Usage of Silver Nanoparticles in Orthodontic Bonding Reagents
Abstract
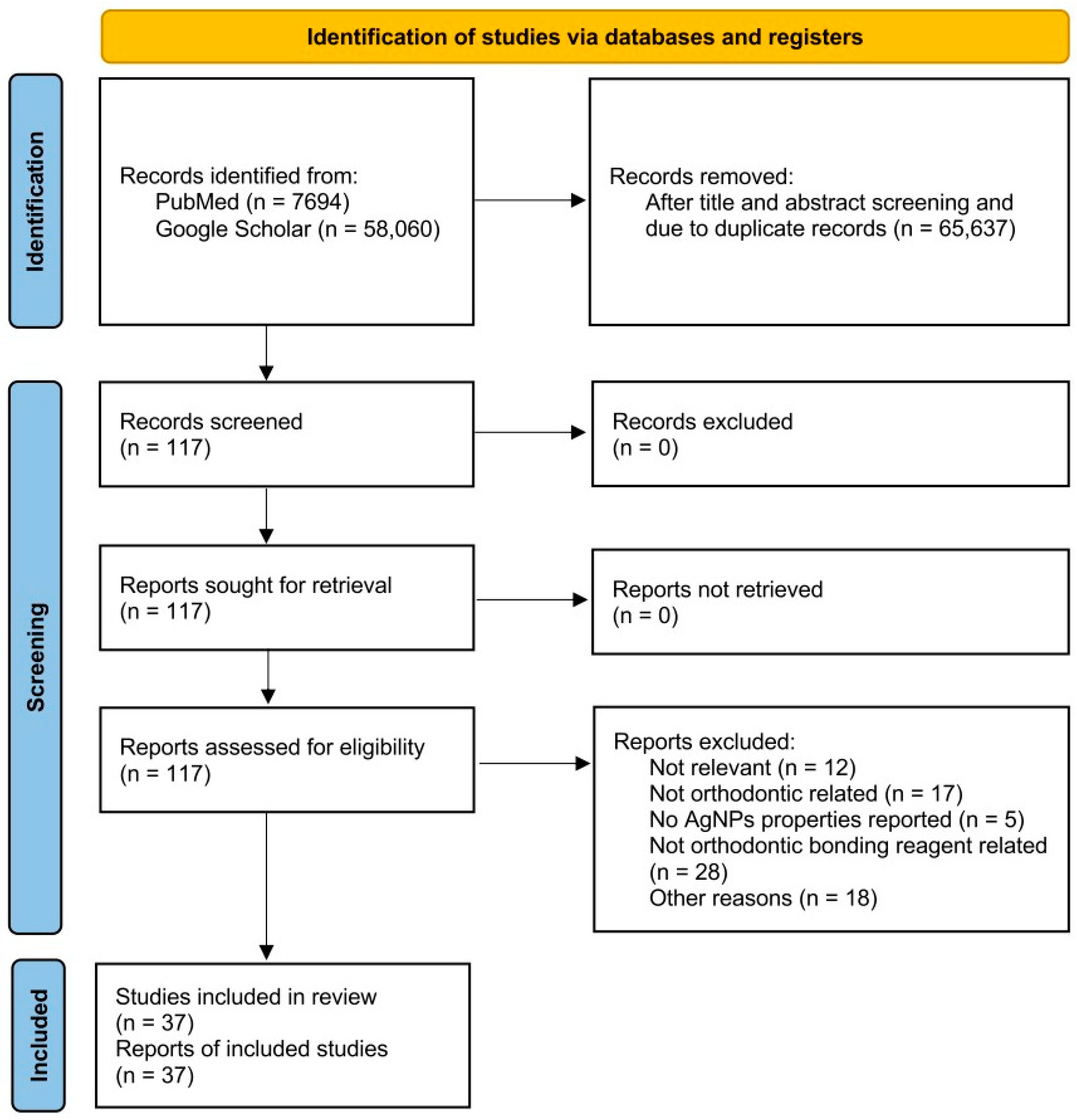
1. Introduction
2. Materials and Methods
3. Nanosilver Particles in Orthodontic Primers
3.1. Antibacterial Effects
| Bonding Reagent | References | Tested Conc. of AgNPs (w/w) | Combinatory Materials | Type of Study | Antibacterial Effect |
|---|---|---|---|---|---|
| TransbondTM XT primer (3M, Monrovia, CA, USA) | Degrazia et al., 2016 [28] | 0.11%, 0.18%, 0.33% | N/A | In Vitro | All Conc. inhibit S. mutans |
| TransbondTM XT primer (3M) | Blocher et al., 2015 [31] | 0.11%, 0.18%, 0.33% | N/A | In Vitro | N/A |
| Universal Bonding (Dentonics Inc., Monroe, LA, USA) | Jenabi et al., 2023 [29] | 0.5%, 1%, 2.5%, 5% | N/A | In Vitro | All Conc. inhibit S. mutans |
| TransbondTM XT primer (3M) | Akhavan et al., 2013 [32] | 1%, 5%, 10% Ag-HA | Doped HA with various concentration of Ag nanoparticle | In Vitro | N/A |
| Single Bond™ Universal Adhesive (3M) | Gilani et al., 2020 [33] | 1%, 5%, 10% Ag-HA | Ag-HA nanoparticle powder | In Vitro | N/A |
3.2. Side Effects
3.2.1. Influence on Shear Bond Strength (SBS)
3.2.2. Discoloration
| Bonding Reagent | References | Tested Conc. of AgNPs (w/w) | Combinatory Materials | Type of Study | Side Effects | ||
|---|---|---|---|---|---|---|---|
| SBS | Cytotoxicity | Discoloration | |||||
| TransbondTM XT primer (3M) | Degrazia et al., 2016 [28] | 0.11%, 0.18%, 0.33% | N/A | In Vitro | Decreased with all Conc. | N/A | N/A |
| TransbondTM XT primer (3M) | Blocher et al., 2015 [31] | 0.11%, 0.18%, 0.33% | N/A | In Vitro | No significant difference | N/A | All Conc. showed silver spots under 10× Magnification |
| Universal Bonding (Dentonics Inc.) | Jenabi et al., 2023 [29] | 0.5%, 1%, 2.5%, 5% | N/A | In Vitro | Dose-dependently decreased * | N/A | N/A |
| TransbondTM XT primer (3M) | Akhavan et al., 2013 [32] | 1%, 5%, 10% Ag-HA | Doped HA with various concentration of Ag nanoparticle | In Vitro | 1%: significantly increased; 5%: no significant difference; 10%: significantly reduced | N/A | N/A |
| Single Bond™ Universal Adhesive (3M) | Gilani et al., 2020 [33] | 1%, 5%, 10% Ag-HA | Ag-HA nanoparticle powder | In Vitro | 1% and 5%: significantly reduced; 10%: no significant difference | N/A | N/A |
3.3. Summary
4. Nanosilver Particles in Orthodontic Composites
4.1. Antibacterial and Anti-Demineralization Effects of AgNPs
| Composite | References | Tested Conc. of AgNPs (w/w) | Type of Study | Antibacterial Effect |
|---|---|---|---|---|
| TransbondTM XT (3M) | Reddy et al., 2016 [41] | 1% | In Vitro | N/A |
| TransbondTM XT (3M) | Eslamian et al., 2020 [35] | 0.3% | In Vitro | Long-lasting antibacterial effect on S. mutans at both 24 h and 30 days |
| TransbondTM XT (3M) | Najafi et al., 2020 [38] | 0.5% | In Vitro | Inhibit demineralization caused by S. mutans and L. casei up to 1.5 mm away from the brackets |
| TransbondTM XT (3M) | Sánchez-Tito et al., 2021 [36] | 0.05%, 0.1%, 0.5%, and 1% | In Vitro | Dose-dependently inhibit S. mutans and L. acidophilus and area of WSL |
| TransbondTM XT (3M) | Sánchez-Tito et al., 2022 [42] | 0.05%, 0.1%, 0.5%, and 1% | In Vitro | N/A |
| TransbondTM XT (3M) | Sánchez-Tito et al., 2023 [37] | 0.05%, 0.1%, 0.5%, and 1% | In Vitro | Does-dependently decrease the depth of the demineralization zone and area of WSL |
| TransbondTM XT (3M)) | Tavakolinejad et al., 2023 [43] | 0.3% | In Vitro | N/A |
| TransbondTM XT (3M) | Sánchez-Tito et al., 2024 [44] | 0.05%, 0.1%, 0.5%, and 1% | In Vitro | N/A |
| TransbondTM XT (3M) | Bahador et al., 2020 [24] | 1%, 5%, 10% | In Vivo (Wistar Rats) | Dose-dependently inhibit S. mutans, S. sanguinis, and L. acidophilus at 24 h |
| Enlight Light Cure Composite (Ormco, Orange, CA, USA) | Mahendra et al., 2022 [39] | 1% | In Vitro | Long-lasting antibacterial effect on S. mutans and L. acidophilus up to 30 days |
