Histological Analysis of Sticky Tooth and Sticky Bone
Abstract
1. Introduction
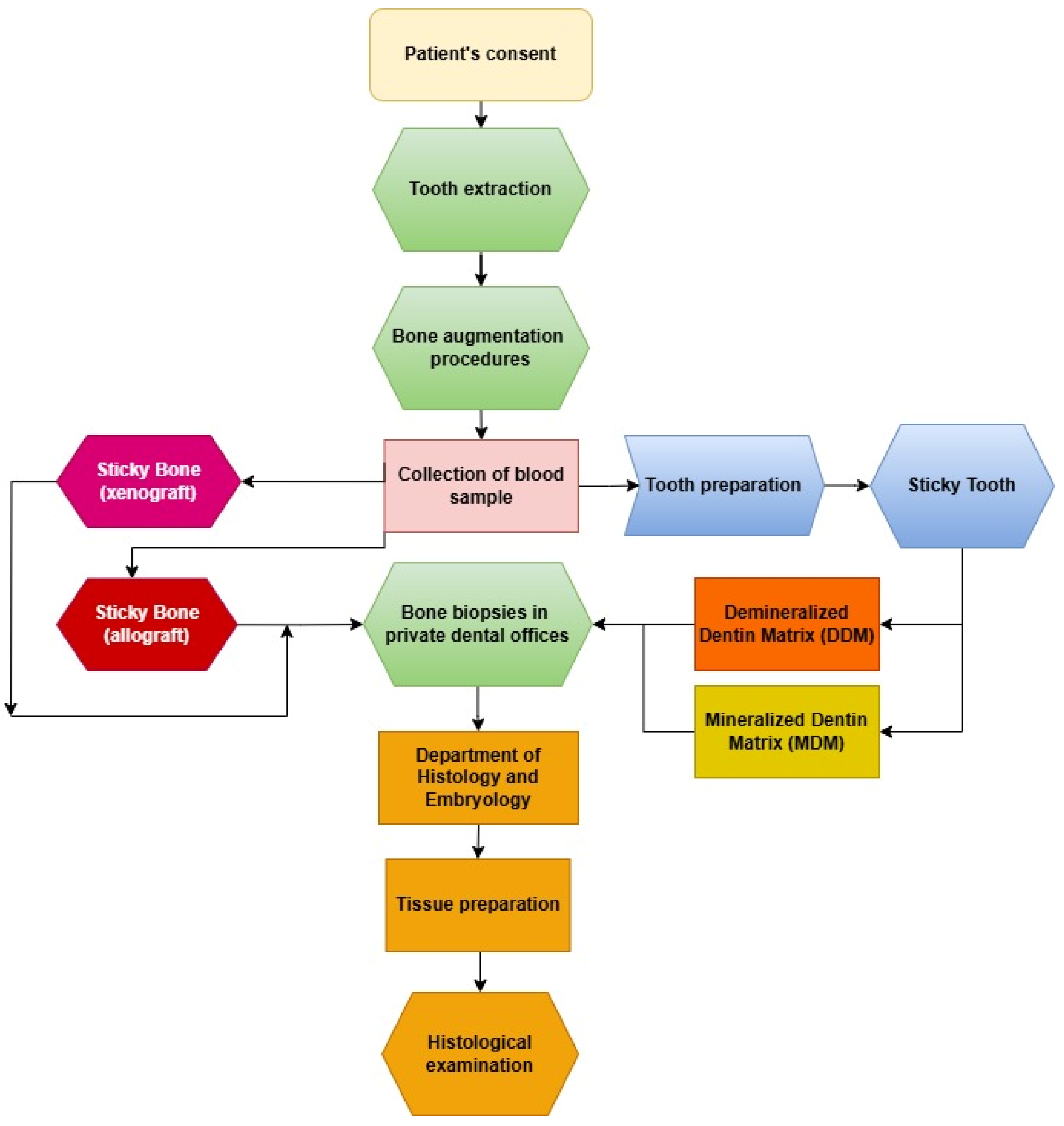
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
- Age ≥ 18 years;
- Consent to participate and attend follow-ups (2 weeks, 4 weeks, and 3–4 months post-surgery);
- Good oral hygiene;
- Single tooth extraction without maxillary sinus communication;
- Clinical and radiological assessment confirming ridge defects.
- Children;
- Systemic diseases;
- Poor oral hygiene;
- Odontogenic infections;
- Mental disorders;
- Smokers;
- Pregnant patients;
- Patients who refused to consent;
- Endodontically treated teeth;
- Oncology patients.
