Abstract
Objectives: Recently, there has been great interest in teeth and their derivatives as suitable substrates for the treatment of alveolar bone defects. This retrospective study evaluates the clinical and radiographic outcomes of implants inserted in a site that underwent GBR procedure using a tooth derivate material. Materials and methods: A total of 21 patients received a GBR using an autologous extracted tooth. Four months after the GBR techniques, the implants were inserted and were followed for an average of 5.28 + −1.10 years after loading. The X-ray was analyzed after a period of 63.36 + −13.2 months for a total follow-up period. Results: A total of 28 implants were inserted. All the implants were clinically functional after the follow-up period. The average bone loss from the X-ray images was 0.1208 + −0.1307. Conclusion: Within the limitations of this study, the use of a tooth as a graft using a tooth transformer device guarantees the production of bone and maintenance over time.
1. Introduction
Bone regeneration, particularly in dentistry, has experienced significant growth in recent decades, with biotechnology playing a crucial role [1,2,3,4].
Various bone substitutes, including allografts, xenografts, and autografts, have been proposed as post-extraction grafting materials to prevent periodontal defects. All studied biomaterials have shown the ability to reduce the bone resorption suffered in the buccal alveolar cortical bone after extraction. Autologous bone remains the gold standard due to its unique combination of osteoinductive, osteoconductive, and osteogenic properties. However, it has its own drawbacks, such as having limited obtainability in intraoral areas, requiring general anesthesia to be obtained from extraoral areas, causing an extra surgical trauma, and having a short resorption time [5,6,7,8,9,10]. Osteoinductive grafting materials stimulate bone formation by inducing the differentiation of multipotent mesenchymal stem cells from the surrounding host bone tissue [10,11,12,13]. Ongoing research is focused on developing biocompatible, cost-effective biomaterials that promote new bone formation with properties like natural bone, while minimizing morbidity and achieving optimal results in the shortest possible time. In recent years, teeth extracted for various reasons have increasingly been used as bone graft materials with high success rates, rather than being discarded as clinical waste. This shift is due to the striking structural similarities between teeth and bone. Both teeth and maxillofacial bones originate from the same neural crest cells and share a comparable composition of type I collagen and hydroxyapatite [14,15,16,17]. Additionally, dentin contains key growth factors such as Transforming Growth Factor-Beta (TGF-β), Bone Morphogenetic Proteins (BMPs), and insulin-like growth factors I and II, all of which play a crucial role in bone regeneration [18,19,20,21,22,23]. Additionally, it offers the advantage of not inducing host tissue reactivity or heterotopic bone formation, which is a crucial safety factor when selecting a graft [24,25,26]. Bone Morphogenetic Protein 2 (BMP-2) plays a crucial role in stimulating undifferentiated mesenchymal and osteoblastic cells, effectively promoting osteogenesis. Recent case reports have documented the successful use of an autologous, partially demineralized dentin matrix in various bone augmentation procedures, demonstrating significant clinical efficacy [27,28,29,30,31]. One of the most important evaluations in dentistry is post-extraction volumetric changes. These changes can hinder implant placement [32,33,34,35]. Numerous studies have evaluated the implant survival rate after 5 or 10 years. The implant survival rates range from 92.9 to 96–24% [36,37,38]. Some authors have instead analyzed peri-implant bone loss over time, estimating approximately 0.4 mm (±0.7) after 12 months [39,40,41]. The most interesting data for comparison with our study are those evaluating peri-implant bone loss in regenerated sites. The average values from these studies range from 2.48 mm (±0.80) to 3.45 mm (±0.63) [41,42,43]. A recent human study has demonstrated high histological and histomorphometrical levels of vital bone formation in GBR procedures. This was achieved using autologous demineralized dentin matrix grafts derived from freshly extracted teeth, processed with the innovative Tooth Transformer device [44,45,46].
A recent study has demonstrated the presence of BMP-2 in the graft material produced from the extracted tooth after treatment with the Tooth Transformer device (Tooth transformer srl Milan—Italy) [47].
The aim of the present study is to evaluate, after GBR therapies, the bone loss around 28 dental implants, from 21 patients, placed after regeneration procedures using an autologous graft derived from extracted teeth, with a follow-up period ranging from 5 to 7 years [48,49,50]. The objectives of the present study were also to evaluate the implant success rate, implant survival rate, and peri-marginal bone loss of implants placed in regenerated bone tissue using an extracted autologous tooth processed with the Tooth Transformer after 5 years [51,52].
2. Materials and Methods
2.1. Study Design and Patient Selection
This study is a retrospective chart review that includes 21 consecutive patients who underwent alveolar bone regeneration procedures using autologous tooth-derived grafts at a single clinic between 2017 and 2020. In total, 28 dental implants were inserted into 21 alveolar bone defects, as some sites required the placement of multiple implants. These patients were followed up for a period ranging from 4 to 5 years.
Inclusion Criteria
Patients included in the study (Table 1) were those who required dental implants after tooth extraction, which resulted in reduced bone volume necessitating regeneration through a Guided Bone Regeneration (GBR) procedure. Alveolar preservation or ridge preservation techniques were used to allow implant placement after hard tissue healing.

Table 1.
Between 2019 and 2021, twenty-one patients were treated with GBR using autologous extracted teeth. Twenty-height implants were inserted and after the healing period they were loaded with prosthesis.
Exclusion Criteria
Patients were excluded if they were smokers, pregnant, or had systemic conditions such as diabetes, cancer, HIV, bone diseases, metabolic diseases, or if they were undergoing treatment with bisphosphonates, immunosuppressive agents, radiotherapy, or chemotherapy.
Surgical Procedures and Follow-Up
In all cases, the defects were covered with an OsseoGuard collagen membrane (Collagen Matrix, Oakland, NJ, USA). Before treatment, each patient underwent comprehensive radiographic evaluation using orthopantomograms (OPGs) and cone beam computed tomography (CBCT) to assess the extent of bone loss and defects.
Postoperative follow-up evaluations were carried out using intraoral radiographs and periodic clinical examinations at 3, 6, and 12 months, and then yearly. The implant survival rate and peri-implant bone loss were assessed by comparing radiographic images taken immediately after implant placement and during follow-ups.
Tooth Transformer Device
The Tooth Transformer device (Tooth Transformer Srl, Milan, Italy) was used to process extracted teeth into graft material. The device follows a standardized protocol, which involves thorough cleaning of extracted teeth to remove restorative materials, cement, and tartar. The teeth are then ground into optimally sized particles and undergo partial demineralization using solutions contained in a single-use kit. The kit includes six liquids organized in one box: one container with H₂O₂, one with HCl, and four with demineralized water [53,54,55,56,57,58,59,60]. This process, lasting approximately 25 min, preserves key growth factors naturally present in dentin, such as Bone Morphogenetic Protein-2 (BMP-2) and Transforming Growth Factor-Beta (TGF-β), ensuring the material retains its osteoinductive and osteoconductive properties, thus promoting bone regeneration and implant integration [61]. Inferential statistics, including paired t-tests or ANOVA, were used to compare bone loss between different time points and to assess correlations between patient demographics, implant type, and bone resorption rates.
Ethical Approval
The clinical study protocol was approved by the Ethics Committee of the University of Chieti on 21 March 2019 and was registered under the number 638—21/3/19.
2.2. Surgical Procedures
Before surgery, patients underwent clinical and radiographic examinations, including orthopantomograms (OPGs), intraoral radiographs, and cone beam computed tomography (CBCT) when necessary. One week prior to regeneration or implant surgery, all patients received a professional oral hygiene session [62,63]. On the day of surgery, local plexus anesthesia was administered using articaine with epinephrine to enhance hemostasis and reduce postoperative discomfort. Teeth were extracted using atraumatic techniques to preserve the surrounding alveolar bone; for complex extractions, piezosurgical instruments were utilized to minimize bone trauma. Immediately after extraction, teeth were thoroughly cleaned [64]. Any restorative materials (such as amalgam, composites, or endodontic sealers), cement, tartar, and residual periodontal ligament tissues were meticulously removed using a high-speed diamond bur under constant irrigation. Teeth were then sectioned into smaller fragments to facilitate the subsequent graft preparation process. The extracted tooth fragments were processed using the Tooth Transformer device (Tooth Transformer Srl, Milan, Italy).