| Light-cured experimental composite adhesive | Ahn et al., 2009 [40] | 0 ppm, 250 ppm, 500 ppm | In Vitro | Antibacterial effect by decreased S. mutans and S. sobrinus adhesion, and prevention of WSL |
| Flow Tain (Reliance Orthodontic Products, Inc., Itasca, IL, USA) | Mirhashemi et al., 2021 [8] | 1%, 2%, 5% | In Vitro | Dose-dependently inhibit S. mutans, S. sanguinis, and L. acidophilus |
| Flow-It™ ALC™ Flowable Dental Composite (Pentron Clinical Technologies LLC, Orange, CA, USA) | Al-Thomali et al., 2022 [45] | 0.05% | In Vitro | N/A |
| Master-Dent® Flow Composite (Dentonics Inc.) | Jenabi et al., 2023 [29] | 0.5%, 1%, 2.5%, and 5% | In Vitro | Dose-dependently inhibit S. mutans |
4.2. Antibacterial Effects of Modified AgNPs
| Bonding Reagent | References | Tested Conc. of AgNPs (w/w) | Combinatory Materials | Type of Study | Antibacterial Effect |
|---|---|---|---|---|---|
| TransbondTM XT | Jia et al., 2023 [46] | NPA: 0.1%, 0.2%, 0.3%, and 0.5% | 50 mM AgNO3 mixed with 50 mg Nacp + 2 mg/mL dopamine hydrochloride | In Vitro | Inhibit S. mutans growth, prevent WSL (Only tested with 0.2%) |
| TransbondTM XT | Sawan et al., 2021 [49] | GNP-Ag: 0.25%, 0.5% | 80 mg AgNO3 mixed with 50 mg GNP solution | In Vitro | Dose-dependently inhibit S. mutans at 24 h and 30 days |
| TransbondTM XT | Kamran et al., 2022 [50] | NSPs-loaded PLGA: 2.5%, 5% | 0.2 mL of 10 mM AgNO3 mixed with 2 mg of 0.5% PVA + 1 mL of NaBH4 + 10 mg of PLGA in 1.5 mL of TCM | In Vitro | Dose-dependently inhibit S. mutans at 24 h and 30 days |
| TransbondTM XT | Sodagar et al., 2016 [52] | Ag-HA NPs: 1%, 5%, and 10% | 100 mg AgNO3 mixed with 1 g HA nano powder | In Vitro | Dose-dependent (5% and 10% are similar) antibacterial effect on S. mutans, L. acidophilus, and S. sanguinis at 3, 15, 30 days; prevent WSL |
| TransbondTM XT | Rajan et al., 2024 [53] | Ag-HA NPs: 2%, 4% (v/v%) | 1 g of nanosized HA powder + Ag in 100 mL of ethanol + NH4H2PO4 + Ammonium hydroxide + AgNO3_Ca(NO3)24H2O | In Vitro | Dose-dependent antibacterial effect on S. aureus, S. mutans, and E. coli |
| TransbondTM XT | Aguiar et al., 2022 [19] | Ag@SiO2NPs: 0.5%, 1%, 3% | Ag@SiO2NPs | In Vitro | Dose-dependent antibacterial effect on S. mutans |
| TransbondTM XT | Almoammar et al., 2024 [47] | ZrO2AgDNP: 2.5%, and 5% | ZrO2AgDNP | In Vitro | Dose-dependent antibacterial effect on S. mutans |
| TransbondTM XT | Uehara et al., 2024 [54] | β-AgVO3: 2.5%, 5% | β-AgVO3 | In Vitro | Dose-dependent antibacterial effect on S. mutans and S. sanguinis |
| No-mix self-cure composite resin (Unite Bonding System; Reliance, USA) | Kachoei et al., 2021 [55] | Ag/ZnO: 5%, 10%, 15%, and 20% | AZ: Ag + ZnO synthesized; AZ: ZnO nanoparticle + AgNO3 solution | In Vitro | All Conc. showed antibacterial activity against S. mutans, S. aureus, E. coli, and L. gasseri; All Conc. has no effect against Candida albicans |
| GC Ortho Connect (GC Orthodontics, Japan) | Seifi et al., 2024 [51] | nBG@Ag: 1%, 3%, 5% | 2000M 2% PEG + di-ammonium hydrogen orthophosphate + AgNO3 | In Vitro | Dose-dependent antibacterial effect on S. mutans |
4.3. Side Effects
4.3.1. Influences on Shear Bond Strength (SBS)
4.3.2. Discoloration
4.3.3. Cytotoxicity
| Composite | References | Tested Conc. of AgNPs (w/w) | Type of Study | Side Effects | ||
|---|---|---|---|---|---|---|
| SBS | Cytotoxicity | Discoloration | ||||
| TransbondTM XT (3M) | Reddy et al., 2016 [41] | 1% | In Vitro | Decreased | N/A | N/A |
| TransbondTM XT (3M) | Eslamian et al., 2020 [35] | 0.3% | In Vitro | Decreased | N/A | N/A |
| TransbondTM XT (3M) | Najafi et al., 2020 [38] | 0.5% | In Vitro | N/A | N/A | N/A |
| TransbondTM XT (3M) | Sánchez-Tito et al., 2021 [36] | 0.05%, 0.1%, 0.5%, and 1% | In Vitro | N/A | N/A | N/A |
| TransbondTM XT (3M) | Sánchez-Tito et al., 2022 [42] | 0.05%, 0.1%, 0.5%, and 1% | In Vitro | N/A | N/A | Dose-dependent enamel discoloration after 6 months |
