Enhancing Bioavailability and Stability of Plant Secondary Metabolites: Formulation and Characterization of Nanophytosomes Encapsulating Red Bryony and Horned Poppy Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of the RB and HP Extracts
2.3. Preparation of the Nanophytosomes
2.4. Evaluation of Drug Loading (DL) and Encapsulation Efficiency (EE)
- RB Concentration: y = 0.0081x + 0.0296 (R2 = 0.9904)
- HP Concentration: y = 0.0057x + 0.0777 (R2 = 0.9957)
2.5. Evaluation of the Physicochemical Properties of the Nanophytosomes
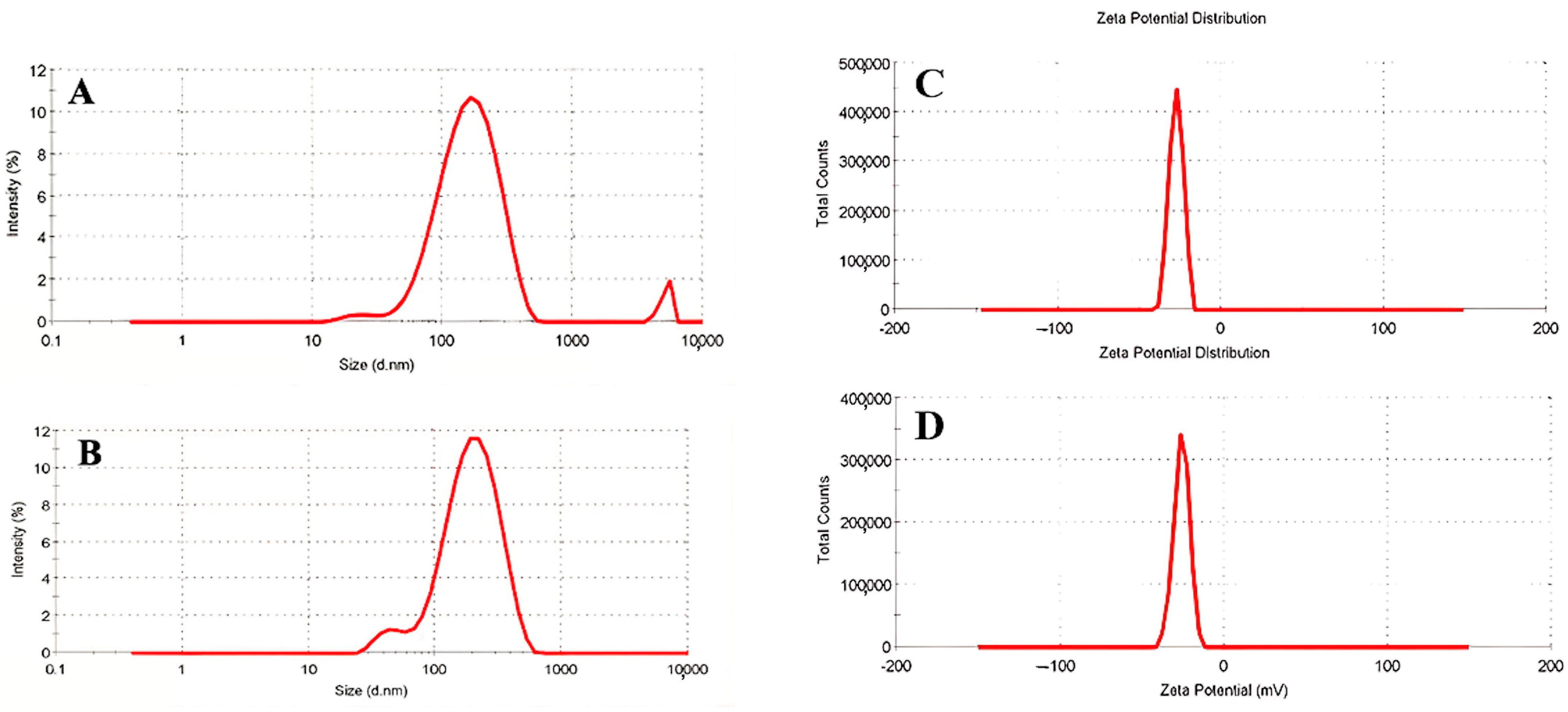
2.5.1. DLS and Zeta Potential Analysis
2.5.2. Scanning Electron Microscopy
2.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Evaluation of the Release Characteristics of the Nanophytosomes
2.7. Stability Studies
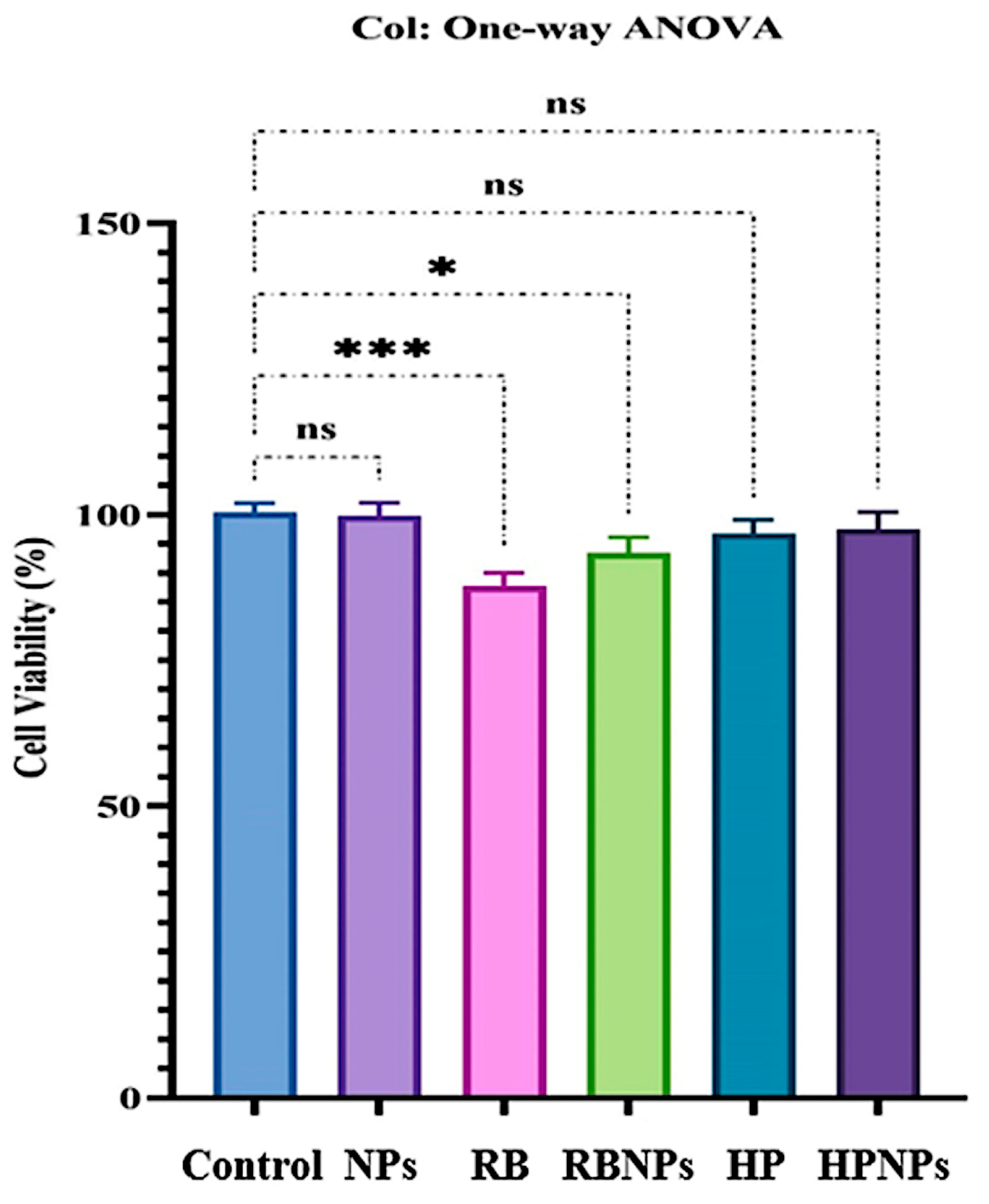
2.8. Cytotoxicity Evaluations
2.9. Statistical Analysis
3. Results and Discussion
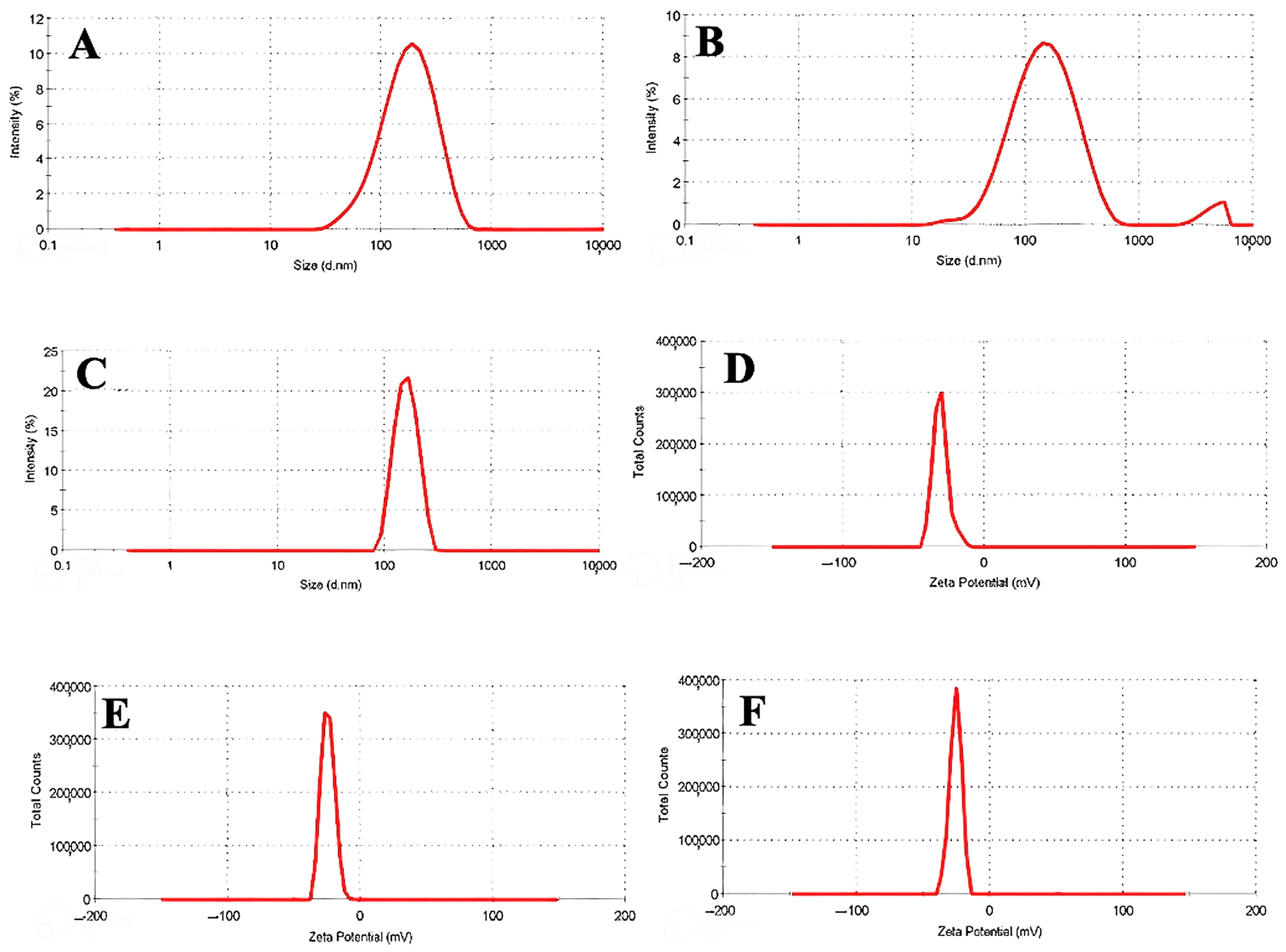
3.1. DLS and Zeta Potential of Nanophytosomes
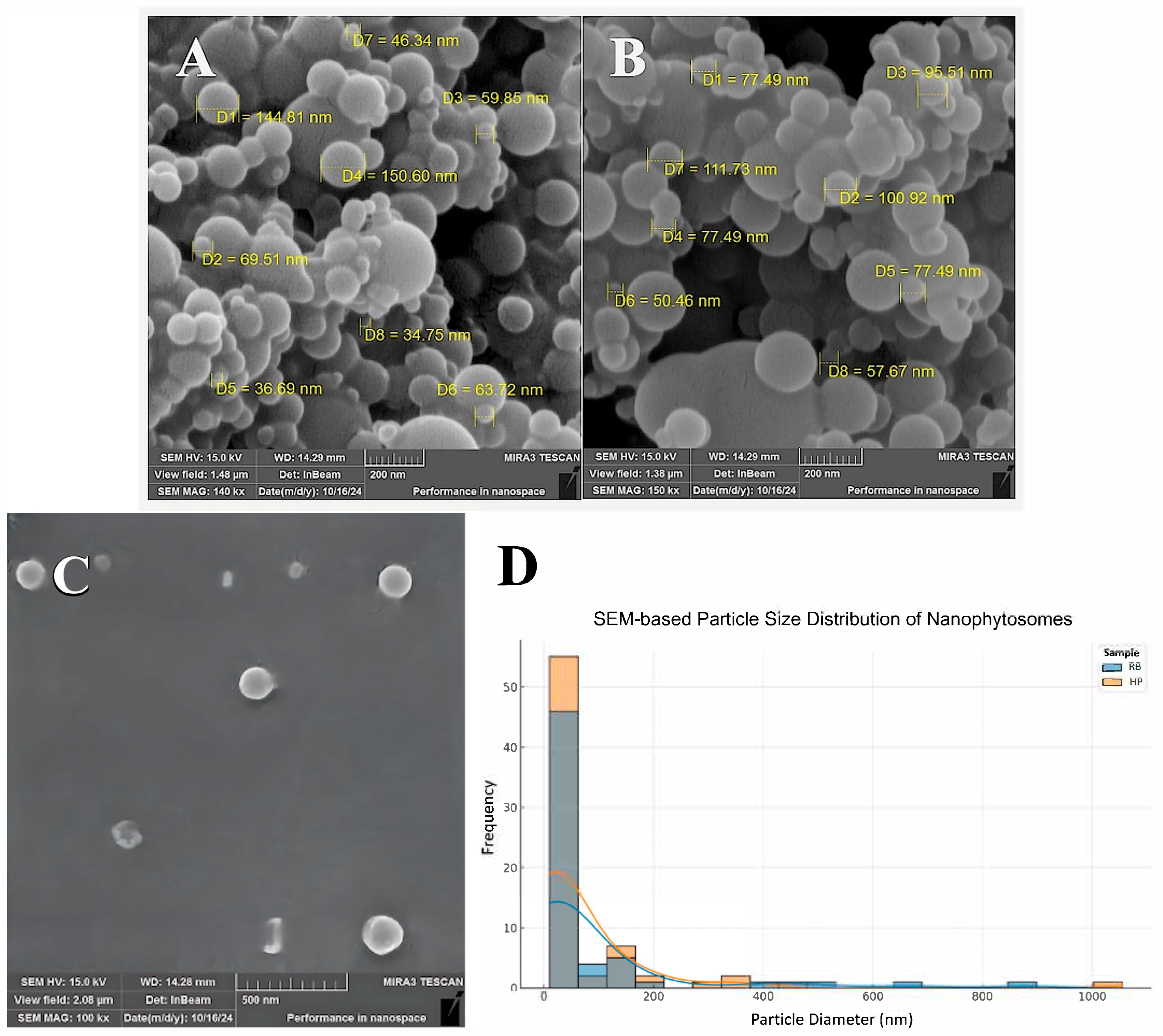
3.2. Scanning Electron Microscopy (SEM)
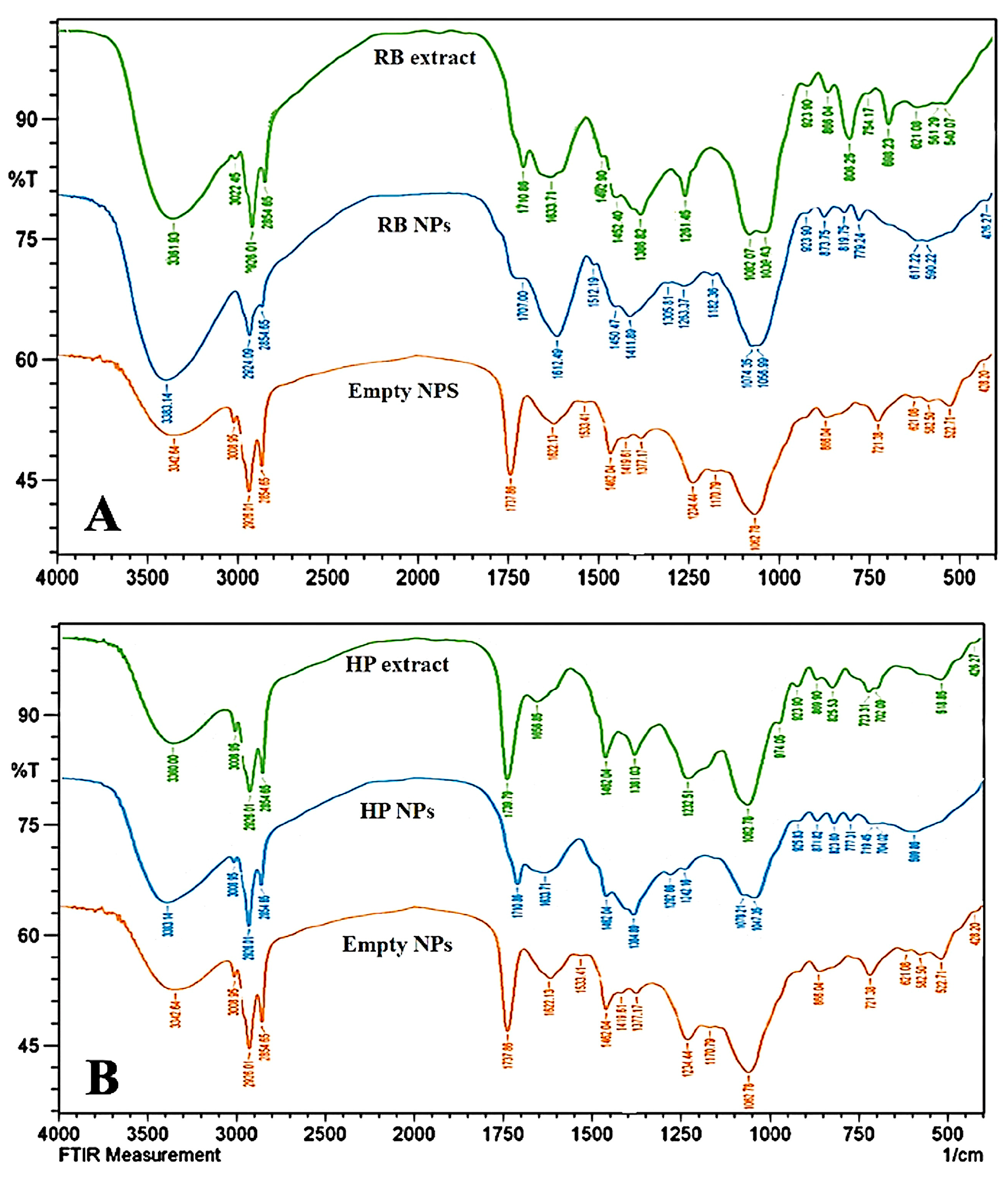
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Drug Loading (DL) and Encapsulation Efficiency (EE) Studies
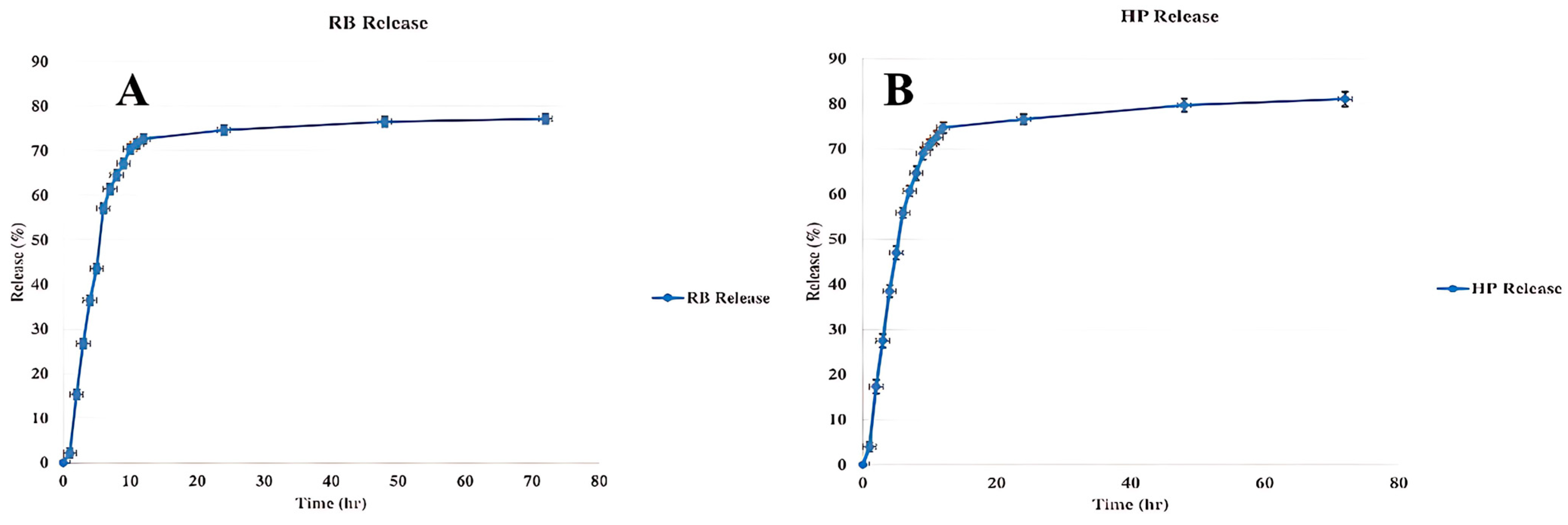
3.5. Release Profile of Nanophytosomes
3.6. Cytotoxicity Evaluations
3.7. Stability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Traditional herbal medicine for the prevention and treatment of cold and flu in the autumn of 2020, overlapped with COVID-19. Nat. Prod. Commun. 2020, 15, 1934578X20951431. [Google Scholar] [CrossRef]
- Rashrash, M.; Schommer, J.C.; Brown, L.M. Prevalence and Predictors of Herbal Medicine Use Among Adults in the United States. J. Patient Exp. 2017, 4, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Salm, S.; Rutz, J.; van den Akker, M.; Blaheta, R.A.; Bachmeier, B.E. Current state of research on the clinical benefits of herbal medicines for non-life-threatening ailments. Front. Pharmacol. 2023, 14, 1234701. [Google Scholar] [CrossRef]
- Benarba, B. Ethnomedicinal study of Bryonia dioica, a plant used as anti-breast cancer herbal therapy in North West Algeria. J. Med. Herbs Ethnomed. 2015, 1, 113–115. [Google Scholar]
- Chekroun, E.; Bechiri, A.; Azzi, R.; Adida, H.; Benariba, N.; Djaziri, R. Antidiabetic activity of two aqueous extracts of two cucurbitaceae: Citrullus colocynthis and Bryonia dioica. Phytothérapie 2017, 15, 57–66. [Google Scholar] [CrossRef]
- Akaberi, T.; Shourgashti, K.; Emami, S.A.; Akaberi, M. Phytochemistry and pharmacology of alkaloids from Glaucium spp. Phytochemistry 2021, 191, 112923. [Google Scholar] [CrossRef]
