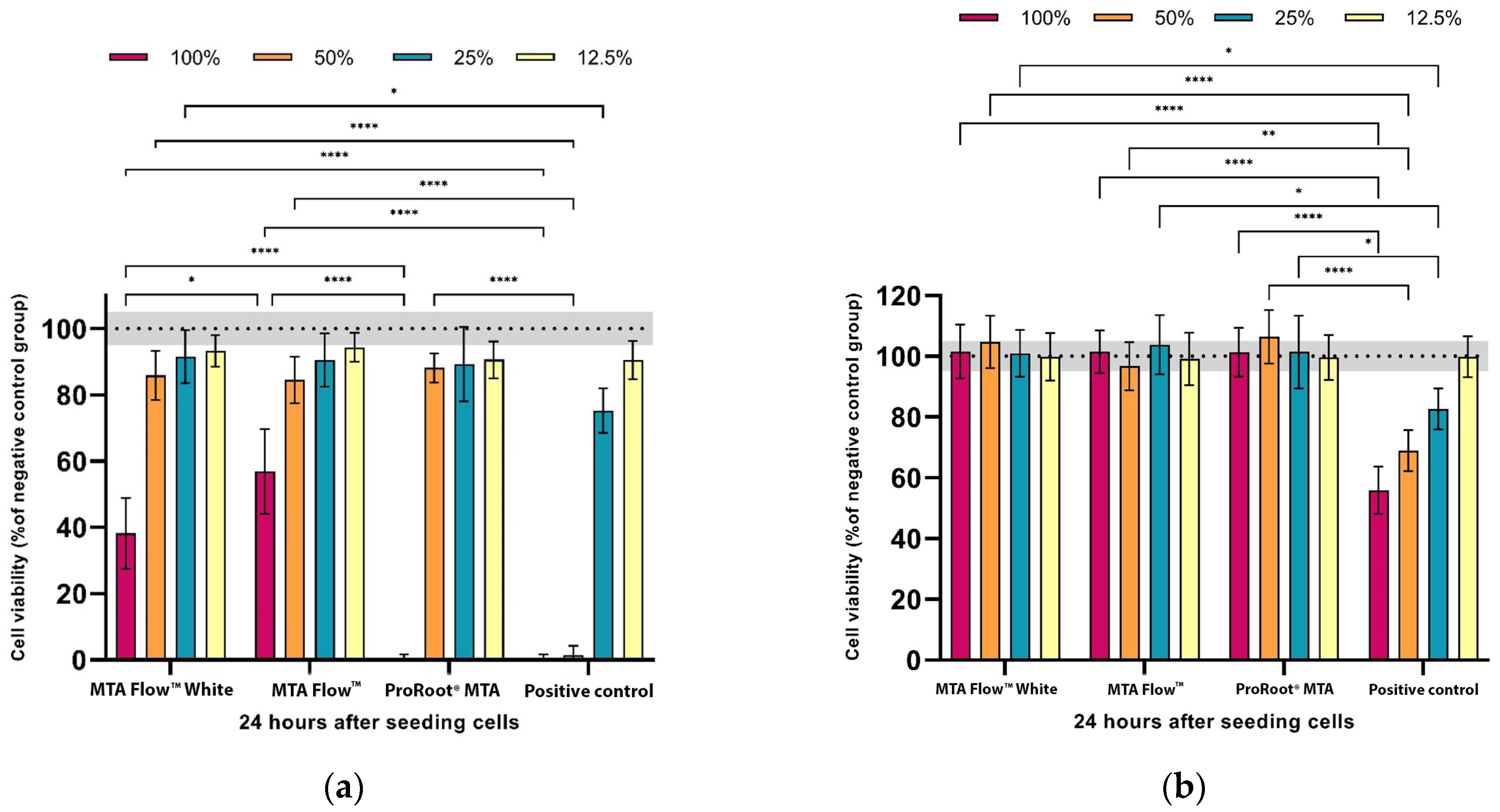
3.2. MTA FlowTM and MTA FlowTM White Cytotoxicity Assay
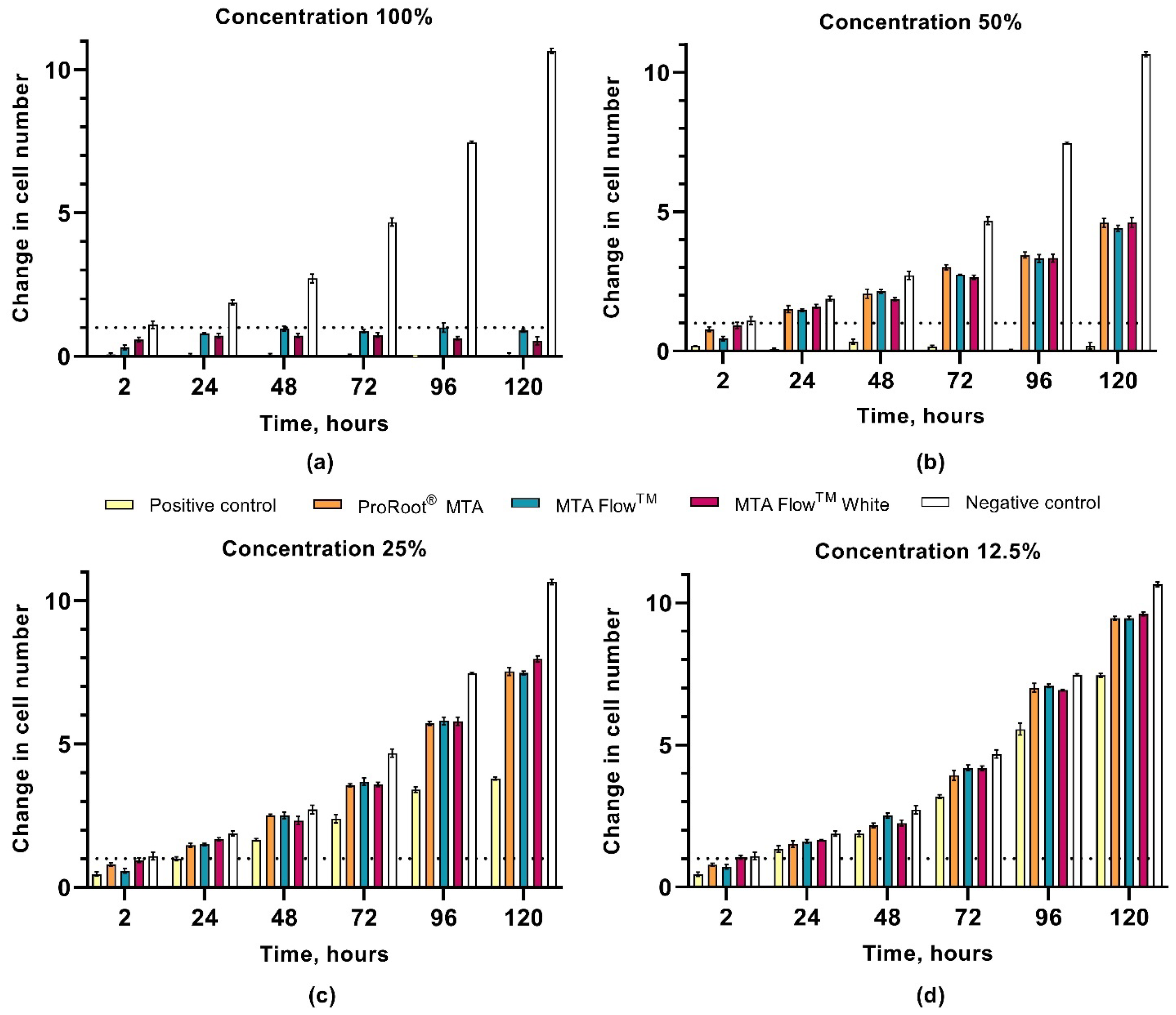
3.3. hDPSCs Proliferation Assay
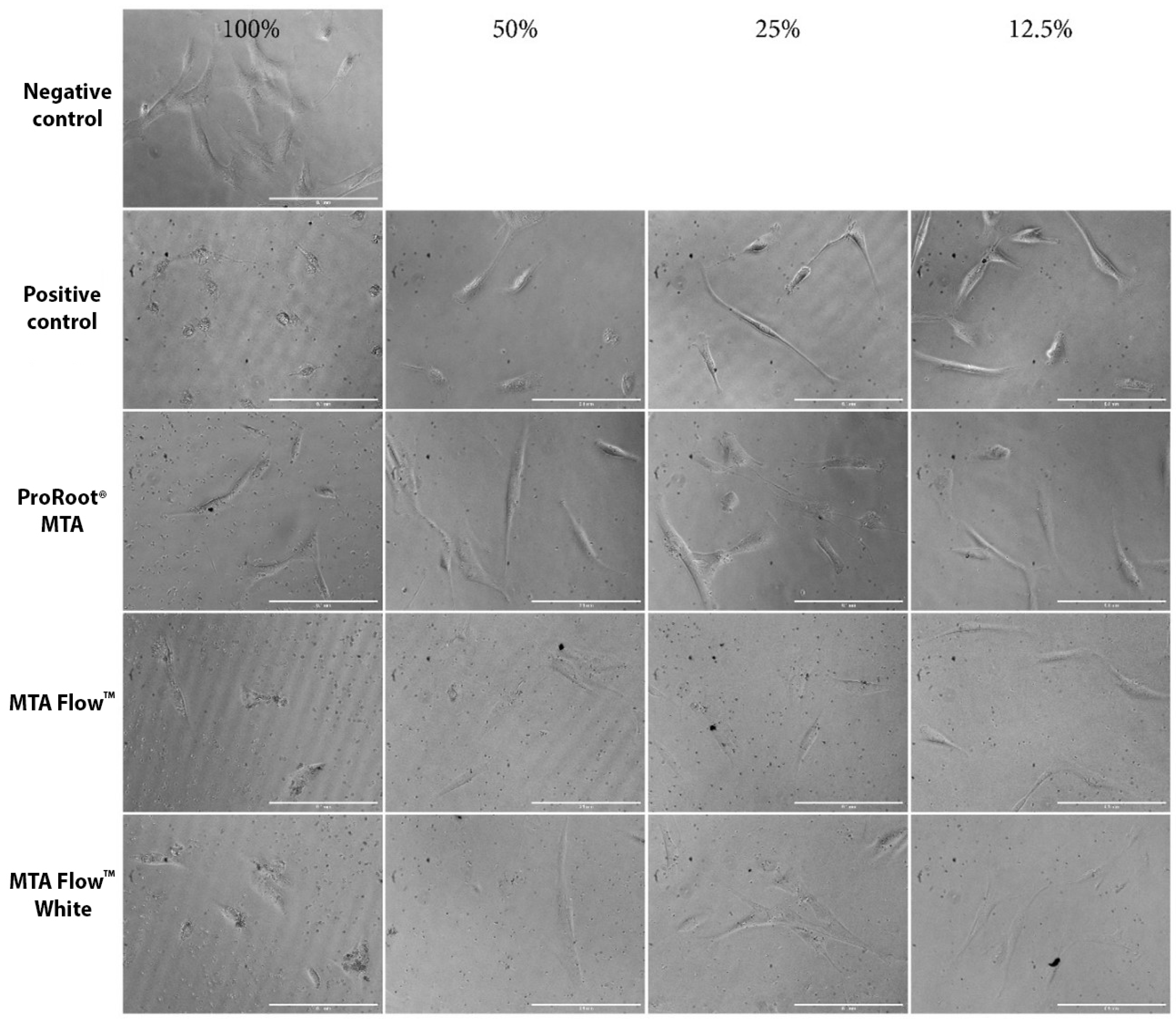
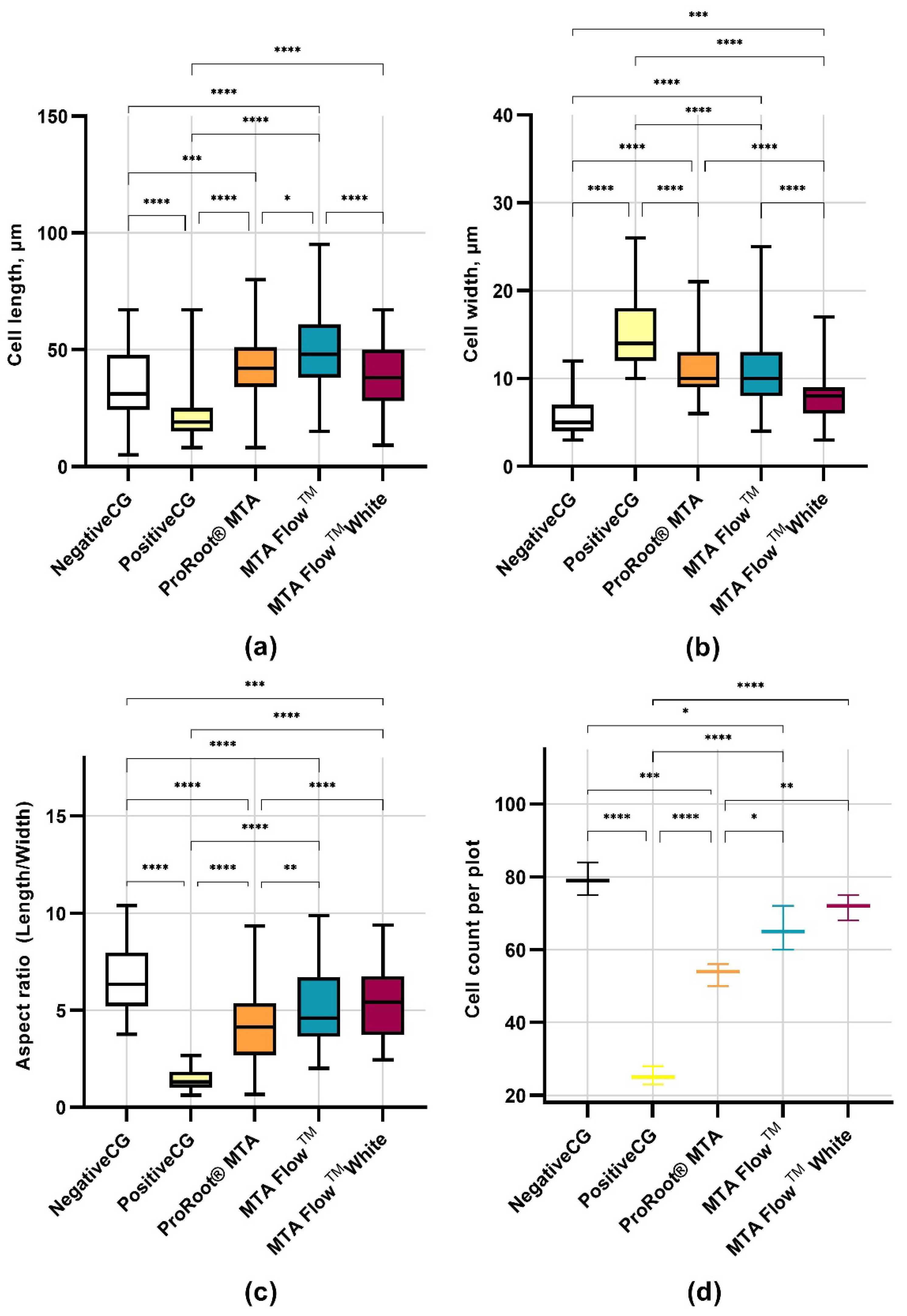
3.4. Human Dental Pulp Stem Cells Morphology Assessment
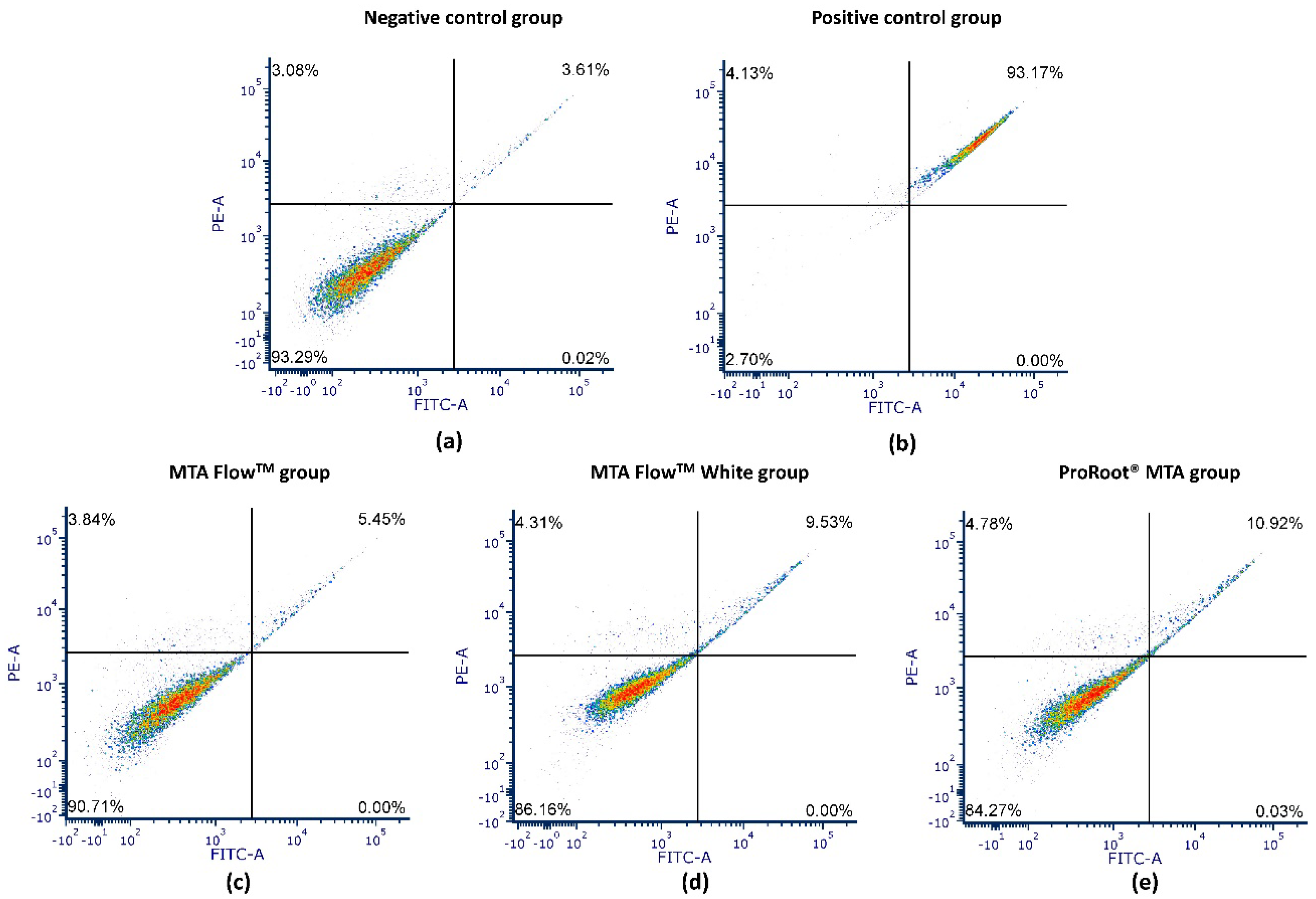
3.5. Annexin V Apoptosis Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hDPSCs | Human dental pulp stem cells |
| MTA | Mineral trioxide aggregate |
| HCSC | Hydraulic Calcium Silicate-Based cement |
| NegativeCG | Negative control group |
| PositveCG | Positive control group |
| ProRootCG | ProRoot® MTA group |
| MFWhite | MTA FlowTM White |
| MF | MTA Flow TM |
References
- Manaspon, C.; Jongwannasiri, C.; Chumprasert, S.; Sa-Ard-Iam, N.; Mahanonda, R.; Pavasant, P.; Porntaveetus, T.; Osathanon, T. Human dental pulp stem cell responses to different dental pulp capping materials. BMC Oral Health 2021, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Birant, S.; Gokalp, M.; Duran, Y.; Koruyucu, M.; Akkoc, T.; Seymen, F. Cytotoxicity of NeoMTA Plus, ProRoot MTA and Biodentine on human dental pulp stem cells. J. Dent. Sci. 2021, 16, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Kunert, M.; Lukomska-Szymanska, M. Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef]
- Kahler, B.; Taha, N.; Lu, J.; Saoud, T. Vital pulp therapy for permanent teeth with diagnosis of irreversible pulpitis: Biological basis and outcome. Aust. Dent. J. 2023, 68, S110–S122. [Google Scholar] [CrossRef]
- Camilleri, J.; Atmeh, A.; Li, X.; Meschi, N. Present status and future directions: Hydraulic materials for endodontic use. Int. Endod. J. 2022, 55, 710–777. [Google Scholar] [CrossRef]
- Duncan, H.F. Present status and future directions—Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55, 497–511. [Google Scholar] [CrossRef]
- Hirschberg, C.S.; Galicia, J.C.; Ruparel, N.B. The American Association of Endodontists AAE Position Statement on Vital Pulp Therapy. J. Endod. 2021, 47, 1340–1344. [Google Scholar] [CrossRef]
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Chua, P.; Elamin, A.D.; Clarke, M.; El-Karim, I.A. Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 556–571. [Google Scholar] [CrossRef]
- Clauder, T. Present status and future directions—Managing perforations. Int. Endod. J. 2022, 55, 872–891. [Google Scholar] [CrossRef]
- Simon, S.; Rilliard, F.; Berdal, A.; Machtou, P. The use of mineral trioxide aggregate in one-visit apexification treatment: A prospective study. Int. Endod. J. 2007, 40, 186–197. [Google Scholar] [CrossRef]
- Fagogeni, I.; Metlerska, J.; Lipski, M.; Falgowski, T.; Maciej, G.; Nowicka, A. Materials used in regenerative endodontic procedures and their impact on tooth discoloration. J. Oral Sci. 2019, 61, 379–385. [Google Scholar] [CrossRef]