2.2.1. Tooth Transformer Device and Graft Preparation
The Tooth Transformer device is an automated system designed to transform autologous teeth into biocompatible graft material. It follows a standardized protocol that includes the following:
- Mechanical grinding of the tooth into particles of optimal size.
- Chemical decontamination and partial demineralization using a series of six solutions contained in a sterile, single-use kit (one container with hydrogen peroxide [H₂O₂], one with hydrochloric acid [HCl], and four with demineralized water).
- Preservation of key growth factors such as Bone Morphogenetic Protein-2 (BMP-2), Transforming Growth Factor-Beta (TGF-β), and Insulin-like Growth Factors (IGFs), essential for bone regeneration.
The entire process takes approximately 25 min, producing sterile, osteoconductive, and osteoinductive graft material ready for immediate clinical use.
Following preparation, the dentin graft material was mixed with the patient’s fresh autologous blood collected directly from the surgical site using sterile syringes. No additional anticoagulant systems were utilized. The blood was gently mixed with the dentin particles to enhance graft handling properties and improve biological integration.
2.2.2. Grafting and Implant Procedures
The dentin–blood mixture was placed into the alveolar defect, ensuring complete filling of the cavity. A resorbable collagen membrane (OsseoGuard, Collagen Matrix, Oakland, NJ, USA) was placed over the graft to stabilize the material and prevent soft tissue invasion during early healing. The flap was repositioned and sutured using non-resorbable sutures to achieve primary closure. Patients were prescribed postoperative antibiotics (Amoxicillin, 1 g twice daily for 7 days) and analgesics (Ibuprofen, 600 mg as needed) and instructed to rinse with chlorhexidine mouthwash to support healing. After a healing period of approximately 4 months, implant surgery was performed. A full-thickness flap was elevated, and implant site preparation was completed using a sequential drilling protocol to achieve primary stability. The implants were placed at the crestal bone level and allowed to osseointegrate before prosthetic loading. Final impressions were taken after implant healing to fabricate full ceramic prostheses (Figure 1 and Figure 2).
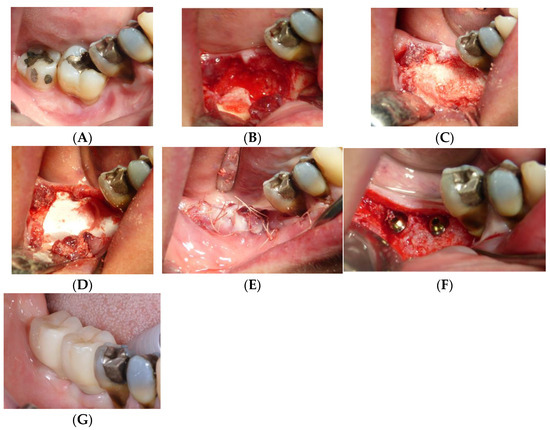
Figure 1.
Operative sequence of a case, from extraction of two lower molars (A) followed by the regenerative procedure (B–E), insertion of the implants (F), and finally insertion of the prosthesis (G).
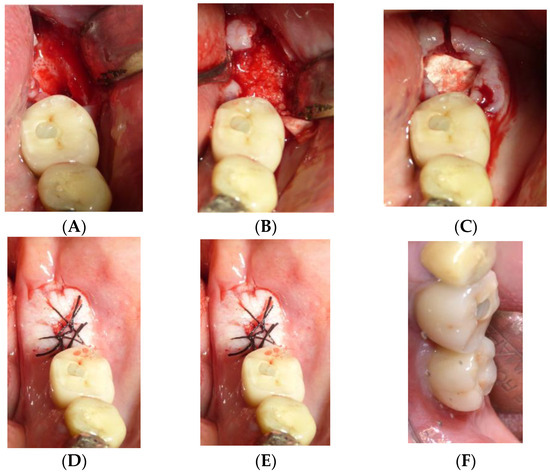
Figure 2.
Operative sequence of another case, from the extraction of one lower molar (A) followed by the regenerative procedure (B–D), insertion of the implants (E), and finally the insertion of the prosthesis (F).
2.3. Follow-Up
Postoperative follow-up assessments were conducted at regular intervals to monitor the stability of the implants and evaluate the bone remodeling process. Patients were scheduled for clinical evaluations for 1 month, 3 months, 6 months, and then annually following implant placement. Each visit included a detailed assessment of soft tissue healing, implant stability, and potential signs of complications such as mucositis or peri-implantitis [65]. Radiographic evaluations, with Irys version 16.0 device (BioNano Genomics, San Diego, CA, USA), played a crucial role in tracking peri-implant bone changes over time [66,67,68,69,70,71]. Digital radiographic software, Mayray hyperion ×5, was employed to analyze bone levels, using the implant collar as a reference point to measure the distance between the implant shoulder and the mesial and distal marginal bone. In addition to conventional radiographs, some cases required three-dimensional imaging with cone beam computed tomography (CBCT) to assess the quality of bone regeneration and verify implant osseointegration [72]. The CBCT scans provided a more comprehensive view of bone density and volumetric changes, particularly in cases where significant ridge augmentation was performed. The stability of the peri-implant bone was evaluated by comparing radiographs taken immediately post-implant placement to those taken at subsequent follow-ups. Any marginal bone loss was recorded and analyzed to determine trends over time [73,74].
2.4. Statistical Analysis
The objectives of this study were to evaluate the implant success rate, implant survival rate, and peri-marginal bone loss after 5 years. The collected data were statistically processed to assess the effectiveness of the Tooth Transformer-derived graft in preserving alveolar bone integrity over the long term.
Descriptive statistics were used to calculate mean values, standard deviations, and ranges for each variable, including peri-implant bone loss, implant success rate, and other clinical outcomes [51,52,75,76,77,78,79,80,81].
A p-value < 0.05 was considered statistically significant [82,83].
The statistical analysis was performed using SPSS software (version X) [48,50,84,85,86,87,88,89,90,91].
3. Results
The sample analyzed consisted of 21 patients and 28 placed implants. The implant success rate was 100% four years after the implant placement phase. Retrospective radiographic evaluations performed on periapical radiographs measured mesial–distal bone levels around dental implants over time. The data indicated that the average mesial bone loss was 0.14302 + −0.0107 and distal bone loss was 0.09934 + −0.0143. The mean peri-implant bone loss, as measured from the radiographic follow-up, was 0.1208 ± 0.1307 mm. This represents a significantly lower resorption rate compared to other studies evaluating implants placed in regenerated bone, where bone loss values ranged from 0.4 mm to 1.6 mm within the first year. A breakdown of the peri-implant bone loss measurements showed that the average mesial bone loss was 0.14302 ± 0.0107 mm, while the distal bone loss was 0.09934 ± 0.0143 mm [92]. These minimal values indicate high stability of the regenerated bone. The reduced resorption may be attributed to the biocompatibility of dentin-based grafts, their slow resorption rate, and their ability to retain bioactive molecules that promote bone remodeling. No signs of inflammation, necrosis, or foreign body reaction were observed at any time point, confirming the high biocompatibility of the autologous dentin graft.
X-ray Follow-ups. As part of the follow-up, X-rays were taken at different time points for the patients to assess the progression of bone resorption and implant stability. Figure 3 shows the X-ray follow-up during the time, showing the extraction and X-ray results from 2021, 2022, and 2024. Figure 4 provides a summary of the average resorption seen in the patient depicted in Figure 3. Figure 5 shows the X-ray follow-up over time, showing the extraction and X-ray results from 2019, 2021, and 2023. Figure 6 summarizes the average resorption observed in the patient depicted in Figure 5 [93].
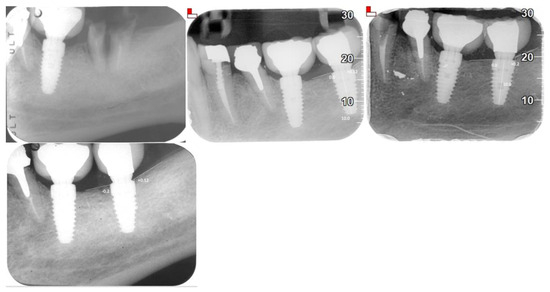
Figure 3.
