Multi-Scale Mechanics of Cryopreserved Human Arterial Allografts Across a Six-Month Period
Abstract
1. Introduction
2. Aim
3. Materials and Methods
3.1. Donor Inclusion and Exclusion Criteria
3.2. Ethics Approval
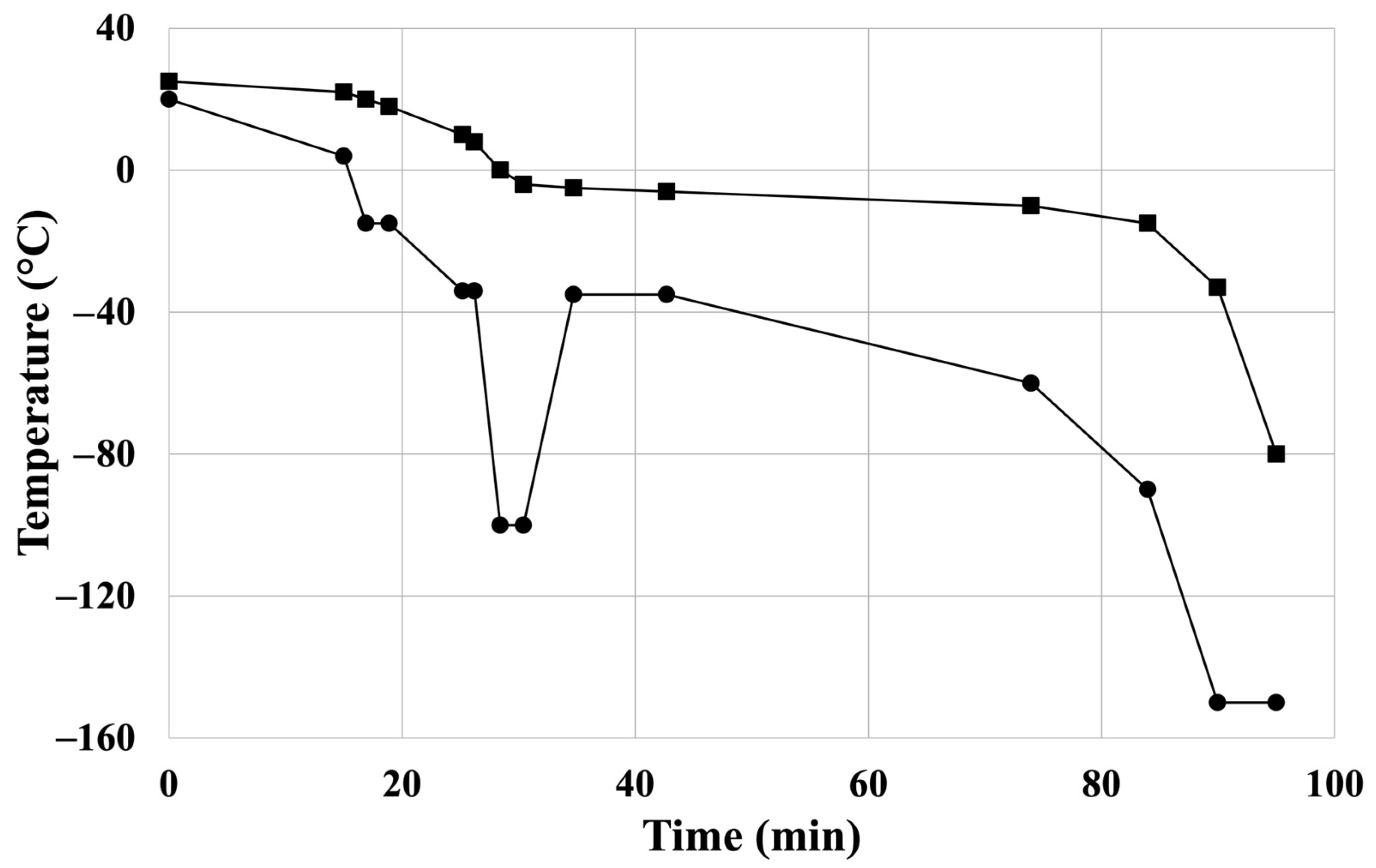
3.3. Cardiovascular Allograft Biobank Protocol: Harvest, Cryopreservation, Storage, and Thawing of CPA Samples
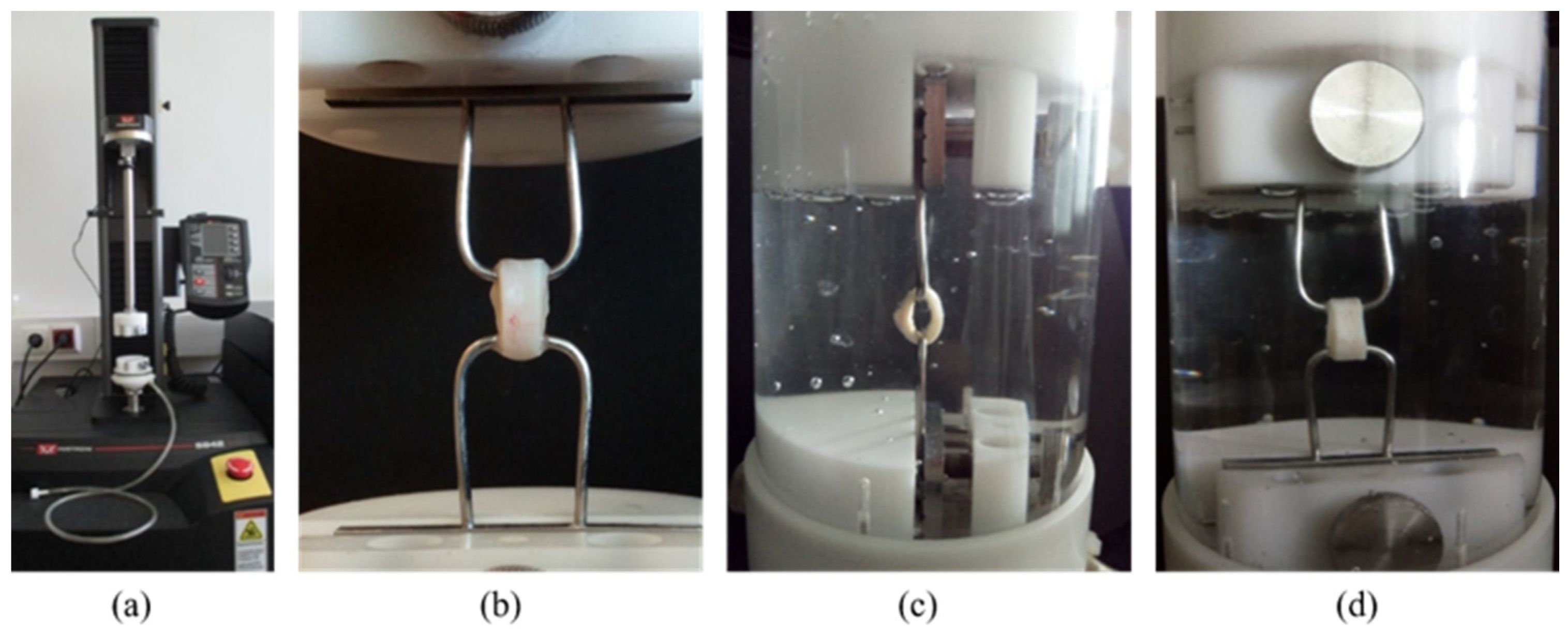