- Berlinck, R.G.; Crnkovic, C.M.; Gubiani, J.R.; Bernardi, D.I.; Ióca, L.P.; Quintana-Bulla, J.I. The isolation of water-soluble natural products–challenges, strategies and perspectives. Nat. Prod. Rep. 2022, 39, 596–669. [Google Scholar] [CrossRef]
- Naeem, A.; Ming, Y.; Pengyi, H.; Jie, K.Y.; Yali, L.; Haiyan, Z.; Shuai, X.; Wenjing, L.; Ling, W.; Xia, Z.M.; et al. The fate of flavonoids after oral administration: A comprehensive overview of its bioavailability. Crit. Rev. Food Sci. Nutr. 2022, 62, 6169–6186. [Google Scholar] [CrossRef]
- Shahzad, N.; Alzahrani, A.R.; Ibrahim, I.A.; Shahid, I.; Alanazi, I.M.; Falemban, A.H.; Imam, M.T.; Mohsin, N.; Azlina, M.F.; Arulselvan, P. Therapeutic strategy of biological macromolecules based natural bioactive compounds of diabetes mellitus and future perspectives: A systemic review. Heliyon 2024, 10, e24207. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-based nanophytosomes as effective drug delivery systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Mahfuz, A.M.; Hossain, M.K.; Khan, M.I.; Hossain, I.; Anik, M.I. Smart drug delivery nanostructured systems for cancer therapy. In New Trends in Smart Nanostructured Biomaterials in Health Sciences; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–39. [Google Scholar]
- Petrovic, S.M.; Barbinta-Patrascu, M.E. Organic and biogenic nanocarriers as bio-friendly systems for bioactive compounds’ delivery: State-of-the art and challenges. Materials 2023, 16, 7550. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.; Rajora, A. Phytosomes: A critical tool for delivery of herbal drugs for cancer: Phytosomes: Advancing Herbal Medicine Delivery. Phytochem. Rev. 2025, 24, 165–195. [Google Scholar] [CrossRef]
- Kapoor, D.U.; Gaur, M.; Parihar, A.; Prajapati, B.G.; Singh, S.; Patel, R.J. Phosphatidylcholine (PCL) fortified nano-phytopharmaceuticals for improvement of therapeutic efficacy. EXCLI J. 2023, 22, 880. [Google Scholar] [PubMed]
- Toma, L.; Deleanu, M.; Sanda, G.M.; Barbălată, T.; Niculescu, L.Ş.; Sima, A.V.; Stancu, C.S. Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders. Int. J. Mol. Sci. 2024, 25, 4162. [Google Scholar] [CrossRef]
- Bernela, M.; Seth, M.; Kaur, N.; Sharma, S.; Pati, P.K. Harnessing the potential of nanobiotechnology in medicinal plants. Ind. Crops Prod. 2023, 194, 116266. [Google Scholar] [CrossRef]
- Freag, M.S.; Elnaggar, Y.S.; Abdallah, O.Y. Lyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: Optimization and ex vivo permeation. Int. J. Nanomed. 2013, 8, 2385–2397. [Google Scholar]