- Nie, E.; Yu, J.; Jiang, R.; Liu, X.; Li, X.; Islam, R.; Alam, M.K. Effectiveness of direct pulp capping bioactive materials in dentin regeneration: A review. Materials 2021, 14, 6811. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Plenk, H.; Kim, S. Periapical Tissue Responses and Cementum Regeneration with Amalgam, SuperEBA, and MTA as Root-End Filling Materials. J. Endod. 2005, 31, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.W.; Koo, J.; Song, M.; Jang, I.H.; Gambarini, G.; Kim, H.-C. Physicochemical Properties and Biocompatibility of Various Bioceramic Root Canal Sealers: In Vitro Study. J. Endod. 2023, 49, 871–879. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium silicate and calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef]
- Camilleri, J. Classification of Hydraulic Cements Used in Dentistry. Front. Dent. Med. 2020, 1, 9. [Google Scholar] [CrossRef]
- Song, W.; Li, S.; Tang, Q.; Chen, L.; Yuan, Z. In vitro biocompatibility and bioactivity of calcium silicate-based bioceramics in endodontics (Review). Int. J. Mol. Med. 2021, 48, 1–22. [Google Scholar] [CrossRef]
- Ohshima, H.; Nakakura-Ohshima, K.; Takeuchi, K.; Hoshino, M.; Takano, Y.; Maeda, T. Pulpal regeneration after cavity preparation, with special reference to close spatio-relationships between odontoblasts and immunocompetent cells. Microsc. Res. Tech. 2003, 60, 483–490. [Google Scholar] [CrossRef]
- Niu, L.-N.; Jiao, K.; Wang, T.-D.; Zhang, W.; Camilleri, J.; Bergeron, B.E.; Feng, H.-L.; Mao, J.; Chen, J.-H.; Pashley, D.H.; et al. A review of the bioactivity of hydraulic calcium silicate cements. J. Dent. 2014, 42, 517–533. [Google Scholar] [CrossRef]
- Koutroulis, A.; Kuehne, S.A.; Cooper, P.R.; Camilleri, J. The role of calcium ion release on biocompatibility and antimicrobial properties of hydraulic cements. Sci. Rep. 2019, 9, 19019. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Q.; Camba, J.; Gu, L.-S.; Bergeron, B.E.; Ricucci, D.; Pashley, D.H.; Tay, F.R.; Niu, L.-N. Mechanism of bioactive molecular extraction from mineralized dentin by calcium hydroxide and tricalcium silicate cement. Dent. Mater. 2018, 34, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Tomson, P.L.; Lumley, P.J.; Smith, A.J.; Cooper, P.R. Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events. Int. Endod. J. 2017, 50, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, F.; Camilleri, J. A critical review of the material properties guiding the clinician’s choice of root canal sealers. Clin. Oral Investig. 2023, 27, 4147–4155. [Google Scholar] [CrossRef]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef]
- Dawood, A.E.; Parashos, P.; Wong, R.H.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part III: Clinical Applications, Drawbacks, and Mechanism of Action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Eren, S.K.; Örs, S.A.; Aksel, H.; Canay, Ş.; Karasan, D. Effect of irrigants on the color stability, solubility, and surface characteristics of calcium-silicate based cements. Restor. Dent. Endod. 2022, 47, e10. [Google Scholar] [CrossRef]
- Felman, D.; Parashos, P. Coronal Tooth Discoloration and White Mineral Trioxide Aggregate. J. Endod. 2013, 39, 484–487. [Google Scholar] [CrossRef]
