The Influence of Drying Time, Application Mode, and Agitation on the Dentin Bond Strength of a Novel Mesoporous Bioactive Glass-Containing Universal Dentin Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Micro-Tensile Bond Strength (μTBS) Assessment
2.3. Failure Mode Analysis
2.4. Field-Emission Scanning Electron Microscopy (FE-SEM) Analysis of the Bonded Interfaces
2.5. Statistical Analysis
3. Results
3.1. μTBS Assessment
3.2. Failure Mode Analysis of the Fractured Surfaces
3.3. FE-SEM Analysis of the Bonded Interfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A0 | Without agitation |
| A1 | With agitation |
| ANOVA | Analysis of variance |
| BAG | Bioactive glass |
| FE-SEM | Field-emission scanning electron microscopy |
| HCA | Hydroxycarbonate apatite |
| HSD | Honestly significant difference |
| MBG | Mesoporous bioactive glass |
| MPa | Megapascal |
| μTBS | Micro-tensile bond strength |
| S | Self-etch |
| SEM | Scanning electron microscopy |
| T | Total-etch |
References
- Hashimoto, M.; Tay, F.R.; Svizero, N.R.; de Gee, A.J.; Feilzer, A.J.; Sano, H.; Kaga, M.; Pashley, D.H. The Effects of Common Errors on Sealing Ability of Total-Etch Adhesives. Dent. Mater. 2006, 22, 560–568. [Google Scholar] [CrossRef]
- Choi, A.N.; Lee, J.H.; Son, S.A.; Jung, K.H.; Kwon, Y.H.; Park, J.K. Effect of Dentin Wetness on the Bond Strength of Universal Adhesives. Materials 2017, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, Y.; Yoshiyama, M.; Donnelly, A.M.; Agee, K.A.; Sword, J.; Tay, F.R.; Pashley, D.H. Effects of Resin Hydrophilicity on Dentin Bond Strength. J. Dent. Res. 2006, 85, 1016–1021. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental Adhesion Review: Aging and Stability of the Bonded Interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef]
- Nonato, R.F.; Moreira, P.H.A.; Silva, D.O.D.; Ferreira, M.W.C.; Reis, A.; Cardenas, A.F.M.; Loguercio, A.D.; Siqueira, F.S.F. Long-Term Evaluation of Bonding Performance of Universal Adhesives Based on Different Dentinal Moisture Levels. J. Adhes. Dent. 2022, 24, 395–406. [Google Scholar] [PubMed]
- Carvalho, R.M.; Tay, F.; Sano, H.; Yoshiyama, M.; Pashley, D.H. Long-Term Mechanical Properties of EDTA-Demineralized Dentin Matrix. J. Adhes. Dent. 2000, 2, 193–199. [Google Scholar] [PubMed]
- Sano, H.; Yoshikawa, T.; Pereira, P.N.R.; Kanemura, N.; Morigami, M.; Tagami, J.; Pashley, D.H. Long-Term Durability of Dentin Bonds Made with a Self-Etching Primer, In Vivo. J. Dent. Res. 1999, 78, 906–911. [Google Scholar] [CrossRef]
- Giannini, M.; Vermelho, P.M.; de Araújo Neto, V.G.; Soto-Montero, J.R. An Update on Universal Adhesives: Indications and Limitations. Curr. Oral Health Rep. 2022, 9, 57–65. [Google Scholar] [CrossRef]