- Kim, S.M.; Jung, J.I.; Chai, C.; Imm, J.Y. Characteristics and glucose uptake promoting effect of chrysin-loaded phytosomes prepared with different phospholipid matrices. Nutrients 2019, 11, 2549. [Google Scholar] [CrossRef]
- Shriram, R.G.; Moin, A.; Alotaibi, H.F.; Khafagy, E.S.; Al Saqr, A.; Abu Lila, A.S.; Charyulu, R.N. Phytosomes as a plausible nano-delivery system for enhanced oral bioavailability and improved hepatoprotective activity of silymarin. Pharmaceuticals 2022, 15, 790. [Google Scholar] [CrossRef]
- Kediso, T.E.; Tolessa, T.; Getachew, F.; Makonnen, E.; Seifu, D. Effect of 70% Ethanol Extract and its Solvent Fractions of Artemisia afra (Jacq. Ex Willd.) against Pentylenetetrazole-Induced Seizure in Mice. Evid.-Based Complement. Altern. Med. 2021, 2021, 6690965. [Google Scholar] [CrossRef]
- Abdulqahar, F.W.; El-Messery, T.M.; Zaky, A.A.; El-Said, M.M. In vitro digestibility of Aucklandia costus-loaded nanophytosomes and their use in yoghurt as a food model. Food Biosci. 2022, 50, 102106. [Google Scholar]
- Hooresfand, Z.; Ghanbarzadeh, S.; Hamishehkar, H. Preparation and characterization of rutin-loaded nanophytosomes. Pharm. Sci. 2015, 21, 145–151. [Google Scholar] [CrossRef]
- Andishmand, H.; Yousefi, M.; Jafari, N.; Azadmard-Damirchi, S.; Homayouni-Rad, A.; Torbati, M.; Hamishehkar, H. Designing and fabrication of colloidal nano-phytosomes with gamma-oryzanol and phosphatidylcholine for encapsulation and delivery of polyphenol-rich extract from pomegranate peel. Int. J. Biol. Macromol. 2024, 256, 128501. [Google Scholar] [CrossRef] [PubMed]
- Karekar, P.; Killedar, S.; More, H. Nanophytosomes Loading Andrographis paniculata Hydroalcoholic Extract: Promising Drug Delivery for Hepatoprotective Efficacy. J. Pharm. Innov. 2023, 18, 1084–1099. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hamishehkar, H.; Piruzifard, M.K. Nanophytosome as a promising carrier for improving cumin essential oil properties. Food Biosci. 2021, 42, 101079. [Google Scholar]
- Phatvej, W. Internalisation and Cytotoxicity of Alkyl-Capped Silicon Quantum Dots (SiDQs) in Various Mammalian Cell Lines. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2015. [Google Scholar]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanophytosomes: A review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Patil, S.; Patil, A.G. The progressive journey of phytosomes in herbal based pharmacotherapeutics. Curr. Bioact. Compd. 2020, 16, 853–886. [Google Scholar] [CrossRef]
