Developing Bioengineered 3D-Printed Composite Scaffolds with Antimicrobial Potential for Bone Tissue Regeneration
Abstract
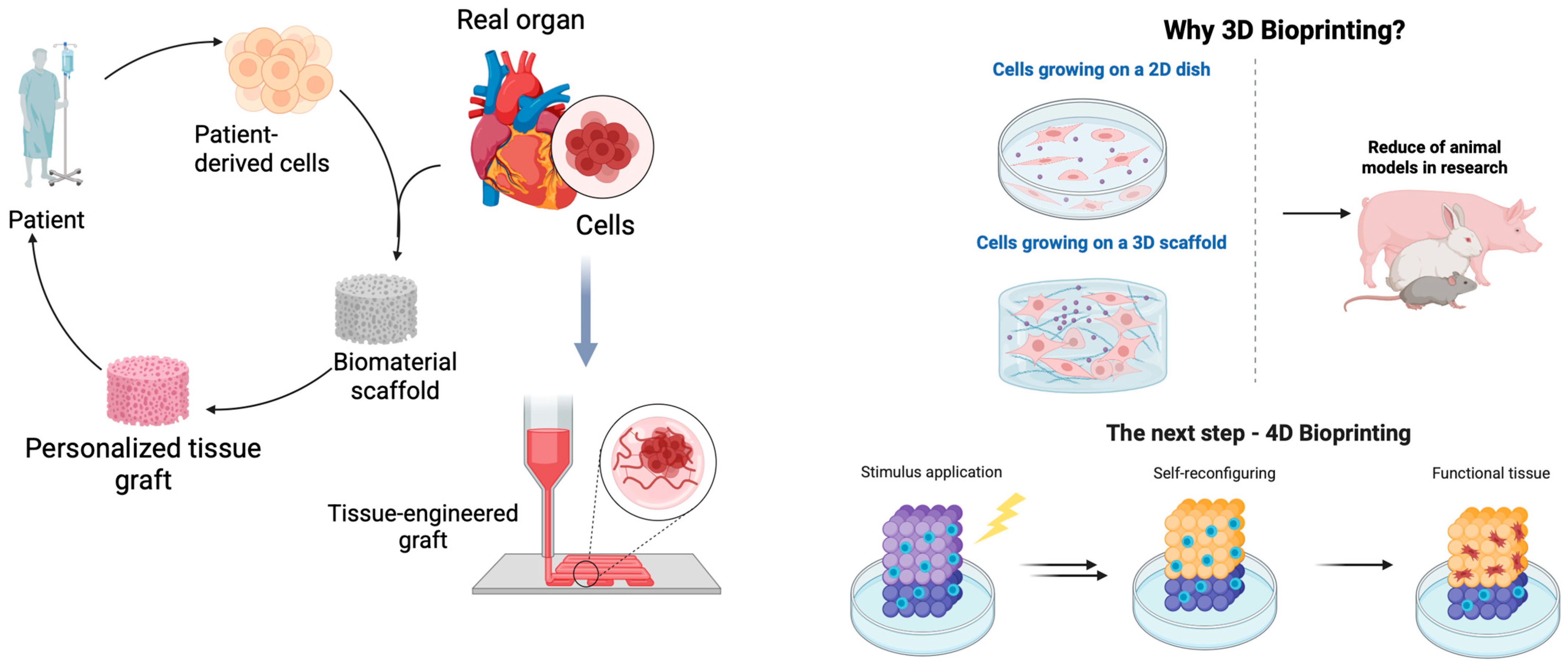
1. Introduction
2. Materials and Methods
2.1. Materials
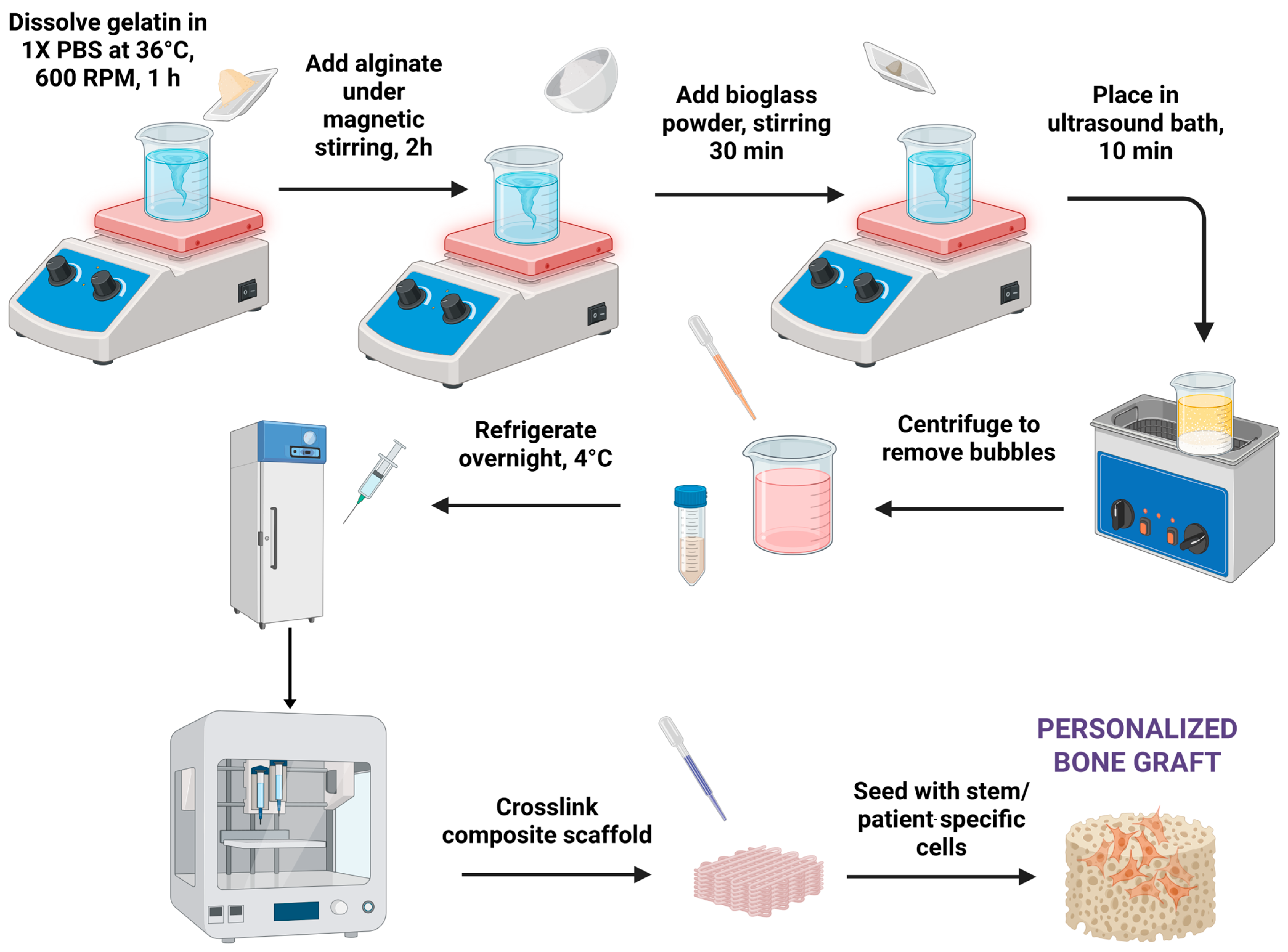
2.2. Hydrogel Synthesis
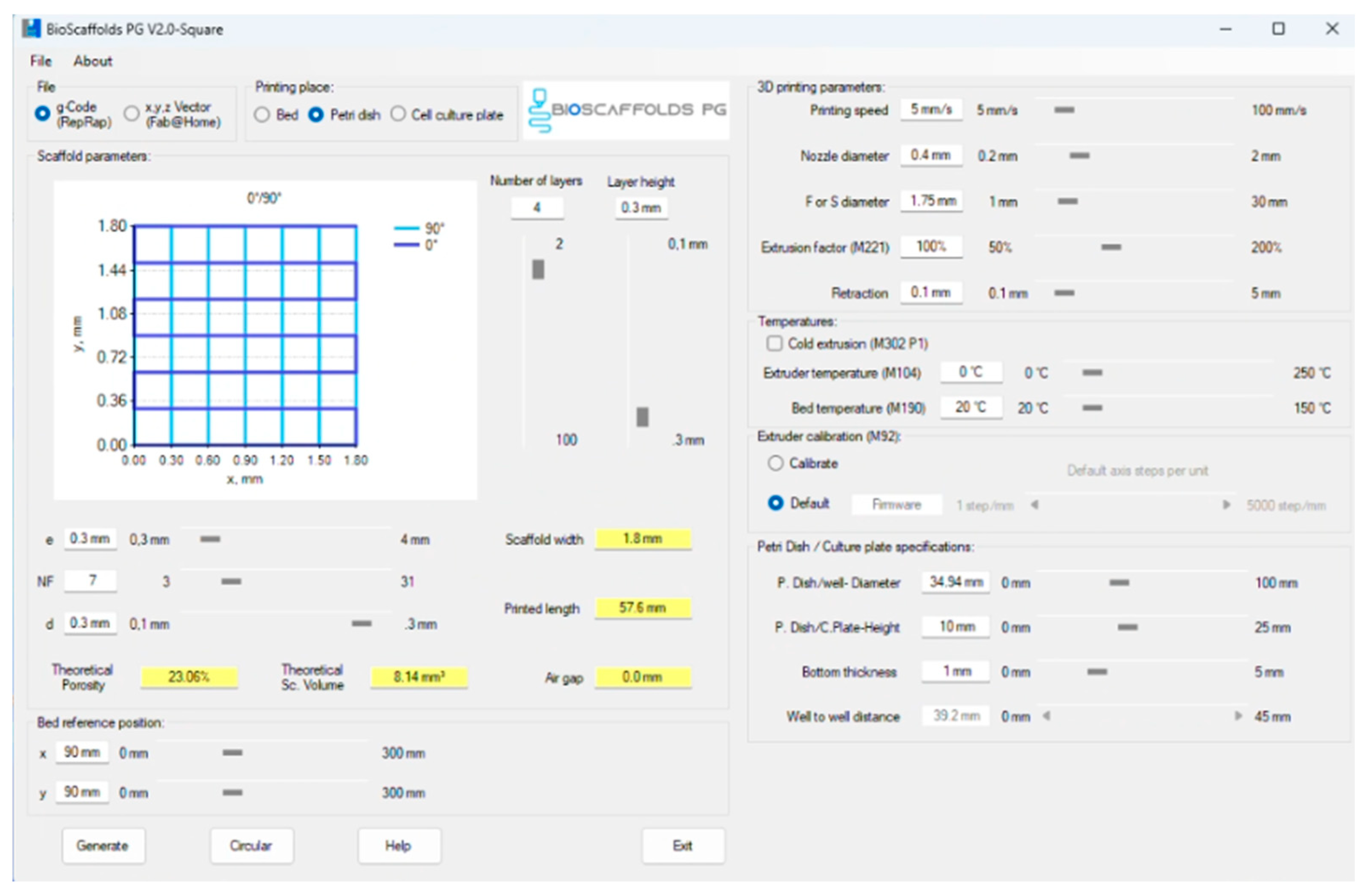
2.3. Three-Dimensional Printing Process
2.4. Characterization Methods
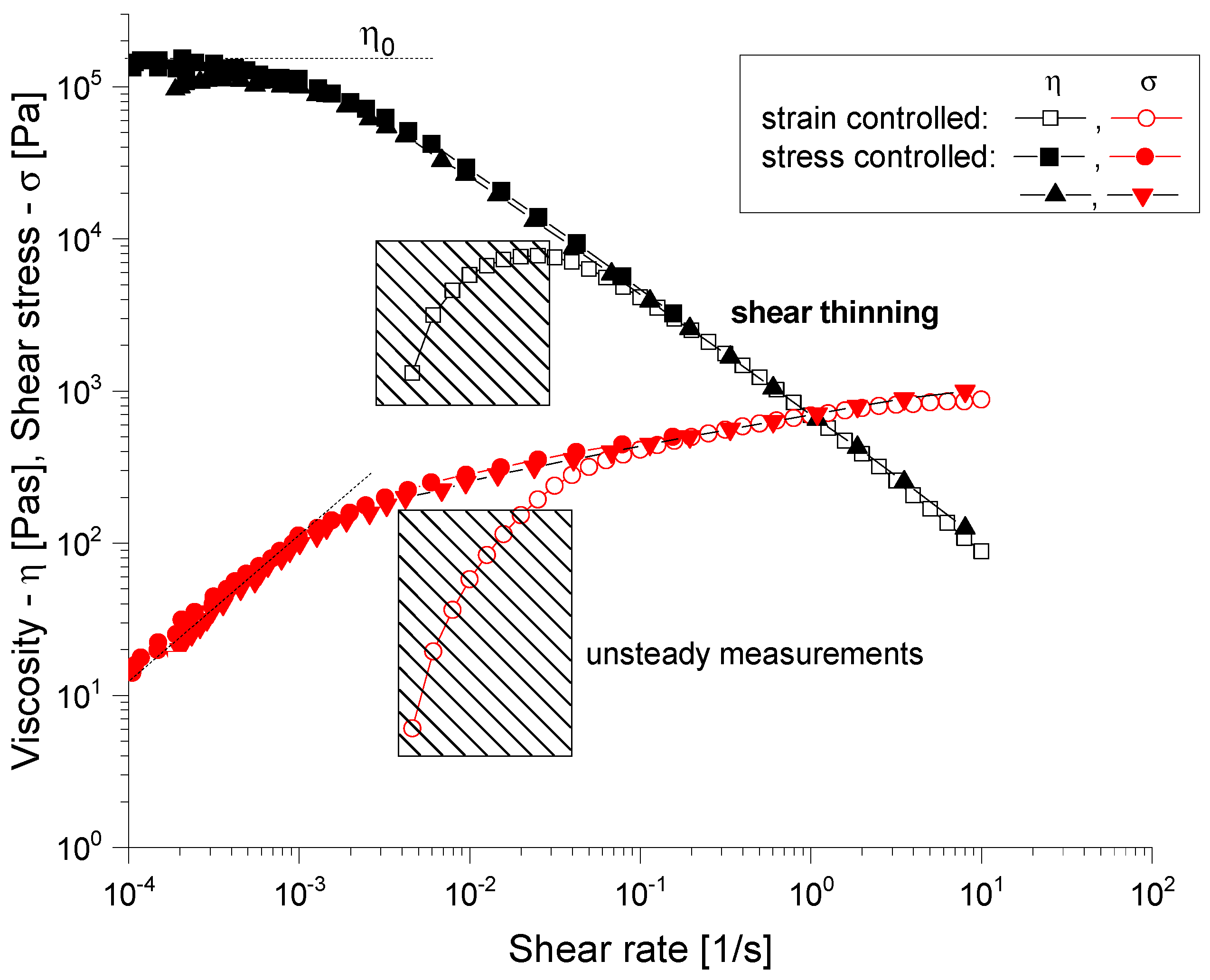
2.4.1. Rheological Evaluation
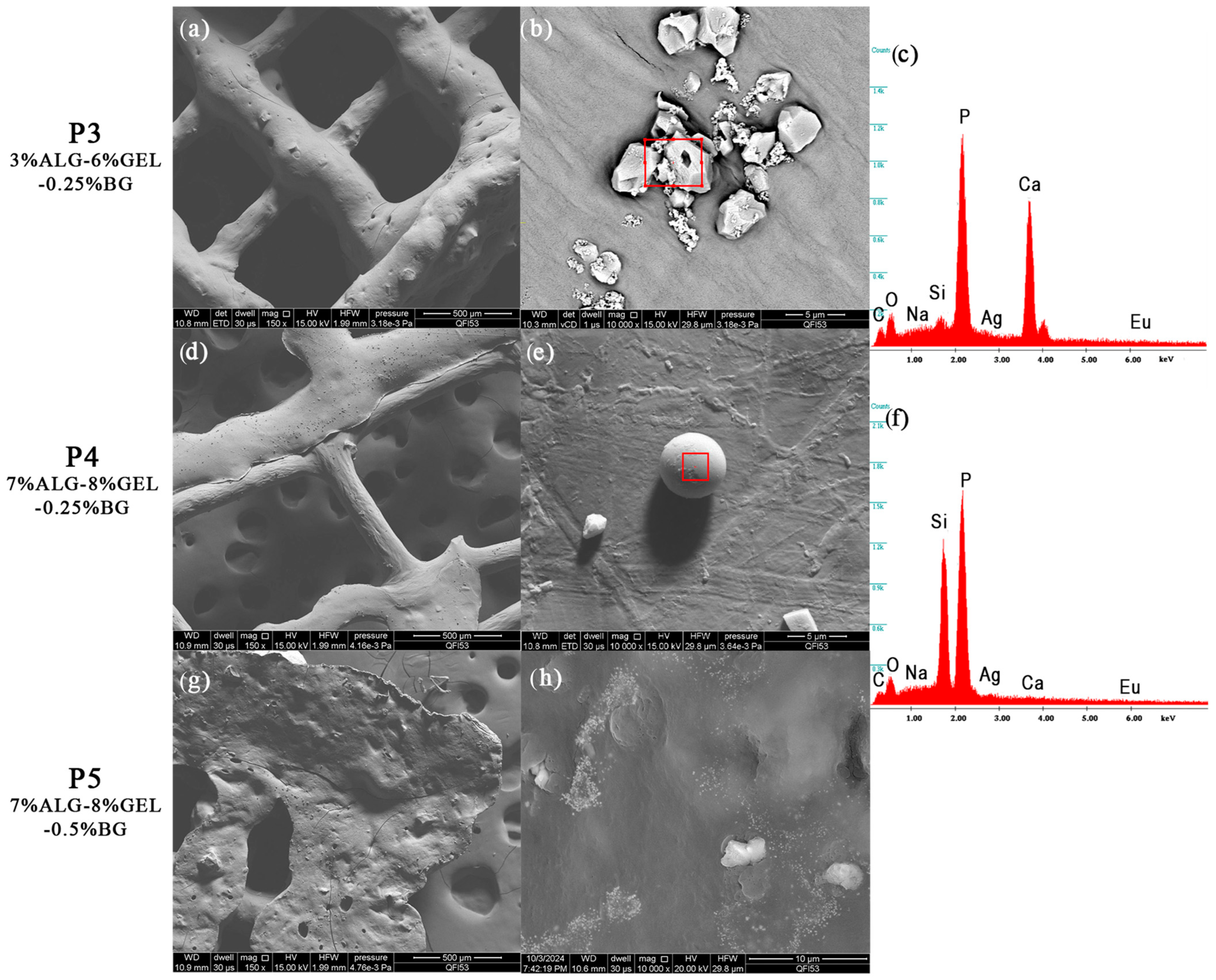
2.4.2. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
2.4.3. Fourier Transform Infrared Spectroscopy
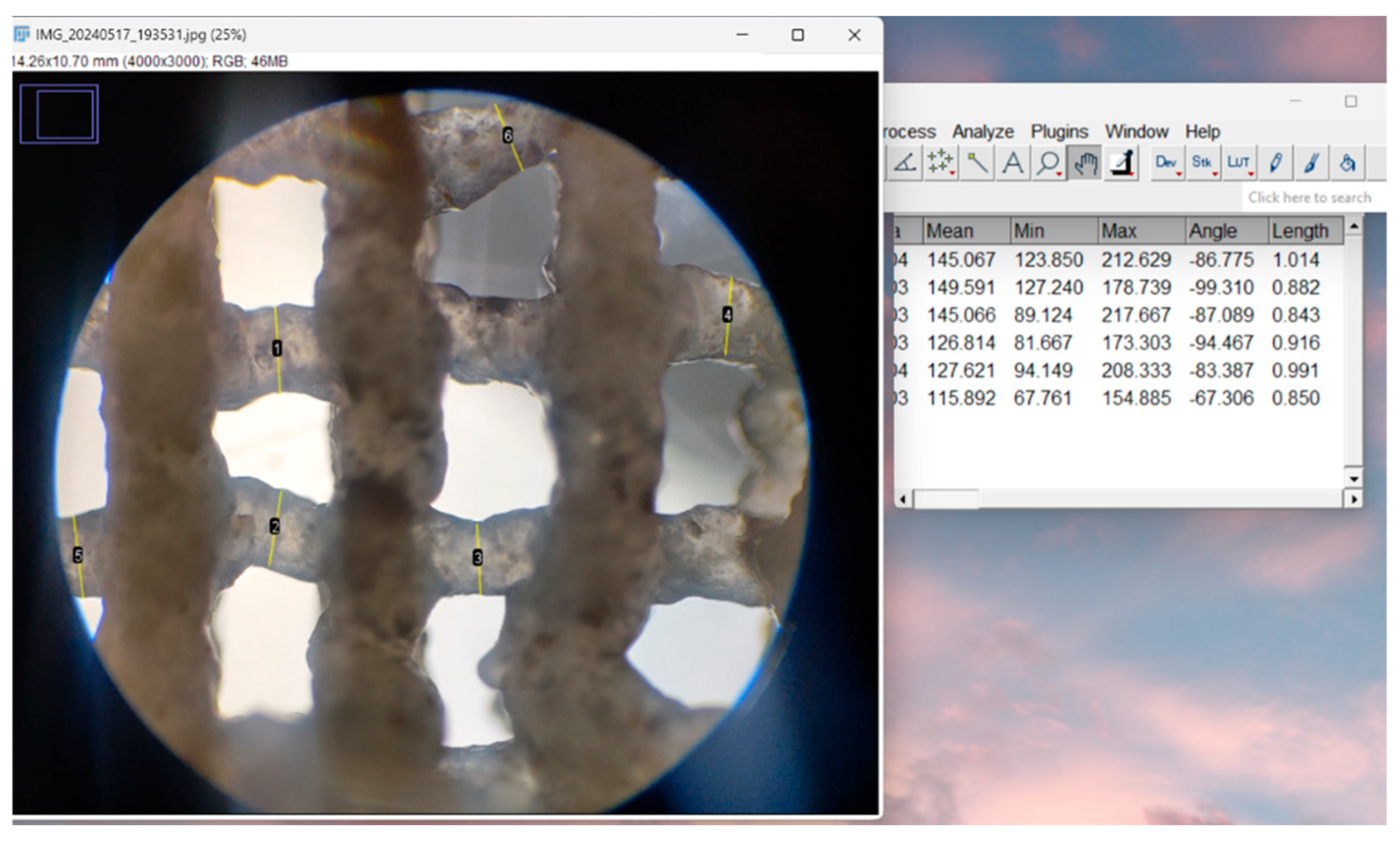
2.4.4. Optical Microscopy—Printing Accuracy
2.4.5. Gravimetric Method
2.4.6. In Vitro Degradation Study
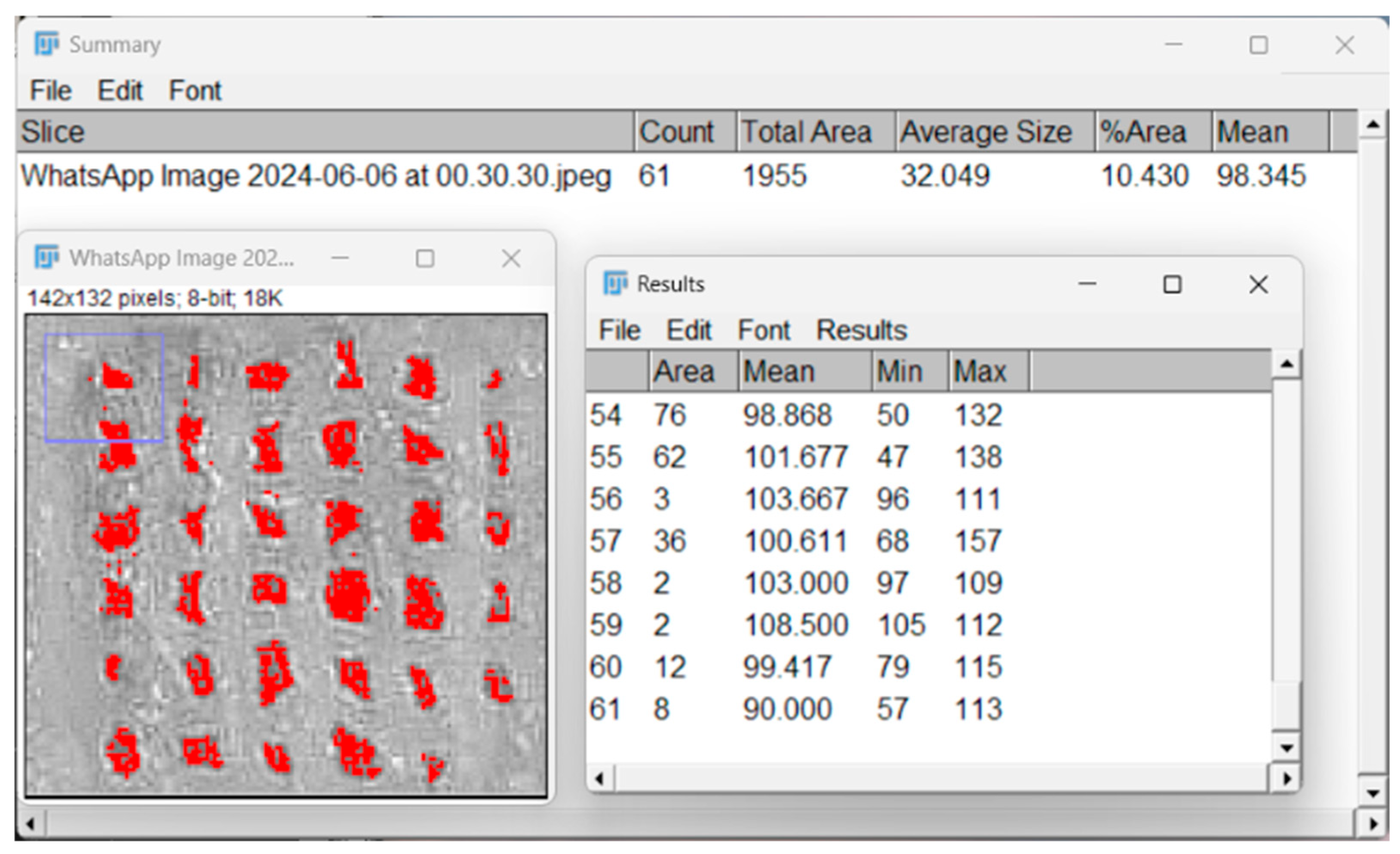
2.4.7. Porosity Evaluation with Particle Analyzer
2.4.8. In Vitro Mineralization
2.4.9. Cell Seeding and LIVE/DEAD Assay
3. Results
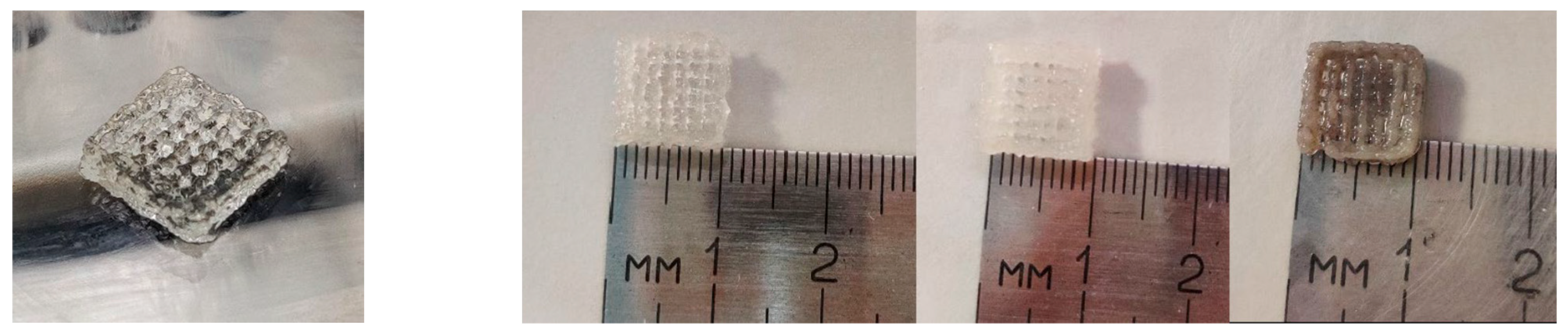
3.1. Three-Dimensional Printing Process
3.2. Characterization Methods
3.2.1. Rheological Evaluation
3.2.2. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
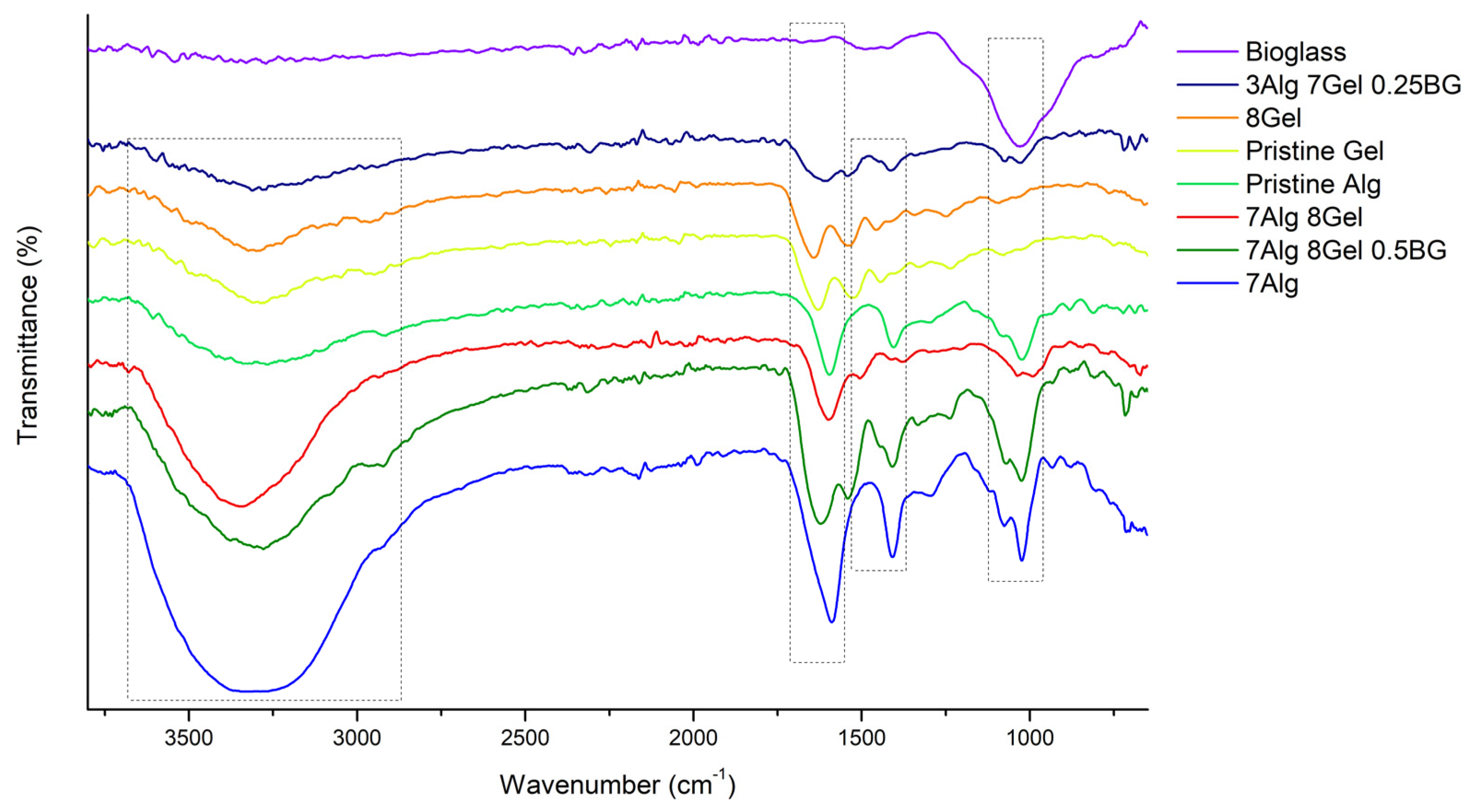
3.2.3. Fourier Transform Infrared Spectroscopy
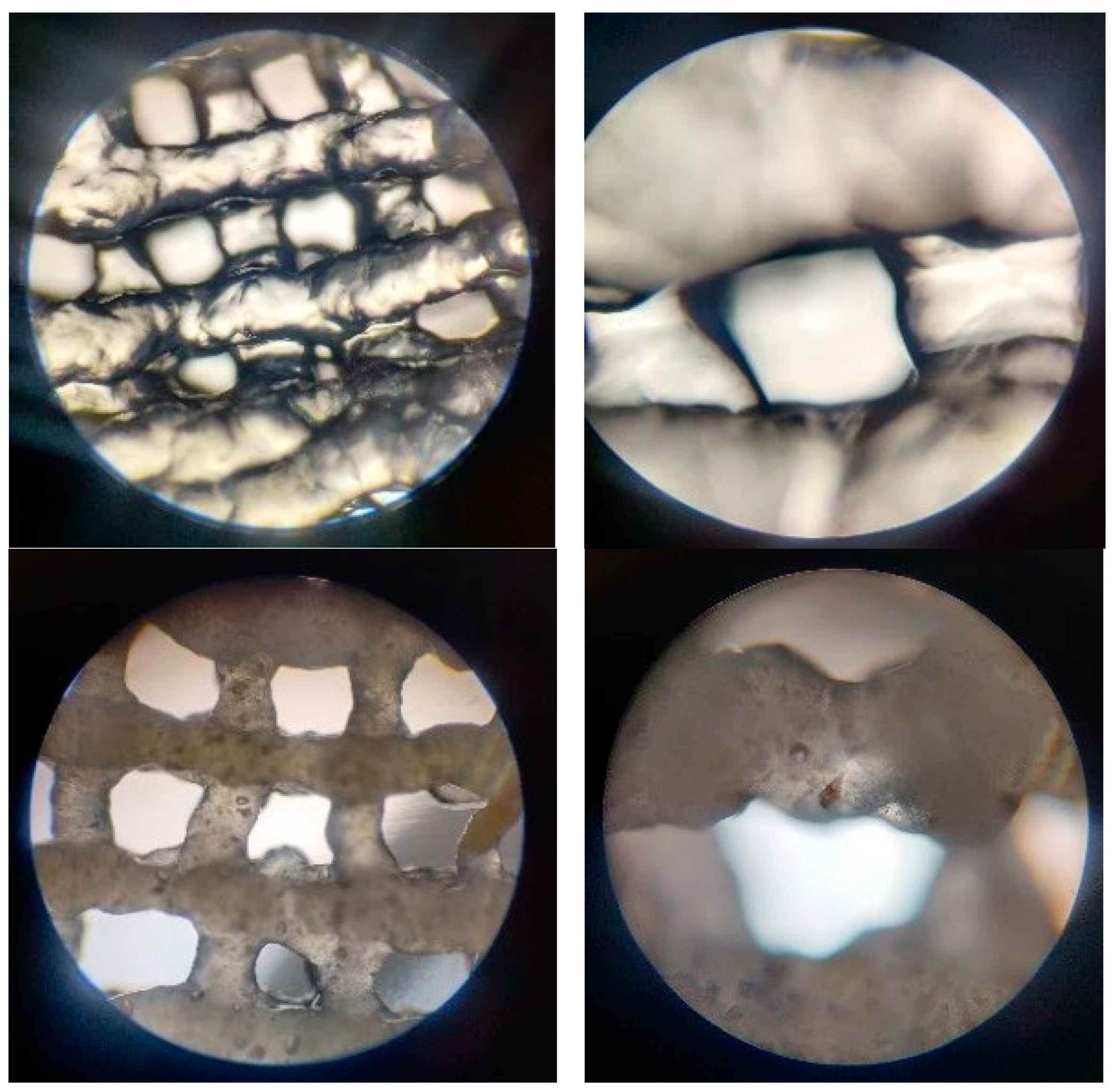
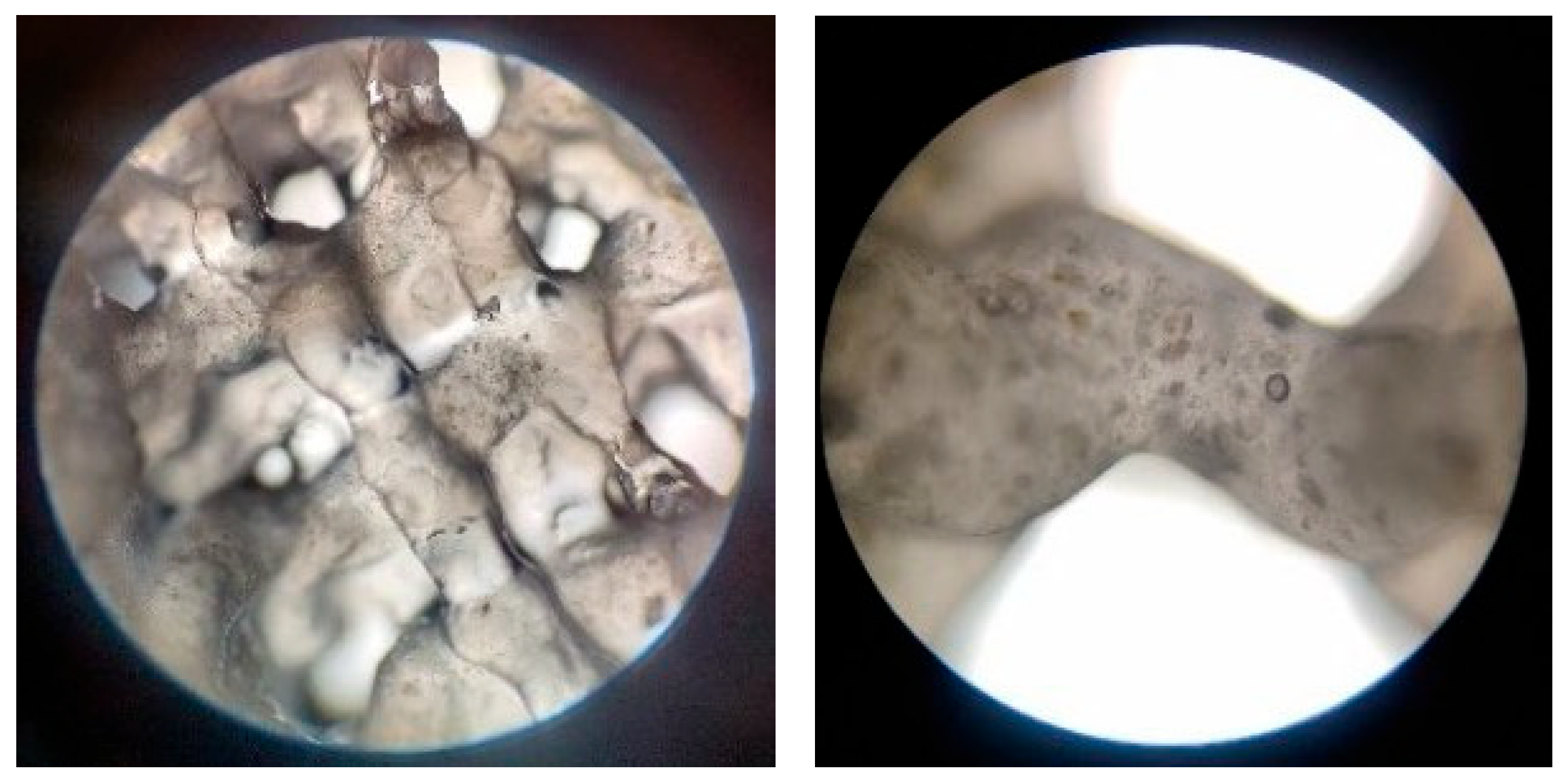
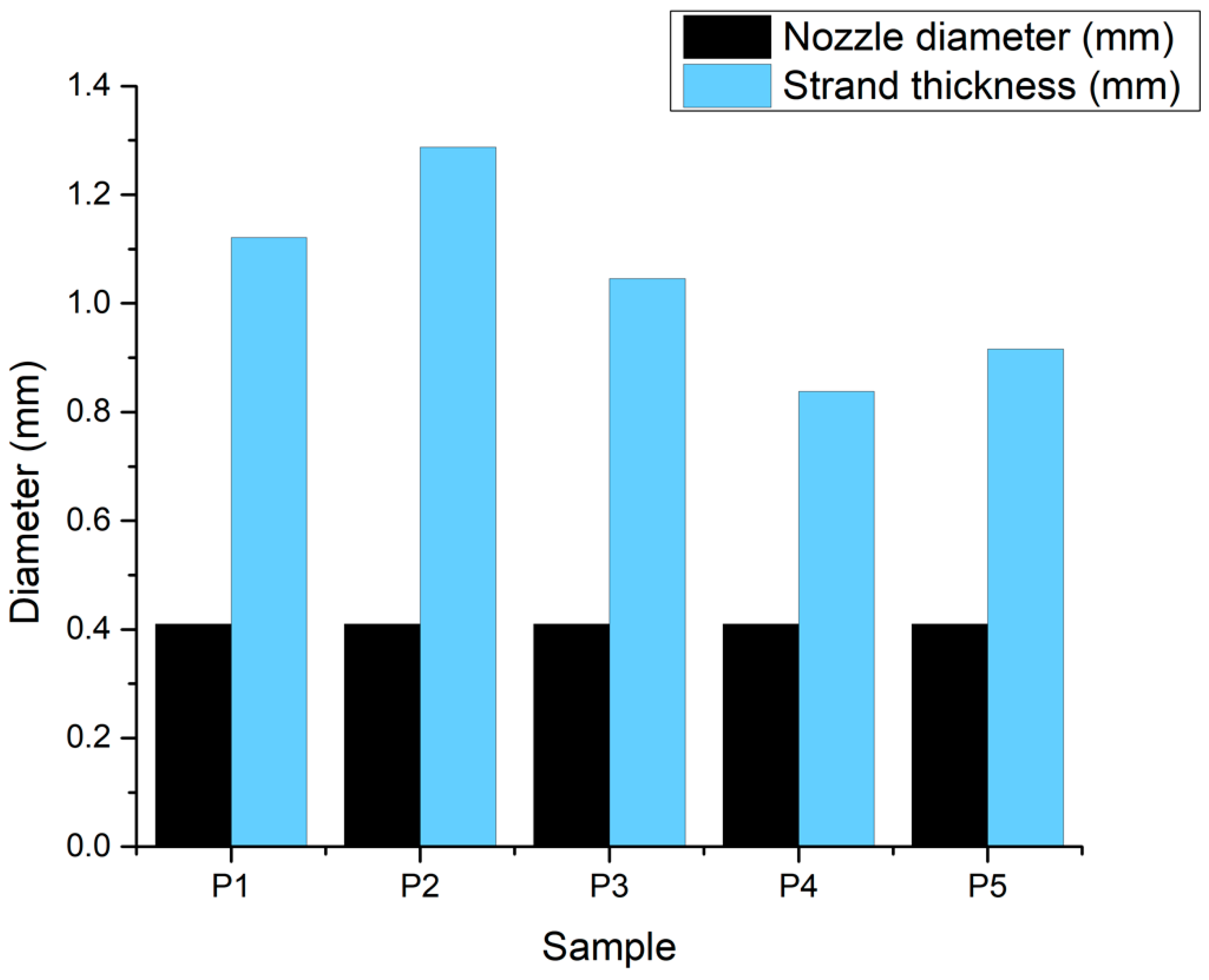
3.2.4. Optical Microscopy—Printing Accuracy
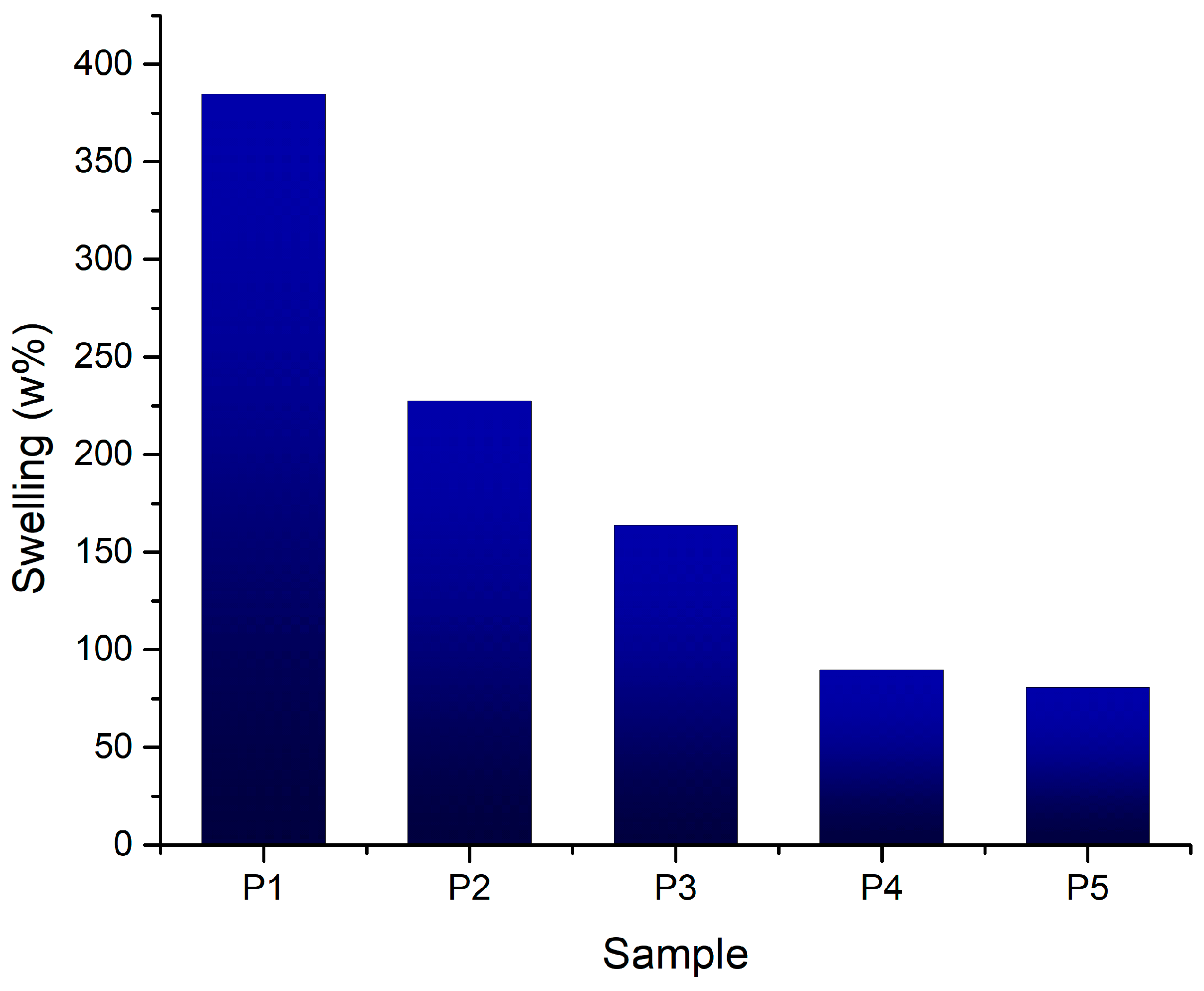
3.2.5. Swelling Degree
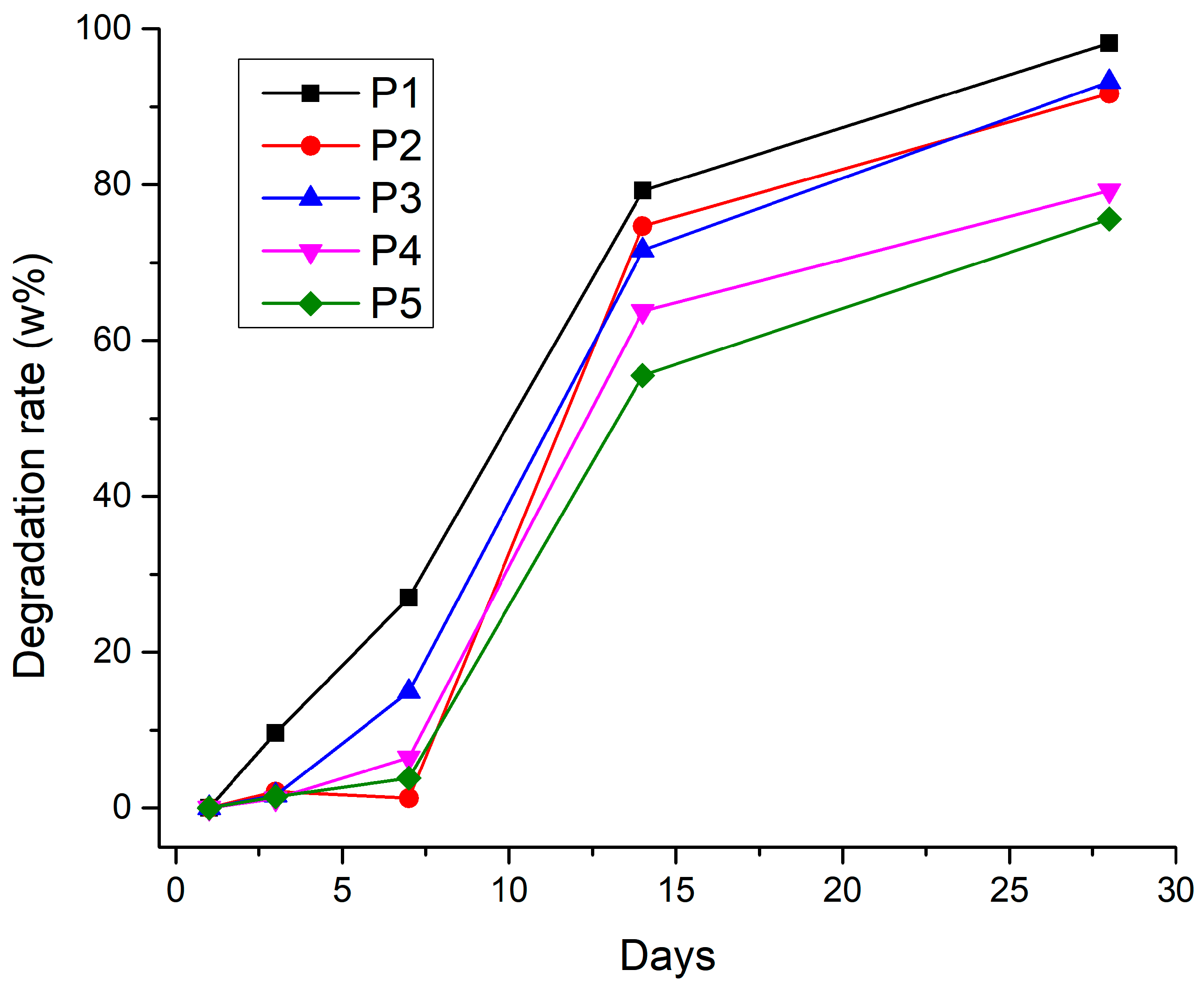
3.2.6. Degradation Rate
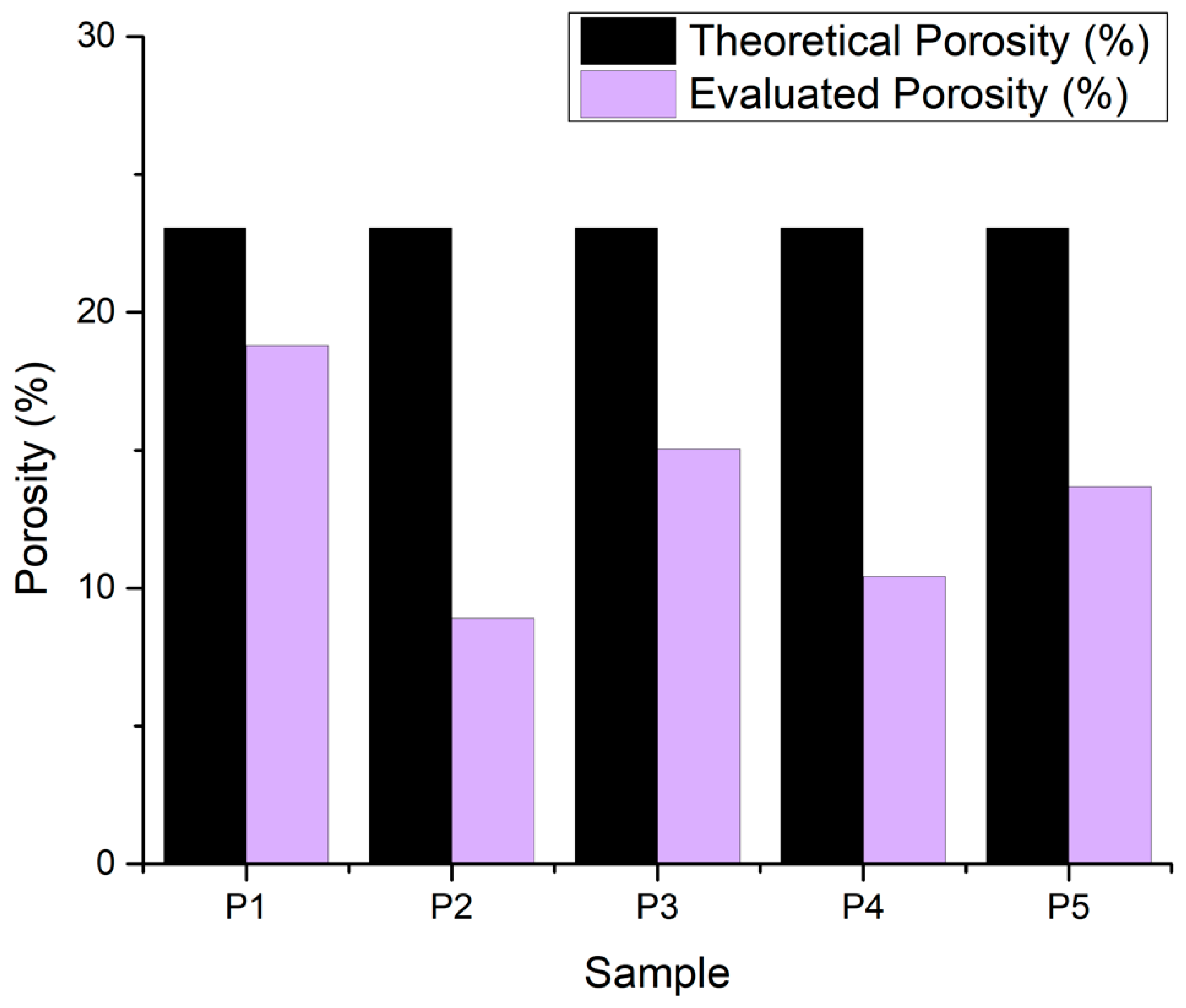
3.2.7. Porosity Evaluation
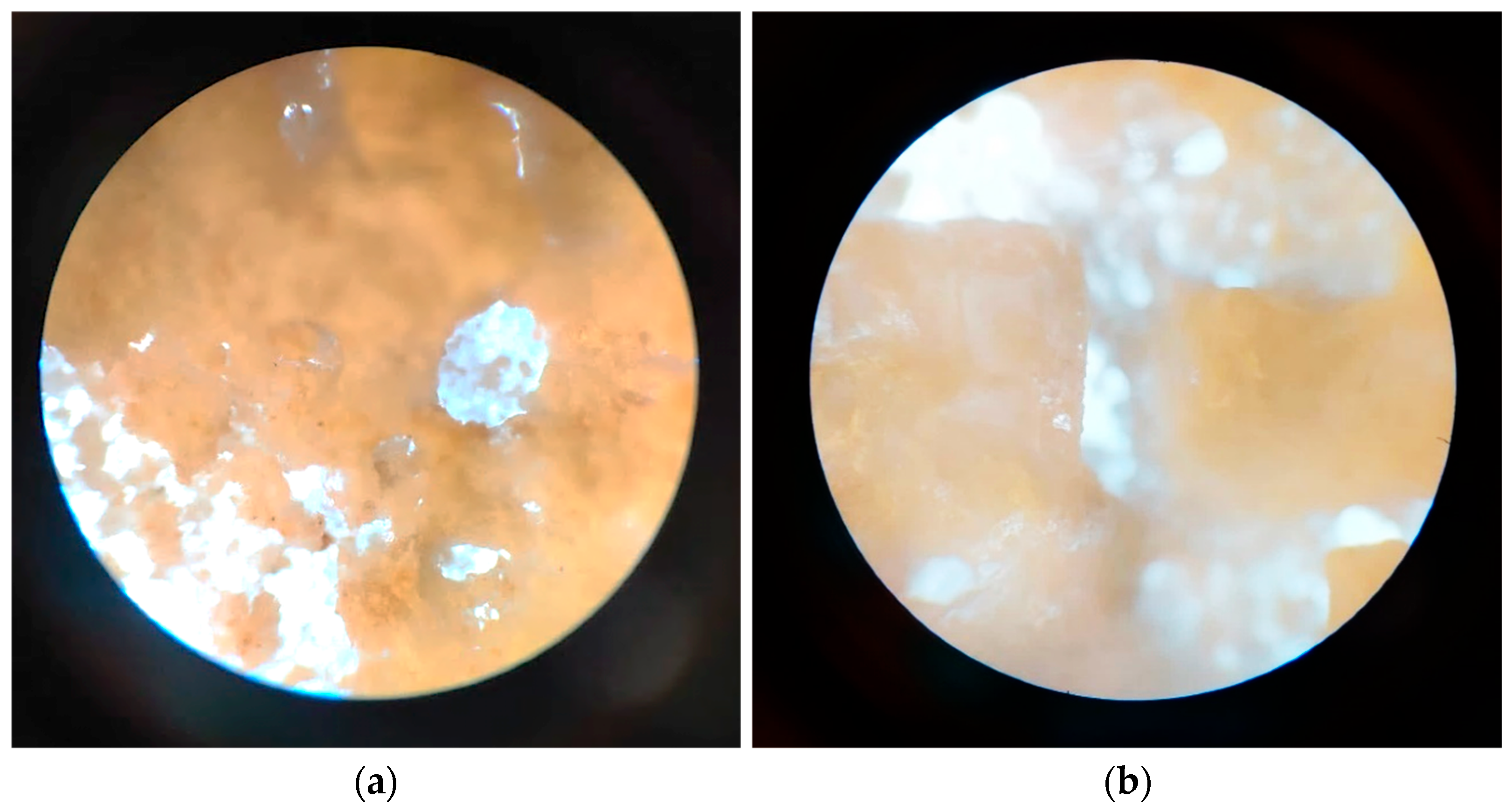
3.2.8. In Vitro Mineralization
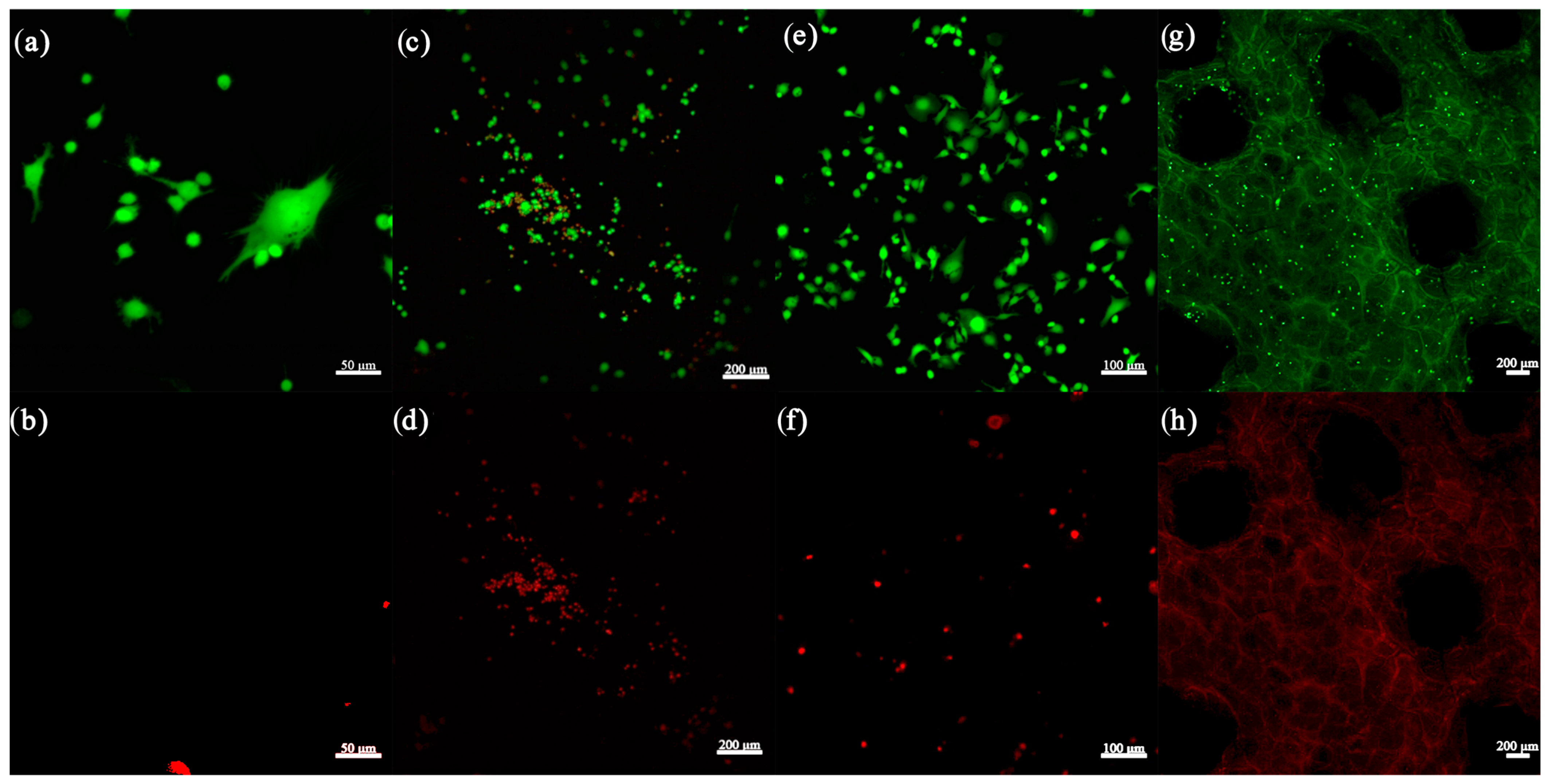
3.2.9. Cell Seeding and LIVE/DEAD Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FTIR | Fourier transform infrared spectroscopy |