| TransbondTM XT (3M) | Sánchez-Tito et al., 2023 [37] | 0.05%, 0.1%, 0.5%, and 1% | In Vitro | N/A | N/A | N/A |
| TransbondTM XT (3M)) | Tavakolinejad et al., 2023 [43] | 0.3% | In Vitro | Decreased | N/A | N/A |
| TransbondTM XT (3M) | Sánchez-Tito et al., 2024 [44] | 0.05%, 0.1%, 0.5%, and 1% | In Vitro | Decreased | N/A | N/A |
| TransbondTM XT (3M) | Bahador et al., 2020 [24] | 1%, 5%, 10% | In Vivo (Wistar Rats) | N/A | N/A | N/A |
| Enlight Light Cure Composite (Ormco) | Mahendra et al., 2022 [39] | 1% | In Vitro | Decreased | N/A | N/A |
| Light-cured experimental composite adhesive | Ahn et al., 2009 [40] | 0 ppm, 250 ppm, 500 ppm | In Vitro | No significant difference | N/A | N/A |
| Flow Tain (Reliance Orthodontic Products, Inc.) | Mirhashemi et al., 2021 [8] | 1%, 2%, 5% | In Vitro | N/A | N/A | N/A |
| Flow-It™ ALC™ Flowable Dental Composite (Pentron Clinical Technologies LLC.) | Al-Thomali et al., 2022 [45] | 0.05% | In Vitro | Increased | N/A | N/A |
| Master-Dent® Flow Composite (Dentonics Inc.) | Jenabi et al., 2023 [29] | 0.5%, 1%, 2.5%, and 5% | In Vitro | Only significant reduction in 5% | N/A | N/A |
| Bonding Reagent | References | Tested Conc. of AgNPs (w/w) | Combinatory Materials | Type of Study | Side Effects | |||
|---|---|---|---|---|---|---|---|---|
| SBS | Cytotoxicity | Discoloration | Surface Roughness | |||||
| TransbondTM XT | Jia et al., 2023 [46] | NPA: 0.1%, 0.2%, 0.3%, & 0.5% | 50 mM AgNO3 mixed with 50 mg Nacp + 2 mg/mL dopamine hydrochloride | In Vitro | 0.1% and 0.2%: met the minimal standard SBS; 0.3% and 0.5%: significantly reduced | All conc. showed greater than 70% cell viability (L929 cells) | N/A | N/A |
| TransbondTM XT | Sawan et al., 2021 [49] | GNP-Ag: 0.25%, 0.5% | 80 mg AgNO3 mixed with 50 mg GNP solution | In Vitro | 0.25%: no significant effect; 0.5%: decreased | 0.25%: >80% HGF survival; 0.5%: <80% HGF survival after 48 h; | N/A | N/A |
| TransbondTM XT | Kamran et al., 2022 [50] | NSPs-loaded PLGA: 2.5%, 5% | 0.2 mL of 10 mM AgNO3 mixed with 2 mg of 0.5% PVA + 1 mL of NaBH4 + 10 mg of PLGA in 1.5 mL of TCM | In Vitro | 2.5%: no significant effect; 5%: decreased | 2.5%: increased HGF viability rate after 24, 48, and 72 h; 5%: decreased HGF viability in 48 h and 72 h | N/A | N/A |
| TransbondTM XT | Sodagar et al., 2016 [52] | Ag-HA NPs: 1%, 5%, and 10% | 100 mg AgNO3 mixed with 1 g HA nano powder | In Vitro | N/A | N/A | N/A | N/A |
| TransbondTM XT | Rajan et al., 2024 [53] | Ag-HA NPs: 2%, 4% (v/v%) | 1 g of nanosized HA powder + Ag in 100 mL of ethanol + NH4H2PO4 + Ammonium hydroxide + AgNO3_Ca(NO3)24H2O | In Vitro | N/A | N/A | N/A | N/A |
| TransbondTM XT | Aguiar et al., 2022 [19] | Ag@SiO2NPs: 0.5%, 1%, 3% | Ag@SiO2NPs | In Vitro | No significant difference to the control; but 3% had significant lower SBS than 1% | N/A | N/A | N/A |
| TransbondTM XT | Almoammar et al., 2024 [47] | ZrO2AgDNP: 2.5%, & 5% | ZrO2AgDNP | In Vitro | Dose-dependent increase in µTBS | N/A | N/A | N/A |
| TransbondTM XT | Uehara et al., 2024 [54] | β-AgVO3: 2.5%, 5% | β-AgVO3 | In Vitro | Decreased in both concentrations regardless of thermocycling | N/A | N/A | Dose-dependently decreased |
| No-mix self-cure composite resin (Unite Bonding System; Reliance, USA) | Kachoei et al., 2021 [55] | Ag/ZnO: 5%, 10%, 15%, and 20% | AZ: Ag + ZnO synthesized; AZ: ZnO nanoparticle + AgNO3 solution | In Vitro | No significant difference | No effect on HGF viability up to 0.1 µg/mL for AZ group, AZ group had lowest viability | N/A | N/A |
| GC Ortho Connect (GC Orthodontics, Japan) | Seifi et al., 2024 [51] | nBG@Ag: 1%, 3%, 5% | 2000 M 2% PEG + di-ammonium hydrogen orthophosphate + AgNO3 | In Vitro | Decreased within clinically acceptable range | No significant effect | N/A | N/A |
4.4. Summary