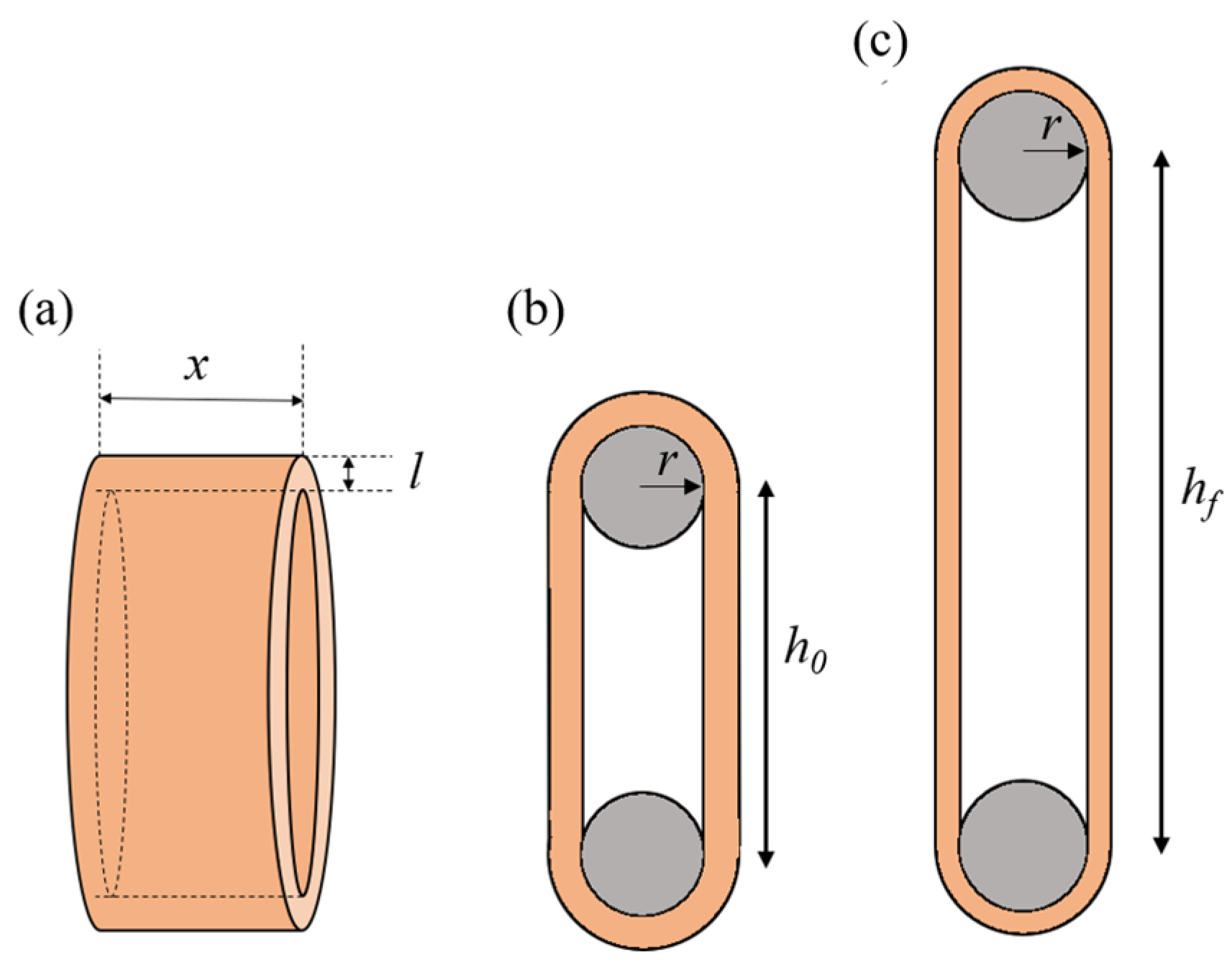
3.4. Uniaxial Ring Test
- Tensile strength ();
- Stretch ratio at maximum load ();
- Extensibility;
- Engineering stress ();
- True stress ().
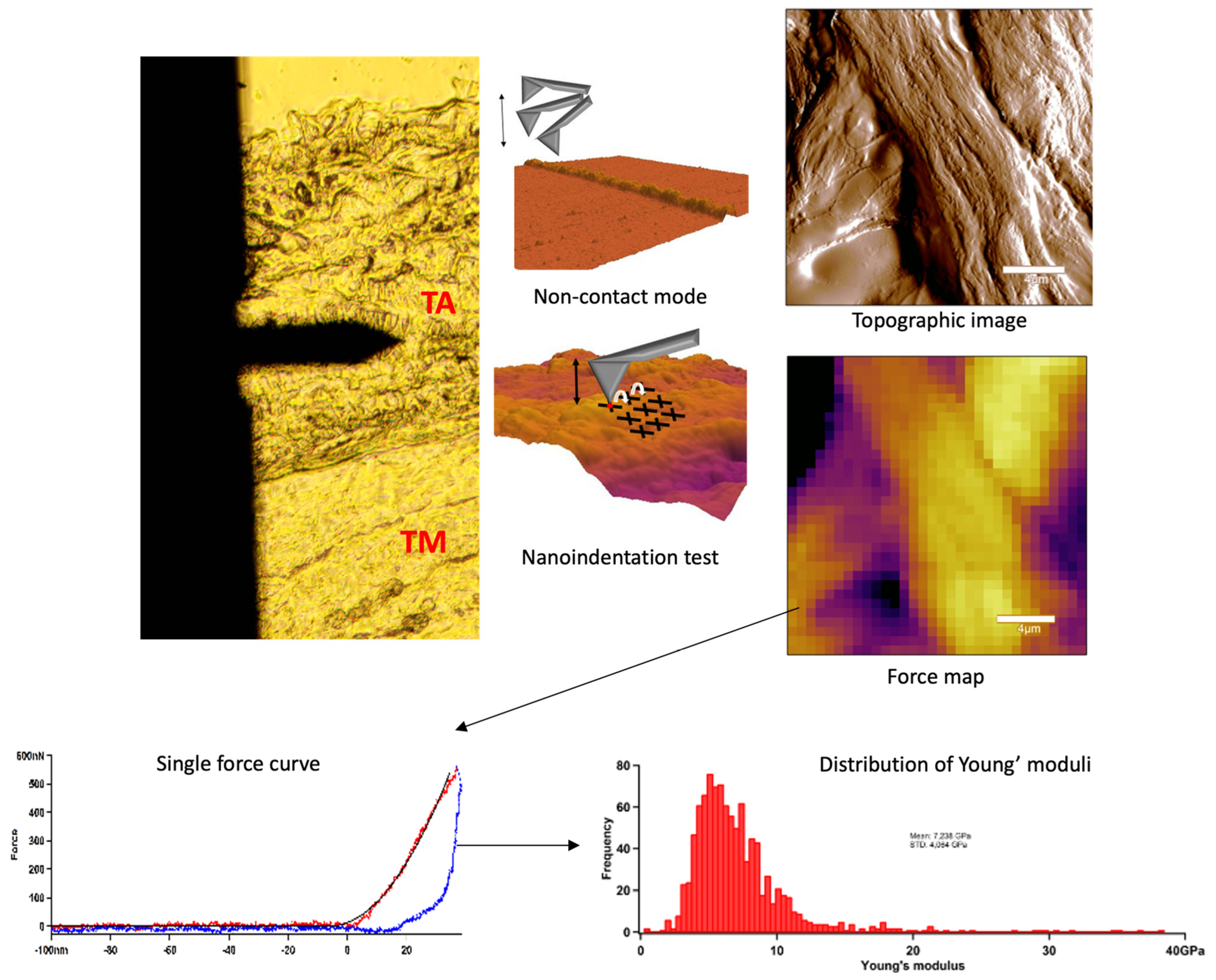
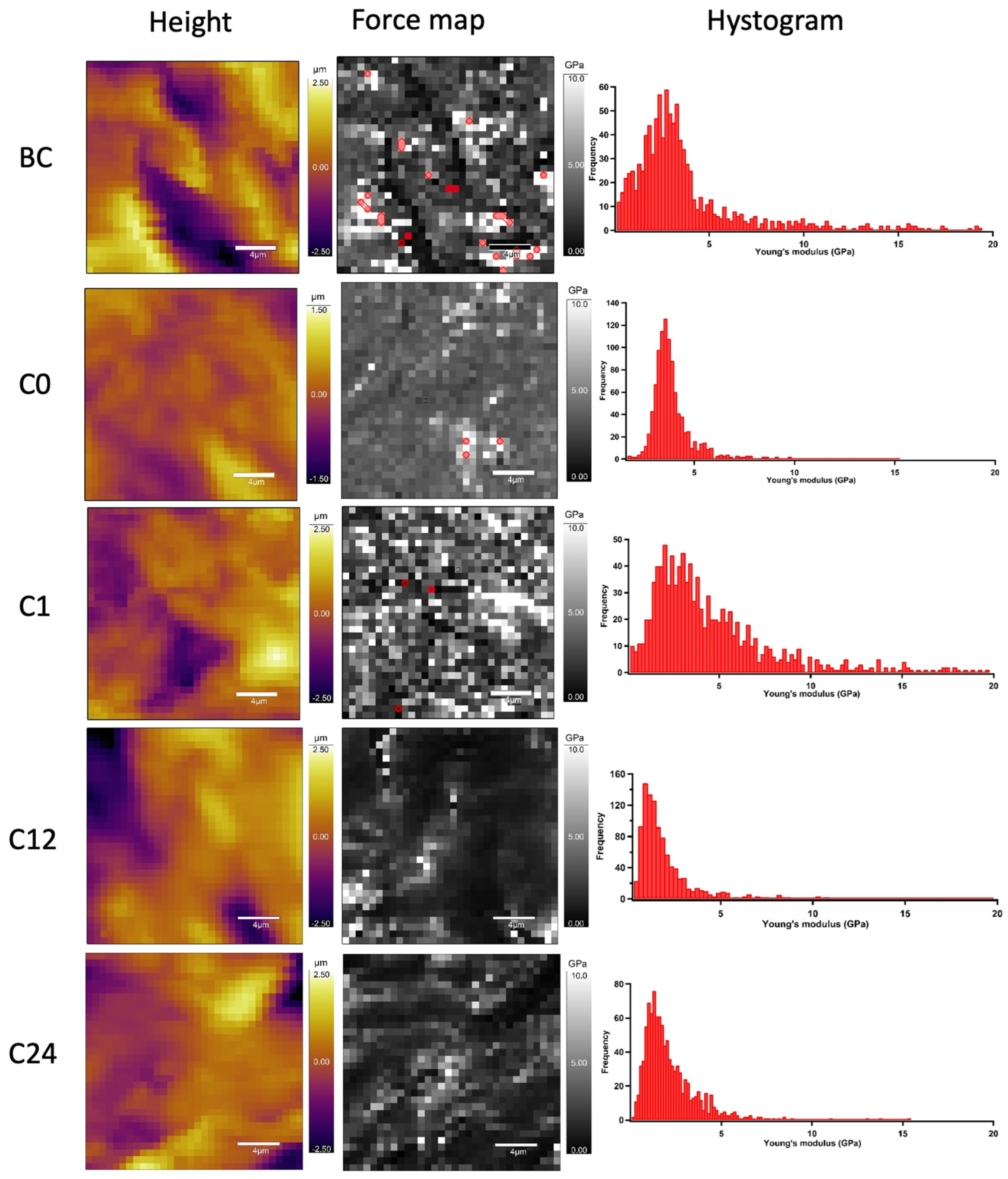
3.5. Atomic Force Microscopy (AFM): Topography and Nanoindentation
Sample Preparation for Nanoindentation Tests
3.6. Statistical Analysis
- If there is no observable trend or pattern in the differences as the means increase.
- If the 95% confidence interval of the mean differences includes the value of zero or the difference is smaller than the measurement variability and precision, or if it is not clinically significant.
- If the 95% limits of agreement are within the range of the measurement variability and precision, or if the difference is not clinically significant.