| SEM | scanning electron microscopy (SEM) |
| EDS | energy-dispersive X-ray spectroscopy |
| SBF | simulated body fluid |
| EBB | extrusion-based bioprinting |
| Alg | alginate |
| Gel | gelatin |
| BG | bioglass |
| DNA | deoxyribonucleic acid |
| hFOB 1.19 | human fetal osteoblastic cells |
| TEOS | tetraethyl orthosilicate Si(OC2H5)4 |
| TEP | triethyl phosphate (C2H5)3PO4 |
| DC | direct current |
| ATR | attenuated total reflection |
| PBS | phosphate-buffered saline |
| DMEM | Dulbecco’s modified Eagle’s medium |
| MTT | 3-(4,5-dimethylthazolk-2-yl)-2,5-diphenyl tetrazolium bromide |
References
- Siracusa, V.; Maimone, G.; Antonelli, V. State-of-Art of Standard and Innovative Materials Used in Cranioplasty. Polymers 2021, 13, 1452. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Byambaa, B.; Morshed, M.; Cheikh, M.I.; Shakoor, R.A.; Mustafy, T.; Marei, H.E. Advances in osteobiologic materials for bone substitutes. J. Tissue Eng. Regen. Med. 2018, 12, 1448–1468. [Google Scholar] [CrossRef]
- Cheng, L.; Shoma, K.; Suresh, S.; He, H.; Rajput, R.S.; Feng, Q.; Ramesh, S.; Wang, Y.; Krishnan, S.; Ostrovidov, S.; et al. 3D Printing of Micro- and Nanoscale Bone Substitutes: A Review on Technical and Translational Perspectives. Int. J. Nanomed. 2021, 16, 4289. [Google Scholar] [CrossRef]
- Konka, J.; Buxadera-Palomero, J.; Espanol, M.; Ginebra, M.P. 3D printing of hierarchical porous biomimetic hydroxyapatite scaffolds: Adding concavities to the convex filaments. Acta Biomater. 2021, 134, 744–759. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-Based Biomaterials in Tissue Engineering and Regenerative Medicine. Mar. Drugs 2023, 21, 189. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Zolfagharian, A.; Bodaghi, M. 4D bioprinting of smart polymers for biomedical applications: Recent progress, challenges, and future perspectives. React. Funct. Polym. 2022, 179, 105374. [Google Scholar] [CrossRef]