5. Nanosilver Particles in Glass Ionomer Cement
5.1. Antibacterial Effects
| Bonding Reagent | References | Tested Conc. of AgNPs (w/w) | Combinatory Materials | Type of Study | Antibacterial Effect |
|---|---|---|---|---|---|
| GIC (Queen Mary University of London) | Paiva et al., 2018 [57] | 0.05%, 0.10%, and 0.50% | N/A | In Vitro | dose-dependent antibacterial effect against S. mutans and E. coli |
| GIC (GC Fuji II) | Jowkar et al., 2019 [62] | 0.1%, 0.2% | N/A | In Vitro | N/A |
| RMGIC (GC LC Fuji) | Wang et al., 2015 [58] | 0.05%, 0.1% | N/A | In Vitro | dose-dependent effects on against S. mutans, total streptococci, and planktonic bacteria; and on reduced WSL |
| RMGIC (GC LC Fuji) | Ding et al., 2021 [61] | 0.15% | AgNPs + 0%, 5%, 10%, 20%, 30% NAC | In Vitro | AgNPs alone group inhibits S. mutans; 20% NAC increased the AgNPs’ effects against S. mutans |
| RMGIC (GC LC Fuji II) | Raghimi et al., 2024 [63] | 0.1%, 0.5%, 1% and 2% | Si-HA-Ag hybrid nanoparticles | In Vitro | N/A |
| RMGIC (GC LC Fuji) | Biglar et al., 2023 [64] | 2%, 5%, 10% | Si-HA-Ag hybrid nanoparticles | In Vitro | N/A |
| RMGIC (GC LC Fuji) | Li et al., 2013 [59] | 1%, 3%, 5%, 10%, 15% | AgNaZr2(PO4)3·H2O, AGP-ZP003 | In Vitro | Dose-dependent antibacterial effect against S. mutans |
| RMGIC (GC LC Fuji) | Li et al., 2015 [60] | 1%, 3%, 5%, 10%, 15% | AgNaZr2(PO4)3·H2O, AGP-ZP003 | In Vivo (SD rats) | Dose-dependent bactericidal effect against S. mutans |
5.2. Side Effects
5.2.1. SBS
5.2.2. Cytotoxicity
5.2.3. Discoloration
| Bonding Reagent | References | Tested Conc. of AgNPs (w/w) | Combinatory Materials | Type of Study | Side Effects | ||
|---|---|---|---|---|---|---|---|
| SBS | Cytotoxicity | Discoloration | |||||
| GIC (Queen Mary University of London) | Paiva et al., 2018 [57] | 0.05%, 0.10%, and 0.50% | N/A | In Vitro | N/A | N/A | N/A |
| GIC (GC Fuji II) | Jowkar et al., 2019 [62] | 0.1%, 0.2% | N/A | In Vitro | dose-dependent increase (to dentin) | N/A | N/A |
| RMGIC (GC LC Fuji) | Wang et al., 2015 [58] | 0.05%, 0.1% | N/A | In Vitro | No significant effect | N/A | No noticeable change |
| RMGIC (GC LC Fuji) | Ding et al., 2021 [61] | 0.15% | AgNPs + 0%, 5%, 10%, 20%, 30% NAC | In Vitro | 0–20% NAC: no effect; 30% NAC: decreased | AgNPs: reduced cell viability; AgNPs + 20%NAC: increased cell viability than AgNPs alone | N/A |
| RMGIC (GC LC Fuji II) | Raghimi et al., 2024 [63] | 0.1%, 0.5%, 1% and 2% | Si-HA-Ag hybrid nanoparticles | In Vitro | N/A | N/A | Dose-dependent increased yellowish-brown |
| RMGIC (GC LC Fuji) | Biglar et al., 2023 [64] | 2%, 5%, 10% | Si-HA-Ag hybrid nanoparticles | In Vitro | 2%: Slightly increased, no significant effect; 5%: Slightly decreased, no significant effect; 10%: Significantly decreased | N/A | N/A |
| RMGIC (GC LC Fuji) | Li et al., 2013 [59] | 1%, 3%, 5%, 10%, 15% | AgNaZr2(PO4)3·H2O, AGP-ZP003 | In Vitro | Dose-dependent decrease and was significant in 15% nanosilver but within the clinical acceptable range | N/A | very light grey color for all conc. |
| RMGIC (GC LC Fuji) | Li et al., 2015 [60] | 1%, 3%, 5%, 10%, 15% | AgNaZr2(PO4)3·H2O, AGP-ZP003 | In Vivo (SD rats) | N/A | N/A | N/A |
5.3. Summary
6. Future Directions
6.1. Limitations of Currently Available Investigations
6.2. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gkantidis, N.; Christou, P.; Topouzelis, N. The orthodontic-periodontic interrelationship in integrated treatment challenges: A systematic review. J. Oral. Rehabil. 2010, 37, 377–390. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Dobrzynski, W.; Zawadzka-Knefel, A.; Janecki, M.; Kurek, K.; Lubojanski, A.; Szymonowicz, M.; Rybak, Z.; Wiglusz, R.J. Nanomaterials Application in Orthodontics. Nanomaterials 2021, 11, 337. [Google Scholar] [CrossRef]
