Comparative Histological Analysis of Dentine-Derived Tooth Grafts in Maxillary vs Mandibular Socket Preservation: A Retrospective Study of 178 Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Inclusion Criteria
- Subjects who underwent surgical intervention for tooth removal and alveolar socket preservation using exclusively a tooth-derived bone substitute (Tooth Transformer®—Tooth Transformer SRL).
- Subjects who did not have any systemic diseases or conditions that could impair bone metabolism.
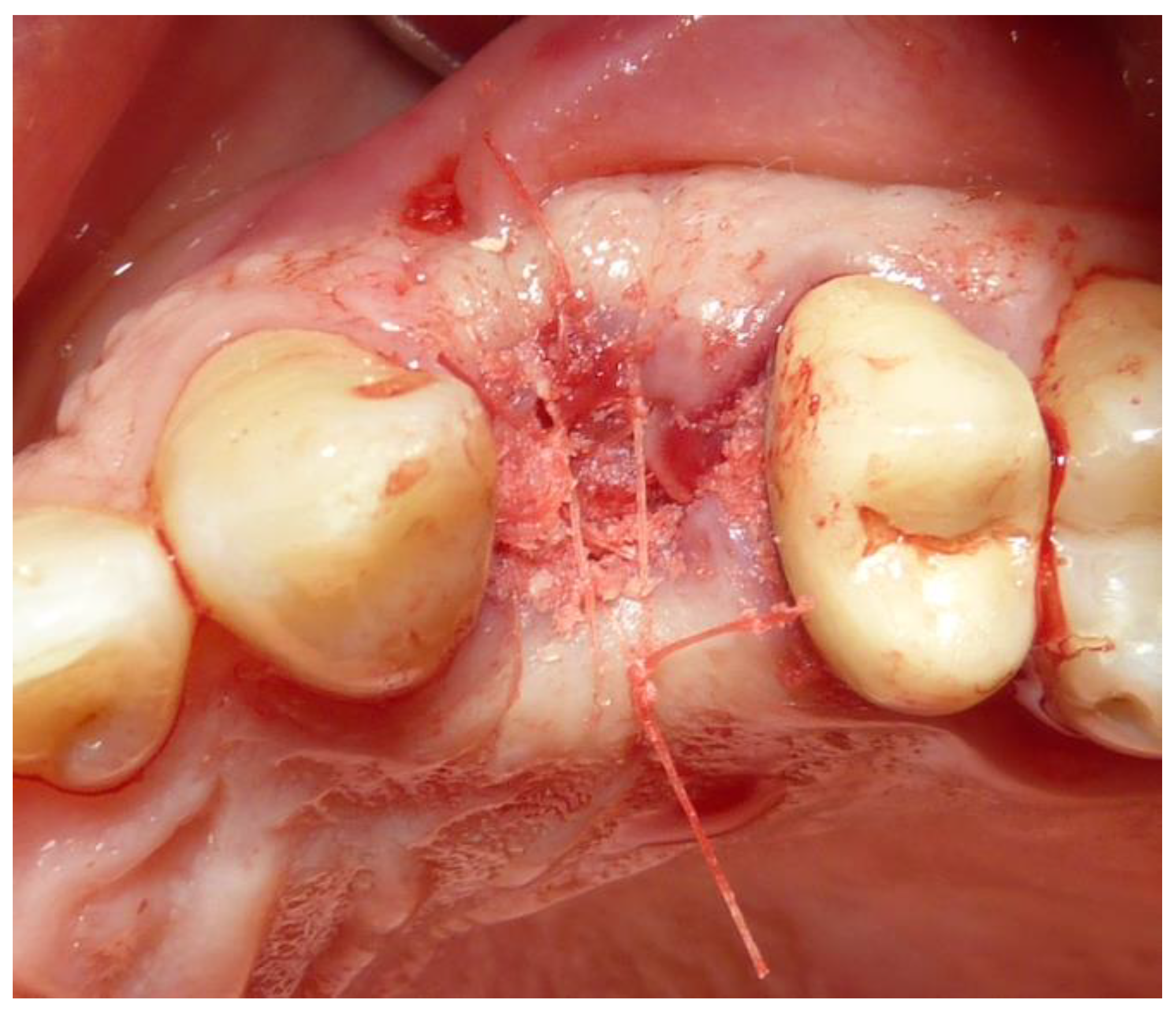
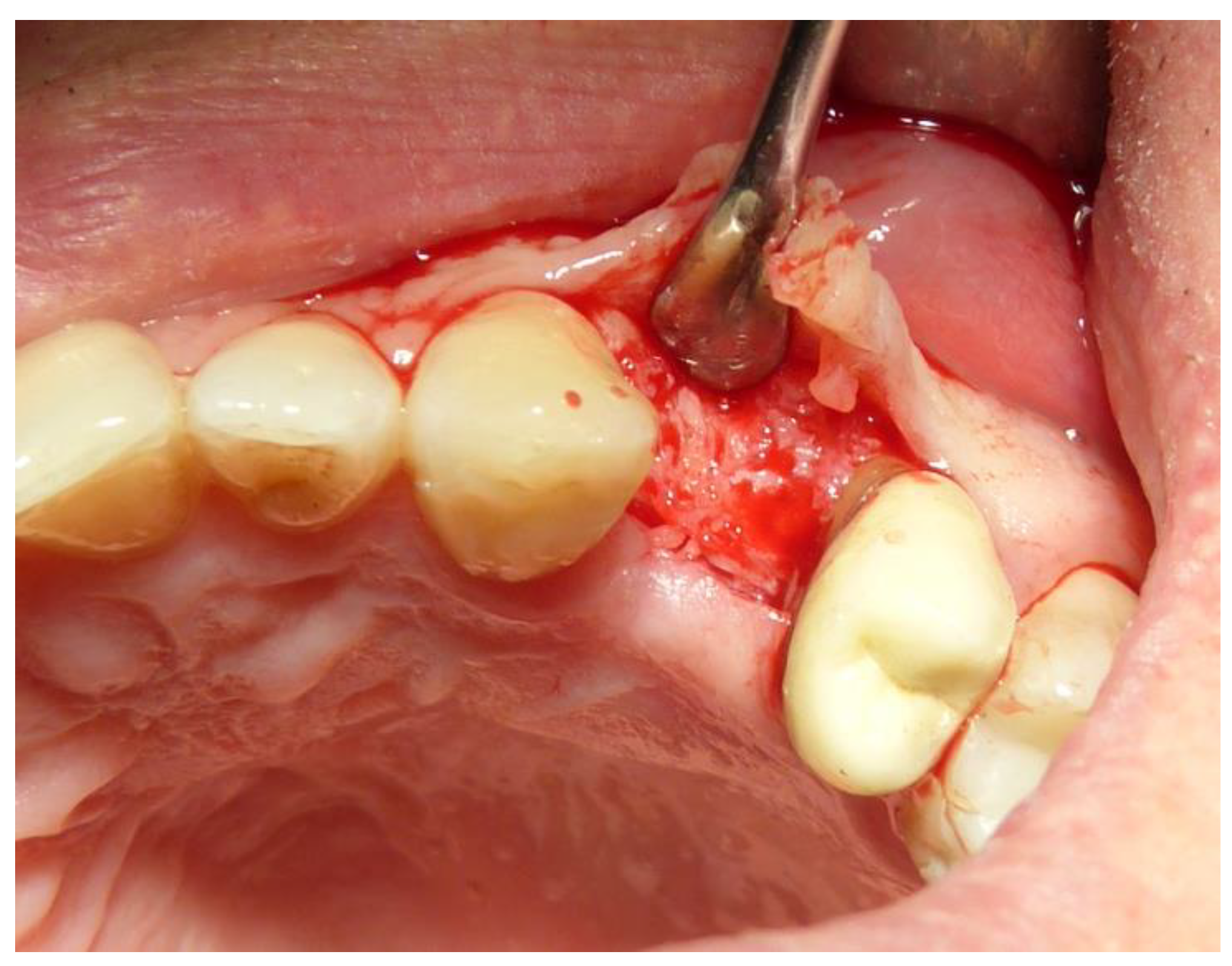
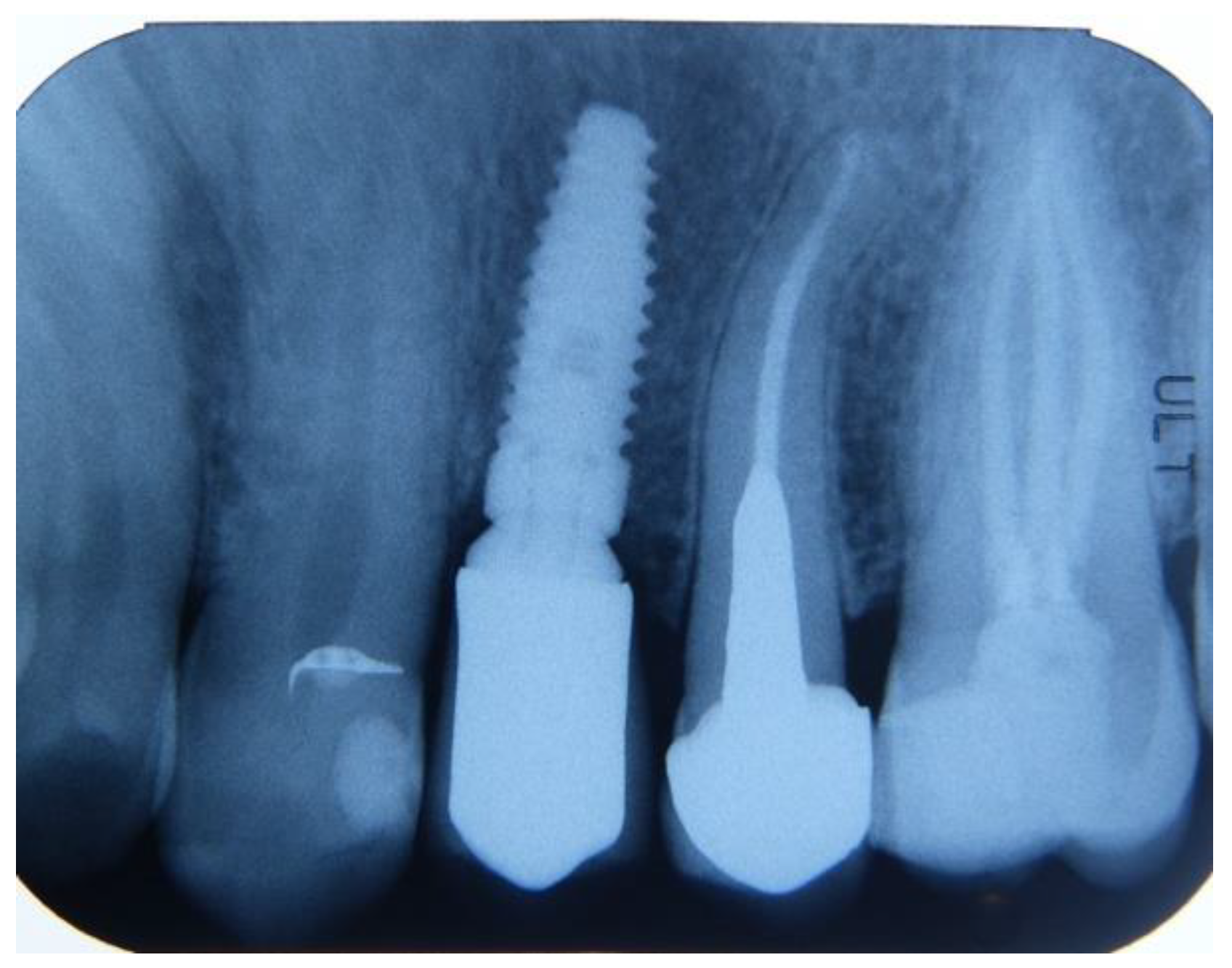
2.3. Preoperative and Surgical Procedures
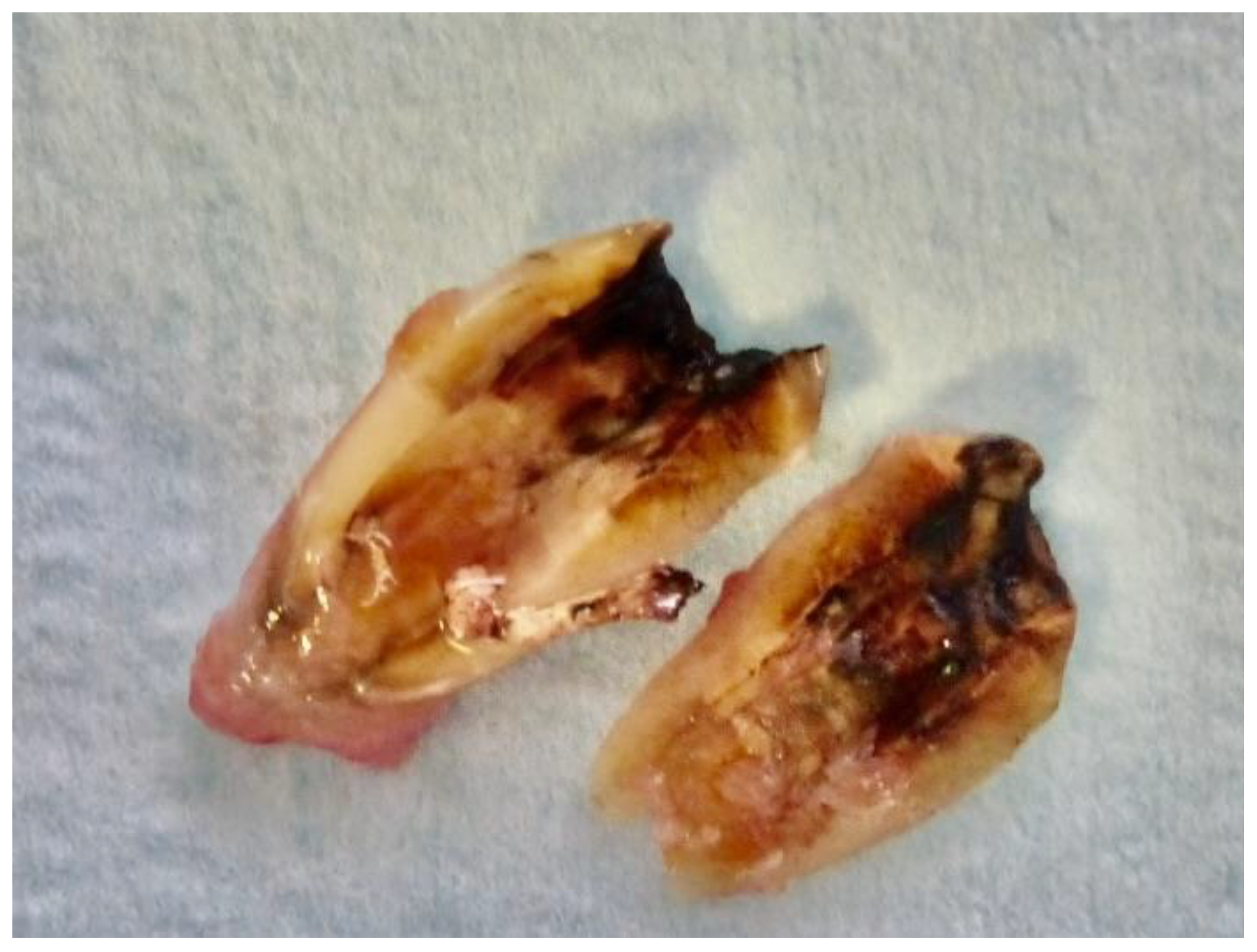
2.4. Sample Preparation
- BV%: the percentage of mineralized tissue, excluding medullary tissues.
- TT%: the percentage of volume occupied by residual graft material, primarily dentin.
- VB%: the percentage of vital bone, excluding medullary tissues.