- Ma, P.; Wu, W.; Wei, Y.; Ren, L.; Lin, S.; Wu, J. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering. Mater. Des. 2021, 207, 109865. [Google Scholar] [CrossRef]
- Mccoy, R.J.; Jungreuthmayer, C.; O’Brien, F.J. Influence of flow rate and scaffold pore size on cell behavior during mechanical stimulation in a flow perfusion bioreactor. Biotechnol. Bioeng. 2012, 109, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Reina-Romo, E.; Papantoniou, I.; Bloemen, V.; Geris, L. Computational Design of Tissue Engineering Scaffolds; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Fairag, R.; Rosenzweig, D.H.; Ramirez-Garcialuna, J.L.; Weber, M.H.; Haglund, L. Three-Dimensional Printed Polylactic Acid Scaffolds Promote Bone-like Matrix Deposition in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 15306–15315. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.G.; Malherbi, M.S.; de Souza, N.S.; César, G.B.; Tominaga, T.T.; Miyahara, R.Y.; Mendonça, P.d.S.B.d.; Faria, D.R.; Rosso, J.M.; Freitas, V.F.; et al. 4th Generation Biomaterials Based on PVDF-Hydroxyapatite Composites Produced by Electrospinning: Processing and Characterization. Polymers 2022, 14, 4190. [Google Scholar] [CrossRef]
- Liu, H.; Gong, Y.; Zhang, K.; Ke, S.; Wang, Y.; Wang, J.; Wang, H. Recent Advances in Decellularized Matrix-Derived Materials for Bioink and 3D Bioprinting. Gels 2023, 9, 195. [Google Scholar] [CrossRef]
- Fournet, M.E.B.; Azaman, F.A.; Gunbay, S.; Chen, Y.Y.; Devine, D.M. Orthopaedic 3D Printing in Orthopaedic Medicine. Polym. -Based Addit. Manuf. 2019, 121–142. [Google Scholar] [CrossRef]
- Hann, S.Y.; Cui, H.; Esworthy, T.; Zhou, X.; Lee, S.-J.; Plesniak, M.W.; Zhang, L.G. Dual 3D printing for vascularized bone tissue regeneration. Acta Biomater. 2021, 123, 263–274. [Google Scholar] [CrossRef]