- Skilbeck, M.G.; Cannon, R.D.; Farella, M.; Mei, L. The effect of surface roughening of orthodontic elastomers on hydrophobicity and in vitro adherence of Streptococcus gordonii. J. Mech. Behav. Biomed. Mater. 2023, 143, 105881. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, T.; Arash, V.; Rabiee, S.M.; Rajabnia, R.; Pourzare, A.; Rakhshan, V. Antimicrobial effect, frictional resistance, and surface roughness of stainless steel orthodontic brackets coated with nanofilms of silver and titanium oxide: A preliminary study. Microsc. Res. Tech. 2017, 80, 599–607. [Google Scholar] [CrossRef]
- Manuelli, M.; Marcolina, M.; Nardi, N.; Bertossi, D.; De Santis, D.; Ricciardi, G.; Luciano, U.; Nocini, R.; Mainardi, A.; Lissoni, A.; et al. Oral mucosal complications in orthodontic treatment. Minerva Stomatol. 2019, 68, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Matasa, C.G. Microbial attack of orthodontic adhesives. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Mirhashemi, A.; Bahador, A.; Sodagar, A.; Pourhajibagher, M.; Amiri, A.; Gholamrezayi, E. Evaluation of antimicrobial properties of nano-silver particles used in orthodontics fixed retainer composites: An experimental in-vitro study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 87–93. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lim, B.S.; Lee, S.J. Prevalence of cariogenic streptococci on incisor brackets detected by polymerase chain reaction. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 736–741. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Rolim, W.R.; Viana, M.M.; Souza, T.R.; Goncalves, F.; Tanaka, C.J.; Bueno-Silva, B.; Seabra, A.B. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J. Dent. 2020, 96, 103327. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Z.; Zara, J.N.; Hsu, C.; Soofer, D.E.; Lee, K.S.; Siu, R.K.; Miller, L.S.; Zhang, X.; Carpenter, D.; et al. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL-lactic-co-glycolic acid)-coated stainless steel. Biomaterials 2012, 33, 8745–8756. [Google Scholar] [CrossRef]
- Zheng, Z.; Yin, W.; Zara, J.N.; Li, W.; Kwak, J.; Mamidi, R.; Lee, M.; Siu, R.K.; Ngo, R.; Wang, J.; et al. The use of BMP-2 coupled—Nanosilver-PLGA composite grafts to induce bone repair in grossly infected segmental defects. Biomaterials 2010, 31, 9293–9300. [Google Scholar] [CrossRef]
- Murphy, M.; Ting, K.; Zhang, X.; Soo, C.; Zheng, Z. Current development of silver nanoparticle preparation, investigation, and application in the field of medicine. J. Nanomater. 2015, 2015, 696918. [Google Scholar] [CrossRef]
- Ali, A.; Ismail, H.; Amin, K. Effect of nanosilver mouthwash on prevention of white spot lesions in patients undergoing fixed orthodontic treatment—A randomized double-blind clinical trial. J. Dent. Sci. 2022, 17, 249–255. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Duran, G.; Galembeck, A.; Cogo-Muller, K.; Franz-Montan, M.; Duran, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Prabha, R.D.; Kandasamy, R.; Sivaraman, U.S.; Nandkumar, M.A.; Nair, P.D. Antibacterial nanosilver coated orthodontic bands with potential implications in dentistry. Indian J. Med. Res. 2016, 144, 580–586. [Google Scholar] [CrossRef]
- Mohamed Hamouda, I. Current perspectives of nanoparticles in medical and dental biomaterials. J. Biomed. Res. 2012, 26, 143–151. [Google Scholar] [CrossRef]
- Aguiar, R.C.O.; Nunes, L.P.; Batista, E.S.; Viana, M.M.; Rodrigues, M.C.; Bueno-Silva, B.; Roscoe, M.G. Experimental composite containing silicon dioxide-coated silver nanoparticles for orthodontic bonding: Antimicrobial activity and shear bond strength. Dent. Press. J. Orthod. 2022, 27, e222116. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnology 2018, 16, 1–28. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. Rsc Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Bahador, A.; Ayatollahi, B.; Akhavan, A.; Pourhajibagher, M.; Kharazifard, M.J.; Sodagar, A. Antimicrobial Efficacy of Silver Nanoparticles Incorporated in an Orthodontic Adhesive: An Animal Study. Front. Dent. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Z.; Yu, M.; Hsu, C.; Berthiaume, E.A.; Pan, H.; Zhang, X.; Stieg, A.Z.; Wu, B.; Wang, H.; et al. Using an Engineered Galvanic Redox System to Generate Positive Surface Potentials that Promote Osteogenic Functions. ACS Appl. Mater. Interfaces 2018, 10, 15449–15460. [Google Scholar] [CrossRef]
