The Influence of Ca on Mechanical Properties of the Mg–Ca–Zn–RE–Zr Alloy for Orthopedic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloy Preparation Through Casting and Heat Treatment
2.2. Microstructural Characterization and Mechanical Properties
- Optical microscopy was conducted using a Leica DMI5000 M, Wetzlar and Mannheim, Germany research microscope.
- EDS and electron microscopy were performed with the Thermo Scientific Quattro C microscope, Brno, Czech Republic.
- strong>∙ For XRD analysis, an X’Pert PRO MPD X-ray, Panalytical, Almelo, The Netherlands, diffractometer was used.
- strong>∙ For spectrometry, two devices were used in parallel for improved accuracy of the chemical composition: the Foundry Master Smart laboratory optical emission spectrometer (Wetzlar and Mannheim, Germany) and the SPECTRO XEPOS energy-dispersive X-ray fluorescence (ED-XRF) spectrometer, (Wetzlar and Mannheim, Germany).
- strong>∙ The UMTR 2M-CTR tribometer, version 1.122.245, was used for surface characterization, and the UMT Test Viewer, CP4–2.16.93+ software was used to assess tribological behavior.
- strong>∙ OriginPro 8.5 was used to process the XRD patterns, and VoltaMaster 4-version 6.0.2.25130 was used to process the electrochemical corrosion tests and surface analysis.
2.3. Characterization of Electrochemical Corrosion Resistance