X-ray follow-up during the time. Extractions from 2021, 2022, and 2024.
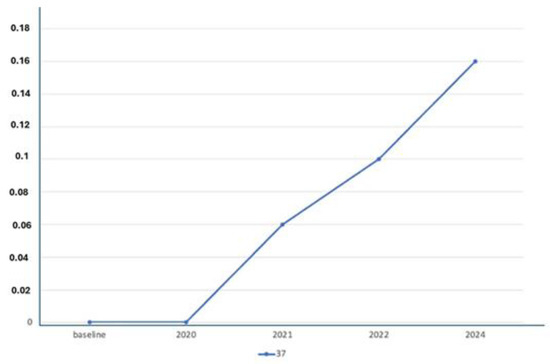
Figure 4.
Average resorption of the patient from Figure 3.
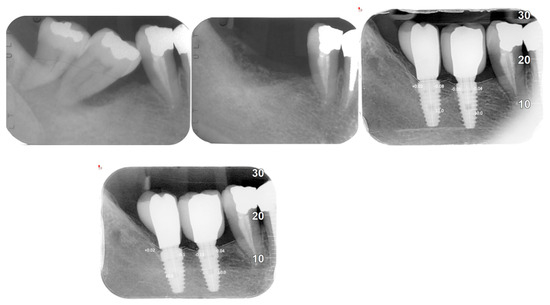
Figure 5.
X-ray follow-up during the time. Extractions from 2019, 2021, and 2023.
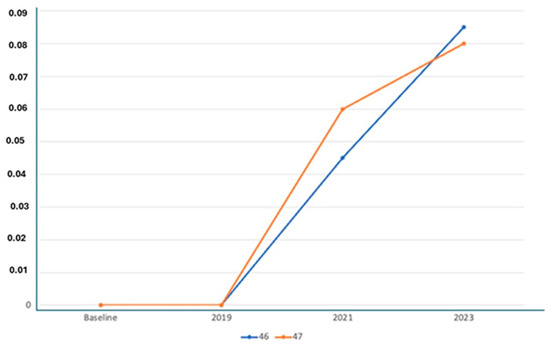
Figure 6.
Average resorption of the patient from Figure 4.
The following tables, Table 2 and Table 3, provide an overview of the patient demographics and implant data used in this study.

Table 2.
Summary of patient demographics and implant data.

Table 3.
Summarized achievements.
4. Discussion
The present study demonstrated a high degree of stability in regenerated bone using dentin, which supported the analyzed implants throughout the entire follow-up period. Histological analysis was performed on bone core biopsies obtained at different time points post-grafting (3, 6, and 12 months). Hematoxylin and eosin (H&E) staining, along with Masson’s trichrome staining, were used to evaluate the structural integration of the dentin graft within the host bone. At 3 months, early signs of new bone formation were visible, with osteoblasts actively depositing osteoid matrix along the surfaces of the dentin granules. Trabecular bone infiltration into the grafted area was evident, indicating the initiation of osteoconduction [94]. At 6 months, histological images revealed an intimate connection between the newly formed bone and the dentin particles, with clear evidence of bone remodeling activity. Osteoclasts were occasionally observed on the dentin surfaces, confirming that the material was undergoing progressive resorption and replacement with new bone. At 12 months, the graft material was almost completely integrated, with mature lamellar bone structures replacing most of the dentin matrix [95]. The amount of bone loss was minimal, which aligns well with findings in the existing literature on the topic [96,97,98]. This is particularly noteworthy considering that bone resorption is one of the most common challenges in implantology. Moreover, the platform-switching technique applied in wide-diameter implants undoubtedly played a critical role in stabilizing the bone and reducing peri-implant bone loss. At the end of the 5-year follow-up, the cumulative implant survival rate was an impressive 100%. In reviewing the literature on implants placed in regenerated sites, the implant survival rate typically ranges between 97% and 100%, with most studies reporting results above 90%. These figures are quite comparable to those observed with implant bones placed in native bone, suggesting that regenerated tissue can offer similar outcomes to native bone in terms of implant survival and follow-up [99,100,101]. This finding supports the clinical application of regenerative techniques, such as the use of dentin grafts, in improving the stability and longevity of dental implants. The classification of defects is another important factor in predicting outcomes. For large defects, a slow-resorbing biomaterial, typically covered with a membrane, has been recommended for optimal healing [102]. This is consistent with the findings of other studies, such as a longitudinal study on bone resorption in implants placed in native bone, where the average resorption value was reported as 1.3 mm. These results underscore the importance of using suitable graft materials and techniques tailored to the size and nature of the defect. Furthermore, a prospective study examining 126 implants placed in defects treated with a Guided Bone Regeneration (GBR) procedure, using xenogeneic material and non-resorbable membranes, showed a 5-year survival rate of 93.1%. While this survival rate is slightly lower than the 100% reported in the current study, it reflects older techniques and materials, highlighting the significant progress made in regenerative implant procedures over the years. Additionally, a randomized study comparing different xenogeneic materials found no significant differences between the two materials under study [103]. After six months of loading, the average peri-implant bone loss at three years was 1.61 mm for the first group and 1.02 mm for the second group, further suggesting that both material types are effective in bone regeneration. Both resorbable and non-resorbable GBR techniques are proven methods that offer high stability for bone tissue, even over extended periods of time [104]. In Naenni’s research, for example, the bone tissue remodeling around implants was 0.23 mm (±0.46) for resorbable membranes and 0.17 mm (±0.28) for non-resorbable membranes, demonstrating minimal bone resorption even at the 5-year mark. These findings suggest that GBR techniques can provide predictable, long-term outcomes in implantology, contributing to the overall success and stability of implants. In a comprehensive analysis conducted in 2021, a total of 483 implants placed after GBR procedures were evaluated. After 12 months of loading, the failure rate was relatively low, with only 10 implants failing (2.3%), and a success rate of 98.2%. The radiographic results indicated an average bone resorption of 0.37 mm (±0.68), with medial resorption at 0.43 mm (±0.83) and distal resorption at 0.23 mm (±0.38). These figures are consistent with the current study’s results, indicating that the use of GBR techniques, particularly when combined with autologous grafts such as dentin, offers stable and predictable outcomes over time [105]. Dentin, being a mineralized tissue like bone, resorbs more slowly than traditional bone chips, ensuring a greater level of osteoconductive stability over time. As highlighted in our histological study, the presence of autologous proteins within dentin, which are homologous to those found in bone tissue, promotes a high level of regeneration. This feature is particularly advantageous as it enhances the integration of the graft with the surrounding bone, minimizing the risk of graft failure. These findings underscore the potential of autologous dentin grafts as a reliable alternative to conventional xenografts and allografts in dental implantology. In addition to their biocompatibility, dentin-based grafts offer the advantage of gradual resorption, which allows for long-term bone regeneration [106]. This characteristic makes them superior to synthetic graft materials, which often carry the risk of immune reactions or slower remodeling. Furthermore, the use of dentin derived from the patient’s own teeth reduces the likelihood of graft rejection, providing an added layer of safety for patients.