- Yin, W.; Zheng, Z.; Liu, Y.; Wang, L.; Shi, C.; Zhang, L.; Liu, S.; Niu, W.; Ting, K.; Bian, Z. Disinfection of infected root canals: Nanosilver has good potential. Small Methods 2019, 3, 1900378. [Google Scholar] [CrossRef]
- Retnaningrum, Y.; Alhasyimi, A.A. Effect of Silver Nanoparticles Synthesized Using Betel Leaf Extract Added into Orthodontic Adhesive on the Bracket’s Tensile Bond Strength. J. Int. Dent. Med. Res. 2021, 14, 474–480. [Google Scholar]
- Degrazia, F.W.; Leitune, V.C.; Garcia, I.M.; Arthur, R.A.; Samuel, S.M.; Collares, F.M. Effect of silver nanoparticles on the physicochemical and antimicrobial properties of an orthodontic adhesive. J. Appl. Oral. Sci. 2016, 24, 404–410. [Google Scholar] [CrossRef]
- Jenabi, N.; Sadeghian, S.; Karimzadeh, F.; Pour, M.S.; Rakhshan, V. Antibacterial activity and shear bond strength of fiber-reinforced composites and bonding agents containing 0.5%, 1%, 2.5%, and 5% silver nanoparticles. Dent. Res. J. 2023, 20, 23. [Google Scholar] [CrossRef]
- Lundstrom, F.; Krasse, B. Caries incidence in orthodontic patients with high levels of Streptococcus mutans. Eur. J. Orthod. 1987, 9, 117–121. [Google Scholar] [CrossRef]
- Blocher, S.; Frankenberger, R.; Hellak, A.; Schauseil, M.; Roggendorf, M.J.; Korbmacher-Steiner, H.M. Effect on enamel shear bond strength of adding microsilver and nanosilver particles to the primer of an orthodontic adhesive. BMC Oral. Health 2015, 15, 42. [Google Scholar] [CrossRef]
- Akhavan, A.; Sodagar, A.; Mojtahedzadeh, F.; Sodagar, K. Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontol. Scand. 2013, 71, 1038–1042. [Google Scholar] [CrossRef]
- Gilani, M.A.H.; Ameli, N.; Ghorbani, R.; Akhavan, A.; Rabiei, A.; Zeinabadi, M.S.; Kameli, S. Effect of Adding Nano Silver-Hydroxyapatite to the Orthodontic Primer on Bracket-Enamel Shear Bond Strength. J. Evol. Med. Dent. Sci. 2020, 9, 3457–3463. [Google Scholar] [CrossRef]
- Grenho, L.; Barros, J.; Ferreira, C.; Santos, V.R.; Monteiro, F.J.; Ferraz, M.P.; Cortes, M.E. In vitro antimicrobial activity and biocompatibility of propolis containing nanohydroxyapatite. Biomed. Mater. 2015, 10, 025004. [Google Scholar] [CrossRef]
- Eslamian, L.; Borzabadi-Farahani, A.; Karimi, S.; Saadat, S.; Badiee, M.R. Evaluation of the Shear Bond Strength and Antibacterial Activity of Orthodontic Adhesive Containing Silver Nanoparticle, an In-Vitro Study. Nanomaterials 2020, 10, 1466. [Google Scholar] [CrossRef]
- Sanchez-Tito, M.; Tay, L.Y. Antibacterial and white spot lesions preventive effect of an orthodontic resin modified with silver-nanoparticles. J. Clin. Exp. Dent. 2021, 13, e685–e691. [Google Scholar] [CrossRef]
- Sanchez-Tito, M.; Castaneda-Via, J.A.; Tay, L.Y. Raman microscopy evaluation of the preventive effect of a modified orthodontic adhesive with silver nanoparticles on the formation of white spot lesions. J. Clin. Exp. Dent. 2023, 15, e706–e713. [Google Scholar] [CrossRef]
- Najafi, H.Z.; Azadeh, N.; Motamedifar, M. Evaluation of the Preventive Effect of Composites Containing Silver and TiO(2) Nanoparticles on Demineralization around Orthodontic Brackets. J. Contemp. Dent. Pract. 2020, 21, 874–879. [Google Scholar] [CrossRef]