4. Results
4.1. Donor Characteristics
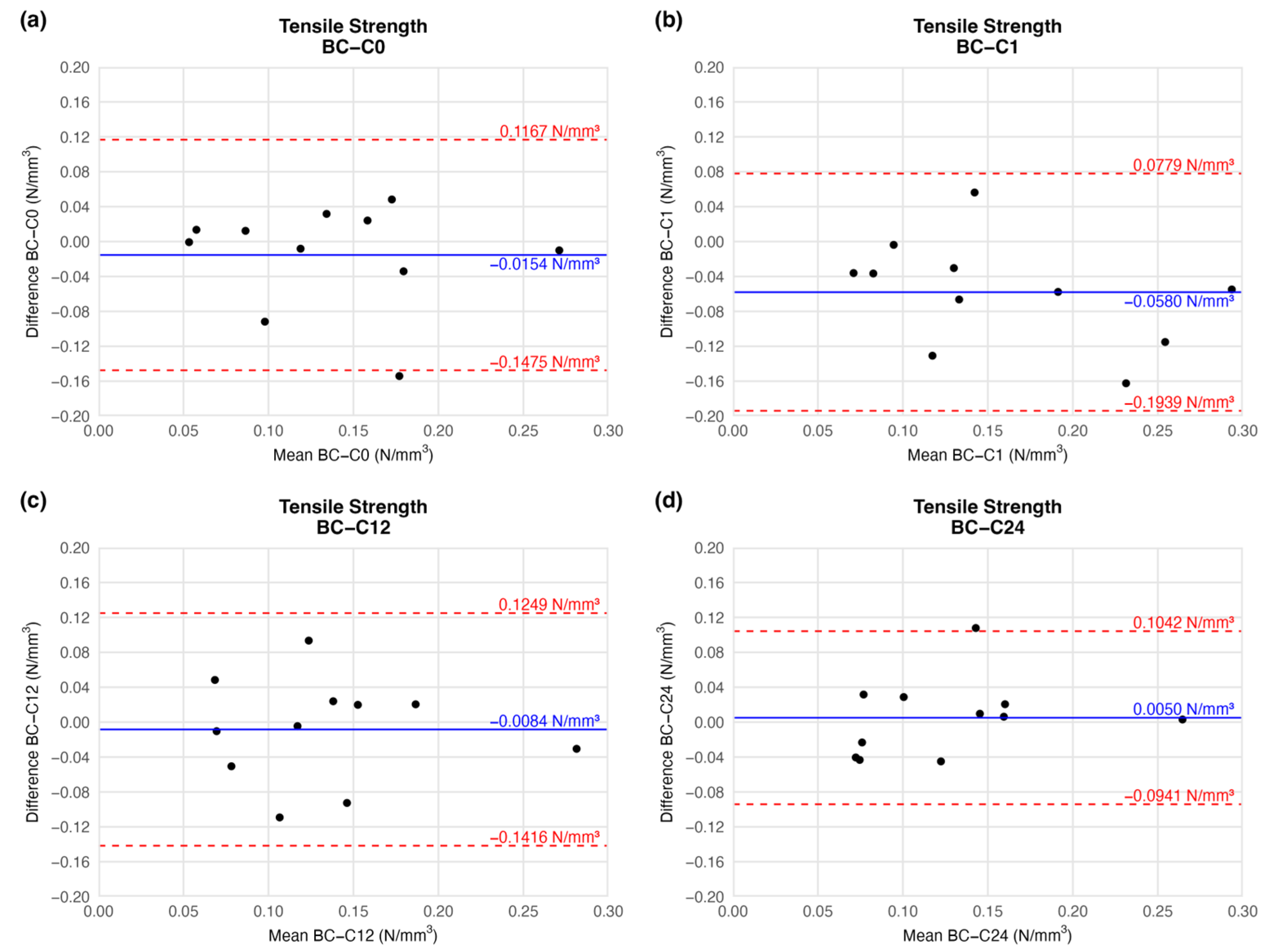
4.1.1. Tensile Strength
4.1.2. Stretch Ratio at Maximum Load
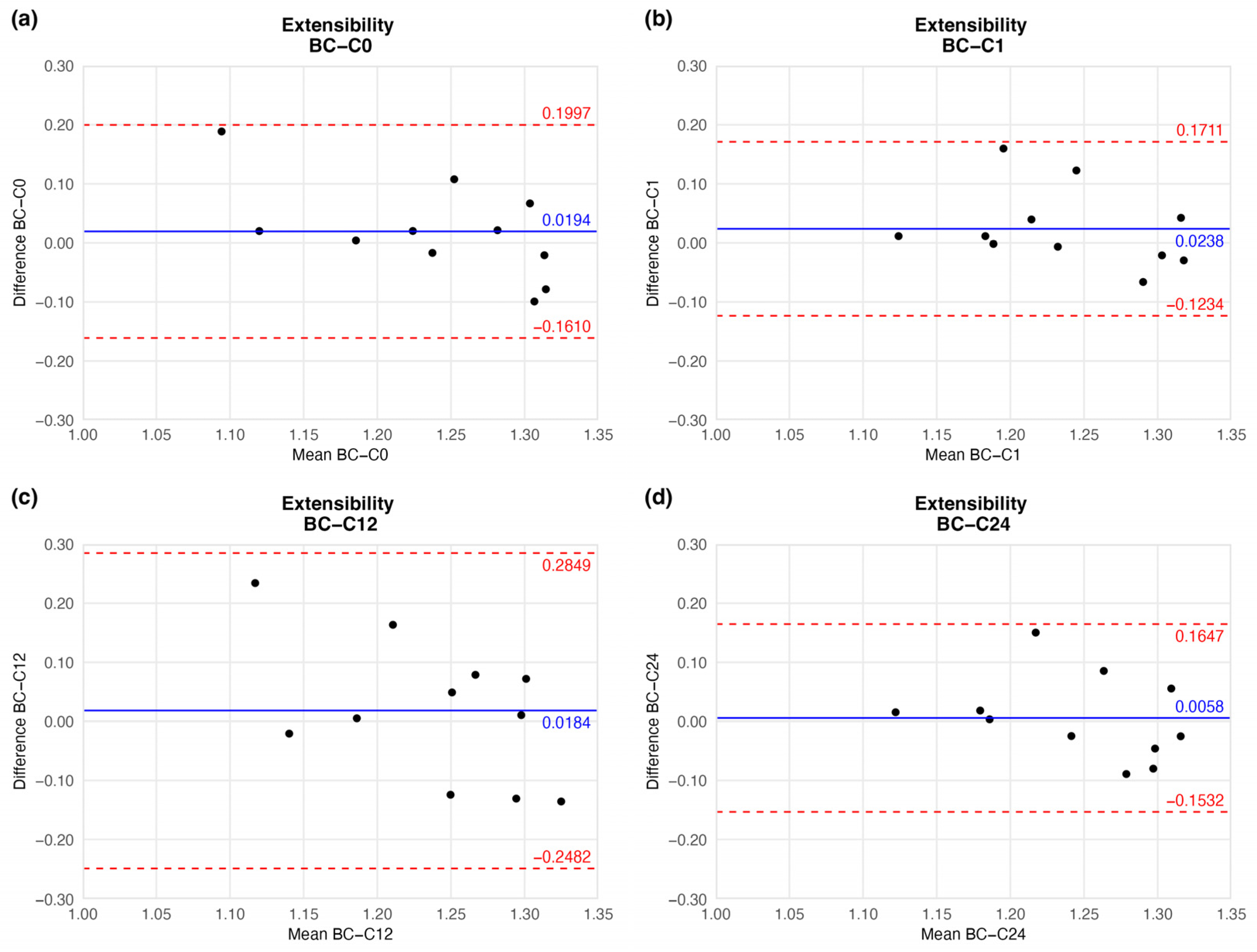
4.1.3. Extensibility
4.1.4. Engineering Stress (Circumferential Stress)
4.1.5. True Stress
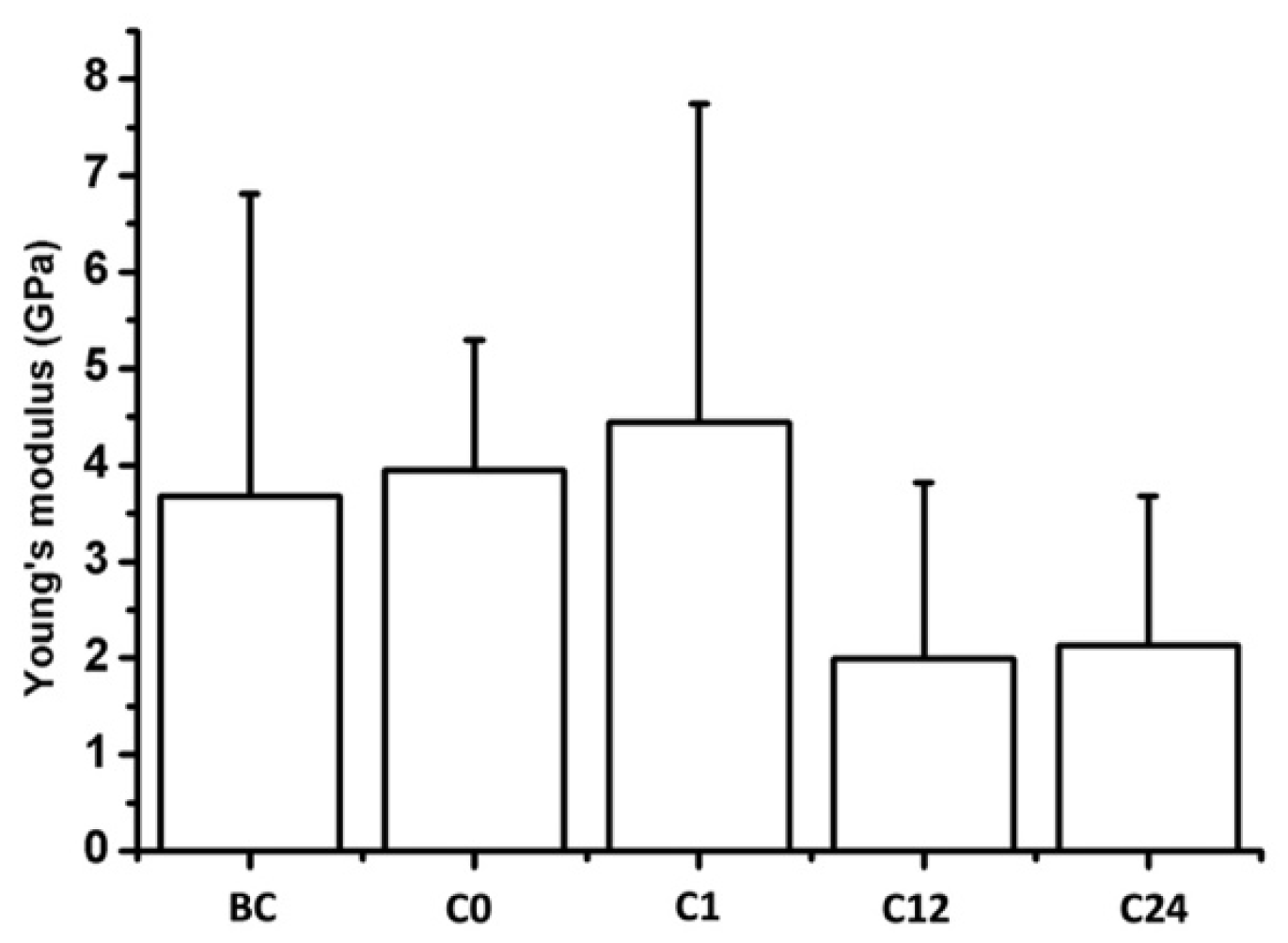
4.2. AFM Nanoindentation
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| AFM | Atomic Force Microscopy |
| BAP | Bland–Altman Plot |
| CP | Cryopreserved |
| CPA | Cryopreserved Vascular Allograft |
| ECM | Extracellular Matrix |
| VA | Vascular Allograft |
| YM | Young’s modulus |
References
- Noel, A.A.; Gloviczki, P.; Cherry, K.J.; Safi, H.; Goldstone, J.; Morasch, M.D.; Johansen, K.H. Abdominal aortic reconstruction in infected fields: Early results of the United States Cryopreserved Aortic Allograft Registry. J. Vasc. Surg. 2002, 35, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Bower, T.C.; Creager, M.A.; Amin-Hanjani, S.; O’gara, P.T.; Lockhart, P.B.; Darouiche, R.O.; Ramlawi, B.; Derdeyn, C.P.; Bolger, A.F.; et al. Vascular Graft Infections, Mycotic Aneurysms, and Endovascular Infections: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e412–e460. [Google Scholar] [CrossRef]
- Batt, M.; Feugier, P.; Camou, F.; Coffy, A.; Senneville, E.; Caillon, J.; Calvet, B.; Chidiac, C.; Laurent, F.; Revest, M.; et al. A Meta-Analysis of Outcomes After In Situ Reconstructions for Aortic Graft Infection. Angiology 2018, 69, 370–379. [Google Scholar] [CrossRef]
- Müller-Schweinitzer, E. Cryopreservation of vascular tissues. Organogenesis 2009, 5, 97–104. [Google Scholar] [CrossRef]
- Jang, T.H. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Farber, A.; Major, K.; Wagner, W.H.; Cohen, J.L.; Cossman, D.V.; Lauterbach, S.R.; Levin, P.M. Cryopreserved saphenous vein allografts in infrainguinal revascularization: Analysis of 240 grafts. J. Vasc. Surg. 2003, 38, 15–21. [Google Scholar] [CrossRef]
- Kieffer, E.; Gomes, D.; Chiche, L.; Fléron, M.H.; Koskas, F.; Bahnini, A. Allograft replacement for infrarenal aortic graft infection: Early and late results in 179 patients. J. Vasc. Surg. 2004, 39, 1009–1017. [Google Scholar] [CrossRef]
- Ben Ahmed, S.; Louvancourt, A.; Daniel, G.; Combe, P.; Duprey, A.; Albertini, J.N.; Favre, J.P.; Rosset, E. Cryopreserved arterial allografts for in situ reconstruction of abdominal aortic native or secondary graft infection. J. Vasc. Surg. 2018, 67, 468–477. [Google Scholar] [CrossRef]
- Nagy, Z.; Oláh, Z.; Kókai, J.; Molnár, A.B.; Laczkó, Á.; Szabó, G.V.; Juhász, V.; Garbaisz, D.; Berczeli, M.; Sztupinszky, Z.; et al. Homograftok szerepe az alsó végtagi érrekonstrukciókban. Magy Sebészet (Hung. J. Surg.) 2017, 70, 5–12. [Google Scholar] [CrossRef]
- Mestres, C.A.; Quintana, E.; Kopjar, T.; Ambrosioni, J.; Almela, M.; Fuster, D.; Ninot, S.; Miró, J.M. Twenty-year experience with cryopreserved arterial allografts for vascular infections. Eur. J. Cardio-Thoracic Surg. 2019, 55, 358–365. [Google Scholar] [CrossRef]