Despite the promising results presented in this study, it is important to acknowledge several limitations. One significant limitation is the relatively small sample size of only 21 patients [107,108]. While this sample size allowed for valuable insights, it may not be large enough to detect small differences in implant survival or peri-implant bone loss. Future research involving larger cohorts and multi-center trials would help to confirm these findings and provide more robust data. Additionally, while the follow-up period of up to 5 years is valuable, a longer observation period, such as 10 years, would be necessary to fully assess the long-term stability of the graft material and implants. Moreover, the study did not address the cost effectiveness of using autologous dentin grafts compared to other commonly used bone grafting materials [109,110,111,112,113,114,115]. This is an important consideration for clinical adoption, as the financial implications of using autologous grafts may influence their widespread implementation. Future studies should explore the economic aspects of dentin-based grafts and assess their viability from a cost–benefit perspective. In conclusion, this study highlights the clinical potential of autologous tooth-derived grafts in alveolar bone regeneration [116,117]. The high biocompatibility and osteoconductivity of dentin, combined with its gradual resorption, make it a promising alternative to synthetic or allogenic bone graft materials [106,118,119]. Given the low rate of peri-implant bone resorption observed in this study, the use of dentin-based grafts can be considered a reliable and cost-effective solution for bone regeneration. Clinically, this technique shows great promise in a variety of implant procedures, including socket preservation, sinus lift, and ridge augmentation. As future research further investigates the molecular mechanisms underlying dentin-mediated bone regeneration, including the potential role of growth factors like BMPs, VEGF, and FGF, the scope of applications for this innovative technique is likely to expand [120]. Results from the radiographic follow-ups confirmed that the average peri-implant bone loss remained minimal throughout the 5-year observation period, with mesial and distal bone levels demonstrating high stability. The consistency of these findings with previous studies on autologous dentin grafts suggests that this approach provides a reliable solution for maintaining peri-implant bone health [121].
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) analysis provided further insights into the microstructural composition of the grafted site. SEM images revealed a dense, interconnected bone network, while EDS confirmed the presence of calcium and phosphorus in ratios like natural bone. These findings highlight the osteoconductive and osteoinductive properties of the Tooth Transformer-processed dentin graft, supporting its long-term role in bone regeneration. This study confirms previous findings that tooth-derived grafts, particularly when processed with the Tooth Transformer device, provide a reliable alternative to traditional bone grafting materials. Additionally, the platform-switching technique used for wide-diameter implants may have further contributed to the preservation of marginal bone levels [122].
The integration of advanced technologies such as digital dentistry and 3D bioprinting could further enhance the outcomes of dentin-based bone augmentation procedures, paving the way for a new era of personalized regenerative dentistry [123,124].
5. Conclusions
This study highlights the viability of autologous tooth-derived grafts for alveolar bone regeneration, suggesting that they could serve as a reliable alternative to traditional graft materials. The findings show minimal bone resorption and excellent implant success over a 5-year period. However, further studies with larger sample sizes are needed to better understand the long-term impact of this innovative regenerative technique using demineralized teeth in oral bone regeneration procedures. The high biocompatibility of autologous tooth-derived grafts is clearly demonstrated by the stability of bone regeneration and the low rate of peri-implant bone resorption observed. The high survival rate of dental implants five years after loading further confirms the potential of tooth-derived grafts in supporting intraoral bone maintenance, preservation, and augmentation.
Author Contributions
Conceptualization, E.M., L.F., A.P. and A.D.I.; methodology, L.F. and F.I.; software, A.D.I., G.M. and G.D.; validation, A.M.I. and E.M.; formal analysis, F.I.; resources, L.F. and E.M.; data curation, G.D.; writing—original draft preparation, A.M.I., A.P. and G.D.; writing—review and editing, A.P., G.M. and A.D.I.; visualization, L.F.; supervision, F.I.; project administration, A.M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The clinical study protocol was approved by the Ethics Committee of the University of Chieti (protocol code 638—21/3/19 and date of approval 21 March 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Abbreviation | Full Term |
| BMP-2 | Bone Morphogenetic Protein 2 |
| CBCT | Cone Beam Computed Tomography |
| CEA | Not explicitly defined in the text (possibly a type of implant or specific protocol) |
| EDS | Energy-dispersive X-ray Spectroscopy |
| FGF | Fibroblast Growth Factor |
| g | gram |
| GBR | Guided Bone Regeneration |
| HCl | Hydrochloric acid |
| H&E | Hematoxylin and Eosin |
| HIV | Human Immunodeficiency Virus |
| H2O2 | Hydrogen peroxide |
| N-RES | Non-Resorbable |
| OPGs | Orthopantomograms |
| PRF | Platelet-Rich Fibrin |
| RES | Resorbable |
| SEM | Scanning Electron Microscopy |
| SPSS | Statistical Package for the Social Sciences |
| TGF-β | Transforming Growth Factor-Beta |
| VEGF | Vascular Endothelial Growth Factor |
| X-ray | Radiograph |
References
- Buser, D.; Urban, I.; Monje, A.; Kunrath, M.F.; Dahlin, C. Guided Bone Regeneration in Implant Dentistry: Basic Principle, Progress over 35 Years, and Recent Research Activities. Periodontol 2000 2023, 93, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Romasco, T.; Tumedei, M.; Inchingolo, F.; Pignatelli, P.; Montesani, L.; Iezzi, G.; Petrini, M.; Piattelli, A.; Di Pietro, N. A Narrative Review on the Effectiveness of Bone Regeneration Procedures with OsteoBiol® Collagenated Porcine Grafts: The Translational Research Experience over 20 Years. J. Funct. Biomater. 2022, 13, 121. [Google Scholar] [CrossRef]
- Mellonig, J.T. Autogenous and Allogeneic Bone Grafts in Periodontal Therapy. Crit. Rev. Oral. Biol. Med. 1992, 3, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Serafini, G.; Lollobrigida, M.; Fortunato, L.; Mazzucchi, G.; Lamazza, L.; Di Nardo, D.; Vozza, I.; Riminucci, M.; De Biase, A. Postextractive Alveolar Ridge Preservation Using L-PRF: Clinical and Histological Evaluation. Case Rep. Dent. 2020, 2020, 5073519. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, G.; Herten, M.; Schwarz, F.; Rothamel, D.; Becker, J. Autogenous Bone Chips: Influence of a New Piezoelectric Device (Piezosurgery) on Chip Morphology, Cell Viability and Differentiation. J. Clin. Periodontol. 2005, 32, 994–999. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The Biology of Bone Grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- González del Pino, J.; Bartolomé del Valle, E.; Graña, G.L.; Villanova, J.F. Free Vascularized Fibular Grafts Have a High Union Rate in Atrophic Nonunions. Clin. Orthop. Relat. Res. 2004, 419, 38–45. [Google Scholar] [CrossRef]
- Duffy, G.P.; Wood, M.B.; Rock, M.G.; Sim, F.H. Vascularized Free Fibular Transfer Combined with Autografting for the Management of Fracture Nonunions Associated with Radiation Therapy. J. Bone Jt. Surg. Am. 2000, 82, 544–554. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Nampo, T.; Watahiki, J.; Enomoto, A.; Taguchi, T.; Ono, M.; Nakano, H.; Yamamoto, G.; Irie, T.; Tachikawa, T.; Maki, K. A New Method for Alveolar Bone Repair Using Extracted Teeth for the Graft Material. J. Periodontol. 2010, 81, 1264–1272. [Google Scholar] [CrossRef]
- Cochran, D.L.; Jones, A.; Heijl, L.; Mellonig, J.T.; Schoolfield, J.; King, G.N. Periodontal Regeneration with a Combination of Enamel Matrix Proteins and Autogenous Bone Grafting. J. Periodontol. 2003, 74, 1269–1281. [Google Scholar] [CrossRef]
- Koga, T.; Minamizato, T.; Kawai, Y.; Miura, K.; I, T.; Nakatani, Y.; Sumita, Y.; Asahina, I. Bone Regeneration Using Dentin Matrix Depends on the Degree of Demineralization and Particle Size. PLoS ONE 2016, 11, e0147235. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, J.; Zhong, X.; He, F.; Wu, X.; Shen, G. Demineralized Dentin Matrix Composite Collagen Material for Bone Tissue Regeneration. J. Biomater. Sci. Polym. Ed. 2013, 24, 1519–1528. [Google Scholar] [CrossRef]
- Fujii, Y.; Hatori, A.; Chikazu, D.; Ogasawara, T. Application of Dental Pulp Stem Cells for Bone and Neural Tissue Regeneration in Oral and Maxillofacial Region. Stem Cells Int. 2023, 2023, 2026572. [Google Scholar] [CrossRef]
- Mancini, A.; Chirico, F.; Colella, G.; Piras, F.; Colonna, V.; Marotti, P.; Carone, C.; Inchingolo, A.D.; Inchingolo, A.M.; Inchingolo, F.; et al. Evaluating the Success Rates and Effectiveness of Surgical and Orthodontic Interventions for Impacted Canines: A Systematic Review of Surgical and Orthodontic Interventions and a Case Series. BMC Oral. Health 2025, 25, 295. [Google Scholar] [CrossRef] [PubMed]
- Laforgia, A.; Inchingolo, A.M.; Inchingolo, F.; Sardano, R.; Trilli, I.; Di Noia, A.; Ferrante, L.; Palermo, A.; Inchingolo, A.D.; Dipalma, G. Paediatric Dental Trauma: Insights from Epidemiological Studies and Management Recommendations. BMC Oral. Health 2025, 25, 6. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, A.M.; Colonna, V.; Marotti, P.; Carone, C.; Ferrante, L.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D. Autologous and Heterologous Minor and Major Bone Regeneration with Platelet-Derived Growth Factors. J. Funct. Biomater. 2025, 16, 16. [Google Scholar] [CrossRef]
- Yoshida, T.; Vivatbutsiri, P.; Morriss-Kay, G.; Saga, Y.; Iseki, S. Cell Lineage in Mammalian Craniofacial Mesenchyme. Mech. Dev. 2008, 125, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Iseki, S.; Maxson, R.E.; Sucov, H.M.; Morriss-Kay, G.M. Tissue Origins and Interactions in the Mammalian Skull Vault. Dev. Biol. 2002, 241, 106–116. [Google Scholar] [CrossRef]
- Kapur, R.P. Colonization of the Murine Hindgut by Sacral Crest-Derived Neural Precursors: Experimental Support for an Evolutionarily Conserved Model. Dev. Biol. 2000, 227, 146–155. [Google Scholar] [CrossRef]
- Materials Design and Application of Demineralized Dentin/Apatite Composite Granules Derived from Human Teeth|Request PDF. Available online: https://www.researchgate.net/publication/287760820_Materials_design_and_application_of_demineralized_dentinapatite_composite_granules_derived_from_human_teeth (accessed on 30 March 2025).