- Cardoso, M.; de Almeida Neves, A.; Mine, A.; Coutinho, E.; Van Landuyt, K.; De Munck, J.; Van Meerbeek, B. Current Aspects on Bonding Effectiveness and Stability in Adhesive Dentistry. Aust. Dent. J. 2011, 56, 31–44. [Google Scholar] [CrossRef]
- Tjäderhane, L. Dentin Bonding: Can We Make It Last? Oper. Dent. 2015, 40, 4–18. [Google Scholar] [CrossRef]
- Choi, K.-K.; Ferracane, J.; Ryu, G.; Choi, S.; Lee, M.; Park, S. Effects of Cavity Configuration on Composite Restoration. Oper. Dent. 2004, 29, 462–469. [Google Scholar] [PubMed]
- Lee, Y.; Park, J.W. Effect of Moisture and Drying Time on the Bond Strength of the One-Step Self-Etching Adhesive System. Restor. Dent. Endod. 2012, 37, 155–159. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.A.; Rangel, P.M.; Barcellos, D.C.; Pagani, C.; Rocha Gomes Torres, C. Bond Strength of Adhesive Systems with Different Solvents to Dry and Wet Dentin. J. Contemp. Dent. Pract. 2013, 14, 9–13. [Google Scholar]
- Stape, T.H.S.; Uctasli, M.; Cibelik, H.S.; Tjäderhane, L.; Tezvergil-Mutluay, A. Dry Bonding to Dentin: Broadening the Moisture Spectrum and Increasing Wettability of Etch-and-Rinse Adhesives. Dent. Mater. 2021, 37, 1676–1687. [Google Scholar] [CrossRef]
- Saeed, N.A.; Tichy, A.; Shimada, Y. Bonding of Universal Adhesives to Bur-Cut Dentin: Effect of Double Application and Dentin Moisture Level. Dent. Mater. J. 2022, 41, 724–730. [Google Scholar] [CrossRef]
- Chiba, Y.; Rikuta, A.; Yasuda, G.; Yamamoto, A.; Takamizawa, T.; Kurokawa, H.; Miyazaki, M. Influence of Moisture Conditions on Dentin Bond Strength of Single-Step Self-Etch Adhesive Systems. J. Oral Sci. 2006, 48, 131–137. [Google Scholar] [CrossRef]
- Jang, J.-H.; Jeon, B.-K.; Mo, S.Y.; Park, M.; Choi, D.; Choi, K.-K.; Kim, D.-S. Effect of Various Agitation Methods on Adhesive Layer Formation of HEMA-Free Universal Dentin Adhesive. Dent. Mater. J. 2019, 38, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Tay, F.R.; King, N.M.; Imazato, S.; Pashley, D.H. Bonding of Mild Self-Etching Primers/Adhesives to Dentin with Thick Smear Layers. Am. J. Dent. 2003, 16, 340–346. [Google Scholar]
- Velasquez, L.M.; Sergent, R.S.; Burgess, J.O.; Mercante, D. Effect of Placement Agitation and Placement Time on the Shear Bond Strength of 3 Self-Etching Adhesives. Oper. Dent. 2006, 31, 426–430. [Google Scholar] [CrossRef]
- Reis, A.; Carrilho, M.; Breschi, L.; Loguercio, A. Overview of Clinical Alternatives to Minimize the Degradation of the Resin-Dentin Bonds. Oper. Dent. 2013, 38, E103–E127. [Google Scholar] [CrossRef]