- Mahendra, T.V.D.; Muddada, V.; Gorantla, S.; Karri, T.; Mulakala, V.; Prasad, R.; Chintala, S.K.; Mounica, K. Evaluation of antibacterial properties and shear bond strength of orthodontic composites containing silver nanoparticles, titanium dioxide nanoparticles and fluoride: An in vitro study. Dental Press. J. Orthod. 2022, 27, e222067. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lee, S.J.; Kook, J.K.; Lim, B.S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent. Mater. 2009, 25, 206–213. [Google Scholar] [CrossRef]
- Reddy, A.K.; Kambalyal, P.B.; Patil, S.R.; Vankhre, M.; Khan, M.Y.; Kumar, T.R. Comparative evaluation and influence on shear bond strength of incorporating silver, zinc oxide, and titanium dioxide nanoparticles in orthodontic adhesive. J. Orthod. Sci. 2016, 5, 127–131. [Google Scholar] [CrossRef]
- Sanchez-Tito, M.; Tay, L.Y. Effect of an orthodontic resin modified with silver-nanoparticles on enamel color change. J. Clin. Exp. Dent. 2022, 14, e241–e246. [Google Scholar] [CrossRef]
- Tavakolinejad, Z.; Mohammadi Kamalabadi, Y.; Salehi, A. Comparison of the Shear Bond Strength of Orthodontic Composites Containing Silver and Amorphous Tricalcium Phosphate Nanoparticles: An ex vivo Study. J. Dent. 2023, 24, 285–292. [Google Scholar] [CrossRef]
- Sanchez-Tito, M.; Tay, L.Y. Effect of the addition of silver nanoparticles on the mechanical properties of an orthodontic adhesive. Saudi Dent. J. 2024, 36, 359–363. [Google Scholar] [CrossRef]
- Al-Thomali, Y. Shear bond strength of orthodontic brackets after adding silver nanoparticles to a nano-bond adhesive at different thermal cycles and cyclic loading-An in vitro study. J. Orthod. Sci. 2022, 11, 28. [Google Scholar] [CrossRef]
- Jia, A.; Wang, P.; Tong, F.; Chen, Z.; Deng, Y.; Yao, H.; Wang, L.; Liu, Y.; Ge, H. Developing a Novel Enamel Adhesive with Amorphous Calcium Phosphate and Silver Nanoparticles to Prevent Demineralization during Orthodontic Treatment. J. Funct. Biomater. 2023, 14, 77. [Google Scholar] [CrossRef]
- Almoammar, S.; Kamran, M.A.; Alnazeh, A.A.; Almagbol, M.; Al Jearah, M.M.; Mannakandath, M.L. Orthodontic adhesive loaded with different proportions of ZrO2 silver-doped nanoparticles: An in vitro muTBS, SEM, EDX, FTIR, and antimicrobial analysis. Microsc. Res. Tech. 2024, 87, 1146–1156. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Sawan, N.M.; AlSagob, E.I.; Ben Gassem, A.A.; Alshami, A.A. Graphene functionalized with nanosilver particle-modified methacrylate-based bonding agent improves antimicrobial capacity and mechanical strength at tooth orthodontic bracket interface. Polym. Compos. 2021, 42, 5850–5858. [Google Scholar] [CrossRef]
- Kamran, M.A.; Alnazeh, A.A.; Hameed, M.S.; Yassin, S.M.; Mannakandath, M.L.; Alshahrani, I. Formulation and clinical performance of nanosilver loaded poly-l-glycolic acid modified orthodontic adhesive for orthodontic bonding. J. Mol. Struct. 2022, 1249, 131490. [Google Scholar] [CrossRef]
- Seifi, M.; Eskandarloo, F.; Amdjadi, P.; Farmany, A. Investigation of mechanical properties, remineralization, antibacterial effect, and cellular toxicity of composite orthodontic adhesive combined with silver-containing nanostructured bioactive glass. BMC Oral. Health 2024, 24, 650. [Google Scholar] [CrossRef]
- Sodagar, A.; Akhavan, A.; Hashemi, E.; Arab, S.; Pourhajibagher, M.; Sodagar, K.; Kharrazifard, M.J.; Bahador, A. Evaluation of the antibacterial activity of a conventional orthodontic composite containing silver/hydroxyapatite nanoparticles. Prog. Orthod. 2016, 17, 40. [Google Scholar] [CrossRef]
- Rajan, K.R.; Nagesh, S.; SP, M. Preparation, Characterization, and Assessment of Antimicrobial Properties of Silver-doped Hydroxyapatite Nanoparticles in Orthodontic Composite. J. Adv. Oral. Res. 2024, 15, 179–185. [Google Scholar] [CrossRef]
- Uehara, L.M.; Teixeira, A.B.V.; Valente, M.; Reis, A.C.D. Mechanical and microbiological properties of orthodontic resin modified with nanostructured silver vanadate decorated with silver nanoparticles (betaAgVO3). J. Dent. 2024, 145, 104836. [Google Scholar] [CrossRef]