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Ten- Heggeler, J.M.; De Van DerWeijden, G.A. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans. A systematic review. Clin. Oral Implants Res. 2011, 22, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Chan, H.L.; Galindo-Moreno, P.; Elnayef, B.; Suarez-Lopez del Amo, F.; Wang, F.; Wang, H.L. Alveolar Bone Architecture: A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Elian, N.; Cho, S.C.; Froum, S.; Smith, R.B.; Tarnow, D.P. A simplified socket classification and repair technique. Pract. Proced. Aesthet. Dent. 2007, 19, 99–104. [Google Scholar]
- Chu, S.J.; Hochman, M.N.; Tarnow, D.P. Subclassification and clinical management of extraction sockets with labial dentoalveolar dehiscence defects. Compend. Contin. Educ. Dent. 2015, 36, 516–522. [Google Scholar]
- Khoury, F.; Hanser, T. Three-Dimensional Vertical Alveolar Ridge Augmentation in the Posterior Maxilla: A 10-year Clinical Study. Int. J. Oral Maxillofac. Implant 2019, 34, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Taschieri, S.; Francetti, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric results after postextraction socket healing with different biomaterials: A systematic review of the literature and meta-analysis. Int. J. Oral Maxilofac. Implants 2017, 32, 1001–1017. [Google Scholar] [CrossRef]
- Yeomans, J.D.; Urist, M.R. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch. Oral Biol. 1967, 12, 999–1008. [Google Scholar] [CrossRef]
- Minetti, E.; Giacometti, E.; Gambardella, U.; Contessi, M.; Ballini, A.; Marenzi, G.; Celko, M.; Mastrangelo, F. Alveolar Socket Preservation with Different Autologous Graft Materials: Preliminary Results of a Multicenter Pilot Study in Human. Materials 2020, 13, 1153. [Google Scholar] [CrossRef]
- Minetti, E.; Celko, M.; Contessi, M.; Carini, F.; Gambardella, U.; Giacometti, E.; Santillana, J.; Beca Campoy, T.; Schmitz, J.H.; Libertucci, M.; et al. Implants Survival Rate in Regenerated Sites with Innovative Graft Biomaterials: 1 Year Follow-Up. Materials 2021, 14, 5292. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Savadori, P.; Barlattani, A.; Franco, R., Jr.; Michele, M.; Gianfreda, F.; Bollero, P. Autologous tooth graft: A histological comparison between dentin mixed with xenograft and dentin alone grafts in socket preservation. J. Biol. Regul. Homeost. Agents 2019, 33 (Suppl. S2), 189. [Google Scholar]
- Minetti, E.; Gianfreda, F.; Palermo, A.; Bollero, P. Autogenous Dentin Particulate Graft for Alveolar Ridge Augmentation with and without Use of Collagen Membrane: Preliminary Histological Analysis on Humans. Materials 2022, 15, 4319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human Dentin-Matrix-Derived Bone Morphogenetic Protein. J. Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Hsu, J.T.; Fuh, L.J.; Peng, S.L.; Huang, H.L.; Tsai, M.T. New classification for bone type at dental implant sites: A dental computed tomography study. BMC Oral Health 2023, 23, 324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hashemi, S.; Tabatabaei, S.; Fathi, A.; Asadinejad, S.M.; Atash, R. Tooth graft: An umbrella overview. Eur. J. Dent. 2023, 18, 041–054. [Google Scholar] [CrossRef]