- Shokouhinejad, N.; Nekoofar, M.H.; Pirmoazen, S.; Shamshiri, A.R.; Dummer, P.M. Evaluation and Comparison of Occurrence of Tooth Discoloration after the Application of Various Calcium Silicate–based Cements: An Ex Vivo Study. J. Endod. 2016, 42, 140–144. [Google Scholar] [CrossRef]
- Yoldaş, S.E.; Bani, M.; Atabek, D.; Bodur, H. Comparison of the Potential Discoloration Effect of Bioaggregate, Biodentine, and White Mineral Trioxide Aggregate on Bovine Teeth: In Vitro Research. J. Endod. 2016, 42, 1815–1818. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J. Endod. 2014, 40, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Shin, Y.-S.; Lee, H.-S.; Kim, S.-O.; Shin, Y.; Jung, I.-Y.; Song, J.S. Color Changes of Teeth after Treatment with Various Mineral Trioxide Aggregate–based Materials: An Ex Vivo Study. J. Endod. 2015, 41, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Condurache-Bota, S.; Tigau, N. The influence of the oxidation degree of bismuth oxide thin films on their optical properties. Rev. Roum. Chim. 2017, 62, 755–760. [Google Scholar]
- Ber, B.S.; Hatton, J.F.; Stewart, G.P. Chemical Modification of ProRoot MTA to Improve Handling Characteristics and Decrease Setting Time. J. Endod. 2007, 33, 1231–1234. [Google Scholar] [CrossRef]
- Kogan, P.; He, J.; Glickman, G.N.; Watanabe, I. The Effects of Various Additives on Setting Properties of MTA. J. Endod. 2006, 32, 569–572. [Google Scholar] [CrossRef]
- Emara, R.; Elhennawy, K.; Schwendicke, F. Effects of calcium silicate cements on dental pulp cells: A systematic review. J. Dent. 2018, 77, 18–36. [Google Scholar] [CrossRef]
- Farrugia, C.; Baca, P.; Camilleri, J.; Moliz, M.T.A. Antimicrobial activity of ProRoot MTA in contact with blood. Sci. Rep. 2017, 7, srep41359. [Google Scholar] [CrossRef]
- Gandolfi, M.; Iezzi, G.; Piattelli, A.; Prati, C.; Scarano, A. Osteoinductive potential and bone-bonding ability of ProRoot MTA, MTA Plus and Biodentine in rabbit intramedullary model: Microchemical characterization and histological analysis. Dent. Mater. 2017, 33, e221–e238. [Google Scholar] [CrossRef]
- Kang, T.-Y.; Choi, J.-W.; Seo, K.-J.; Kim, K.-M.; Kwon, J.-S. Physical, Chemical, Mechanical, and Biological Properties of Four Different Commercial Root-End Filling Materials: A Comparative Study. Materials 2021, 14, 1693. [Google Scholar] [CrossRef]
- Brizuela, C.; Ormeño, A.; Cabrera, C.; Cabezas, R.; Silva, C.I.; Ramírez, V.; Mercade, M. Direct Pulp Capping with Calcium Hydroxide, Mineral Trioxide Aggregate, and Biodentine in Permanent Young Teeth with Caries: A Randomized Clinical Trial. J. Endod. 2017, 43, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Parinyaprom, N.; Nirunsittirat, A.; Chuveera, P.; Na Lampang, S.; Srisuwan, T.; Sastraruji, T.; Bua-On, P.; Simprasert, S.; Khoipanich, I.; Sutharaphan, T.; et al. Outcomes of Direct Pulp Capping by Using Either ProRoot Mineral Trioxide Aggregate or Biodentine in Permanent Teeth with Carious Pulp Exposure in 6- to 18-Year-Old Patients: A Randomized Controlled Trial. J. Endod. 2017, 44, 341–348. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Orangi, J.; Lotfi, M.; Soukup, J.W.; Garcia-Godoy, F.; Sheibani, N. Effect of particle size on calcium release and elevation of pH of endodontic cements. Dent. Traumatol. 2015, 31, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Gokturk, H.; Ozkocak, I. The effect of different chelators on the dislodgement resistance of MTA Repair HP, MTA Angelus, and MTA Flow. Odontology 2022, 110, 20–26. [Google Scholar] [CrossRef]