- Bhargav, E.; Koteshwara, K.B.; Reddy, Y.P.; Sowmya, C.; Ramalingam, P. Development of Curcumin nanophytosomes surface functionalized with Chondroitin sulfate-A for treating k1 Plasmodium falciparum drug-resistant malaria. J. Drug Deliv. Sci. Technol. 2023, 87, 104788. [Google Scholar] [CrossRef]
- Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Cinci, L.; Landucci, E.; Bilia, A.R.; Bergonzi, M.C. Formulation of nanomicelles to improve the solubility and the oral absorption of silymarin. Molecules 2019, 24, 1688. [Google Scholar] [CrossRef]
- Ortega-Pérez, L.G.; Ayala-Ruiz, L.A.; Magaña-Rodríguez, O.R.; Piñón-Simental, J.S.; Aguilera-Méndez, A.; Godínez-Hernández, D.; Rios-Chavez, P. Development and Evaluation of Phytosomes Containing Callistemon citrinus Leaf Extract: A Preclinical Approach for the Treatment of Obesity in a Rodent Model. Pharmaceutics 2023, 15, 2178. [Google Scholar] [CrossRef]
- Munot, N.; Kandekar, U.; Giram, P.S.; Khot, K.; Patil, A.; Cavalu, S. A comparative study of quercetin-loaded nanocochleates and liposomes: Formulation, characterization, assessment of degradation and in vitro anticancer potential. Pharmaceutics 2022, 14, 1601. [Google Scholar] [CrossRef]
- Ali, M.M.; Shoukri, R.A.; Yousry, C. Effect of particle size versus surface charge on the brain targeting behavior of elastic nanovesicles: In-vitro characterization, comparison between I-optimal and D-optimal statistical optimization and in-vivo pharmacokinetic evaluation. J. Drug Deliv. Sci. Technol. 2024, 96, 105693. [Google Scholar] [CrossRef]
- Miranda-Linares, V.; Quintanar-Guerrero, D.; Del Real, A.; Zambrano-Zaragoza, M.L. Spray-drying method for the encapsulation of a functionalized ingredient in alginate-pectin nano-and microparticles loaded with distinct natural actives: Stability and antioxidant effect. Food Hydrocoll. 2020, 101, 105560. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, W.; Feng, X.; Zhou, G.; Zhang, M.; Zeng, L.; Hu, X.; Liu, Z.; Song, H. A comparative study on the preparation and evaluation of solubilizing systems for silymarin. Drug Deliv. Transl. Res. 2024, 14, 1616–1634. [Google Scholar] [CrossRef]
- Liu, N.; Park, H.J. Chitosan-coated nanoliposome as vitamin E carrier. J. Microencapsul. 2009, 26, 235–242. [Google Scholar] [CrossRef]
- Karekar, P.; Killedar, S.; More, H.; Shaikh, A.; Joshi, S.; Waghmare, S.; Walvekar, A.; Buchade, R.; Patil, S. Accelerated Stability Studies of Mucuna prureins Hydroalcoholic Extract Phytosome Formulation, and Evaluation of its Capsule Dosage Form. Biol. Forum Int. J. 2023, 15, 1135–1139. [Google Scholar]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols–A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Pham, B.T. Preparation and physicochemical evaluation of hydrogel containing quercetin phytosomes. Pharm. Sci. Asia 2021, 48, 122–138. [Google Scholar]