2.3. Preparation of Graft (In Vivo)
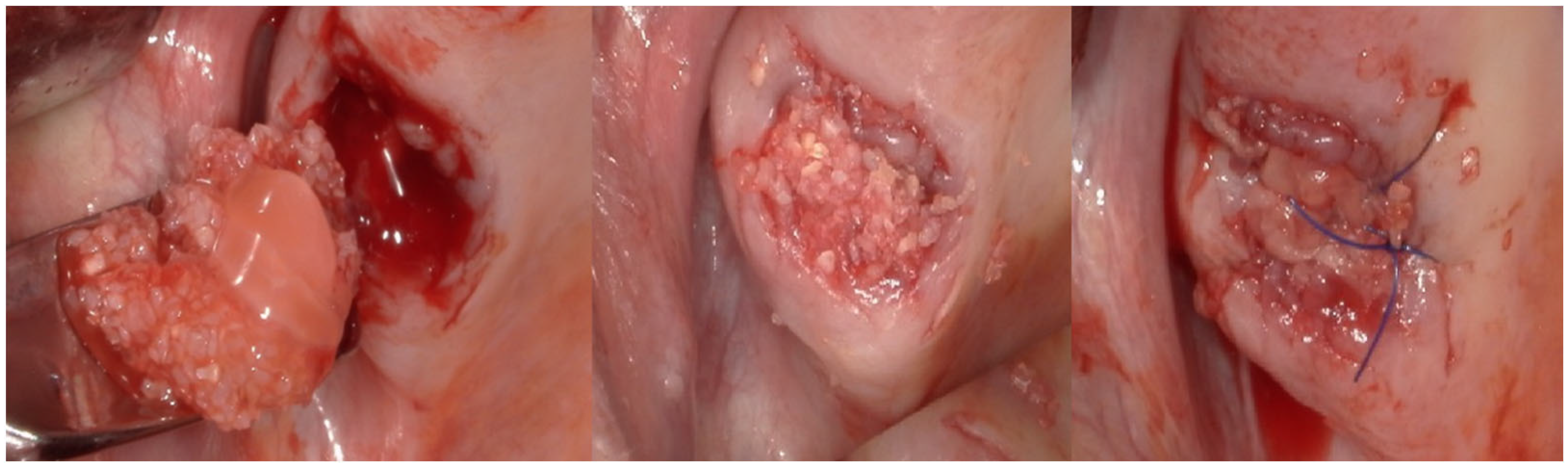
2.4. Surgical Procedure (In Vivo)
2.5. Bone Biopsies (In Vivo)
2.6. Histological Procedure (In Vitro)
2.7. Quantitative Histological Analysis
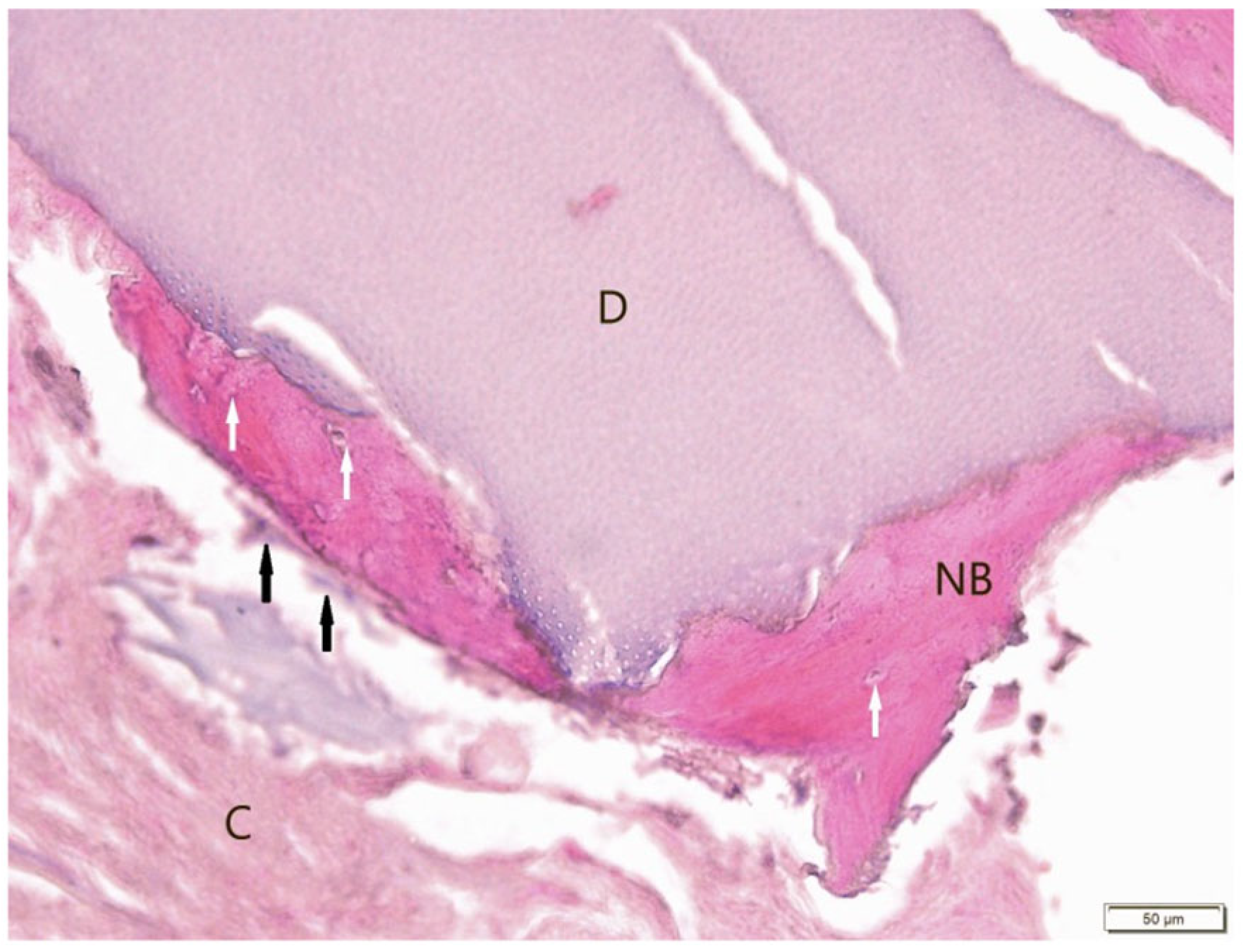
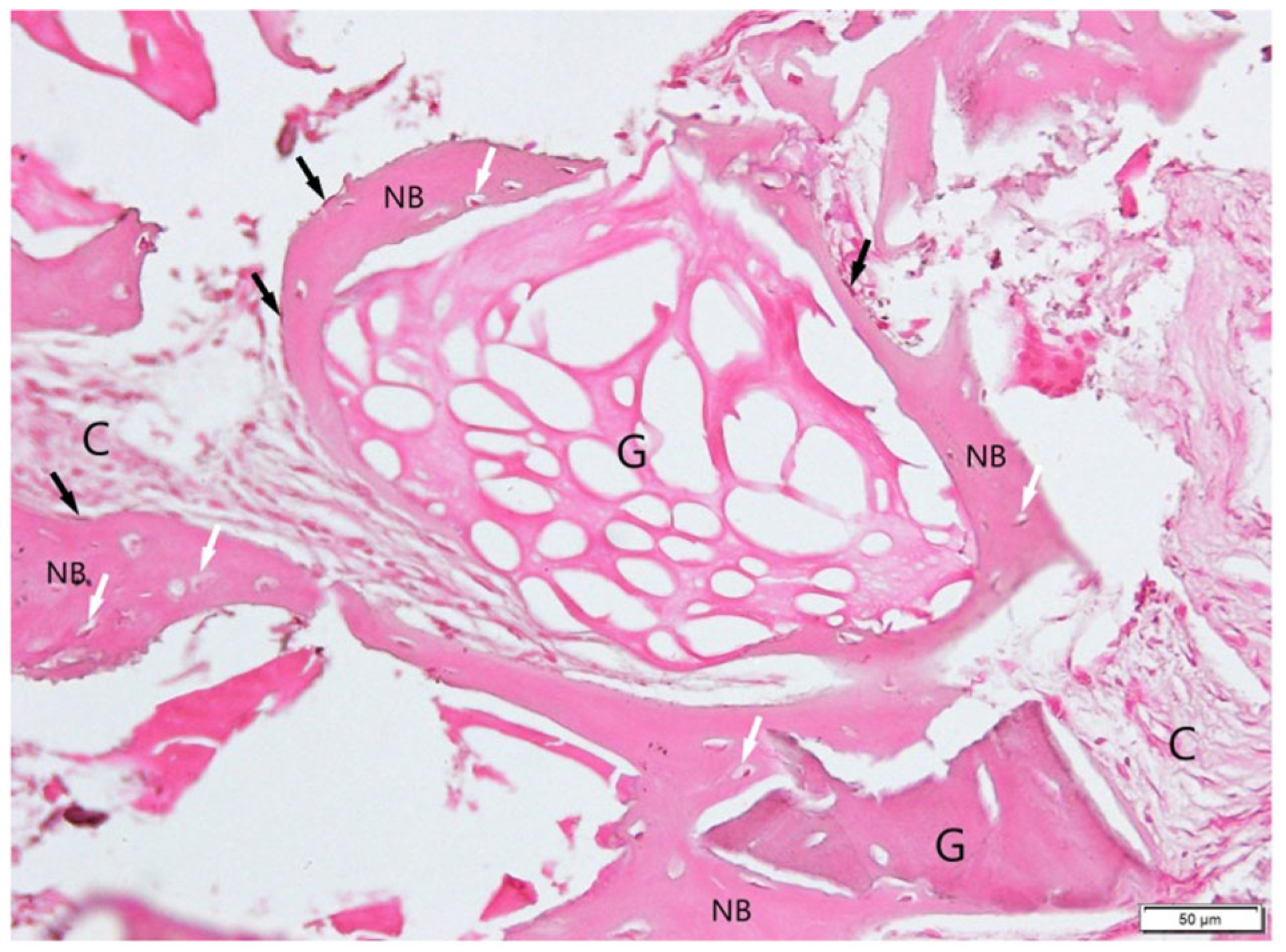
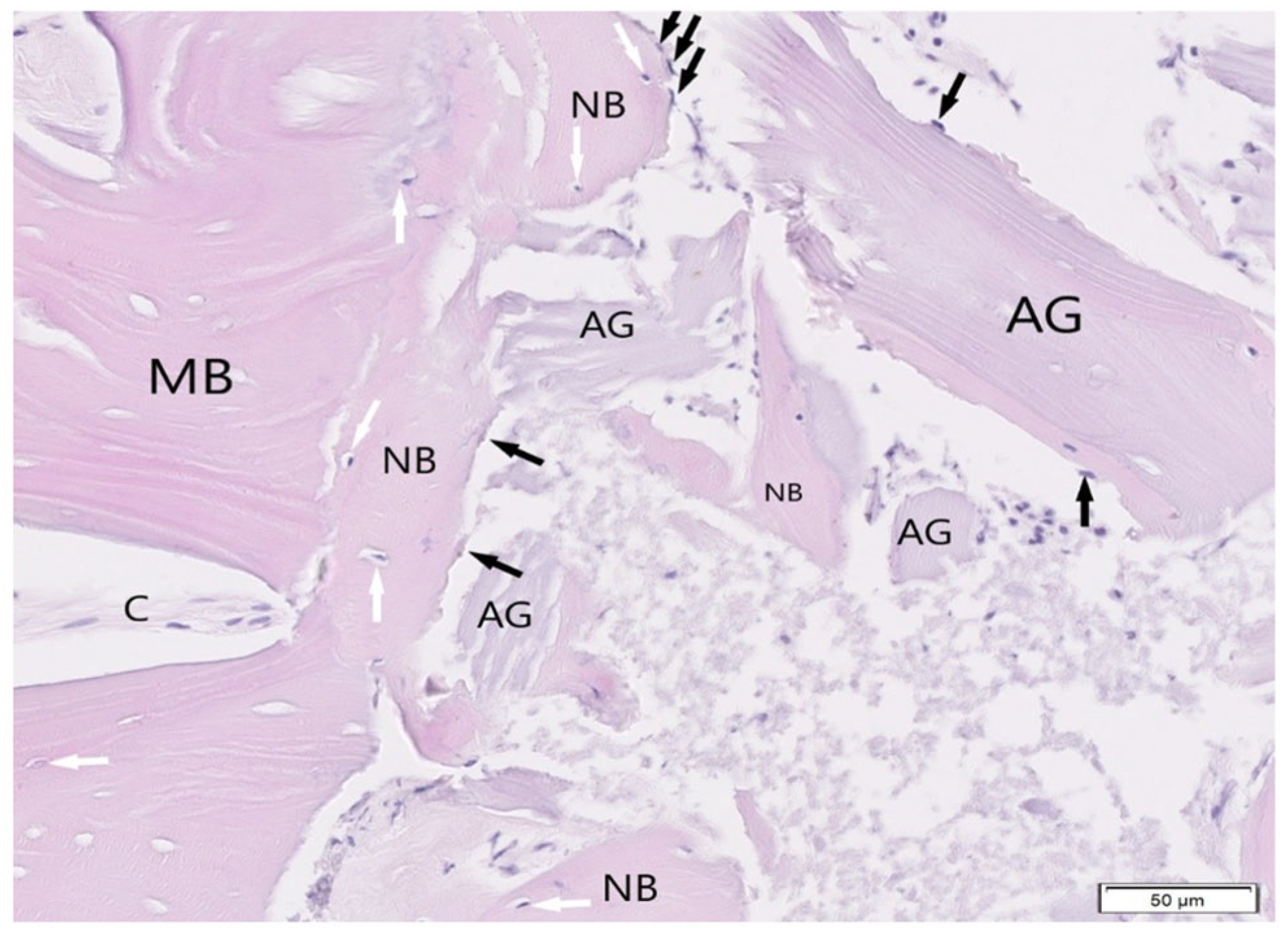
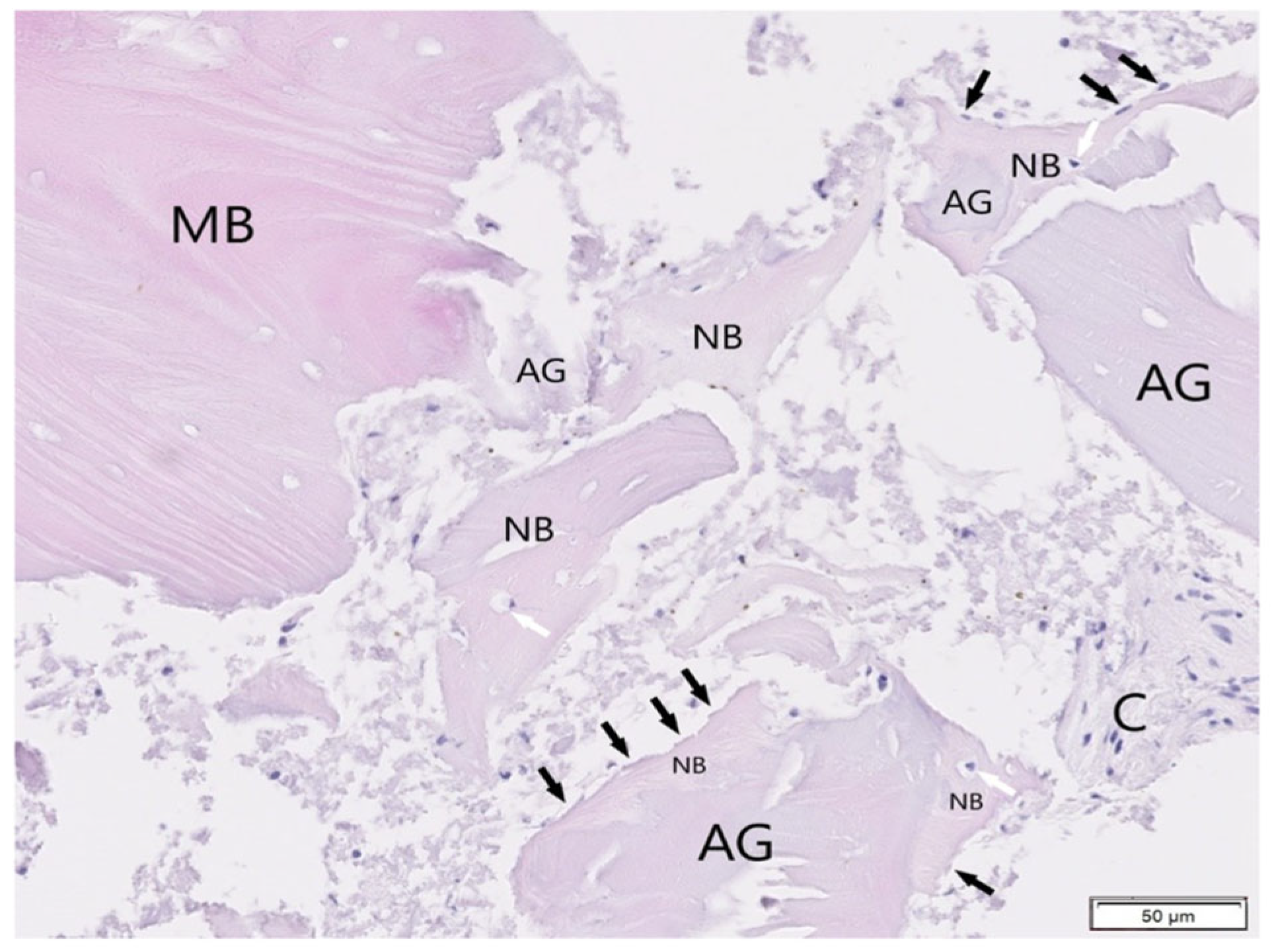
3. Results
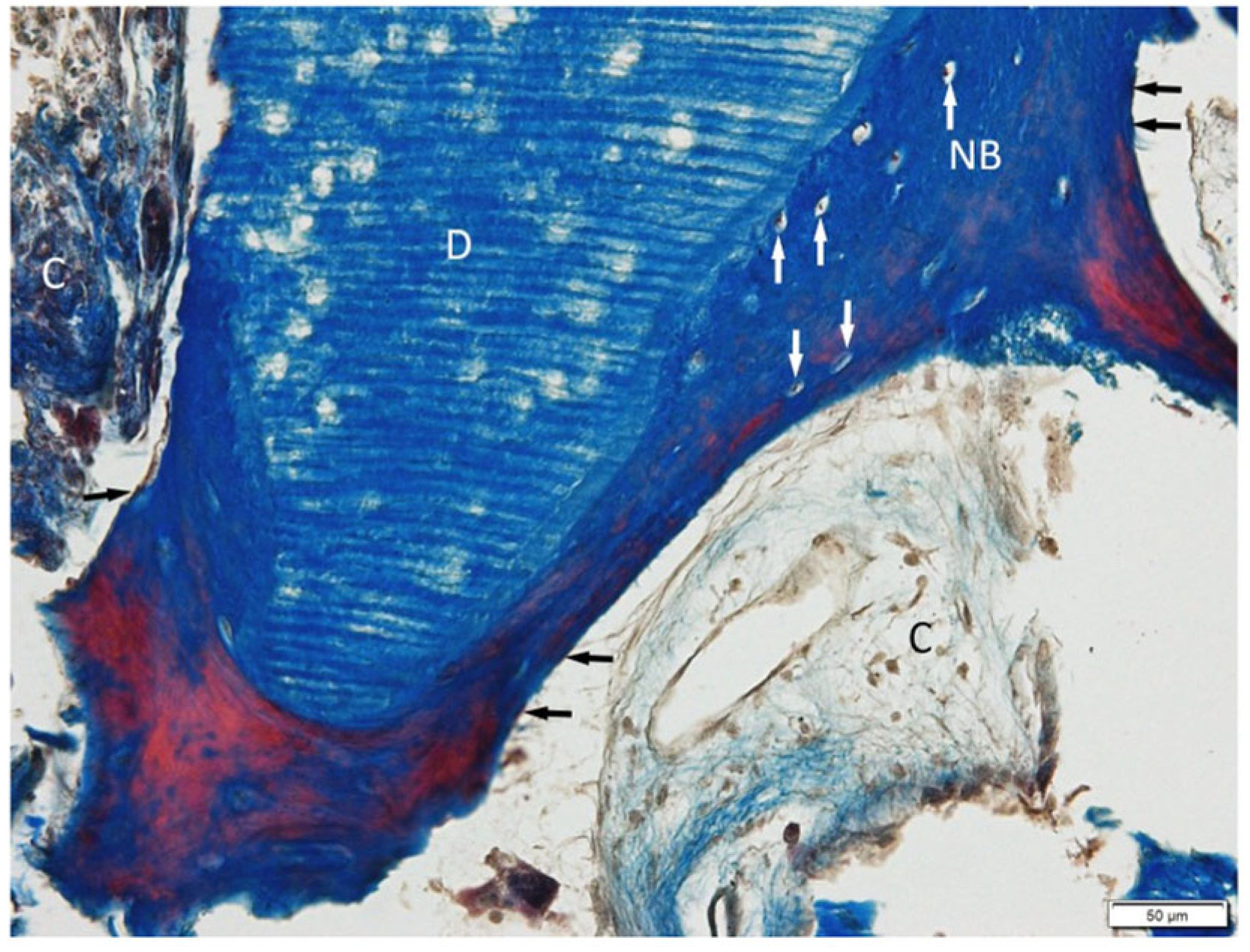
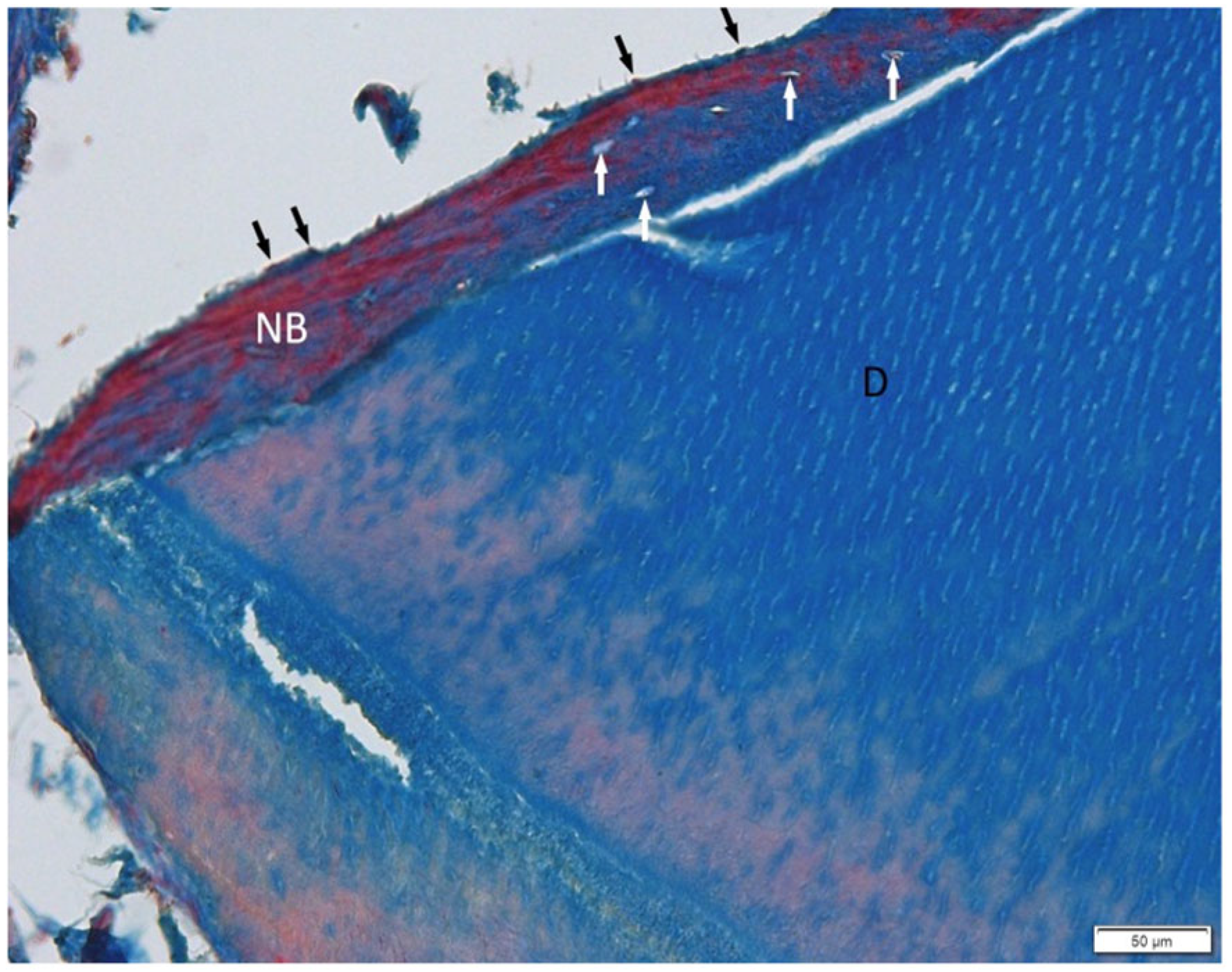
3.1. Histological Examination
3.1.1. BonMaker ST
3.1.2. Smart Dentin Grinder ST
3.1.3. Bio-Gen Mix Xenograft SB
3.1.4. BioBank Allograft SB
3.2. Histological Evaluation of Graft Integration with Bone Tissue
3.3. Evaluation of Healing Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornelius Timothius, C.J.; Kilic, H.N.; Gandhi, K.K.; Kakar, A.; John, V. Particulate Bone Graft Materials for Periodontal and Implant Surgery: A Narrative Review and Case Series. Dent. Rev. 2023, 3, 100068. [Google Scholar] [CrossRef]
- Pandit, N.; Pandit, I. Autogenous Bone Grafts in Periodontal Practice: A Literature Review. J. Int. Clin. Dent. Res. Organ. 2016, 8, 27. [Google Scholar] [CrossRef]
- Dłucik, R.; Orzechowska-Wylęgała, B.; Dłucik, D.; Bogus, K. Histological Examination of Tooth-Derived Biomaterials Obtained from Different Devices. Expert Rev. Med. Devices 2023, 20, 979–988. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Sahin, D.; Sader, R.; Becker, J.; Schwarz, F. Efficacy of Autogenous Teeth for the Reconstruction of Alveolar Ridge Deficiencies: A Systematic Review. Clin. Oral Investig. 2019, 23, 4263–4287. [Google Scholar] [CrossRef]
- Janjua, O.S.; Qureshi, S.M.; Shaikh, M.S.; Alnazzawi, A.; Rodriguez-Lozano, F.J.; Pecci-Lloret, M.P.; Zafar, M.S. Autogenous Tooth Bone Grafts for Repair and Regeneration of Maxillofacial Defects: A Narrative Review. Int. J. Environ. Res. Public. Health 2022, 19, 3690. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Gianfreda, F.; Bollero, P.; Annicchiarico, C.; Daniele, M.; Padula, R.; Mastrangelo, F. Comparative Histological Analysis of Dentine-Derived Tooth Grafts in Maxillary vs. Mandibular Socket Preservation: A Retrospective Study of 178 Cases. Dent. J. 2024, 12, 320. [Google Scholar] [CrossRef]