- Irmak, Ö.; Yaman, B.C.; Orhan, E.O.; Ozer, F.; Blatz, M.B. Effect of Rubbing Force Magnitude on Bond Strength of Universal Adhesives Applied in Self-Etch Mode. Dent. Mater. J. 2018, 37, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J. Universal Adhesives: A Literature Review. J. Esthet. Restor. Dent. 2019, 31, 331–350. [Google Scholar]
- Yoshida, Y.; Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Torii, Y.; Ogawa, T.; Osaka, A.; Van Meerbeek, B. Self-Assembled Nano-Layering at the Adhesive Interface. J. Dent. Res. 2012, 91, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Viana, Í.E.L.; Borges, R.; Marchi, J.; Feitosa, S.; Marques, M.M.; Scaramucci, T. A 58S Bioactive Glass for Dentin Hypersensitivity and Erosive Tooth Wear: An In Vitro Study. J. Dent. 2022, 127, 104343. [Google Scholar] [CrossRef]
- Lee, E.M.R.; Borges, R.; Marchi, J.; de Paula Eduardo, C.; Marques, M.M. Bioactive Glass and High-Intensity Lasers as a Promising Treatment for Dentin Hypersensitivity: An In Vitro Study. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.Z.; Follak, A.; da Rosa, L.S.; Montagner, A.F.; Lenzi, T.L.; Rocha, R.O. Bovine Tooth Is a Substitute for Human Tooth on Bond Strength Studies: A Systematic Review and Meta-Analysis of In Vitro Studies. Dent. Mater. 2016, 32, 1385–1393. [Google Scholar] [CrossRef]
- Al-Zain, A.O.; Albuqayli, A.; Albogami, A.; Alkudsi, A.; Alwabiri, M.; Koshak, A.T.; Alsefri, H.; Munchow, E.A. Effect of Double Adhesive Layer Application on Micro-Tensile Dentin Bond Strength of a Universal Adhesive. Front. Dent. Med. 2024, 5, 1484498. [Google Scholar] [CrossRef]
- Samy, F.M.; El-Kholany, N.R.; Hamama, H.H. Evaluation of Bond Durability of Different Self-Adhesive Bioactive Restorative Systems to Dentin. Sci. Rep. 2025, 15, 3667. [Google Scholar] [CrossRef]
- Kumagai, R.Y.; Hirata, R.; Pereira, P.N.; Reis, A.F. Moist vs Over-Dried Etched Dentin: FE-SEM/TEM and Bond Strength Evaluation of Resin-Dentin Interfaces Produced by Universal Adhesives. J. Esthet. Restor. Dent. 2020, 32, 325–332. [Google Scholar] [CrossRef]
- Werle, S.B.; Steglich, A.; Soares, F.Z.; Rocha, R.O. Effect of Prolonged Air Drying on the Bond Strength of Adhesive Systems to Dentin. Gen. Dent. 2015, 63, 68–72. [Google Scholar]
- Pashley, D.H.; Carvalho, R.M.; Sano, H.; Nakajima, M.; Yoshiyama, M.; Shono, Y.; Tay, F. The Microtensile Bond Test: A Review. J. Adhes. Dent. 1999, 1, 299–309. [Google Scholar] [PubMed]
- Perdigao, J.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G.; Lopes, A. Field Emission SEM Comparison of Four Postfixation Drying Techniques for Human Dentin. J. Biomed. Mater. Res. 1995, 29, 1111–1120. [Google Scholar] [CrossRef]