- Guevara-Noriega, K.A.; Lucar-Lopez, G.A.; Pomar, J.L. Cryopreserved Allografts for Treatment of Chronic Limb-Threatening Ischemia in Patients Without Autologous Saphenous Veins. Ann. Vasc. Surg. 2019, 60, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Furlough, C.L.; Jain, A.K.; Ho, K.J.; Rodriguez, H.E.; Tomita, T.M.; Eskandari, M.K. Peripheral artery reconstructions using cryopreserved arterial allografts in infected fields. J. Vasc. Surg. 2019, 70, 562–568. [Google Scholar] [CrossRef]
- Couture, T.; Gaudric, J.; Du Montcel, S.T.; Jayet, J.; Verscheure, D.; Davaine, J.M.; Jarraya, M.; Chiche, L.; Koskas, F. Short and Mid Term Outcomes of Cryopreserved Abdominal Aortic Allografts Used as a Substitute for Infected Prosthetic Grafts in 200 Patients. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 89–97. [Google Scholar] [CrossRef]
- Brown, K.E.; Heyer, K.; Rodriguez, H.; Eskandari, M.K.; Pearce, W.H.; Morasch, M.D. Arterial reconstruction with cryopreserved human allografts in the setting of infection: A single-center experience with midterm follow-up. J. Vasc. Surg. 2009, 49, 660–666. [Google Scholar] [CrossRef]
- Golemovic, M.; Skific, M.; Haluzan, D.; Pavic, P.; Golubic Cepulic, B. Ten-year experience with cryopreserved vascular allografts in the Croatian Cardiovascular Tissue Bank. Cell Tissue Bank. 2022, 23, 807–824. [Google Scholar] [CrossRef]
- Castier, Y.; Paraskevas, N.; Maury, J.M.; Karsenti, A.; Cerceau, O.; Legendre, A.F.; Duprey, A.; Cerceau, P.; Francis, F.; Leseche, G. Cryopreserved arterial allograft reconstruction for infected peripheral bypass. Ann. Vasc. Surg. 2010, 24, 994–999. [Google Scholar] [CrossRef]
- Masmejan, S.; Deslarzes-Dubuis, C.; Petitprez, S.; Longchamp, A.; Haller, C.; Saucy, F.; Corpataux, J.M.; Déglise, S. Ten Year Experience of Using Cryopreserved Arterial Allografts for Distal Bypass in Critical Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 823–831. [Google Scholar] [CrossRef]
- Tatar, A.R.; Derycke, L.; Cochennec, F.; Jaziri, A.; Desgranges, P.; Touma, J. Unmet Needs in Cryopreserved Arterial Allograft Implantation for Peripheral Vascular Graft Infections. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 788–789. [Google Scholar] [CrossRef]
- Weiss, S.; Bachofen, B.; Widmer, M.K.; Makaloski, V.; Schmidli, J.; Wyss, T.R. Long-term results of cryopreserved allografts in aortoiliac graft infections. J. Vasc. Surg. 2021, 74, 268–275. [Google Scholar] [CrossRef]
- Couture, T.; Gaudric, J.; Davaine, J.M.; Jayet, J.; Chiche, L.; Jarraya, M.; Koskas, F. Results of cryopreserved arterial allograft replacement for thoracic and thoracoabdominal aortic infections. J. Vasc. Surg. 2021, 73, 626–634. [Google Scholar] [CrossRef]