- Dłucik, R.; Orzechowska-Wylęgala, B.; Dłucik, D.; Puzzolo, D.; Micali, A. Socket Preservation or Guided Bone Regeneration—A Case Report. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2021, 49, 153–157. [Google Scholar]
- Wushou, A.; Zheng, Y.; Han, Y.; Yang, Z.-C.; Han, F.-K. The Use of Autogenous Tooth Bone Graft Powder in the Treatment of Osseous Defects after Impacted Mandibular Third Molar Extraction: A Prospective Split-Mouth Clinical Pilot Study. BMC Oral Health 2022, 22, 433. [Google Scholar] [CrossRef]
- Sun, H.; Yin, X.; Yang, C.; Kuang, H.; Luo, W. Advances in Autogenous Dentin Matrix Graft as a Promising Biomaterial for Guided Bone Regeneration in Maxillofacial Region: A Review. Medicine 2024, 103, e39422. [Google Scholar] [CrossRef]
- Olchowy, A.; Olchowy, C.; Zawiślak, I.; Matys, J.; Dobrzyński, M. Revolutionizing Bone Regeneration with Grinder-Based Dentin Biomaterial: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 9583. [Google Scholar] [CrossRef]
- Cervera-Maillo, J.M.; Morales-Schwarz, D.; Morales-Melendez, H.; Mahesh, L.; Calvo-Guirado, J.L. Autologous Tooth Dentin Graft: A Retrospective Study in Humans. Medicina 2021, 58, 56. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchi, G.; Mariano, A.; Serafini, G.; Lamazza, L.; Scotto d’Abusco, A.; De Biase, A.; Lollobrigida, M. Osteoinductive Properties of Autologous Dentin: An Ex Vivo Study on Extracted Teeth. J. Funct. Biomater. 2024, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Al-Quisi, A.F.; Abdulkareem, A.A. Efficacy of Autogenous Dentin Biomaterial on Alveolar Ridge Preservation: A Randomized Controlled Clinical Trial. BioMed Res. Int. 2023, 2023, 7932432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Bao, J.; Fu, X.; Wu, Y.; Yang, S.; Ren, X.; Fang, X.; Yuan, Q.; Xie, Z.; Seriwatanachai, D. The Healing Capacity and Osteogenesis Pattern of Demineralized Dentin Matrix (DDM)-Fibrin Glue (FG) Compound. Sci. Rep. 2023, 13, 13140. [Google Scholar] [CrossRef]