- Sravya, V.; Deepa, V.L.; Lalitha, P.L.; Komandla, D.R.; Bollu, I.P.; Dalavai, P. Role of phosphate-buffered saline on push-out bond strength of MTA FlowTM and BiodentineTM after acid challenge: An in vitro study. J. Conserv. Dent. 2022, 25, 264–268. [Google Scholar]
- Pelepenko, L.E.; Saavedra, F.; Antunes, T.B.M.; Bombarda, G.F.; Gomes, B.P.F.A.; Zaia, A.A.; Camilleri, J.; Marciano, M.A. Physicochemical, antimicrobial, and biological properties of White-MTAFlow. Clin. Oral Investig. 2020, 25, 663–672. [Google Scholar] [CrossRef]
- Bueno, C.R.E.; Vasques, A.M.V.; Cury, M.T.S.; Sivieri-Araújo, G.; Jacinto, R.C.; Gomes-Filho, J.E.; Cintra, L.T.A.; Dezan-Júnior, E. Biocompatibility and biomineralization assessment of mineral trioxide aggregate flow. Clin. Oral Investig. 2019, 23, 169–177. [Google Scholar] [CrossRef]
- Abedi-Amin, A.; Luzi, A.; Giovarruscio, M.; Paolone, G.; Darvizeh, A.; Agulló, V.V.; Sauro, S. Innovative root-end filling materials based on calcium-silicates and calcium-phosphates. J. Mater. Sci. Mater. Med. 2017, 28, 31. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices–Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
- Alksne, M.; Simoliunas, E.; Kalvaityte, M.; Skliutas, E.; Rinkunaite, I.; Gendviliene, I.; Baltriukiene, D.; Rutkunas, V.; Bukelskiene, V. The effect of larger than cell diameter polylactic acid surface patterns on osteogenic differentiation of rat dental pulp stem cells. J. Biomed. Mater. Res. Part A 2019, 107, 174–186. [Google Scholar] [CrossRef]
- Benetti, F. Cytotoxicity and biocompatibility of a new bioceramic endodontic sealer containing calcium hydroxide. Braz. Oral Res. 2019, 33, e042. [Google Scholar] [CrossRef]
- Escobar-García, D.M.; Medina-Rosas, M.G.; González-Amaro, A.M.; Méndez-González, V.; Flores, H.; Pozos-Guillén, A. MTA-Based Cements: Biocompatibility and Effects on the Gene Expression of Collagen Type 1 and TGF- β 1. BioMed Res. Int. 2022, 2022, 2204698. [Google Scholar] [CrossRef] [PubMed]
- Bossù, M.; Mancini, P.; Bruni, E.; Uccelletti, D.; Preziosi, A.; Rulli, M.; Relucenti, M.; Donfrancesco, O.; Iaculli, F.; Di Giorgio, G.; et al. Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases. Biology 2021, 10, 470. [Google Scholar] [CrossRef]
- Pedano, M.S.; Li, X.; Li, S.; Sun, Z.; Cokic, S.M.; Putzeys, E.; Yoshihara, K.; Yoshida, Y.; Chen, Z.; Van Landuyt, K.; et al. Freshly-mixed and setting calcium-silicate cements stimulate human dental pulp cells. Dent. Mater. 2018, 34, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Meraji, N.; Nekoofar, M.H.; Yazdi, K.A.; Sharifian, M.R.; Fakhari, N.; Camilleri, J. Bonding to caries affected dentine. Dent. Mater. 2018, 34, e236–e245. [Google Scholar] [CrossRef]