- González-Gay, M.; López-Martínez, R.; Busto-Suárez, S.; Riedemann-Wistuba, M.E.; Menéndez-Herrero, M.Á.; Álvarez-Marcos, F.; Alonso-Pérez, M.; Alonso-Arias, R. Immunological Aspects Involved in the Degeneration of Cryopreserved Arterial Allografts. Front. Surg. 2020, 7, 616654. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Delay, C.; Girsowicz, E.; Chenesseau, B.; Bonnin, E.; Ghariani, M.Z.; Thaveau, F.; Georg, Y.; Geny, B.; Chakfe, N. Cryopreserved Cadaveric Arterial Allograft for Arterial Reconstruction in Patients with Prosthetic Infection. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 636–644. [Google Scholar] [CrossRef]
- Wang, C.-C.; Lopez-Valdes, S.; Lin, T.-L.; Yap, A.; Yong, C.-C.; Li, W.-F.; Wang, S.-H.; Lin, C.-C.; Liu, Y.-W.; Lin, T.-S.; et al. Outcomes of long storage times for cryopreserved vascular grafts in outflow reconstruction in living donor liver transplantation. Liver Transplant. 2014, 20, 173–181. [Google Scholar] [CrossRef]
- Hidi, L.; Komorowicz, E.; Kovács, G.I.; Szeberin, Z.; Garbaisz, D.; Nikolova, N.; Tenekedjiev, K.; Szabó, L.; Kolev, K.; Sótonyi, P. Cryopreservation moderates the thrombogenicity of arterial allografts during storage. PLoS ONE 2021, 16, e0255114. [Google Scholar] [CrossRef]
- Macrae, R.A.; Miller, K.; Doyle, B.J. Methods in Mechanical Testing of Arterial Tissue: A Review. Strain 2016, 52, 380–399. [Google Scholar] [CrossRef]
- Grant, C.A.; Twigg, P.C. Pseudostatic and dynamic nanomechanics of the tunica adventitia in elastic arteries using atomic force microscopy. ACS Nano 2013, 7, 456–464. [Google Scholar] [CrossRef]
- Hungarian National Blood Transfusion Service. Legislation of Organ and Tissue Transplantation; Hungarian National Blood Transfusion Service: Karolina út, Hungary, 2020. [Google Scholar]
- Asgari, M.; Latifi, N.; Giovanniello, F.; Espinosa, H.D.; Amabili, M. Revealing Layer-Specific Ultrastructure and Nanomechanics of Fibrillar Collagen in Human Aorta via Atomic Force Microscopy Testing: Implications on Tissue Mechanics at Macroscopic Scale. Adv. NanoBiomed Res. 2022, 2, 2100159. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G.; Martin Bland, J.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Schenke-Layland, K.; Madershahian, N.; Riemann, I.; Starcher, B.; Halbhuber, K.-J.J.; König, K.; Stock, U.A. Impact of cryopreservation on extracellular matrix structures of heart valve leaflets. Ann. Thorac. Surg. 2006, 81, 918–926. [Google Scholar] [CrossRef]
- Schenke-Layland, K.; Xie, J.; Heydarkhan-Hagvall, S.; Hamm-Alvarez, S.F.; Stock, U.A.; Brockbank, K.G.M.; MacLellan, W.R. Optimized Preservation of Extracellular Matrix in Cardiac Tissues: Implications for Long-Term Graft Durability. Ann. Thorac. Surg. 2007, 83, 1641–1650. [Google Scholar] [CrossRef]
- Pegg, D.E.; Wusteman, M.C.; Boylan, S. Fractures in Cryopreserved Elastic Arteries. Cryobiology 1997, 34, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Rendal, E.; Martínez Santos, M.V.; Rodriguez, M.; Sánchez, J.; Segura, R.; Matheu, G.; Filgueira, P.; Pértega, S.; Andión, C. Effects of cryopreservation and thawing on the structure of vascular segment. Transplant. Proc. 2004, 36, 3283–3287. [Google Scholar] [CrossRef]