- Park, I.S.; Kim, H.J.; Kwon, J.; Kim, D.S. Comparative In Vitro Study of Sol–Gel-Derived Bioactive Glasses Incorporated into Dentin Adhesives: Effects on Remineralization and Mechanical Properties of Dentin. J. Funct. Biomater. 2025, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jang, J.H.; Woo, S.U.; Choi, K.K.; Kim, S.Y.; Ferracane, J.L.; Kim, D.S. Effect of Novel Bioactive Glass-Containing Dentin Adhesive on the Permeability of Demineralized Dentin. Materials 2021, 14, 5423. [Google Scholar] [CrossRef] [PubMed]
- Oltramare, R.; Par, M.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Short- and Long-Term Dentin Bond Strength of Bioactive Glass-Modified Dental Adhesives. Nanomaterials 2021, 11, 1894. [Google Scholar] [CrossRef]
- Da Rosa, W.L.D.O.; Piva, E.; da Silva, A.F. Bond Strength of Universal Adhesives: A Systematic Review and Meta-Analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef]
- Li, H.; Burrow, M.; Tyas, M. Nanoleakage Patterns of Four Dentin Bonding Systems. Dent. Mater. 2000, 16, 48–56. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Adhesion to Enamel and Dentin: Current Status and Future Challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar]
- Saikaew, P.; Senawongse, P.; AFM, A.; Sano, H.; Harnirattisai, C. Effect of Smear Layer and Surface Roughness on Resin-Dentin Bond Strength of Self-Etching Adhesives. Dent. Mater. J. 2018, 37, 973–980. [Google Scholar] [CrossRef]
- Reis, A.; Pellizzaro, A.; Dal-Bianco, K.; Gomes, O.; Patzlaff, R.; Loguercio, A.D. Impact of Adhesive Application to Wet and Dry Dentin on Long-Term Resin-Dentin Bond Strengths. Oper. Dent. 2007, 32, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Ciucchi, B.; Sano, H.; Horner, J.A. Permeability of Dentin to Adhesive Agents. Quintessence Int. 1993, 24, 618–631. [Google Scholar] [PubMed]
- Senawongse, P.; Srihanon, A.; Muangmingsuk, A.; Harnirattisai, C. Effect of Dentine Smear Layer on the Performance of Self-Etching Adhesive Systems: A Micro-Tensile Bond Strength Study. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.; Stanislawczuk, R.; Zander-Grande, C.; Gagler, D.; Reis, A.; Loguercio, A.D. Bond Strength and Quality of the Hybrid Layer of One-Step Self-Etch Adhesives Applied with Agitation on Dentin. Oper. Dent. 2010, 35, 211–219. [Google Scholar] [CrossRef]
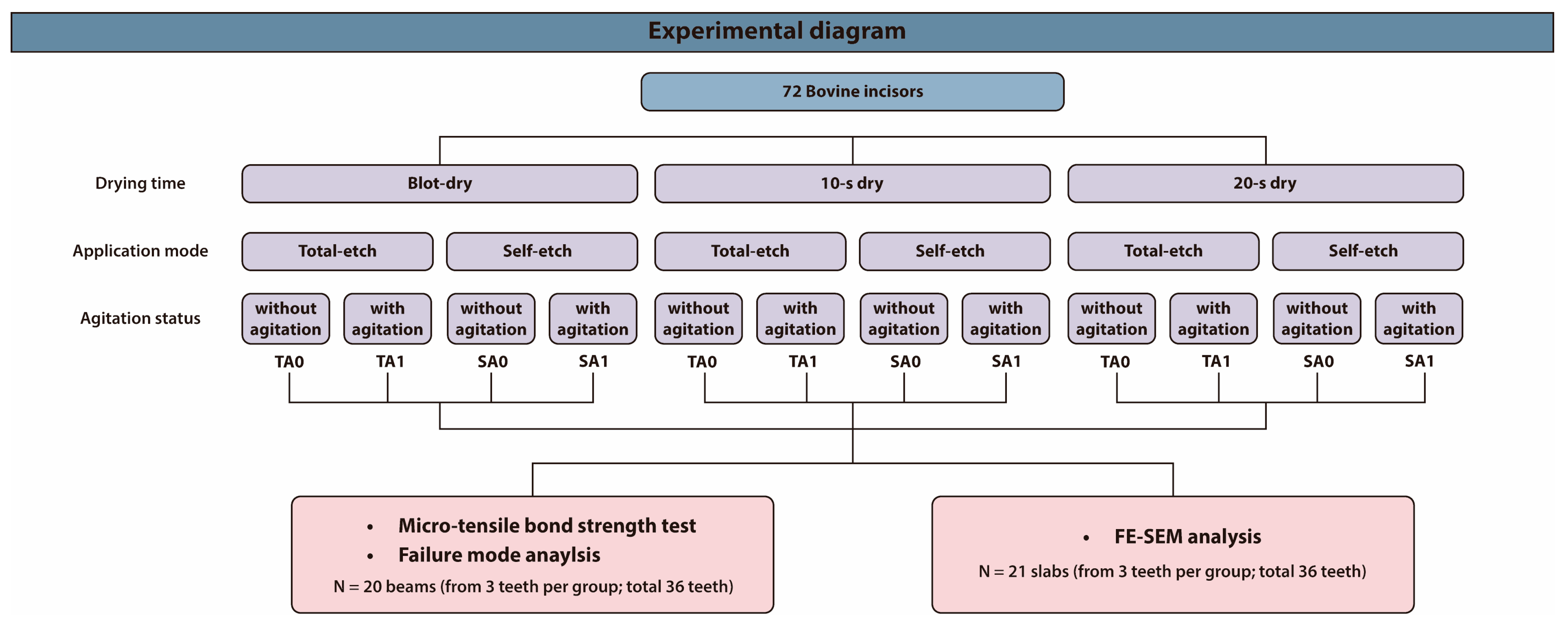



| Groups | Subgroup | Procedure |
|---|---|---|
| Drying time | Blot-dry | The dentin surface was air-dried for 2 s with a three-way syringe at a pressure of 1 bar, with the air nozzle positioned at a 45° angle and 1.5 cm away from the dentin surface. |
| 10 s dry | The dentin surface was air-dried for 10 s with a three-way syringe at a pressure of 1 bar, with the air nozzle positioned at a 45° angle and 1.5 cm away from the dentin surface. | |