- Bhargav, A.; Sanjairaj, V.; Rosa, V.; Feng, L.W.; Fuh YH, J. Applications of Additive Manufacturing in Dentistry: A Review; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Kaasi, A.; Kharmandayan, P. Additive Manufacturing in Medicine. In Encyclopedia of Smart Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 300–320. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef]
- Ng, W.L.; Chan, A.; Ong, Y.S.; Chua, C.K. Deep Learning for Fabrication and Maturation of 3D Bioprinted Tissues and Organs; Taylor and Francis Ltd.: Abingdon, UK, 2020. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D Bioprinting: From Printing Methods to Biomedical Applications; Shenyang Pharmaceutical University: Shenyang, China, 2020. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Peng, W.; Ozbolat, V. Application areas of 3D bioprinting. Drug Discov. Today 2016, 21, 1257–1271. [Google Scholar] [CrossRef]
- Dikyol, C.; Altunbek, M.; Bartolo, P.; Koc, B. Multimaterial bioprinting approaches and their implementations for vascular and vascularized tissues. Bioprinting 2021, 24, e00159. [Google Scholar] [CrossRef]
- Simcock, I.C.; Shelmerdine, S.C.; Langan, D.; Anna, G.; Sebire, N.J.; Arthurs, O.J. Micro-CT yields high image quality in human fetal post-mortem imaging despite maceration. BMC Med. Imaging 2021, 21, 128. [Google Scholar] [CrossRef]
- Fahimipour, F.; Rasoulianboroujeni, M.; Dashtimoghadam, E.; Khoshroo, K.; Tahriri, M.; Bastami, F.; Lobner, D.; Tayebi, L. 3D printed TCP-based scaffold incorporating VEGF-loaded PLGA microspheres for craniofacial tissue engineering. Dent. Mater. 2017, 33, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Gorroñogoitia, I.; Urtaza, U.; Zubiarrain-Laserna, A.; Alonso-Varona, A.; Zaldua, A.M. A Study of the Printability of Alginate-Based Bioinks by 3D Bioprinting for Articular Cartilage Tissue Engineering. Polymers 2022, 14, 354. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate Hydrogels for Bone Tissue Engineering, from Injectables to Bioprinting: A Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Tariq, A.; Bhawani, S.A.; Alotaibi, K.M.; Moheman, A. Smart biopolymers and their applications. Smart Polym. Nanocompos. Biomed. Environ. Appl. 2021, 145–167. [Google Scholar] [CrossRef]
- Smith, G.N.; Brok, E.; Schmiele, M.; Mortensen, K.; Bouwman, W.G.; Duif, C.P.; Hassenkam, T.; Alm, M.; Thomsen, P.; Arleth, L. The microscopic distribution of hydrophilic polymers in interpenetrating polymer networks (IPNs) of medical grade silicone. Polymer 2021, 224, 123671. [Google Scholar] [CrossRef]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing from nature: Biopolymers and biocomposites as smart wound care materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef]
- Hajikhani, A.; Scocozza, F.; Conti, M.; Marino, M.; Auricchio, F.; Wriggers, P. Experimental characterization and computational modeling of hydrogel cross-linking for bioprinting applications. Int. J. Artif. Organs 2019, 42, 548–557. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, A.; Motan, D.; Bhattacharya, P.; McNabb, A.; Skerry, T.M.; Lacroix, D. Heterogeneity in The Mechanical Properties of Integrins Determines Mechanotransduction Dynamics in Bone Osteoblasts. Sci. Rep. 2019, 9, 13113. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication and apatite formation. J. Biomed. Mater. Res. A 2014, 102, 254–274. [Google Scholar] [CrossRef] [PubMed]