- Mori, G.G.; Teixeira, L.M.; de Oliveira, D.L.; Jacomini, L.M.; da Silva, S.R. Biocompatibility evaluation of biodentine in subcutaneous tissue of rats. J. Endod. 2014, 40, 1485–1488. [Google Scholar] [CrossRef]
- Pedano, M.S.; Li, X.; Yoshihara, K.; Van Landuyt, K.; Van Meerbeek, B. Cytotoxicity and bioactivity of dental pulp-capping agents towards human tooth-pulp cells: A systematic review of in-vitro studies and meta-analysis of randomized and controlled clinical trials. Materials 2020, 13, 2670. [Google Scholar] [CrossRef]
- Ghoddusi, J.; Afshari, J.T.; Donyavi, Z.; Brook, A.; Disfani, R.; Esmaeelzadeh, M. Cytotoxic effect of a new endodontic cement and mineral trioxide aggregate on L929 line culture. Iran Endod J. 2008, 3, 17–23. [Google Scholar]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; De Stefano Dorigo, E.; Prati, C. Biocompatibility and bioactivity of set MTA Flow compared to other calcium silicate-based cements. Clin. Oral Investig. 2011, 15, 661–668. [Google Scholar]
- Kim, M.; Yang, W.; Kim, H.; Ko, H. Comparison of the biological properties of ProRoot MTA, OrthoMTA, and endocem MTA cements. J. Endod. 2014, 40, 1649–1653. [Google Scholar] [CrossRef]
- Guimarães, B.M.; Vivan, R.R.; Piazza, B.; Alcalde, M.P.; Bramante, C.M.; Duarte, M.A.H. Chemical-physical Properties and Apatite-forming Ability of Mineral Trioxide Aggregate Flow. J. Endod. 2017, 43, 1692–1696. [Google Scholar] [CrossRef]
- Hansen, S.W.; Marshall, J.G.; Sedgley, C.M. Comparison of Intracanal EndoSequence Root Repair Material and ProRoot MTA to Induce pH Changes in Simulated Root Resorption Defects over 4 Weeks in Matched Pairs of Human Teeth. J. Endod. 2011, 37, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.E.; Frangou-Polyzois, M.J.; Polyzois, G.L. In vitro biocompatibility of denture relining materials. Gerodontology 2006, 23, 17–22. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Camilleri, J. The chemical composition of mineral trioxide aggregate. J. Conserv. Dent. 2008, 11, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-N.; Son, H.-J.; Noh, H.-J.; Koh, J.-T.; Chang, H.-S.; Hwang, I.-N.; Hwang, Y.-C.; Oh, W.-M. Cytotoxicity of Newly Developed Ortho MTA Root-end Filling Materials. J. Endod. 2012, 38, 1627–1630. [Google Scholar] [CrossRef]
- da Fonseca, T.S.; da Silva, G.F.; Tanomaru-Filho, M.; Sasso-Cerri, E.; Guerreiro-Tanomaru, J.M.; Cerri, P.S. In vivo evaluation of the inflammatory response and IL-6 immunoexpression promoted by Biodentine and MTA Angelus. Int. Endod. J. 2015, 49, 145–153. [Google Scholar] [CrossRef]
- Khandelwal, A.; Janani, K.; Teja, K.; Jose, J.; Battineni, G.; Riccitiello, F.; Valletta, A.; Palanivelu, A.; Spagnuolo, G. Review Article Periapical Healing following Root Canal Treatment Using Different Endodontic Sealers: A Systematic Review. BioMed Res. Int. 2022, 2022, 3569281. [Google Scholar] [CrossRef]