- Pukacki, F.; Jankowski, T.; Gabriel, M.; Oszkinis, G.; Krasinski, Z.; Zapalski, S. The mechanical properties of fresh and cryopreserved arterial homografts. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 21–24. [Google Scholar] [CrossRef]
- Novotny, R.; Mericka, P.; Chlupac, J.; Matejka, R.; Kristek, J.; Marada, T.; Konarik, M.; Ivak, P.; Sterba, L.; Hlubocky, J.; et al. The Effect of Different Thawing Rates on Cryopreserved Human Iliac Arteries Allograft’s Structural Damage and Mechanical Properties. Biomed. Res. Int. 2020, 2020, 6545190. [Google Scholar] [CrossRef] [PubMed]
- Langerak, S.E.; Groenink, M.; Van der Wall, E.E.; Wassenaar, C.; Vanbavel, E.; Van Baal, M.C.; Spaan, J.A.E. Impact of current cryopreservation procedures on mechanical and functional properties of human aortic homografts. Transpl. Int. 2001, 14, 248–255. [Google Scholar] [CrossRef]
- O’Leary, S.A.; Doyle, B.J.; McGloughlin, T.M. The impact of long term freezing on the mechanical properties of porcine aortic tissue. J. Mech. Behav. Biomed. Mater. 2014, 37, 165–173. [Google Scholar] [CrossRef]
- Gross, R.E.; Hurwitt, E.S.; Bill, A.H.; Peirce, E.C. Preliminary Observations on the Use of Human Arterial Grafts in the Treatment of Certain Cardiovascular Defects. N. Engl. J. Med. 1948, 239, 578–579. [Google Scholar] [CrossRef]
- Hemmasizadeh, A.; Darvish, K.; Autieri, M. Characterization of Changes to the Mechanical Properties of Arteries due to Cold Storage Using Nanoindentation Tests. Ann. Biomed. Eng. 2013, 40, 1434–1442. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Fazekas, G. DSC analysis of cryopreservation on the structure of porcine aortic biograft as a function of storage time. J. Therm. Anal. Calorim. 2022, 147, 10411–10417. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Benkő, L.; Fazekas, G. Evaluation of the aortic wall structural alteration following cryopreservation in 1 year follow-up period. J. Therm. Anal. Calorim. 2023, 148, 13313–13320. [Google Scholar] [CrossRef]
- Cai, G.; Lai, B.; Hong, H.; Lin, P.; Chen, W.; Zhu, Z.; Chen, H. Effects of cryopreservation on excretory function, cellular adhesion molecules and vessel lumen formation in human umbilical vein endothelial cells. Mol. Med. Rep. 2017, 16, 547–552. [Google Scholar] [CrossRef]
- Wang, P.; Shu, Z.; He, L.; Chen, S.; Wang, Y.; Li Wang, X. The structural and cellular viability in cryopreserved rabbit carotid arteries. J. Surg. Res. 2006, 131, 241–251. [Google Scholar] [CrossRef]



| Characteristic | n = 11 | |
|---|---|---|
| Cause of death | Trauma | 3 (27.27%) |
| Cerebral ischaemia | 2 (18.18%) | |
| Cerebral haemorrhage | 6 (54.55%) | |
| Age (years) | 45.00 (33.00–50.50) | |
| Female sex | 3 (27.27%) | |
| Body mass index (BMI kg/m2) | 26.30 (23.65–27.80) | |
| Medical history | Hypertension | 3 (27.27%) |
| Diabetes | 1 (9.09%) | |
| Pulmonary disease (COPD) | 1 (9.09%) | |