- OCP registration.
- Linear anodic polarization: Potential range (−300 to +300) mV relative to the open-circuit potential, with a potential scan rate of dE/dt = 0.1 mV/s.
- Cyclic polarization: Potential range (−500 to +2200) mV, with a potential scan rate of 10 mV/s.
3. Results
3.1. Microstructural Analysis
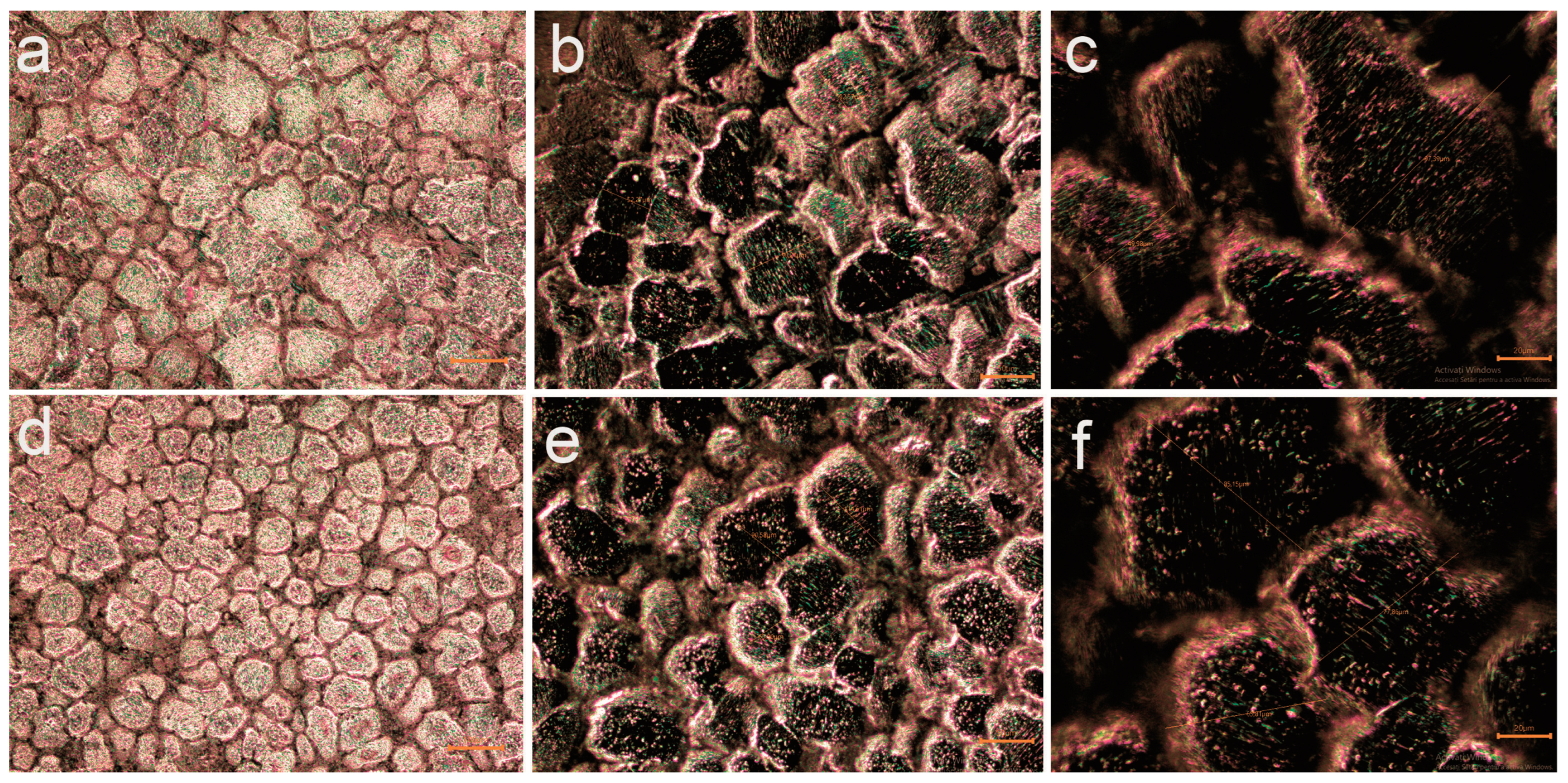
3.1.1. Optical Microscopy
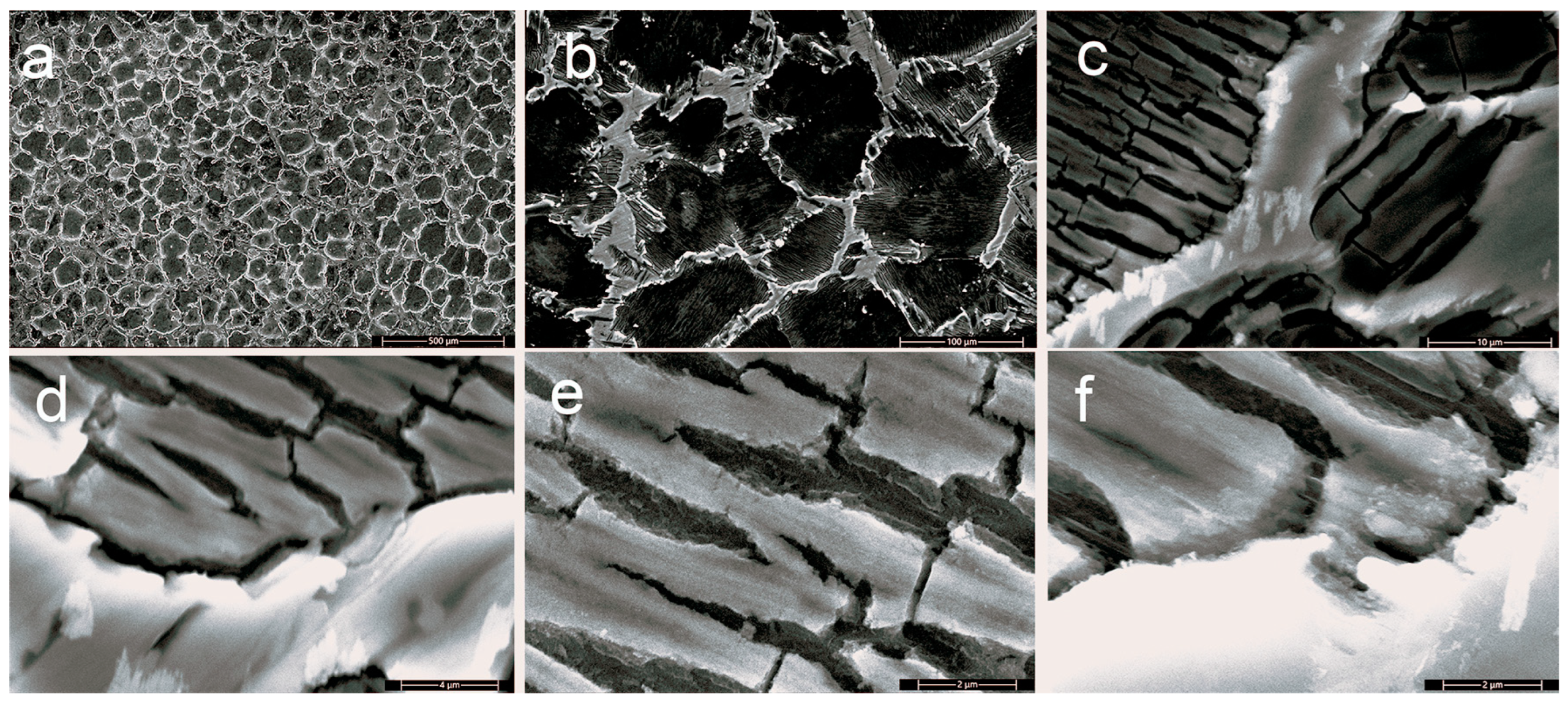
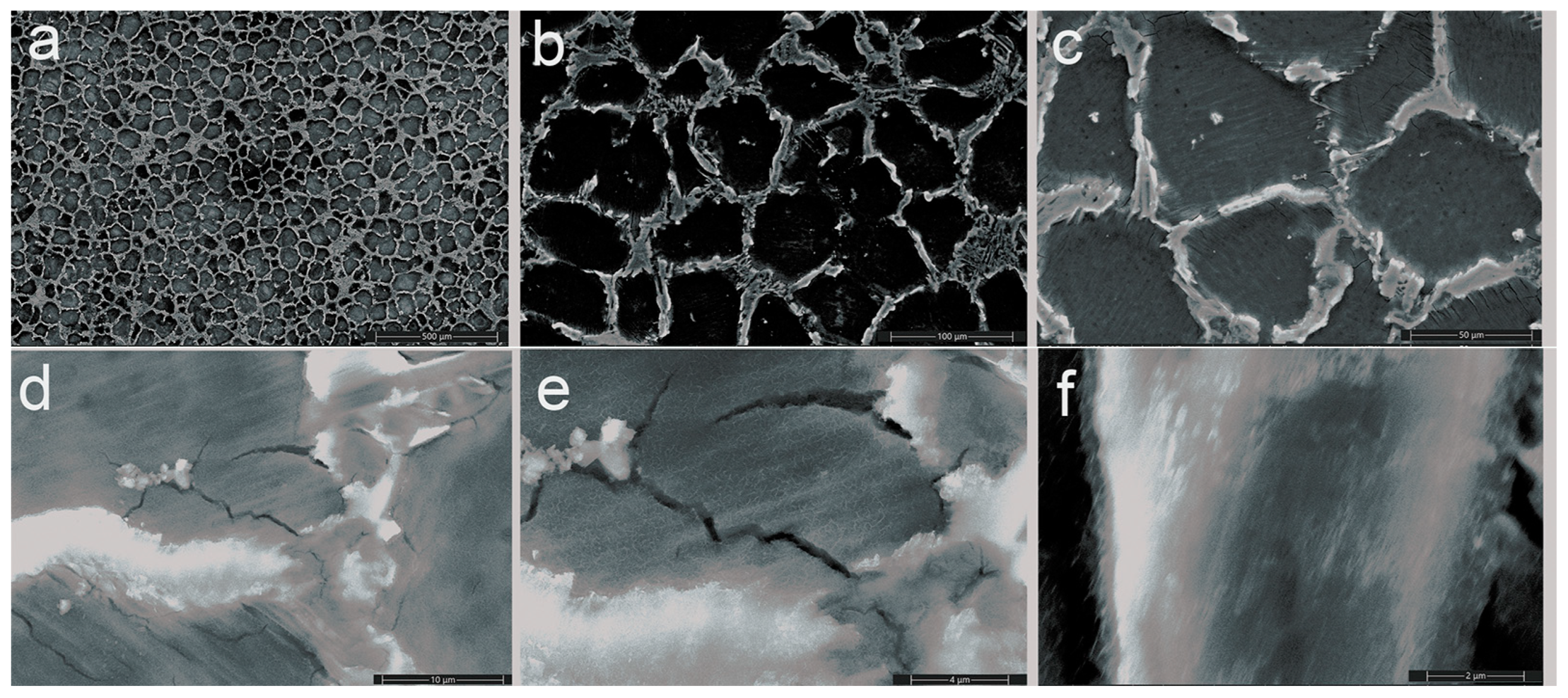
3.1.2. Scanning Electron Microscopy (SEM)