- AlBakhakh, B.; Al-Saedi, A.; Al-Taee, R.; Nahidh, M.; Testarelli, L. Rapid Apical Healing with Simple Obturation Technique in Response to a Calcium Silicate-Based Filling Material. Int. J. Dent. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; Lozano, A.; López-García, S.; García-Bernal, D.; Sanz, J.L.; Guerrero-Gironés, J.; Llena, C.; Forner, L.; Melo, M. Biomineralization potential and biological properties of a new tantalum oxide (Ta2O5)–containing calcium silicate cement. Clin. Oral Investig. 2022, 26, 1427–1441. [Google Scholar] [CrossRef]
- Tanomaru-Filho, M.; Andrade, A.S.; Rodrigues, E.M.; Viola, K.S.; Faria, G.; Camilleri, J.; Guerreiro-Tanomaru, J.M. Biocompatibility and mineralized nodule formation of Neo MTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int. Endod. J. 2017, 50, e31–e39. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Marciano, M.A.; Shelton, R.M.; Camilleri, J. Leaching and cytotoxicity of bismuth oxide in ProRoot MTA—A laboratory investigation. Int. Endod. J. 2024, 57, 1293–1314. [Google Scholar] [CrossRef]
- Song, M.; Lee, S.-M.; Bang, J.-Y.; Kim, R.H.; Kwak, S.W.; Kim, H.-C. Chemomechanical Properties and Biocompatibility of Various Premixed Putty-type Bioactive Ceramic Cements. J. Endod. 2023, 49, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Margunato, S.; Taşlı, P.N.; Aydın, S.; Kazandağ, M.K.; Şahin, F. In Vitro Evaluation of ProRoot MTA, Biodentine, and MM-MTA on Human Alveolar Bone Marrow Stem Cells in Terms of Biocompatibility and Mineralization. J. Endod. 2015, 41, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, D.; Song, D.; Kim, H.-M.; Kim, S.-Y. Biocompatibility and Bioactivity of Set Direct Pulp Capping Materials on Human Dental Pulp Stem Cells. Materials 2020, 13, 3925. [Google Scholar] [CrossRef]
- Taha, N.A.; About, I.; Sedgley, C.M.; Messer, H.H. Conservative Management of Mature Permanent Teeth with Carious Pulp Exposure. J. Endod. 2020, 46, S33–S41. [Google Scholar] [CrossRef]
- Mondelli, J.A.S.; Hoshino, R.A.; Weckwerth, P.H.; Cerri, P.S.; Leonardo, R.T.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; da Silva, G.F. Biocompatibility of mineral trioxide aggregate flow and biodentine. Int. Endod. J. 2019, 52, 193–200. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Saavedra, F.; Bombarda, G.F.; Gomes, B.P.F.d.A.; De-Jesus-Soares, A.; Zaia, A.A.; Duarte, M.A.H.; Tanomaru-Filho, M.; Marciano, M.A. Dental discoloration caused by grey-mtaflow cement: Analysis of its physicochemical, biological and antimicrobial properties. J. Appl. Oral Sci. 2020, 28, e20200269. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; Senna, P.M.; De-Deus, G.; Zaia, A.A. Cytocompatibility of Biodentine using a three-dimensional cell culture model. Int. Endod. J. 2016, 49, 574–580. [Google Scholar] [CrossRef]
- Kotova, A.V.; Lobov, A.A.; Dombrovskaya, J.A.; Sannikova, V.Y.; Ryumina, N.A.; Klausen, P.; Shavarda, A.L.; Malashicheva, A.B.; Enukashvily, N.I. Comparative Analysis of Dental Pulp and Periodontal Stem Cells: Differences in Morphology, Functionality, Osteogenic Differentiation and Proteome. Biomedicines 2021, 9, 1606. [Google Scholar] [CrossRef]