- Murata, M.; Akazawa, T.; Mitsugi, M.; Arafat, M.; Um, I.-W.; Minamida, Y.; Kim, K.-W.; Kim, Y.-K.; Sun, Y.; Qi, C. Autograft of Dentin Materials for Bone Regeneration. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; InTech: Houston TX, USA, 2013; ISBN 978-953-51-1051-4. [Google Scholar]
- Murata, M.; Akazawa, T.; Mitsugi, M.; Um, I.W.; Kim, K.W.; Kim, Y.K. (PDF) Human Dentin as Novel Biomaterial for Bone Regeneration. In Biomaterials: Physics and Chemistry; ResearchGate: Berlin, Germany, 2011. [Google Scholar]
- Murata, M.; Kabir, M.A.; Hirose, Y.; Ochi, M.; Okubo, N.; Akazawa, T.; Kashiwazaki, H. Histological Evidences of Autograft of Dentin/Cementum Granules into Unhealed Socket at 5 Months after Tooth Extraction for Implant Placement. J. Funct. Biomater. 2022, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Um, I.-W. Advances in Oral Tissue Engineering; Quintessence Books; Quintessence Pub: Batavia, IL, USA, 2014. [Google Scholar]
- Murata: Advances in Biomaterials Sciences and Biomedical...-Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=Advances%20in%20Biomaterials%20Science%20and%20Biomedical%20Applications&author=M.%20Murata&author=T.%20Akazawa&author=M.%20Mitsugi&author=M.A.%20Kabir&author=Y.%20Minamida&publication_year=2013& (accessed on 30 March 2025).
- Kim, E.-S. Autogenous Fresh Demineralized Tooth Graft Prepared at Chairside for Dental Implant. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 8. [Google Scholar] [CrossRef][Green Version]
- Esposito, M.; Grusovin, M.G.; Rees, J.; Karasoulos, D.; Felice, P.; Alissa, R.; Worthington, H.V.; Coulthard, P. Interventions for Replacing Missing Teeth: Augmentation Procedures of the Maxillary Sinus. Cochrane Database Syst. Rev. 2010, CD008397. [Google Scholar] [CrossRef]
- Kim, E.-S.; Kang, J.-Y.; Kim, J.-J.; Kim, K.-W.; Lee, E.-Y. Space Maintenance in Autogenous Fresh Demineralized Tooth Blocks with Platelet-Rich Plasma for Maxillary Sinus Bone Formation: A Prospective Study. Springerplus 2016, 5, 274. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Polizzi, E.M.; Gherlone, E.F. Tissue Remodeling after Bone Expansion in Grafted and Ungrafted Sockets. Int. J. Oral. Maxillofac. Implant. 2014, 29, 699–704. [Google Scholar] [CrossRef]
- Crespi, R.; Capparè, P.; Gherlone, E. Dental Implants Placed in Extraction Sites Grafted with Different Bone Substitutes: Radiographic Evaluation at 24 Months. J. Periodontol. 2009, 80, 1616–1621. [Google Scholar] [CrossRef]
- Bittner, N.; Planzos, L.; Volchonok, A.; Tarnow, D.; Schulze-Späte, U. Evaluation of Horizontal and Vertical Buccal Ridge Dimensional Changes After Immediate Implant Placement and Immediate Temporization with and Without Bone Augmentation Procedures: Short-Term, 1-Year Results. A Randomized Controlled Clinical Trial. Int. J. Periodontics Restor. Dent. 2020, 40, 83–93. [Google Scholar] [CrossRef]
- Clementini, M.; Agostinelli, A.; Castelluzzo, W.; Cugnata, F.; Vignoletti, F.; De Sanctis, M. The Effect of Immediate Implant Placement on Alveolar Ridge Preservation Compared to Spontaneous Healing after Tooth Extraction: Radiographic Results of a Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2019, 46, 776–786. [Google Scholar] [CrossRef]
- Strauss, F.J.; Fukuba, S.; Naenni, N.; Jung, R.; Jonker, B.; Wolvius, E.; Pijpe, J. Alveolar Ridge Changes 1-Year after Early Implant Placement, with or without Alveolar Ridge Preservation at Single-Implant Sites in the Aesthetic Region: A Secondary Analysis of Radiographic and Profilometric Outcomes from a Randomized Controlled Trial. Clin. Implant. Dent. Relat. Res. 2024, 26, 356–368. [Google Scholar] [CrossRef]
- Mardas, N.; Trullenque-Eriksson, A.; MacBeth, N.; Petrie, A.; Donos, N. Does Ridge Preservation Following Tooth Extraction Improve Implant Treatment Outcomes: A Systematic Review: Group 4: Therapeutic Concepts & Methods. Clin. Oral. Implant. Res. 2015, 26 (Suppl. S11), 180–201. [Google Scholar] [CrossRef]
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic Review of the Survival Rate and the Incidence of Biological, Technical, and Aesthetic Complications of Single Crowns on Implants Reported in Longitudinal Studies with a Mean Follow-up of 5 Years. Clin. Oral. Implant. Res. 2012, 23 (Suppl. S6), 2–21. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, I.; deLlanos-Lanchares, H.; Brizuela-Velasco, A.; Alvarez-Riesgo, J.-A.; Llorente-Pendas, S.; Herrero-Climent, M.; Alvarez-Arenal, A. Complications of Fixed Full-Arch Implant-Supported Metal-Ceramic Prostheses. Int. J. Env. Res. Public. Health 2020, 17, 4250. [Google Scholar] [CrossRef]
- Compton, S.M.; Clark, D.; Chan, S.; Kuc, I.; Wubie, B.A.; Levin, L. Dental Implants in the Elderly Population: A Long-Term Follow-Up. Int. J. Oral. Maxillofac. Implant. 2017, 32, 164–170. [Google Scholar] [CrossRef]
- Manz, M.C. Factors Associated with Radiographic Vertical Bone Loss around Implants Placed in a Clinical Study. Ann. Periodontol. 2000, 5, 137–151. [Google Scholar] [CrossRef]
- Maiorana, C.; Poli, P.P.; Deflorian, M.; Testori, T.; Mandelli, F.; Nagursky, H.; Vinci, R. Alveolar Socket Preservation with Demineralised Bovine Bone Mineral and a Collagen Matrix. J. Periodontal Implant. Sci. 2017, 47, 194–210. [Google Scholar] [CrossRef]
- Minetti, E.; Corbella, S.; Taschieri, S.; Canullo, L. Tooth as Graft Material: Histologic Study. Clin. Implant. Dent. Relat. Res. 2022, 24, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Hämmerle, C.H.F.; Jung, R.E.; Feloutzis, A. A Systematic Review of the Survival of Implants in Bone Sites Augmented with Barrier Membranes (Guided Bone Regeneration) in Partially Edentulous Patients. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 226–231; discussion 232–233. [Google Scholar] [CrossRef] [PubMed]
- Memè, L.; Bambini, F.; Pizzolante, T.; Sampalmieri, F.; Bianchi, A.; Mummolo, S. Evaluation of a Single Non-Surgical Approach in the Management of Peri-Implantitis: Glycine Powder Air-Polishing versus Ultrasonic Device. Oral. Implantol. A J. Innov. Adv. Tech. Oral. Health 2024, 16, 67–78. [Google Scholar] [CrossRef]