- Dłucik, R.; Orzechowska-Wylęgała, B.; Dłucik, D.; Puzzolo, D.; Santoro, G.; Micali, A.; Testagrossa, B.; Acri, G. Comparison of Clinical Efficacy of Three Different Dentin Matrix Biomaterials Obtained from Different Devices. Expert Rev. Med. Devices 2023, 20, 313–327. [Google Scholar] [CrossRef]
- Murata, M.; Hirose, Y.; Ochi, M.; Tazaki, J.; Okubo, N.; Akazawa, T. Twenty Years-Passed Case of Demineralized Dentin Matrix Autograft for Sinus Bone Augmentation—A First Case of Dentin Graft in Human. J. Clin. Exp. Dent. 2023, 15, e861–e865. [Google Scholar] [CrossRef]
- Anido-Anido, A.; Souza-Junior, E.J.; Brandt, W.C.; Silva, E.; Alonso, R.C.B.; Zaia, A.; Puppin-Rontani, R.M.; Sinhoreti, M.A.C. Dental Adhesives Containing Alternative Photoinitiators: Degree of Conversion and Cytotoxicity. Dent. Mater. 2012, 28, e1. [Google Scholar] [CrossRef]
- Amid, R.; Kheiri, A.; Kheiri, L.; Kadkhodazadeh, M.; Ekhlasmandkermani, M. Structural and Chemical Features of Xenograft Bone Substitutes: A Systematic Review of In Vitro Studies. Biotechnol. Appl. Biochem. 2021, 68, 1432–1452. [Google Scholar] [CrossRef]
- Cowan, P.T.; Launico, M.V.; Kahai, P. Anatomy, Bones. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef]
- Nagaraj, A.; Shetty, M.; Thomas, B.; Hegde, R. Ridge Augmentation Using Autograft and Xenograft Versus Xenograft Alone With Simultaneous Implant Placement: A Randomised Clinical Trial. Acta Medica Bulg. 2024, 51 (Suppl. 2), 83–89. [Google Scholar] [CrossRef]
- Khan, R.S.; Aslam, M.; Ucer, C.; Wright, S. Success of Xenografts in Alveolar Ridge Preservation Based on Histomorphometric Outcomes. Dent. J. 2023, 11, 215. [Google Scholar] [CrossRef]
- Nishimura, D.A.; Iida, C.; Carneiro, A.L.E.; Arita, E.S.; Costa, C.; Cortes, A.R.G. Digital Workflow for Alveolar Ridge Preservation With Equine-Derived Bone Graft and Subsequent Implant Rehabilitation. J. Oral Implantol. 2021, 47, 159–167. [Google Scholar] [CrossRef]