- Umebayashi, M.; Ohba, S.; Kurogi, T.; Noda, S.; Asahina, I. Full regeneration of maxillary alveolar bone using autogenous partially demineralized dentin matrix and particulate cancellous bone and marrow for implant-supported full arch rehabilitation. J. Oral Implantol. 2020, 46, 122–127. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Qi, Y.; Ma, X.; Qiao, S.; Cai, H.; Zhao, B.C.; Jiang, H.B.; Lee, E.-S. Comparison of Autogenous Tooth Materials and Other Bone Grafts. Tissue Eng. Regen. Med. 2021, 18, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ku, J.K.; Um, I.W.; Seok, H.; Leem, D.H. Impact of Autogenous Demineralized Dentin Matrix on Mandibular Second Molar after Third Molar Extraction: Retrospective Study. J. Funct. Biomater. 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.K.; Kim, S.G.; Oh, J.S.; Jin, S.C.; Son, J.S.; Kim, S.Y.; Lim, S.Y. Analysis of the Inorganic Component of Autogenous Tooth Bone Graft Material. J. Nanosci. Nanotechnol. 2011, 11, 7442–7445. [Google Scholar] [CrossRef]
- Kotze, M.J.; Bütow, K.W.; Olorunju, S.A.; Kotze, H.F. A comparison of mandibular and maxillary alveolar osteogenesis over six weeks: A radiological examination. Head Face Med. 2021, 10, 50. [Google Scholar] [CrossRef]
- Huja, S.S.; Fernandez, S.A.; Hill, K.J.; Li, Y. Remodeling dynamics in the alveolar process and basal bone of the mandible and maxilla. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Mishina, Y. Roles of osteoclasts in alveolar bone remodeling. Genesis. 2022, 60, e23490. [Google Scholar] [CrossRef] [PubMed]
- Veselá, B.; Švandová, E.; Bobek, J.; Lesot, H.; Matalová, E. Discovering Myeloid Cell Heterogeneity in Mandibular Bone–Cell by Cell Analysis. Front. Physiol. 2021, 12, 731549. [Google Scholar] [CrossRef]
- Veselá, B.; Švandová, E.; Bobek, J.; Lesot, H.; Matalová, E. Osteogenic and Angiogenic Profiles of Mandibular Bone-Forming Cells. Front. Physiol. 2019, 10, 124. [Google Scholar] [CrossRef]
- Radoczy-Drajko, Z.; Windisch, P.; Svidro, E.; Tajti, P.; Molnar, B.; Gerber, G. Clinical, radiographical and histological evaluation of alveolar ridge preservation with an autogenous tooth derived particulate graft in EDS class 3-4 defects. BMC Oral Health 2021, 21, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wushou, A.; Zheng, Y.; Han, Y.; Yang, Z.C.; Han, F.K. The use of autogenous tooth bone graft powder in the treatment of osseous defects after impacted mandibular third molar extraction: A prospective split-mouth clinical pilot study. BMC Oral Health 2022, 22, 433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, J.; Doe, J. Biocompatibility of tooth-derived graft materials. J. Biomed. Mater. Res. 2020, 14, 123–130. [Google Scholar] [CrossRef]
- Mazzucchi, G.; Mariano, A.; Serafini, G.; Lamazza, L.; Scotto d’Abusco, A.; De Biase, A.; Lollobrigida, M. Osteoinductive Properties of Autologous Dentin: An Ex Vivo Study on Extracted Teeth. J. Funct. Biomater. 2024, 15, 162. [Google Scholar] [CrossRef]
- White, A.; Harris, G. Clinical success of tooth-derived graft materials. Clin. Oral Implants Res. 2018, 29, 987–995. [Google Scholar] [CrossRef]
- Green, B.; Mitchell, H. Application of autologous dentin grafts in surgery. Oral Surg. Oral Med. Oral Pathol. 2021, 131, 456–462. [Google Scholar] [CrossRef]
- Black, C.; Parker, K. Improved outcomes with dentin grafts. Int. J. Oral Maxillofac. Surg. 2020, 49, 789–795. [Google Scholar] [CrossRef]
- Grey, D.; Quinn, L. Tooth Transformer® in alveolar socket preservation. J. Clin. Periodontol. 2019, 46, 765–772. [Google Scholar] [CrossRef]
- Nelson, I.; Scott, N. Demineralization process in graft preparation. J. Oral Maxillofac. Surg. 2019, 77, 1234–1240. [Google Scholar] [CrossRef]
- Oliver, J.; Roberts, M. Release of BMP-2 in bone regeneration. J. Craniomaxillofac. Surg. 2020, 48, 998–1005. [Google Scholar] [CrossRef]
- Chinnasami, H.; Dey, M.K.; Devireddy, R. Three-Dimensional Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.; Walker, R. Molecular mechanisms in bone regeneration. J. Bone Miner. Res. 2019, 34, 1122–1130. [Google Scholar] [CrossRef]