- Sanz, J.L.; Soler-Doria, A.; López-García, S.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Lozano, A.; Llena, C.; Forner, L.; Guerrero-Gironés, J.; Melo, M. Comparative Biological Properties and Mineralization Potential of 3 Endodontic Materials for Vital Pulp Therapy: Theracal PT, Theracal LC, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2021, 47, 1896–1906. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Castelo-Baz, P.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int. Endod. J. 2017, 50 (Suppl. S2), e63–e72. [Google Scholar] [CrossRef]
- Paranjpe, A.; Zhang, H.; Johnson, J.D. Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. J. Endod. 2010, 36, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, Y.; Zhang, M.; Xu, J.; Zhang, C.; Li, J. Effect of Biodentine on Odonto/Osteogenic Differentiation of Human Dental Pulp Stem Cells. Bioengineering 2023, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-G.; Lee, D.; Kim, Y.-M.; Song, D.; Kim, S.-Y. Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells. Materials 2019, 12, 2482. [Google Scholar] [CrossRef]
- Fridland, M.; Rosado, R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J. Endod. 2003, 29, 814–817. [Google Scholar] [CrossRef]
- Jaroch, K.; Taczyńska, P.; Czechowska, M.; Bogusiewicz, J.; Łuczykowski, K.; Burlikowska, K.; Bojko, B. One extraction tool for in vitro-in vivo extrapolation? SPME-based metabolomics of in vitro 2D, 3D, and in vivo mouse melanoma models. J. Pharm. Anal. 2021, 11, 667–674. [Google Scholar] [CrossRef]
- Goldberg, M. The Dental Pulp: Biology, Pathology, and Regenerative Therapies; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Yu, C.; Abbott, P. An overview of the dental pulp: Its functions and responses to injury. Aust. Dent. J. 2007, 52, S4–S6. [Google Scholar] [CrossRef]
- Rohanová, D.; Boccaccini, A.R.; Horkavcová, D.; Bozděchová, P.; Bezdička, P.; Častorálová, M. Is non-buffered DMEM solution a suitable medium for in vitro bioactivity tests? J. Mater. Chem. B 2014, 2, 5068–5076. [Google Scholar] [CrossRef]
- Thompson, J.G.; McMahon, A.; Gilliam, K.J. Unique Buffering System for Cell Culture Media and Gamete and Embryo Culture Media and Methods. U.S. Patent No. US20140255907A1, 11 September 2014. [Google Scholar]
- Michl, J.; Park, K.C.; Swietach, P. Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun. Biol. 2019, 2, 144. [Google Scholar] [CrossRef]
| Material Type | Material | Powder Composition | Liquid | Manufacturer |
|---|---|---|---|---|
| Portland cement | ProRoot® MTA | Portland cement, calcium sulfate dihydrate, tetracalcium aluminoferrite, gypsum, calcium oxide, bismuth oxide | Distilled water | Dentsply Tulsa Dental, Tulsa, OK, USA |
| Type IV HCSC | MTA Flow TM | di- and tricalcium silicate, calcium sulfate, silica, bismuth trioxide | Water, Water-soluble silicone-based gel | Ultradent Products, Inc., South Jordan, UT, USA |
| MTA FlowTM White | di- and tricalcium silicate, calcium sulfate, silica, tantalum oxide |
| Parameter | Description | ||
|---|---|---|---|
| Cement Type | ProRoot® MTA | MTA Flow™ | MTA Flow™ White |