| Smoking | 4 (36.36%) | |
| Blood group | A | 5 (45.46%) |
| B | 3 (27.27%) | |
| AB | 0 (0%) | |
| 0 | 3 (27.27%) | |
| Rh+ | 10 (90.91%) | |
| Rh− | 1 (9.09%) | |
| Elapsed Weeks | MD ± SD | CI 95% LL | CI 95% UL | |
|---|---|---|---|---|
| Tensile strength (N/mm3) | C0 | −0.0154 ± 0.0593 | −0.1475 | 0.1167 |
| C1 | −0.0580 ± 0.0610 | −0.1939 | 0.0779 | |
| C12 | −0.0084 ± 0.0598 | −0.1416 | 0.1249 | |
| C24 | 0.0050 ± 0.0445 | −0.0941 | 0.1042 | |
| Stretch ratio at maximum load | C0 | −0.0906 ± 0.2789 | −0.7120 | 0.5307 |
| C1 | −0.0393 ± 0.2496 | −0.5956 | 0.5169 | |
| C12 | −0.0846 ± 0.3083 | −0.7714 | 0.6023 | |
| C24 | 0.0101 ± 0.3176 | −0.6976 | 0.7178 | |
| Extensibility | C0 | 0.0194 ± 0.0809 | −0.1610 | 0.1997 |
| C1 | 0.0238 ± 0.0661 | −0.1234 | 0.1711 | |
| C12 | 0.0184 ± 0.1196 | −0.2482 | 0.2849 | |
| C24 | 0.0058 ± 0.0713 | −0.1532 | 0.1647 | |
| Engineering stress (N/mm2) | C0 | −0.1377 ± 0.4365 | −1.1103 | 0.8348 |
| C1 | −0.5966 ± 0.6623 | −2.0722 | 0.8790 | |
| C12 | −0.0760 ± 0.5236 | −1.2428 | 1.0907 | |
| C24 | 0.0260 ± 0.4509 | −0.9788 | 1.0307 | |
| True stress (N/mm2) | C0 | −0.4268 ± 1.1754 | −3.0458 | 2.1923 |
| C1 | −1.2918 ± 1.4919 | −4.6160 | 2.0324 | |
| C12 | −0.2774 ± 1.2944 | −3.1614 | 2.6067 | |
| C24 | 0.0281 ± 1.0271 | −2.2605 | 2.3167 |
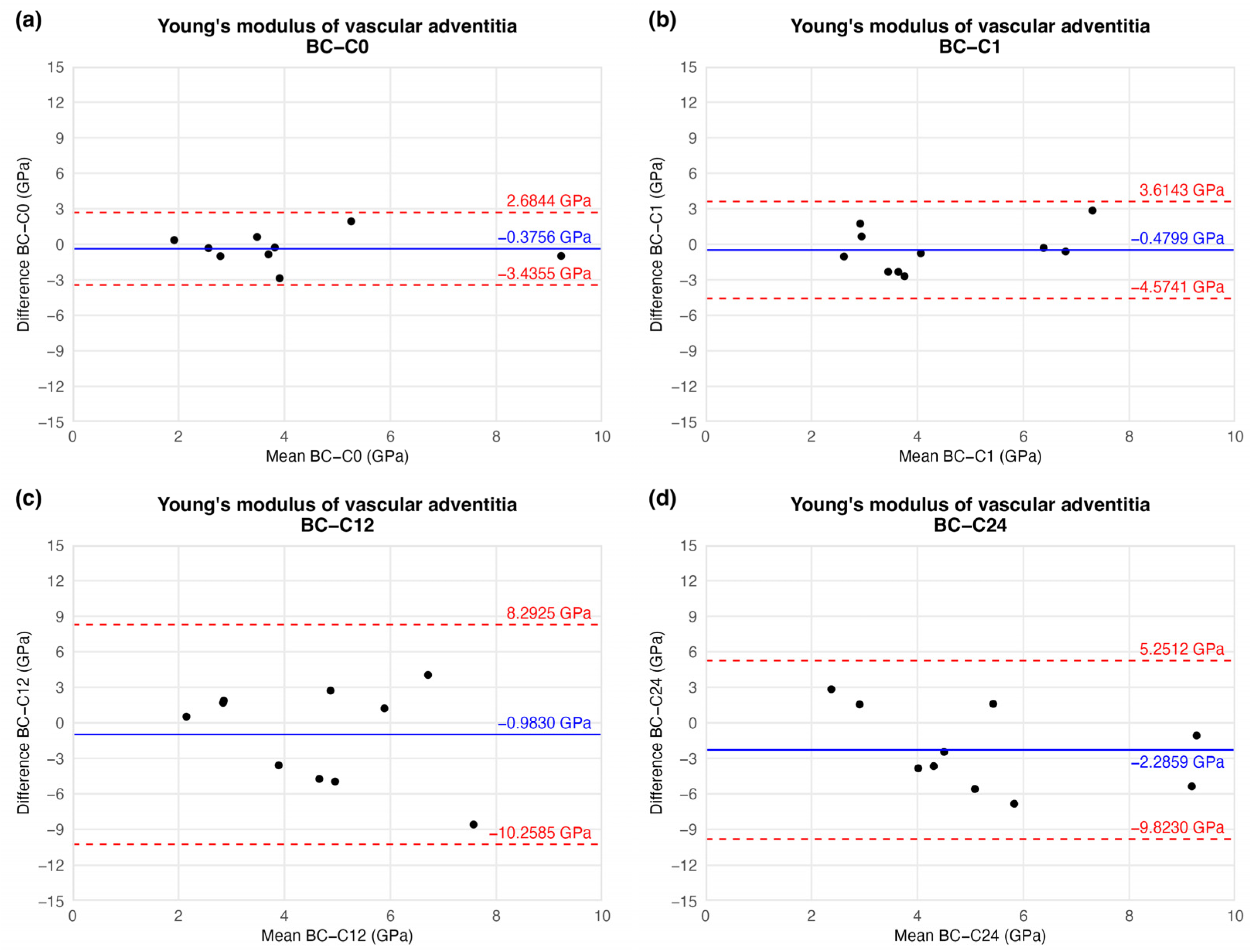
| Elapsed Weeks | MD ± SD | CI 95% LL | CI 95% UL | |
|---|---|---|---|---|
| Young’s modulus (GPa) | C0 | −0.3756 ± 1.3269 | −3.4355 | 2.6844 |
| C1 | −0.4799 ± 1.8099 | −4.5742 | 3.6143 | |
| C12 | −0.9830 ± 4.1629 | −10.2585 | 8.2925 | |
| C24 | −2.2859 ± 3.3827 | −9.8230 | 5.2512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, G.I.; Hidi, L.; Forró, E.; Haluszka, D.; Veres, D.S.; Gyurok, G.P.; Kőszegi, A.; Fintha, A.; Kellermayer, M.; Sótonyi, P. Multi-Scale Mechanics of Cryopreserved Human Arterial Allografts Across a Six-Month Period. J. Funct. Biomater. 2025, 16, 198. https://doi.org/10.3390/jfb16060198
Kovács GI, Hidi L, Forró E, Haluszka D, Veres DS, Gyurok GP, Kőszegi A, Fintha A, Kellermayer M, Sótonyi P. Multi-Scale Mechanics of Cryopreserved Human Arterial Allografts Across a Six-Month Period. Journal of Functional Biomaterials. 2025; 16(6):198. https://doi.org/10.3390/jfb16060198
Chicago/Turabian StyleKovács, Gergely Imre, László Hidi, Evelin Forró, Dóra Haluszka, Dániel Sándor Veres, Gergő Péter Gyurok, Andrea Kőszegi, Attila Fintha, Miklós Kellermayer, and Péter Sótonyi. 2025. "Multi-Scale Mechanics of Cryopreserved Human Arterial Allografts Across a Six-Month Period" Journal of Functional Biomaterials 16, no. 6: 198. https://doi.org/10.3390/jfb16060198
APA StyleKovács, G. I., Hidi, L., Forró, E., Haluszka, D., Veres, D. S., Gyurok, G. P., Kőszegi, A., Fintha, A., Kellermayer, M., & Sótonyi, P. (2025). Multi-Scale Mechanics of Cryopreserved Human Arterial Allografts Across a Six-Month Period. Journal of Functional Biomaterials, 16(6), 198. https://doi.org/10.3390/jfb16060198