- Sayardoust, S.; Norstedt, W.; Shah, F.A. The Long-Term Impact of Alveolar Ridge Preservation with Xenograft Bone Mineral on Peri-Implant Health after 5 Years in Function: A Retrospective Cohort Study of 108 Patients Assessed Clinically and Radiologically. Clin. Exp. Dent. Res. 2022, 8, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Goutam, M.; Batra, N.; Jyothirmayee, K.; Bagrecha, N.; Deshmukh, P.; Malik, S. A Comparison of Xenograft Graft Material and Synthetic Bioactive Glass Allograft in Immediate Dental Implant Patients. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. 1), S980–S982. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-C. Comparison of Clinical Parameters in Dental Implant Therapy between Implant Site Development Using Porcine- and Bovine-Derived Xenografts. Technologies 2021, 9, 72. [Google Scholar] [CrossRef]
- Kormas, I.; Pedercini, A.; Alassy, H.; Wolff, L.F. The Use of Biocompatible Membranes in Oral Surgery: The Past, Present & Future Directions. A Narrative Review. Membranes 2022, 12, 841. [Google Scholar] [CrossRef]
- Ciszyński, M.; Dominiak, S.; Dominiak, M.; Gedrange, T.; Hadzik, J. Allogenic Bone Graft in Dentistry: A Review of Current Trends and Developments. Int. J. Mol. Sci. 2023, 24, 16598. [Google Scholar] [CrossRef]
- Mitton, D.; Rappeneau, J.; Bardonnet, R. Effect of a Supercritical CO2 Based Treatment on Mechanical Properties of Human Cancellous Bone. Eur. J. Orthop. Surg. Traumatol. 2005, 15, 264–269. [Google Scholar] [CrossRef]
- Faces, J.; Poirier, B.; Barbier, Y.; Frayssinet, P.; Joffret, M.-L.; Majewski, W.; Bonel, G.; Larzul, D. Viral Inactivation of Human Bone Tissue Using Supercritical Fluid Extraction. ASAIO J. 1998, 44, 289–293. [Google Scholar] [CrossRef]
- Zwittnig, K.; Mukaddam, K.; Vegh, D.; Herber, V.; Jakse, N.; Schlenke, P.; Zrnc, T.A.; Payer, M. Platelet-Rich Fibrin in Oral Surgery and Implantology: A Narrative Review. Transfus. Med. Hemotherapy Off. Organ Dtsch. Ges. Transfusionsmedizin Immunhamatol. 2023, 50, 348–359. [Google Scholar] [CrossRef]
- Skurska, A.; Chwiedosik, M.; Ślebioda, Z. Adjunctive Use of Platelet-Rich Fibrin in Surgical Treatment of Furcation Defects: A Systematic Review. Adv. Med. Sci. 2023, 68, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Neal, T.W.; Sullivan, S.R.; Cannon, S.; Spresser, W.; Schlieve, T. Platelet Rich Fibrin: A Literature Review of Applications in Oral and Maxillofacial Surgery. J. Oral Maxillofac. Anesth. 2024, 3, 6. [Google Scholar] [CrossRef]