| Preparation consistency * | 0.5 g powder + 0.167 mL liquid (putty consistency, 3:1) | 0.26 g powder + 3 drops (0.17 mL) gel (thick consistency, 3:2) | |
| Total volume of material | 1.3 mL | ||
| Application surface | 50 mm diameter sterile glass plates | ||
| Material compaction | Homogeneously distributed using a sterile cotton swab | ||
| Medium volume added | 13 mL of growth medium | ||
| Surface area-to-volume ratio | 126 mm2/mL (compliant with ISO 10993-12:2021) | ||
| Extraction conditions | 48 h at 37 °C, 95% humidity, CO2-free incubator with soda lime | ||
| Post-processing | Centrifuged (15,000 RPM), filtered (0.22 µm), diluted to 100%, 50%, 25%, 12.5% | ||
| Extraction medium | α-MEM + 10% FBS + 1% penicillin/streptomycin | ||
| Effect | F (DFn, DFd) | Partial η2 | 95% CI |
|---|---|---|---|
| Cement type | F(8, 288) = 5792.35 | 0.994 | [0.007–0.058] |
| Leachate concentration | F(4, 288) = 9521.55 | 0.992 | [0.007–0.058] |
| Time × Cement type | F(40, 288) = 1046.82 | 0.993 | [0.077–0.176] |
| Time × Concentration | F(20, 288) = 886.62 | 0.984 | [0.032–0.109] |
| Cement type × Concentration | F(32, 288) = 426.64 | 0.979 | [0.059–0.151] |
| Time × Cement × Concentration | F(160, 288) = 139.73 | 0.987 | [0.296–0.421] |
| Group | Viable Cells | Late Apoptotic Cells | Necrotic Cells |
|---|---|---|---|
| Negative control | 86.46 ± 6.07 A | 6.87 ± 2.69 A | 6.68 ± 3.75 A |
| Positive control | 4.47 ± 1.32 B | 89.24 ± 1.8 B | 6.29 ± 0.49 A |
| ProRoot® MTA | 84.77 ± 2.15 A | 8.93 ± 1.03 A | 6.30 ± 1.51 A |
| MTA Flow TM | 88.15 ± 0.79 A | 5.29 ± 0.41 A | 6.56 ± 0.87 A |
| MTA FlowTM White | 84.90 ± 1.21 A | 9.55 ± 0.63 A | 5.56 ± 0.69 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tušas, P.; Camilleri, J.; Alksnė, M.; Šimoliūnas, E.; Drukteinis, S.; Urbonė, E.M.; Bukelskienė, V.; Rutkūnas, V.; Pečiulienė, V. Biocompatibility of Hydraulic Calcium Silicate-Based Cement MTA FlowTM on Human Dental Pulp Stem Cells In Vitro. J. Funct. Biomater. 2025, 16, 252. https://doi.org/10.3390/jfb16070252
Tušas P, Camilleri J, Alksnė M, Šimoliūnas E, Drukteinis S, Urbonė EM, Bukelskienė V, Rutkūnas V, Pečiulienė V. Biocompatibility of Hydraulic Calcium Silicate-Based Cement MTA FlowTM on Human Dental Pulp Stem Cells In Vitro. Journal of Functional Biomaterials. 2025; 16(7):252. https://doi.org/10.3390/jfb16070252
Chicago/Turabian StyleTušas, Paulius, Josette Camilleri, Milda Alksnė, Egidijus Šimoliūnas, Saulius Drukteinis, Eglė Marija Urbonė, Virginija Bukelskienė, Vygandas Rutkūnas, and Vytautė Pečiulienė. 2025. "Biocompatibility of Hydraulic Calcium Silicate-Based Cement MTA FlowTM on Human Dental Pulp Stem Cells In Vitro" Journal of Functional Biomaterials 16, no. 7: 252. https://doi.org/10.3390/jfb16070252
APA StyleTušas, P., Camilleri, J., Alksnė, M., Šimoliūnas, E., Drukteinis, S., Urbonė, E. M., Bukelskienė, V., Rutkūnas, V., & Pečiulienė, V. (2025). Biocompatibility of Hydraulic Calcium Silicate-Based Cement MTA FlowTM on Human Dental Pulp Stem Cells In Vitro. Journal of Functional Biomaterials, 16(7), 252. https://doi.org/10.3390/jfb16070252