- Kumar, A.; Kumar, S.; Sharma, D.; Singh, P. Preparation and Characterization of Curcuma Longa Extract-Loaded Ethosome. In Tissue Scaffolds; Springer: New York, NY, USA, 2022; pp. 451–458. [Google Scholar]
- Kumar, N.; Rai, A.; Reddy, N.D.; Shenoy, R.R.; Mudgal, J.; Bansal, P.; Mudgal, P.P.; Arumugam, K.; Udupa, N.; Sharma, N.; et al. Improved in vitro and in vivo hepatoprotective effects of liposomal silymarin in alcohol-induced hepatotoxicity in Wistar rats. Pharmacol. Rep. 2019, 71, 703–712. [Google Scholar] [CrossRef]
- Alexander, A.; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Chung, I.M.; Kirthi, A.V.; Marimuthu, S.; Anbarasan, K. Antiplasmodial activity of eco-friendly synthesized palladium nanoparticles using Eclipta prostrata extract against Plasmodium berghei in Swiss albino mice. Parasitol. Res. 2015, 114, 1397–1406. [Google Scholar] [CrossRef]
- Liu, J.; Wang, A.Q.; Zhang, C.; Feng, L. Study on the relationship between pyrolysis volatile products and structure of Shengli lignite using TG-FTIR-GC/MS. J. Therm. Anal. Calorim. 2022, 147, 8261–8269. [Google Scholar] [CrossRef]
- Siregar, M.A.; Santri, J.A.; Aksani, D.; Maas, A.; Nurudin, M. Analysis of FTIR spectroscopic data to observe hydrophilic and hydrophobic levels of peat in hemic and sapric maturity. IOP Conf. Ser. Earth Environ. Sci. 2022, 1025, 012026. [Google Scholar] [CrossRef]
- Banou, P.; Boyatzis, S.; Choulis, K.; Theodorakopoulos, C.; Alexopoulou, A. Oil Media on Paper: Investigating the Interaction of Cold-Pressed Linseed Oil with Paper Supports with FTIR Analysis. Polymers 2023, 15, 2567. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, A.; Cao, D.; Wei, Y.; Ding, L. Differences in macromolecular structure evolution during the pyrolysis of vitrinite and inertinite based on in situ FTIR and XRD measurements. Energies 2022, 15, 5334. [Google Scholar] [CrossRef]
- Kim, S. Characterization of mechanical, thermal and rheological properties of silica-based nanocomposite filled thermoplastic polyurethane film. Macromol. Res. 2024, 32, 727–743. [Google Scholar] [CrossRef]
- Banoth, R.K.; Thatikonda, A.S. Fourier-transform infrared spectroscopy studies and evaluation of total phenolics flavonoids and antioxidant activity of Cleome gynandra. Asian J. Pharm. Clin. Res. 2019, 12, 214–218. [Google Scholar] [CrossRef]