- Tony, J.B.; Parthasarathy, H.; Tadepalli, A.; Ponnaiyan, D.; Alamoudi, A.; Kamil, M.A.; Alzahrani, K.J.; Alsharif, K.F.; Halawani, I.F.; Alnfiai, M.M.; et al. CBCT Evaluation of Sticky Bone in Horizontal Ridge Augmentation with and without Collagen Membrane—A Randomized Parallel Arm Clinical Trial. J. Funct. Biomater. 2022, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Sareen, V.; K, S.; Saxena, I.; Selvaraj, U.; P, V.; Chauhan, S.; M, G. Role of Sticky Bone in the Management of Various Alveolar Bone Defects: A Systematic Review. Cureus 2024, 16, e63561. [Google Scholar] [CrossRef]
- Wushou, A.; Luo, Y.; Cheng, Q.-T.; Yang, Z.-C. Using Autogenous Tooth Sticky Bone Graft Repair Mandibular Third Molar Dentigerous Cyst Osseous Defects. BMC Oral Health 2024, 24, 39. [Google Scholar] [CrossRef]
- Couso-Queiruga, E.; Stuhr, S.; Tattan, M.; Chambrone, L.; Avila-Ortiz, G. Post-Extraction Dimensional Changes: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2021, 48, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Janaphan, K.; Buranawat, B.; Sathitthammaphon, T.; Hanroongsri, J.; Ardsungnoen, S. Horizontal Ridge Augmentation Using Sticky Bone with Platelet-Rich Fibrin (PRF) in Anterior Maxilla Clinical and Histologic Evidence: A Case Report. Oral Sci. Rep. 2024, 45, 72–78. [Google Scholar] [CrossRef]
- Van Orten, A.; Goetz, W.; Bilhan, H. Tooth-Derived Granules in Combination with Platelet-Rich Fibrin (“Sticky Tooth”) in Socket Preservation: A Histological Evaluation. Dent. J. 2022, 10, 29. [Google Scholar] [CrossRef]
- Schwimer, C.; Firlej, M.; Pohl, S.; Martin, R.; Gluckman, H.; Huwais, S.; Neiva, R. Selective Preservation of Tooth (SPOT): A Step-by-Step Protocol for a Precise, Reproducible, Socket-Shield Technique. Compend. Contin. Educ. Dent. 2024, 45, 350–357. [Google Scholar]