- Favia, G.; Corsalini, M.; Di Venere, D.; Pettini, F.; Favia, G.; Capodiferro, S.; Maiorano, E. Immunohistochemical Evaluation of Neuroreceptors in Healthy and Pathological Temporo-Mandibular Joint. Int. J. Med. Sci. 2013, 10, 1698–1701. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Pezzolla, C.; Patano, A.; Ceci, S.; Ciocia, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Cardarelli, F.; Piras, F.; et al. Experimental Analysis of the Use of Cranial Electromyography in Athletes and Clinical Implications. Int. J. Env. Res. Public. Health 2022, 19, 7975. [Google Scholar] [CrossRef] [PubMed]
- Khouly, I.; Veitz-Keenan, A. Insufficient Evidence for Sinus Lifts over Short Implants for Dental Implant Rehabilitation. Evid. Based Dent. 2015, 16, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Moscatelli, M.; Mariotti, G.; Pagliaro, U.; Raffaelli, E.; Nieri, M. Comparing Membranes and Bone Substitutes in a One-Stage Procedure for Horizontal Bone Augmentation. A Double-Blind Randomised Controlled Trial. Eur. J. Oral. Implant. 2015, 8, 271–281. [Google Scholar]
- Naenni, N.; Stucki, L.; Hüsler, J.; Schneider, D.; Hämmerle, C.H.F.; Jung, R.E.; Thoma, D.S. Implants Sites with Concomitant Bone Regeneration Using a Resorbable or Non-Resorbable Membrane Result in Stable Marginal Bone Levels and Similar Profilometric Outcomes over 5 Years. Clin. Oral. Implant. Res. 2021, 32, 893–904. [Google Scholar] [CrossRef]
- Minetti, E.; Celko, M.; Contessi, M.; Carini, F.; Gambardella, U.; Giacometti, E.; Santillana, J.; Beca Campoy, T.; Schmitz, J.H.; Libertucci, M.; et al. Implants Survival Rate in Regenerated Sites with Innovative Graft Biomaterials: 1 Year Follow-Up. Materials 2021, 14, 5292. [Google Scholar] [CrossRef]
- Amruthesh, S. Dentistry and Ayurveda—IV: Classification and Management of Common Oral Diseases. Indian. J. Dent. Res. 2008, 19, 52–61. [Google Scholar] [CrossRef]
- Anavi, Y.; Gal, G.; Silfen, R.; Calderon, S. Palatal Rotation-Advancement Flap for Delayed Repair of Oroantral Fistula: A Retrospective Evaluation of 63 Cases. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontology 2003, 96, 527–534. [Google Scholar] [CrossRef]
- Abreu, R.R.; Rocha, R.L.; Lamounier, J.A.; Guerra, A.F.M. Etiology, Clinical Manifestations and Concurrent Findings in Mouth-Breathing Children. J. Pediatr. (Rio J.) 2008, 84, 529–535. [Google Scholar] [CrossRef]
- Aguilera, S.B.; Brown, L.; Perico, V.A. Aesthetic Treatment of Bruxism. J. Clin. Aesthet. Dermatol. 2017, 10, 49–55. [Google Scholar]
- Agustin, T.P.; Sutadi, H.; Bachtiar, B.M.; Rizal, M.F. Proportion of Streptococcus Mutans, Streptococcus Sanguinis, and Candida Albicans in Early Childhood Caries: Evaluation by qPCR. Open Dent. J. 2024, 18, e18742106290568. [Google Scholar] [CrossRef]
- Aizenbud, D.; Peled, M.; Figueroa, A.A. A Combined Orthodontic and Surgical Approach in Osteogenesis Imperfecta and Severe Class III Malocclusion: Case Report. J. Oral. Maxillofac. Surg. 2008, 66, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, V.; Myasoedova, V.A.; Vinci, M.C.; Rondinelli, M.; Songia, P.; Massaiu, I.; Cosentino, N.; Moschetta, D.; Valerio, V.; Ciccarelli, M.; et al. The Role of Glycemic Variability in Cardiovascular Disorders. Int. J. Mol. Sci. 2021, 22, 8393. [Google Scholar] [CrossRef] [PubMed]
- AlSarhan, M.; AlJasser, R.; AlOraini, S.; Alotaibi, D.H.; Alsinaidi, A.A.; Habib, S.R. Relationship of Self-Perceived Stress and Expression of Salivary Cortisol in Relation to Gender and Academic Levels among Dental Students. TODENTJ 2024, 18, e18742106282255. [Google Scholar] [CrossRef]
- Alzahabi, R.; Becker, M.W. The Association between Media Multitasking, Task-Switching, and Dual-Task Performance. J. Exp. Psychol. Hum. Percept. Perform. 2013, 39, 1485–1495. [Google Scholar] [CrossRef]
- Amruthesh, S. Dentistry and Ayurveda--III (Basics-Ama, Immunity, Ojas, Rasas, Etiopathogenesis and Prevention). Indian. J. Dent. Res. 2007, 18, 112–119. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Semjonova, A.; Inchingolo, A.M.; Patano, A.; Coloccia, G.; Ceci, S.; Marinelli, G.; Di Pede, C.; Ciocia, A.M.; et al. Oralbiotica/Oralbiotics: The Impact of Oral Microbiota on Dental Health and Demineralization: A Systematic Review of the Literature. Children 2022, 9, 1014. [Google Scholar] [CrossRef]
- Magrin, G.L.; Rafael, S.N.F.; Passoni, B.B.; Magini, R.S.; Benfatti, C.A.M.; Gruber, R.; Peruzzo, D.C. Clinical and Tomographic Comparison of Dental Implants Placed by Guided Virtual Surgery versus Conventional Technique: A Split-Mouth Randomized Clinical Trial. J. Clin. Periodontol. 2020, 47, 120–128. [Google Scholar] [CrossRef]
- Kernen, F.; Kramer, J.; Wanner, L.; Wismeijer, D.; Nelson, K.; Flügge, T. A Review of Virtual Planning Software for Guided Implant Surgery-Data Import and Visualization, Drill Guide Design and Manufacturing. BMC Oral. Health 2020, 20, 251. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Mancini, A.; Inchingolo, F.; Inchingolo, A.D.; Di Venere, D.; Dipalma, G.; et al. Damage from Carbonated Soft Drinks on Enamel: A Systematic Review. Nutrients 2023, 15, 1785. [Google Scholar] [CrossRef]
- Bevilacqua, L.; Lorenzon, M.G.; Bjedov, M.; Costantinides, F.; Angerame, D.; Maglione, M. Evaluation of the Efficacy of Inter-Dental Brush and Dental Floss for Peri-Implant Mucositis: A Crossover Randomized Clinical Trial. Int. J. Dent. Hyg. 2024, 22, 779–788. [Google Scholar] [CrossRef]
- Bae, S.-H.; Fabry, D. Assessing the Relationships between Nurse Work Hours/Overtime and Nurse and Patient Outcomes: Systematic Literature Review. Nurs. Outlook 2014, 62, 138–156. [Google Scholar] [CrossRef]
- Banning, J.A. Chronic fatigue and shift work. Can. Nurse 1991, 87, 3. [Google Scholar] [PubMed]
- Barton, J.; Spelten, E.R.; Smith, L.R.; Totterdell, P.A.; Folkard, S. A Classification of Nursing and Midwifery Shift Systems. Int. J. Nurs. Stud. 1993, 30, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Beckman, R.J.; Hutton, S.; Czekanski, E.; Vance, K.; Mohr, D.C. A Comparison of Shift Length and Nursing and Quality Outcomes in Acute Inpatient Mental Health Units. J. Nurs. Adm. 2022, 52, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Hobbs, B.B. Impact of Shift Work on the Health and Safety of Nurses and Patients. Clin. J. Oncol. Nurs. 2006, 10, 465–471. [Google Scholar] [CrossRef]
- Blake, N. Anmf Participates in International Nursing Symposium on Shift Work. Aust. Nurs. Midwifery J. 2017, 24, 11. [Google Scholar]
- Cassetta, M.; Di Giorgio, R.; Barbato, E. Are Intraoral Radiographs Accurate in Determining the Peri-Implant Marginal Bone Level? Int. J. Oral. Maxillofac. Implant. 2018, 33, 847–852. [Google Scholar] [CrossRef]