- Ul Haq, T.; Ullah, R. Green synthesis and characterization of gold nanophytosomes (Au-Nanophytosomes) using stem extract of Euphorbia neriifolia L. and evaluation of their antibacterial and antifungal potential. Int. J. Nanosci. 2022, 21, 2250008. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Souto, E.B.; Silva, A.M. Nanophytosomes: A novel approach for the delivery of herbal drugs. In Systems of Nanovesicular Drug Delivery; Academic Press: Cambridge, MA, USA, 2022; pp. 239–257. [Google Scholar]
- Alvarado-Noguez, M.L.; Matías-Reyes, A.E.; Pérez-González, M.; Tomás, S.A.; Hernández-Aguilar, C.; Domínguez-Pacheco, F.A.; Arenas-Alatorre, J.A.; Cruz-Orea, A.; Carbajal-Tinoco, M.D.; Galot-Linaldi, J.; et al. Processing and physicochemical properties of magnetite nanophytosomes coated with curcuma longa l. extract. Materials 2023, 16, 3020. [Google Scholar] [CrossRef]
- Karavelioglu, Z.; Cakir-Koc, R. Preparation of chitosan nanophytosomes as Ginkgo Biloba extract carrier: In vitro neuroprotective effect on oxidative stress-induced human neuroblastoma cells (SH-SY5Y). Int. J. Biol. Macromol. 2021, 192, 675–683. [Google Scholar] [CrossRef]
- Madzharova, F.; Chatterley, A.S.; Roeters, S.J.; Weidner, T. Probing Backbone Coupling within Hydrated Proteins with Two-Color 2D Infrared Spectroscopy. J. Phys. Chem. Lett. 2024, 15, 4933–4939. [Google Scholar] [CrossRef]
- Kumbhar, S.; Salunke, M.; Wakure, B. Nanostructured Phytosomes: Revolutionizing Herbal Compound Delivery, Therapeutic Applications, Current Achievements and Future Perspectives. Res. J. Pharm. Technol. 2025, 18, 1899–1905. [Google Scholar] [CrossRef]
- Pooja, D.; Bikkina, D.J.; Kulhari, H.; Nikhila, N.; Chinde, S.; Raghavendra, Y.M.; Sreedhar, B.; Tiwari, A.K. Fabrication, characterization and bioevaluation of silibinin loaded chitosan nanophytosomes. Int. J. Biol. Macromol. 2014, 69, 267–273. [Google Scholar] [CrossRef]
- Solanki, R.; Patel, S. Preparation, characterization and in vitro anticancer efficacy of biotin-conjugated, silibinin loaded bovine serum albumin nanophytosomes. Food Biosci. 2023, 56, 103150. [Google Scholar]
- Zong, L.; Li, C.; Zhong, Y.; Shi, J.; Yuan, Z.; Wang, X. FTIR microspectroscopic investigation of Lactobacillus paracasei apoptosis induced by cisplatin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119542. [Google Scholar] [CrossRef]
- Saražin, J.; Poljanšek, I.; Pizzi, A.; Šernek, M. Curing kinetics of tannin and lignin biobased adhesives determined by DSC and ABES. J. Renew. Mater. 2022, 10, 2117–2131. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Alzahrani, E.; Saeed, A.; Al-Ghamdi, S.A.; Alwafi, R.; Abo-Dief, H.M.; Yassin, A.Y. Exploring the influence of sudan IV Azo dye on the structural, optical, and dispersion characteristics of PVA/Su-IV composites. Phys. Scr. 2024, 99, 105991. [Google Scholar] [CrossRef]