- Firlej, M.; Schwimer, C.; Martin, R.; Gluckman, H.; Guentsch, A.; Neiva, R. Guided Selective Preservation of Tooth (SPOT) Protocol: Fully Guided Socket-Shield Technique. Compend. Contin. Educ. Dent. 2025, 46, 30–37. [Google Scholar]
- Grawish, M.E.; Grawish, L.M.; Grawish, H.M.; Grawish, M.M.; Holiel, A.A.; Sultan, N.; El-Negoly, S.A. Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review. Tissue Eng. Regen. Med. 2022, 19, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Choi, H.; Sohn, D.-S. Optimizing Bone Regeneration with Demineralized Dentin-Derived Graft Material: Impact of Demineralization Duration in a Rabbit Calvaria Model. J. Funct. Biomater. 2024, 15, 331. [Google Scholar] [CrossRef]
- Khurshid, Z.; Adanir, N.; Ratnayake, J.; Dias, G.; Cooper, P.R. Demineralized Dentin Matrix for Bone Regeneration in Dentistry: A Critical Update. Saudi Dent. J. 2024, 36, 443–450. [Google Scholar] [CrossRef]
- Kamal, M.; Andersson, L.; Tolba, R.; Al-Asfour, A.; Bartella, A.K.; Gremse, F.; Rosenhain, S.; Hölzle, F.; Kessler, P.; Lethaus, B. Bone Regeneration Using Composite Non-Demineralized Xenogenic Dentin with Beta-Tricalcium Phosphate in Experimental Alveolar Cleft Repair in a Rabbit Model. J. Transl. Med. 2017, 15, 263. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part I: Technological Concepts and Evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part II: Platelet-Related Biologic Features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L.; et al. Use of Platelet-Rich Fibrin in Regenerative Dentistry: A Systematic Review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kadokura, H.; Iso, E.; Tsuchiya, T.; Yokose, S. Effect of Cells Derived from Periodontal Ligament Tissue on Bone Formation. In Vivo 2024, 38, 1594–1600. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.-H.; Um, I.-W.; Cho, W.-J. Guided Bone Regeneration Using Demineralized Dentin Matrix: Long-Term Follow-Up. J. Oral Maxillofac. Surg. 2016, 74, 515.e1–515.e9. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Sohn, D.