| 20 s dry | The dentin surface was air-dried for 20 s with a three-way syringe at a pressure of 1 bar, with the air nozzle positioned at a 45° angle and 1.5 cm away from the dentin surface. | |
| Application mode | Total-etch | The dentin surfaces were treated with 35% phosphoric acid for 15 s, followed by thorough water rinsing for 30 s with a three-way syringe. |
| Self-etch | The adhesive was applied directly to the dentin surfaces. | |
| Agitation | With agitation | The adhesive was agitated actively for 10 s with a micro-brush under manual pressure. |
| Without agitation | The adhesive was applied passively for 3 s with a micro-brush and left undisturbed for 5 s. |
| Product | Composition |
|---|---|
| Hi-Bond Universal (MEDICLUS, Cheongju, Republic of Korea) | Mesoporous bioactive glass, 10-MDP, Bis-GMA, HEMA, water, ethanol, silane coupling agent, photo-initiator, accelerators, etc. |
| Any-Com (MEDICLUS, Cheongju, Republic of Korea) | Bis-GMA, UDMA, TEGDMA, barium glass, silicon dioxide, photo-initiators, accelerators, other additives. |
| ANOVA Table | F Value | p Value |
|---|---|---|
| Drying time | 53.82 | <0.0001 |
| Agitation | 29.70 | <0.0001 |
| Application mode (Mode) | 31.63 | <0.0001 |
| Drying time × Agitation | 5.779 | 0.0052 |
| Drying time × Mode | 4.375 | 0.0171 |
| Agitation × Mode | 0.08004 | 0.7783 |
| Drying time × Agitation × Mode | 0.2454 | 0.7832 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.; Kim, J.; Choi, D.; Kim, D.-S. The Influence of Drying Time, Application Mode, and Agitation on the Dentin Bond Strength of a Novel Mesoporous Bioactive Glass-Containing Universal Dentin Adhesive. J. Funct. Biomater. 2025, 16, 247. https://doi.org/10.3390/jfb16070247
Kwon J, Kim J, Choi D, Kim D-S. The Influence of Drying Time, Application Mode, and Agitation on the Dentin Bond Strength of a Novel Mesoporous Bioactive Glass-Containing Universal Dentin Adhesive. Journal of Functional Biomaterials. 2025; 16(7):247. https://doi.org/10.3390/jfb16070247
Chicago/Turabian StyleKwon, Jiyoung, Jungwon Kim, Dongseok Choi, and Duck-Su Kim. 2025. "The Influence of Drying Time, Application Mode, and Agitation on the Dentin Bond Strength of a Novel Mesoporous Bioactive Glass-Containing Universal Dentin Adhesive" Journal of Functional Biomaterials 16, no. 7: 247. https://doi.org/10.3390/jfb16070247
APA StyleKwon, J., Kim, J., Choi, D., & Kim, D.-S. (2025). The Influence of Drying Time, Application Mode, and Agitation on the Dentin Bond Strength of a Novel Mesoporous Bioactive Glass-Containing Universal Dentin Adhesive. Journal of Functional Biomaterials, 16(7), 247. https://doi.org/10.3390/jfb16070247