- Trandaș, A.-N.; Trifan, A.; Ungureanu, A.-D.; Dusciuc, A.; Opriș, M.; Milea, O.-C.; Banciu, A.; Constantinoiu, I.; Busuioc, C.; Isopencu, G.-O. Properties of europium and silver doped bioglass thin films obtained by two deposition methods: Biointerfaces for bioinert implants. Mater. Chem. Phys. 2023, 309, 128396. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, L.; Liu, X.; Wu, S.; Peng, S.; Shuai, C. Silver-doped bioglass modified scaffolds: A sustained antibacterial efficacy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112425. [Google Scholar] [CrossRef]
- Unal, F.; Tasar, C.; Ercan, B. Fabrication and in vitro characterization of antibacterial magneto-luminescent core-shell bioactive glass nanoparticles. Ceram. Int. 2023, 49, 20118–20126. [Google Scholar] [CrossRef]
- Wu, L.; Yang, F.; Xue, Y.; Gu, R.; Liu, H.; Xia, D.; Liu, Y. The biological functions of europium-containing biomaterials: A systematic review. Mater. Today Bio 2023, 19, 100595. [Google Scholar] [CrossRef]
- Flores-Jacobo, A.; Aguilar-Reyes, E.A.; León-Patiño, C.A. Effect of Dopants on the Physical, Mechanical, and Biological Properties of Porous Scaffolds for Bone Tissue Engineering. Biomed. Mater. Devices 2022, 1, 234–255. [Google Scholar] [CrossRef]
- ASTM D4440-15; Standard Test Method for Plastics: Dynamic Mechanical Properties Melth Rheology, Book of Standards Volume: 08.02, ICS Code: 83.080.20. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Facts, Q.; Em, E. LIVE/DEAD® Viability/Cytotoxicity Kit *for Mammalian Cells*, 2005. Available online: https://www.thermofisher.cn/order/catalog/product/L3224 (accessed on 18 February 2025).
- Ranmuthu, C.D.S.; Ranmuthu, C.K.I.; Russell, J.C.; Singhania, D.; Khan, W.S. Evaluating the effect of non-cellular bioactive glass-containing scaffolds on osteogenesis and angiogenesis in in vivo animal bone defect models. Front. Bioeng. Biotechnol. 2020, 8, 430. [Google Scholar] [CrossRef]