-S.; Kim, G.; Park, I. Comparative Histomorphometric Evaluation of Bone Regeneration with Different Preparations of Xenogeneic Tooth Block Bone. Int. J. Oral Maxillofac. Implants 2019, 34, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Valentini, P.; Bosshardt, D.D. 20-Year Follow-up in Maxillary Sinus Floor Elevation Using Bovine-Derived Bone Mineral: A Case Report with Histologic and Histomorphometric Evaluation. Int. J. Oral Maxillofac. Implants 2018, 33, 1345–1350. [Google Scholar] [CrossRef] [PubMed]






| Graph 3. | Type of Biomaterial | Vitamin D3 Level * | Calcium Level * | Creatinine Level * | Total Cholesterol * | ALT Level * | Bone Density * |
|---|---|---|---|---|---|---|---|
| 1 | ST-DDM | 46.3 ng/mL | 9.15 mg/dL | 0.90 mg/dL | 189 mg/dL | 21 U/L | D2 |
| 2 | ST-MDM | 45.2 ng/mL | 8.94 mg/dL | 0.98 mg/dL | 196 mg/dL | 19 U/L | D2 |
| 3 | SB Xenograft | 40.1 ng/mL | 10.3 mg/dL | 1.02 mg/dL | 185 mg/dL | 18 U/L | D2 |
| 4 | SB Allograft | 42.8 ng/mL | 9.40 mg/dL | 0.94 mg/dL | 191 mg/dL | 22 U/L | D2 |
| Group | Percentage of Graft Material Embedded in Bone | Percentage of Graft Material Unintegrated within Connective Tissue |
|---|---|---|
| C1 (SB Xenograft) | 56.25% | 43.75% |
| C2 (SB Allograft) | 58.30% | 41.70% |
| ST-DDM | 75.86% | 24.14% 1 |
| ST-MDM | 69.10% 2,3 | 30.90% 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dłucik, R.; Firlej, M.; Bogus, K.; Dłucik, D.; Orzechowska-Wylęgała, B. Histological Analysis of Sticky Tooth and Sticky Bone. J. Funct. Biomater. 2025, 16, 233. https://doi.org/10.3390/jfb16070233
Dłucik R, Firlej M, Bogus K, Dłucik D, Orzechowska-Wylęgała B. Histological Analysis of Sticky Tooth and Sticky Bone. Journal of Functional Biomaterials. 2025; 16(7):233. https://doi.org/10.3390/jfb16070233
Chicago/Turabian StyleDłucik, Robert, Marcel Firlej, Katarzyna Bogus, Daniel Dłucik, and Bogusława Orzechowska-Wylęgała. 2025. "Histological Analysis of Sticky Tooth and Sticky Bone" Journal of Functional Biomaterials 16, no. 7: 233. https://doi.org/10.3390/jfb16070233
APA StyleDłucik, R., Firlej, M., Bogus, K., Dłucik, D., & Orzechowska-Wylęgała, B. (2025). Histological Analysis of Sticky Tooth and Sticky Bone. Journal of Functional Biomaterials, 16(7), 233. https://doi.org/10.3390/jfb16070233