- Al-Saleem, M.S.; Basudan, O.A.; Salem, W.M.; El-Gamal, A.A.; Nasr, F.A.; Abdel-Mageed, W.M.; Alqasoumi, S.I. Alkaloids and phenolic constituents from Glaucium corniculatum. Biochem. Syst. Ecol. 2024, 112, 104780. [Google Scholar] [CrossRef]
- Hssaini, L.; Razouk, R.; Bouslihim, Y. Rapid prediction of fig phenolic acids and flavonoids using mid-infrared spectroscopy combined with partial least square regression. Front. Plant Sci. 2022, 13, 782159. [Google Scholar] [CrossRef]
- Dehghani, F.; Mosleh-Shirazi, S.; Shafiee, M.; Kasaee, S.R.; Amani, A.M. Antiviral and antioxidant properties of green synthesized gold nanophytosomes using Glaucium flavum leaf extract. Appl. Nanosci. 2023, 13, 4395–4405. [Google Scholar] [CrossRef]
- Molaveisi, M.; Taheri, R.A.; Dehnad, D. Innovative application of the Echinacea purpurea (L.) extract-phospholipid phytosomes embedded within Alyssum homolocarpum seed gum film for enhancing the shelf life of chicken meat. Food Biosci. 2022, 50, 102020. [Google Scholar] [CrossRef]
- Koppula, S.; Shaik, B.; Maddi, S. Phytosomes as a New Frontier and Emerging Nanotechnology Platform for Phytopharmaceuticals: Therapeutic and Clinical Applications. Phytother. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, Y.; Chen, Q.; He, Y.; Wang, H.; Yang, L.; Guo, S.; Meng, Z.; Cui, J.; Xue, M.; et al. Monodisperse microparticles loaded with the self-assembled berberine-phospholipid complex-based phytosomes for improving oral bioavailability and enhancing hypoglycemic efficiency. Eur. J. Pharm. Biopharm. 2016, 103, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Parham, S.S.; Nur, H. Plant extracts: Antioxidant and antimicrobial properties and their effect of nanoencapsulation. In Nanotechnology in Herbal Medicine; Woodhead Publishing: Sawston, UK, 2023; pp. 195–219. [Google Scholar]
- Mane, V.; Killedar, S.; More, H.; Tare, H. Preclinical study on camellia sinensis extract-loaded nanophytosomes for enhancement of memory-boosting activity: Optimization by central composite design. Future J. Pharm. Sci. 2024, 10, 66. [Google Scholar] [CrossRef]
- Gómez-Lázaro, L.; Martín-Sabroso, C.; Aparicio-Blanco, J.; Torres-Suárez, A.I. Assessment of In Vitro Release Testing Methods for Colloidal Drug Carriers: The Lack of Standardized Protocols. Pharmaceutics 2024, 16, 103. [Google Scholar] [CrossRef]