- Li, F.; Song, X.; Sun, T.; Mi, L.; Liu, J.; Xia, X.; Bai, N.; Li, X. Alginate/Gelatin Hydrogel Scaffold Containing nCeO2 as a Potential Osteogenic Nanomaterial for Bone Tissue Engineering. Int. J. Nanomed. 2022, 17, 6561. [Google Scholar] [CrossRef] [PubMed]
- Karvinen, J.; Kellomäki, M. Design aspects and characterization of hydrogel-based bioinks for extrusion-based bioprinting. Bioprinting 2023, 32, e00274. [Google Scholar] [CrossRef]
- Kaliampakou, C.; Lagopati, N.; Pavlatou, E.A.; Charitidis, C.A. Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior. Gels 2023, 9, 857. [Google Scholar] [CrossRef] [PubMed]
| Bioglass Composition (mol%) | |||||
|---|---|---|---|---|---|
| 65.0 | 4.5 | 2.5 | 24.0 | 1.0 | 3.0 |
| SiO2 | P2O5 | Na2O | CaO | Ag2O | Eu2O3 |
| Sample | Hydrogel Composition | pH | Pressure (kPa) | Nozzle Diameter | Printing Speed | Layers | Crosslinking Time | ||
|---|---|---|---|---|---|---|---|---|---|
| Alginate | Gelatin | Eu-Doped BG | |||||||
| P1 | 3% | 7% | 8 | 25 | 22 G | 20 mm/s | 4 | 5 min | |
| P2 | 7% | 8% | 7 | 135 | |||||
| P3 | 3% | 6% | 0.25% | 8 | 170 | ||||
| P4 | 7% | 8% | 0.50% | 8 | 210 | ||||
| P5 | 7% | 8% | 0.25% | 8 | 225 | ||||
| Order | Reagent | Quantities for 100 mL SBF 1.5X |
|---|---|---|
| #0 | Ultra-pure water | 75 mL |
| #1 | NaCl | 1.1994 g |
| #2 | NaHCO3 | 0.0525 g |
| #3 | KCl | 0.0336 g |
| #4 | K2HPO4·3H2O | 0.0342 g |
| #5 | MgCl2·6H2O | 0.0458 g |
| #6 | 1 kmol/m3 HCl | 6 cm3 |
| #7 | CaCl2 | 0.0417 g |
| #8 | Na2SO4 | 0.0107 g |
| #9 | (CH2OH)3CNH2 | 0.9086 g |
| #10 | 1 kmol/m3 HCl | Appropriate amount for adjusting pH |
| Alg | Gel | BG | Bands (cm−1) | Correlated Bonds |
|---|---|---|---|---|
| 8% | 1631.48 | Amide II | ||
| 1545.67 | Amide I | |||
| 1414.53 | (CO3)−2 | |||
| 7% | 3325.64 | O-H | ||
| 1594.84 | O-C-O | |||
| 1414.53 | (CO3)−2 | |||
| 7% | 8% | 3325.64 | O-H | |
| 1631.48 | Amide II | |||