- Gher, M.E. Changing Concepts. The Effects of Occlusion on Periodontitis. Dent. Clin. North. Am. 1998, 42, 285–299. [Google Scholar] [CrossRef]
- Serio, F.G.; Hawley, C.E. Periodontal Trauma and Mobility. Diagnosis and Treatment Planning. Dent. Clin. North. Am. 1999, 43, 37–44. [Google Scholar] [CrossRef]
- Anger, J.T.; Weinberg, A.; Suttorp, M.J.; Litwin, M.S.; Shekelle, P.G. Outcomes of Intravesical Botulinum Toxin for Idiopathic Overactive Bladder Symptoms: A Systematic Review of the Literature. J. Urol. 2010, 183, 2258–2264. [Google Scholar] [CrossRef]
- Aravinth, V.; Aswath Narayanan, M.B.; Ramesh Kumar, S.G.; Selvamary, A.L.; Sujatha, A. Comparative Evaluation of Salt Water Rinse with Chlorhexidine against Oral Microbes: A School-Based Randomized Controlled Trial. J. Indian. Soc. Pedod. Prev. Dent. 2017, 35, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Kaur, N.; Kaur, G.; Garg, U. Knowledge, Awareness, and Attitude in Using Dental Implants as an Option in Replacing Missing Teeth Among Dental Patients: Survey-Based Research in a Dental Teaching Hospital in Derabassi, Punjab. Cureus 2022, 14, e27127. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Cantore, S.; Inchingolo, A.; Inchingolo, F.; Di Domenico, M.; Quagliuolo, L.; Boccellino, M. Another Look at Dietary Polyphenols: Challenges in Cancer Prevention and Treatment. Curr. Med. Chem. 2022, 29, 1061–1082. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Alsabeeha, N.H.; Payne, A.G.; Ali, S.; Faggion, C.M.J.; Esposito, M. Interventions for Replacing Missing Teeth: Alveolar Ridge Preservation Techniques for Dental Implant Site Development. Cochrane Database Syst. Rev. 2021, 4, CD010176. [Google Scholar] [CrossRef]
- Avvanzo, P.; Ciavarella, D.; Avvanzo, A.; Giannone, N.; Carella, M.; Lo Muzio, L. Immediate Placement and Temporization of Implants: Three- to Five-Year Retrospective Results. J. Oral. Implant. 2009, 35, 136–142. [Google Scholar] [CrossRef]
- Benvenuti, M.; Wright, M.; Naslund, J.; Miers, A.C. How Technology Use Is Changing Adolescents’ Behaviors and Their Social, Physical, and Cognitive Development. Curr. Psychol. 2023, 42, 16466–16469. [Google Scholar] [CrossRef]
- Bertelli, M.; Bonetti, G.; Donato, K.; Medori, M.C.; Dhuli, K.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; et al. In Memory of Professor Derek Pheby. Clin. Ter. 2023, 174, 227–229. [Google Scholar] [CrossRef]
- Memè, L.; Pizzolante, T.; Saggiomo, A.P.; Plaku, D.; Inchingolo, A.D.; Inchingolo, F.; Rastelli, S. The Use of Ozone Therapy for the Treatment and Post-Surgical Management of Patients Treated with Bilateral Extraction of the Included Third Mandibular Molars. Oral. Implantol. A J. Innov. Adv. Tech. Oral. Health 2024, 16, 124–132. [Google Scholar] [CrossRef]
- Menon, R.K.; Gomez, A.; Brandt, B.W.; Leung, Y.Y.; Gopinath, D.; Watt, R.M.; Crielaard, W.; Nelson, K.E.; Botelho, M.G. Long-Term Impact of Oral Surgery with or without Amoxicillin on the Oral Microbiome-A Prospective Cohort Study. Sci. Rep. 2019, 9, 18761. [Google Scholar] [CrossRef]
- Minervini, G.; Del Mondo, D.; Russo, D.; Cervino, G.; D’Amico, C.; Fiorillo, L. Stem Cells in Temporomandibular Joint Engineering: State of Art and Future Persectives. J. Craniofac. Surg. 2022, 33, 2181–2187. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Mehta, V.; Fiorillo, L.; Badnjević, A.; Cervino, G.; Cicciù, M. Gaucher: A Systematic Review on Oral and Radiological Aspects. Medicina 2023, 59, 670. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Crimi, S.; Badnjević, A.; Cervino, G.; Bianchi, A.; Cicciù, M. Correlation between Temporomandibular Disorders (TMD) and Posture Evaluated Trough the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): A Systematic Review with Meta-Analysis. J. Clin. Med. 2023, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Economic Inequalities and Temporomandibular Disorders: A Systematic Review with Meta-Analysis. J. Oral. Rehabil. 2023, 50, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Russo, D.; Herford, A.S.; Gorassini, F.; Meto, A.; D’Amico, C.; Cervino, G.; Cicciù, M.; Fiorillo, L. Teledentistry in the Management of Patients with Dental and Temporomandibular Disorders. Biomed. Res. Int. 2022, 2022, 7091153. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Inchingolo, A.D.; Patano, A.; Viapiano, F.; Ciocia, A.M.; de Ruvo, E.; Mancini, A.; Inchingolo, F.; Sauro, S.; et al. Autologous Tooth for Bone Regeneration: Dimensional Examination of Tooth Transformer® Granules. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5421–5430. [Google Scholar] [CrossRef] [PubMed]
- Moergel, M.; Rocha, S.; Messias, A.; Nicolau, P.; Guerra, F.; Wagner, W. Radiographic Evaluation of Conical Tapered Platform-switched Implants in the Posterior Mandible: 1-year Results of a Two-center Prospective Study. Clin. Oral. Implant. Res. 2016, 27, 686–693. [Google Scholar] [CrossRef]
- Duarte, F.; Ramos, C.; Santos-Marino, J.; Martínez-Rodriguez, N.; Barona-Dorado, C.; Martínez-González, J.M. Bone Resorption Assessment Following Zygomatic Implants Surgery over 10 Years of Follow-Up. J. Clin. Med. 2025, 14, 989. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Rumpler, M.; Würger, T.; Roschger, P.; Zwettler, E.; Sturmlechner, I.; Altmann, P.; Fratzl, P.; Rogers, M.J.; Klaushofer, K. Osteoclasts on Bone and Dentin in Vitro: Mechanism of Trail Formation and Comparison of Resorption Behavior. Calcif. Tissue Int. 2013, 93, 526–539. [Google Scholar] [CrossRef]
- Buser, D.; Dula, K.; Lang, N.P.; Nyman, S. Long-Term Stability of Osseointegrated Implants in Bone Regenerated with the Membrane Technique. 5-Year Results of a Prospective Study with 12 Implants. Clin. Oral. Implant. Res. 1996, 7, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Simion, M.; Jovanovic, S.A.; Tinti, C.; Benfenati, S.P. Long-Term Evaluation of Osseointegrated Implants Inserted at the Time or after Vertical Ridge Augmentation. A Retrospective Study on 123 Implants with 1–5 Year Follow-Up. Clin. Oral. Implant. Res. 2001, 12, 35–45. [Google Scholar] [CrossRef]
- Canullo, L.; Sisti, A. Early Implant Loading after Vertical Ridge Augmentation (VRA) Using e-PTFE Titanium-Reinforced Membrane and Nano-Structured Hydroxyapatite: 2-Year Prospective Study. Eur. J. Oral. Implant. 2010, 3, 59–69. [Google Scholar]