- Elmi, N.; Ghanbarzadeh, B.; Ayaseh, A.; Sahraee, S.; Heshmati, M.K.; Hoseini, M.; Pezeshki, A. Physical properties and stability of quercetin loaded niosomes: Stabilizing effects of phytosterol and polyethylene glycol in orange juice model. J. Food Eng. 2021, 296, 110463. [Google Scholar] [CrossRef]
- Sardarabadi, H.; Darvishi, M.H.; Zohrab, F.; Javadi, H. Nanophytomedicine: A promising practical approach in phytotherapy. Phytother. Res. 2024, 38, 3607–3644. [Google Scholar] [CrossRef]
- Frosi, I.; Ferron, L.; Colombo, R.; Papetti, A. Natural carriers: Recent advances in their use to improve the stability and bioaccessibility of food active compounds. Crit. Rev. Food Sci. Nutr. 2024, 64, 5700–5718. [Google Scholar] [CrossRef]
- Roy, D.; Nagori, M.; Jain, V.; Pal, P. Development, Characterization and Evaluation of Phytosomal Gel of Curcumin for the Treatment of Topical Fungal Infection. Int. J. Newgen Res. Pharm. Healthc. 2023, 1, 62–69. [Google Scholar]
- Fernandes, F.; Dias-Teixeira, M.; Delerue-Matos, C.; Grosso, C. Critical review of lipid-based nanophytosomes as carriers of neuroprotective drugs and extracts. Nanomaterials 2021, 11, 563. [Google Scholar] [CrossRef]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the flavonoid quercetin with chitosan-coated nano-liposomes. LWT-Food Sci. Technol. 2017, 85, 37–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olfati, A.; Karimi, N.; Arkan, E.; Zhaleh, M.; Mozafari, M.R. Enhancing Bioavailability and Stability of Plant Secondary Metabolites: Formulation and Characterization of Nanophytosomes Encapsulating Red Bryony and Horned Poppy Extracts. J. Funct. Biomater. 2025, 16, 194. https://doi.org/10.3390/jfb16060194
Olfati A, Karimi N, Arkan E, Zhaleh M, Mozafari MR. Enhancing Bioavailability and Stability of Plant Secondary Metabolites: Formulation and Characterization of Nanophytosomes Encapsulating Red Bryony and Horned Poppy Extracts. Journal of Functional Biomaterials. 2025; 16(6):194. https://doi.org/10.3390/jfb16060194
Chicago/Turabian StyleOlfati, Atoosa, Naser Karimi, Elham Arkan, Mohsen Zhaleh, and M. R. Mozafari. 2025. "Enhancing Bioavailability and Stability of Plant Secondary Metabolites: Formulation and Characterization of Nanophytosomes Encapsulating Red Bryony and Horned Poppy Extracts" Journal of Functional Biomaterials 16, no. 6: 194. https://doi.org/10.3390/jfb16060194
APA StyleOlfati, A., Karimi, N., Arkan, E., Zhaleh, M., & Mozafari, M. R. (2025). Enhancing Bioavailability and Stability of Plant Secondary Metabolites: Formulation and Characterization of Nanophytosomes Encapsulating Red Bryony and Horned Poppy Extracts. Journal of Functional Biomaterials, 16(6), 194. https://doi.org/10.3390/jfb16060194