| 1545.67 | Amide I | |||
| 1442.49 | C-H | |||
| 3% | 7% | 0.25% | 1594.84 | O-C-O |
| 1414.53 | (CO3)−2 | |||
| 1078.98 | Si-O-Si | |||
| 1031.73 | Si-O-Si | |||
| 7% | 8% | 0.50% | 3299.61 | O-H |
| 1629.55 | Amide II | |||
| 1526.38 | Amide I | |||
| 1414.53 | (CO3)−2 | |||
| 1078.98 | Si-O-Si | |||
| 1031.73 | Si-O-Si |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifan, A.; Liciu, E.; Busuioc, C.; Stancu, I.-C.; Banciu, A.; Nicolae, C.; Dragomir, M.; Cristea, D.-D.; Sabău, R.-E.; Nițulescu, D.-A.; et al. Developing Bioengineered 3D-Printed Composite Scaffolds with Antimicrobial Potential for Bone Tissue Regeneration. J. Funct. Biomater. 2025, 16, 227. https://doi.org/10.3390/jfb16060227
Trifan A, Liciu E, Busuioc C, Stancu I-C, Banciu A, Nicolae C, Dragomir M, Cristea D-D, Sabău R-E, Nițulescu D-A, et al. Developing Bioengineered 3D-Printed Composite Scaffolds with Antimicrobial Potential for Bone Tissue Regeneration. Journal of Functional Biomaterials. 2025; 16(6):227. https://doi.org/10.3390/jfb16060227
Chicago/Turabian StyleTrifan, Andreea, Eduard Liciu, Cristina Busuioc, Izabela-Cristina Stancu, Adela Banciu, Carmen Nicolae, Mihai Dragomir, Doru-Daniel Cristea, Rosina-Elena Sabău, David-Andrei Nițulescu, and et al. 2025. "Developing Bioengineered 3D-Printed Composite Scaffolds with Antimicrobial Potential for Bone Tissue Regeneration" Journal of Functional Biomaterials 16, no. 6: 227. https://doi.org/10.3390/jfb16060227
APA StyleTrifan, A., Liciu, E., Busuioc, C., Stancu, I.-C., Banciu, A., Nicolae, C., Dragomir, M., Cristea, D.-D., Sabău, R.-E., Nițulescu, D.-A., & Paraschiv, A. (2025). Developing Bioengineered 3D-Printed Composite Scaffolds with Antimicrobial Potential for Bone Tissue Regeneration. Journal of Functional Biomaterials, 16(6), 227. https://doi.org/10.3390/jfb16060227