- Canullo, L.; Iannello, G.; Peñarocha, M.; Garcia, B. Impact of Implant Diameter on Bone Level Changes around Platform Switched Implants: Preliminary Results of 18 Months Follow-up a Prospective Randomized Match-Paired Controlled Trial. Clin. Oral. Implant. Res. 2012, 23, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Wagner, W.; Wiltfang, J.; Nicolau, P.; Moergel, M.; Messias, A.; Behrens, E.; Guerra, F. Effect of Platform Switching on Crestal Bone Levels around Implants in the Posterior Mandible: 3 Years Results from a Multicentre Randomized Clinical Trial. J. Clin. Periodontol. 2016, 43, 374–382. [Google Scholar] [CrossRef]
- Cumbo, C.; Marigo, L.; Somma, F.; La Torre, G.; Minciacchi, I.; D’Addona, A. Implant Platform Switching Concept: A Literature Review. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 392–397. [Google Scholar] [PubMed]
- Moon, S.-Y.; Lim, Y.-J.; Kim, M.-J.; Kwon, H.-B. Three-Dimensional Finite Element Analysis of Platform Switched Implant. J. Adv. Prosthodont. 2017, 9, 31–37. [Google Scholar] [CrossRef]
- Chiapasco, M.; Tommasato, G.; Palombo, D.; Del Fabbro, M. A Retrospective 10-Year Mean Follow-up of Implants Placed in Ridges Grafted Using Autogenous Mandibular Blocks Covered with Bovine Bone Mineral and Collagen Membrane. Clin. Oral. Implant. Res. 2020, 31, 328–340. [Google Scholar] [CrossRef]
- Tang, Y.L.; Yuan, J.; Song, Y.L.; Ma, W.; Chao, X.; Li, D.H. Ridge Expansion Alone or in Combination with Guided Bone Regeneration to Facilitate Implant Placement in Narrow Alveolar Ridges: A Retrospective Study. Clin. Oral Implant. Res. 2024, 26, 204–211. [Google Scholar] [CrossRef]
- Naenni, N.; Schneider, D.; Jung, R.E.; Hüsler, J.; Hämmerle, C.H.F.; Thoma, D.S. Randomized Clinical Study Assessing Two Membranes for Guided Bone Regeneration of Peri-Implant Bone Defects: Clinical and Histological Outcomes at 6 Months. Clin. Oral. Implant. Res. 2017, 28, 1309–1317. [Google Scholar] [CrossRef]
- Comparative Histological Analysis of Dentine-Derived Tooth Grafts in Maxillary vs Mandibular Socket Preservation: A Retrospective Study of 178 Cases. Available online: https://www.mdpi.com/2304-6767/12/10/320 (accessed on 30 March 2025).
- Pang, K.-M.; Um, I.-W.; Kim, Y.-K.; Woo, J.-M.; Kim, S.-M.; Lee, J.-H. Autogenous Demineralized Dentin Matrix from Extracted Tooth for the Augmentation of Alveolar Bone Defect: A Prospective Randomized Clinical Trial in Comparison with Anorganic Bovine Bone. Clin. Oral. Implant. Res. 2017, 28, 809–815. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Piras, F.; Ferrante, L.; Mancini, A.; Palermo, A.; Inchingolo, A.D.; Dipalma, G. Management of Patients Receiving Anticoagulation Therapy in Dental Practice: A Systematic Review. Healthcare 2024, 12, 1537. [Google Scholar] [CrossRef] [PubMed]
- Vervaeke, S.; Collaert, B.; Cosyn, J.; Deschepper, E.; De Bruyn, H. A Multifactorial Analysis to Identify Predictors of Implant Failure and Peri-Implant Bone Loss. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. S1), e298–e307. [Google Scholar] [CrossRef]
- DeLuca, S.; Zarb, G. The Effect of Smoking on Osseointegrated Dental Implants. Part II: Peri-Implant Bone Loss. Int. J. Prosthodont. 2006, 19, 560–566. [Google Scholar]
- Wennström, J.L.; Ekestubbe, A.; Gröndahl, K.; Karlsson, S.; Lindhe, J. Oral Rehabilitation with Implant-Supported Fixed Partial Dentures in Periodontitis-Susceptible Subjects. A 5-Year Prospective Study. J. Clin. Periodontol. 2004, 31, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Casu, C.; Mannu, C. Atypical Afta Major Healing after Photodynamic Therapy. Case Rep. Dent. 2017, 2017, 8517470. [Google Scholar] [CrossRef] [PubMed]
- Cecchinato, D.; Olsson, C.; Lindhe, J. Submerged or Non-Submerged Healing of Endosseous Implants to Be Used in the Rehabilitation of Partially Dentate Patients. J. Clin. Periodontol. 2004, 31, 299–308. [Google Scholar] [CrossRef]
- Krennmair, G.; Krainhöfner, M.; Weinländer, M.; Piehslinger, E. Provisional Implants for Immediate Restoration of Partially Edentulous Jaws: A Clinical Study. Int. J. Oral. Maxillofac. Implant. 2008, 23, 717–725. [Google Scholar]
- del Castillo, R.; Drago, C. Indexing and Provisional Restoration of Single Implants. J. Oral. Maxillofac. Surg. 2005, 63, 11–21. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. [Google Scholar] [CrossRef]
- Palla, G.; Fischer, D.S.; Regev, A.; Theis, F.J. Spatial Components of Molecular Tissue Biology. Nat. Biotechnol. 2022, 40, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Olchowy, A.; Olchowy, C.; Zawiślak, I.; Matys, J.; Dobrzyński, M. Revolutionizing Bone Regeneration with Grinder-Based Dentin Biomaterial: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 9583. [Google Scholar] [CrossRef]
- Bakhshalian, N.; Hooshmand, S.; Campbell, S.C.; Brummel-Smith, K.; Arjmandi, B.H. Biocompatibility and Microstructural Analysis of Osteopromotive Property of Allogenic Demineralized Dentin Matrix|Request PDF. Int. J. Oral Maxillofac. Implant. 2024, 28, 1655. [Google Scholar] [CrossRef]
- Manes, T.J.; DeGenova, D.T.; Taylor, B.C.; Patel, J.N. Far posterior approach for rib fracture fixation: Surgical technique and tips. JBJS Essent Surg Tech. 2024, 14, e23.00094. [Google Scholar] [CrossRef]
- Mourad, K.E.; Rashed, N.H.A.H.; Altonbary, G.Y.; Fattah Hegazy, S.A. Five Years of Radiographic Evaluation for the Peri-Implant Bone Changes of All-on-Four Implant Prostheses Constructed from Different Framework Materials Using Different Digital Construction Techniques. BMC Oral. Health 2024, 24, 910. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-Y.; Kim, E.-S.; Kim, K.-W. Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy Studies on Processed Tooth Graft Material by Vacuum-Ultrasonic Acceleration. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 103–110. [Google Scholar] [CrossRef]
- Sun, H.; Yin, X.; Yang, C.; Kuang, H.; Luo, W. Advances in Autogenous Dentin Matrix Graft as a Promising Biomaterial for Guided Bone Regeneration in Maxillofacial Region: A Review. Medicine 2024, 103, e39422. [Google Scholar] [CrossRef]
- Sapoznikov, L.; Humphrey, M. (PDF) Progress in Dentin-Derived Bone Graft Materials: A New Xenogeneic Dentin-Derived Material with Retained Organic Component Allows for Broader and Easier Application. Cells 2024, 13, 1806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).