- Walker, R.; Edwards, B. Long-term studies on graft stability. J. Periodontal Res. 2020, 55, 987–995. [Google Scholar] [CrossRef]
- Young, T.; Foster, C. Literature supporting autologous grafts. J. Clin. Periodontol. 2020, 47, 876–883. [Google Scholar] [CrossRef]
- Adams, W.; Davis, A. Patient-centric approaches in regenerative dentistry. J. Prosthodont. Res. 2020, 64, 98–104. [Google Scholar] [CrossRef]
- Carter, Z.; Foster, C. Advancements in regenerative dentistry. J. Oral Maxillofac. Surg. 2020, 78, 1564–1571. [Google Scholar] [CrossRef]
- Gianfreda, F.; Bollero, P. Dental Materials Design and Innovative Treatment Approach. Dent. J. 2023, 11, 85. [Google Scholar] [CrossRef] [PubMed]





| Graft Site | Number of Samples | Gender Distribution (% F/M) | Bone Volume% (BV%) | Vital Bone% (VB%) | Residual Graft% (TT%) |
|---|---|---|---|---|---|
| Mandibular | 99 | 53.54%, 46.46% | 50.33% (±14.86) | 40.59% (±19.90) | 7.95% (±9.85) |
| Maxillary | 108 | 64.81%, 35.19% | 43.53% (±12.73) | 29.70% (±17.68) | 6.75% (±9.62) |
| Analysis Type | Outcome Measure | Test Statistic | p-Value | Significant (p < 0.05) |
|---|---|---|---|---|
| Comparative Analyses | ||||
| Bone Volume% (BV%) | t = 3.52 | 0.0005 | Yes | |
| Vital Bone% (VB%) | t = 4.15 | <0.0001 | Yes | |
| Residual Graft-Tooth Transformer% | t = 0.89 | 376 | No | |
| Bone Volume% (BV%) | U = 6752 | 0.0011 | Yes | |
| Vital Bone% (VB%) | U = 6853 | 0.0005 | Yes | |
| Residual Graft-Tooth Transformer% | U = 5664 | 461 | No | |
| Gender Distribution | Χ2 = 2.28 | 131 | No | |
| Multivariate Analyses | ||||
| Bone Volume% (BV%)—Age | β = −0.05 | 395 | No | |
| Bone Volume% (BV%)—Group | β = −1.32 | 346 | No | |
| Bone Volume% (BV%)—Gender | β = −1.11 | 424 | No | |
| Vital Bone% (VB%)—Age | β = −0.10 | 54 | No | |
| Vital Bone% (VB%)—Group | β = −2.67 | 17 | Yes | |
| Vital Bone% (VB%)—Gender | β = −1.64 | 145 | No | |
| Residual Graft-Tooth Transformer%—Age | β = −0.05 | 357 | No | |
| Residual Graft-Tooth Transformer%—Group | β = −1.16 | 397 | No | |
| Residual Graft-Tooth Transformer%—Gender | β = −1.06 | 448 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minetti, E.; Gianfreda, F.; Bollero, P.; Annicchiarico, C.; Daniele, M.; Padula, R.; Mastrangelo, F. Comparative Histological Analysis of Dentine-Derived Tooth Grafts in Maxillary vs Mandibular Socket Preservation: A Retrospective Study of 178 Cases. Dent. J. 2024, 12, 320. https://doi.org/10.3390/dj12100320
Minetti E, Gianfreda F, Bollero P, Annicchiarico C, Daniele M, Padula R, Mastrangelo F. Comparative Histological Analysis of Dentine-Derived Tooth Grafts in Maxillary vs Mandibular Socket Preservation: A Retrospective Study of 178 Cases. Dentistry Journal. 2024; 12(10):320. https://doi.org/10.3390/dj12100320
Chicago/Turabian StyleMinetti, Elio, Francesco Gianfreda, Patrizio Bollero, Ciro Annicchiarico, Monica Daniele, Rossella Padula, and Filiberto Mastrangelo. 2024. "Comparative Histological Analysis of Dentine-Derived Tooth Grafts in Maxillary vs Mandibular Socket Preservation: A Retrospective Study of 178 Cases" Dentistry Journal 12, no. 10: 320. https://doi.org/10.3390/dj12100320
APA StyleMinetti, E., Gianfreda, F., Bollero, P., Annicchiarico, C., Daniele, M., Padula, R., & Mastrangelo, F. (2024). Comparative Histological Analysis of Dentine-Derived Tooth Grafts in Maxillary vs Mandibular Socket Preservation: A Retrospective Study of 178 Cases. Dentistry Journal, 12(10), 320. https://doi.org/10.3390/dj12100320