- Kachoei, M.; Divband, B.; Rahbar, M.; Esmaeilzadeh, M.; Ghanizadeh, M.; Alam, M. A Novel Developed Bioactive Composite Resin Containing Silver/Zinc Oxide (Ag/ZnO) Nanoparticles as an Antimicrobial Material against Streptococcus mutans, Lactobacillus, and Candida albicans. Evid. Based Complement. Altern. Med. 2021, 2021, 4743411. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Fidalgo, T.K.S.; da Costa, L.P.; Maia, L.C.; Balan, L.; Anselme, K.; Ploux, L.; Thire, R. Antibacterial properties and compressive strength of new one-step preparation silver nanoparticles in glass ionomer cements (NanoAg-GIC). J. Dent. 2018, 69, 102–109. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Wang, Y. Antibacterial orthodontic cement to combat biofilm and white spot lesions. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 974–981. [Google Scholar] [CrossRef]
- Li, F.; Li, Z.; Liu, G.; He, H. Long-term antibacterial properties and bond strength of experimental nano silver-containing orthodontic cements. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 849–855. [Google Scholar] [CrossRef]
- Li, F.; Fang, M.; Peng, Y.; Zhang, J. Antibacterial properties of nano silver-containing orthodontic cements in the rat caries disease model. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 1291–1296. [Google Scholar] [CrossRef]
- Ding, R.; Qian, Y.; Chen, M.; Yi, J.; Zhao, Z. The effect of N-acetylcysteine on the antibacterial capability and biocompatibility of nano silver-containing orthodontic cement. Angle Orthod. 2021, 91, 515–521. [Google Scholar] [CrossRef]
- Jowkar, Z.; Jowkar, M.; Shafiei, F. Mechanical and dentin bond strength properties of the nanosilver enriched glass ionomer cement. J. Clin. Exp. Dent. 2019, 11, e275–e281. [Google Scholar] [CrossRef]
- Raghimi, E.C.; Biglar, N.; Sadighian, S.; Karamitanha, F.; Nouri, A.; Nourian, A. Compressive strength and fluoride release profile of a glass ionomer cement reinforced with silver-hydroxyapatite-silica hybrid nanoparticles: An in vitro study. Int. Orthod. 2024, 22, 100871. [Google Scholar] [CrossRef]
- Biglar, N.; Chaychi Raghimi, E.; Sadighian, S.; Karamitanha, F.; Zajkani, E.; Nourian, A. Effect of incorporating silica-hydroxyapatite-silver hybrid nanoparticles into the resin-modified glass ionomer on the adhesive remnant index score and shear bond strength of orthodontic metal brackets: An in vitro study. Int. Orthod. 2023, 21, 100761. [Google Scholar] [CrossRef] [PubMed]
- Shokeen, B.; Viloria, E.; Duong, E.; Rizvi, M.; Murillo, G.; Mullen, J.; Shi, B.; Dinis, M.; Li, H.; Tran, N.C.; et al. The impact of fixed orthodontic appliances and clear aligners on the oral microbiome and the association with clinical parameters: A longitudinal comparative study. Am. J. Orthod. Dentofac. Orthop. 2022, 161, e475–e485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.J.; Niu, M.; Shakir, Z.; Hwang, G.; Chung, C.-H.; Wolff, M.S.; Zheng, Z.; Li, C. Usage of Silver Nanoparticles in Orthodontic Bonding Reagents. J. Funct. Biomater. 2025, 16, 244. https://doi.org/10.3390/jfb16070244
Lee JJ, Niu M, Shakir Z, Hwang G, Chung C-H, Wolff MS, Zheng Z, Li C. Usage of Silver Nanoparticles in Orthodontic Bonding Reagents. Journal of Functional Biomaterials. 2025; 16(7):244. https://doi.org/10.3390/jfb16070244
Chicago/Turabian StyleLee, Janet Jisoo, Meigan Niu, Zinah Shakir, Geelsu Hwang, Chun-Hsi Chung, Mark S. Wolff, Zhong Zheng, and Chenshuang Li. 2025. "Usage of Silver Nanoparticles in Orthodontic Bonding Reagents" Journal of Functional Biomaterials 16, no. 7: 244. https://doi.org/10.3390/jfb16070244
APA StyleLee, J. J., Niu, M., Shakir, Z., Hwang, G., Chung, C.-H., Wolff, M. S., Zheng, Z., & Li, C. (2025). Usage of Silver Nanoparticles in Orthodontic Bonding Reagents. Journal of Functional Biomaterials, 16(7), 244. https://doi.org/10.3390/jfb16070244