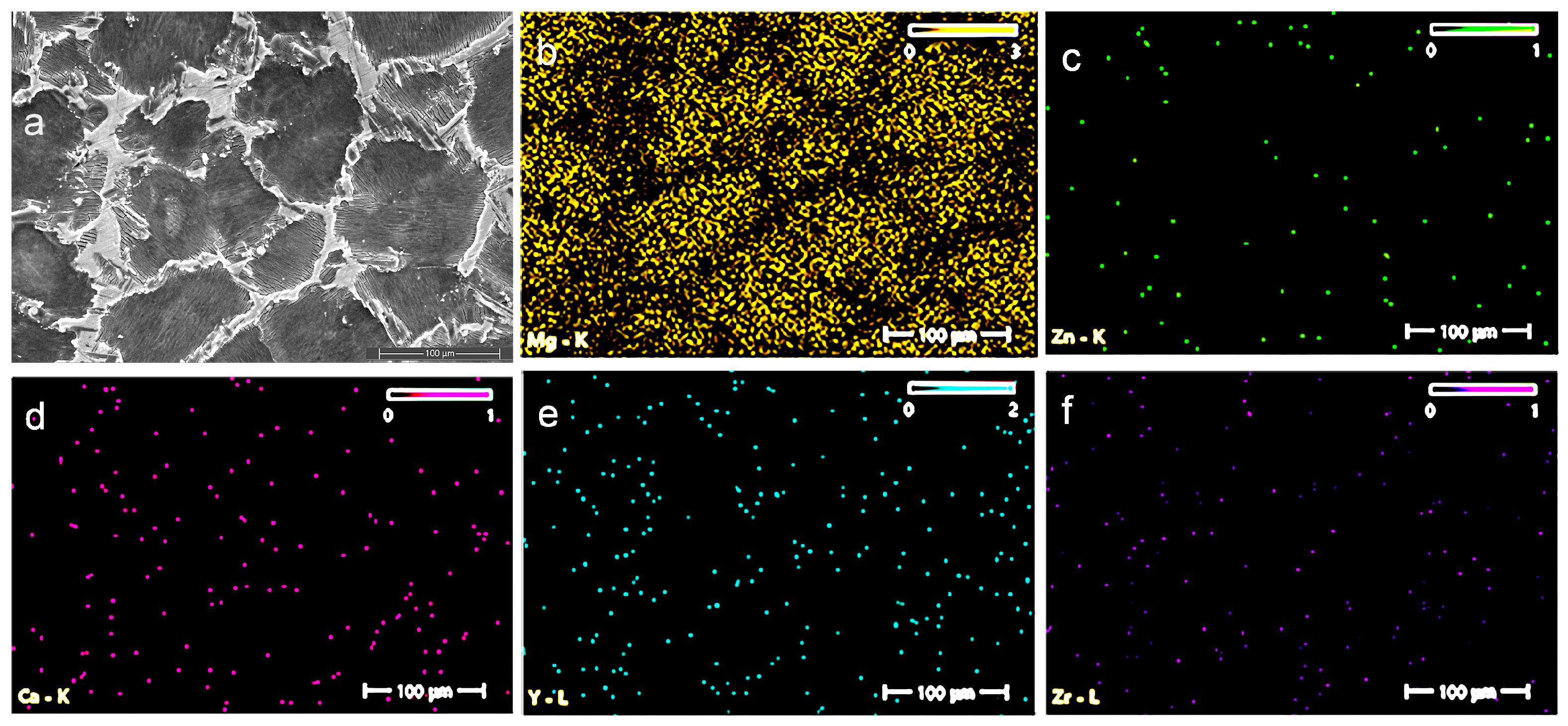
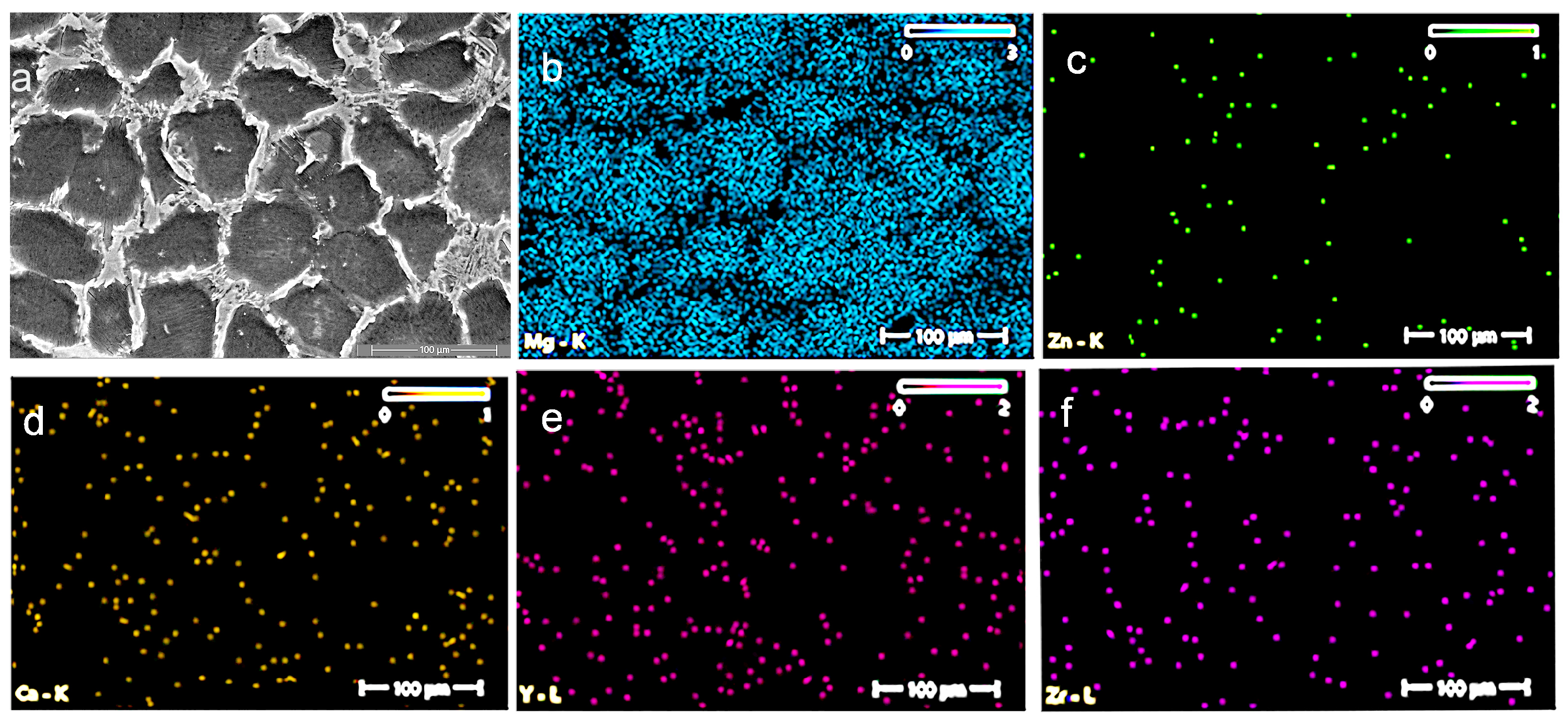
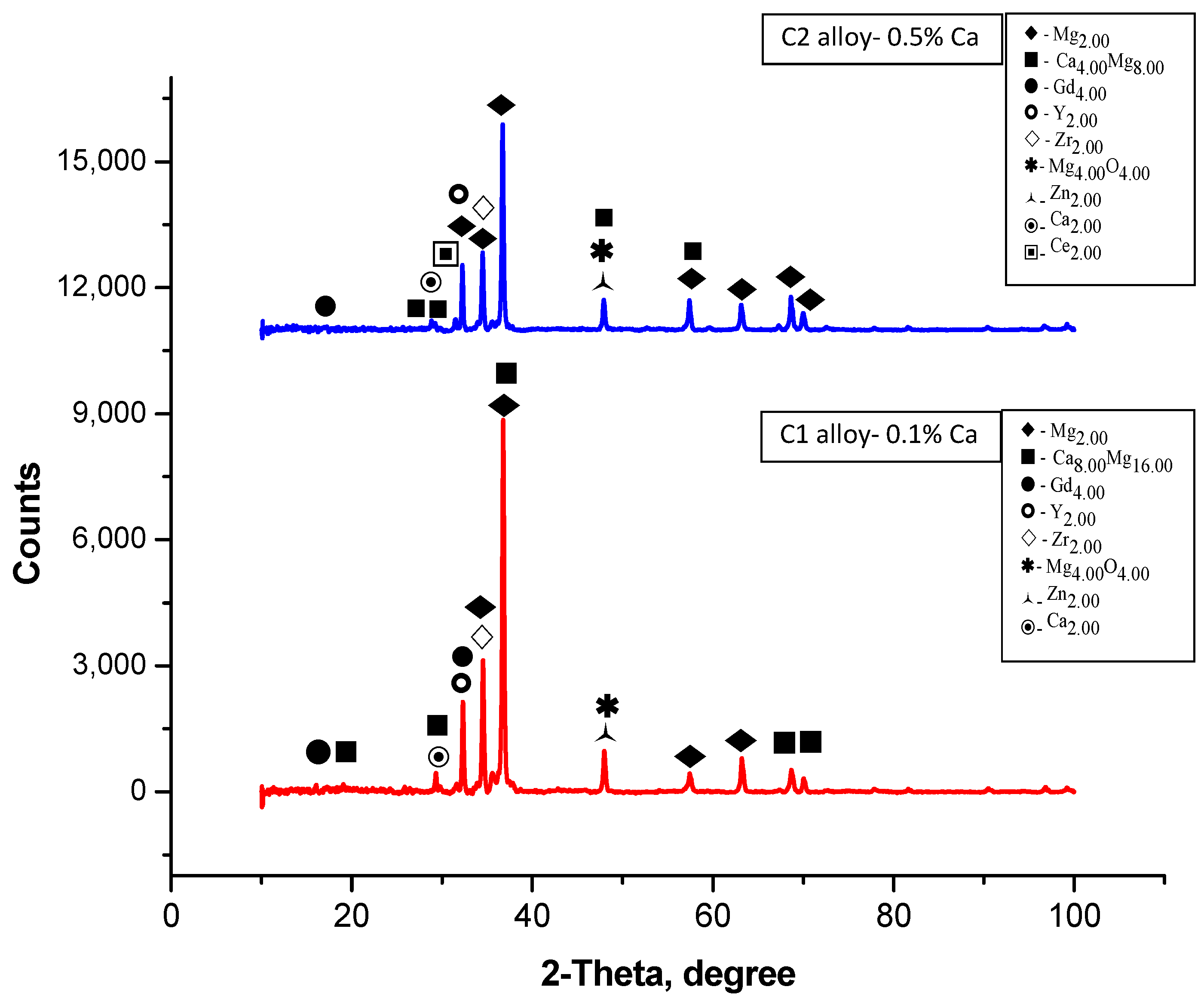
3.2. Energy-Dispersive X-Ray Spectroscopy (EDS) and X-Ray Difraction (XRD)
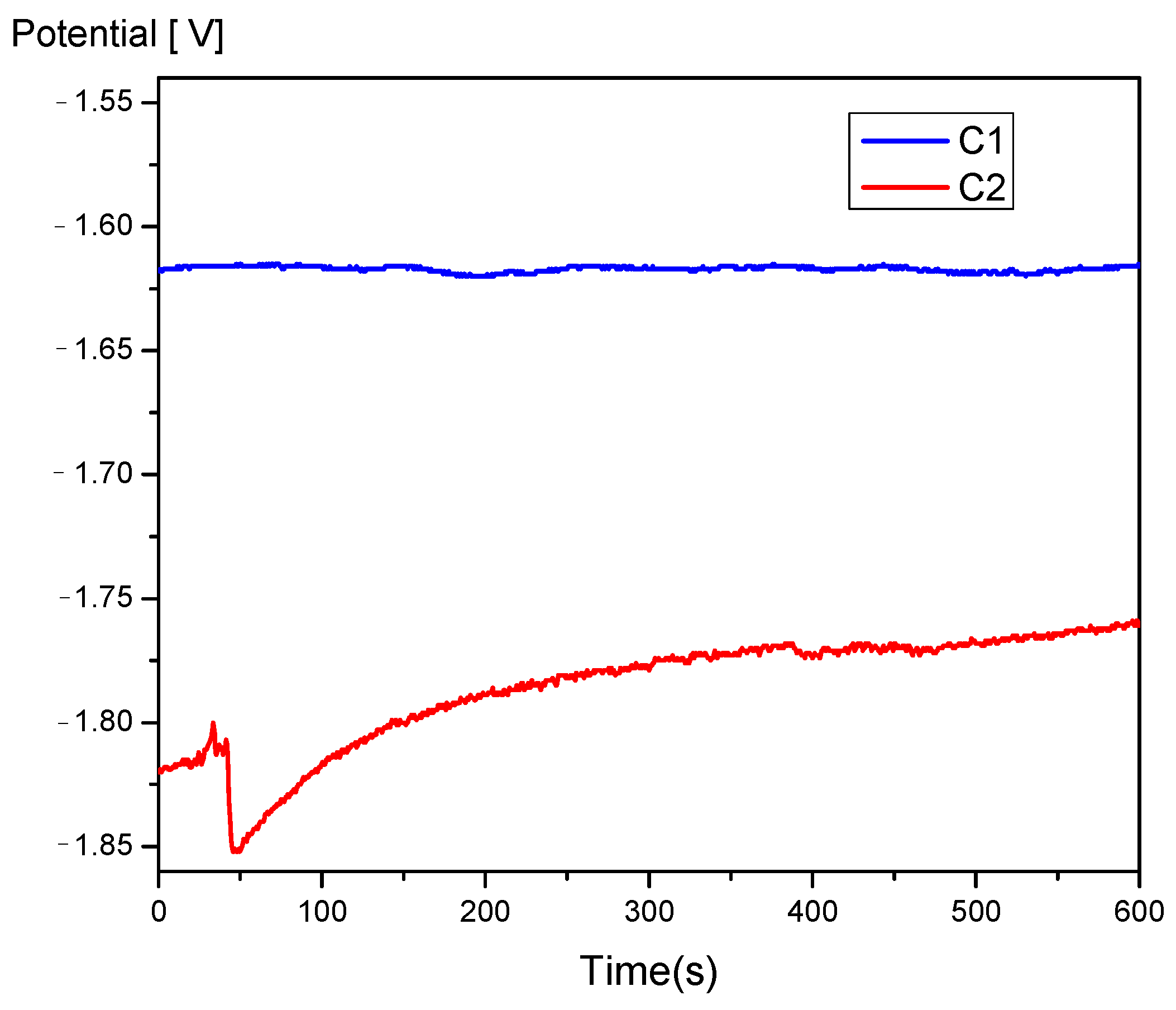
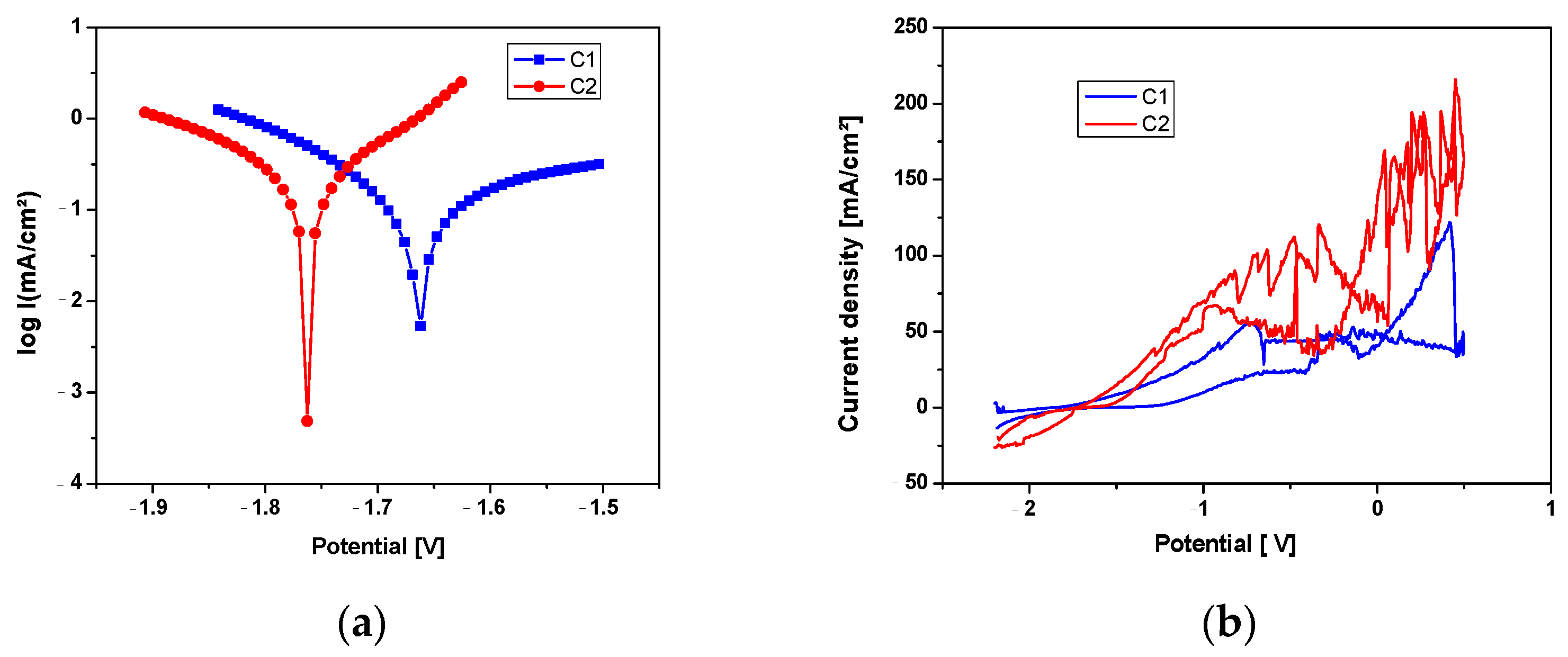
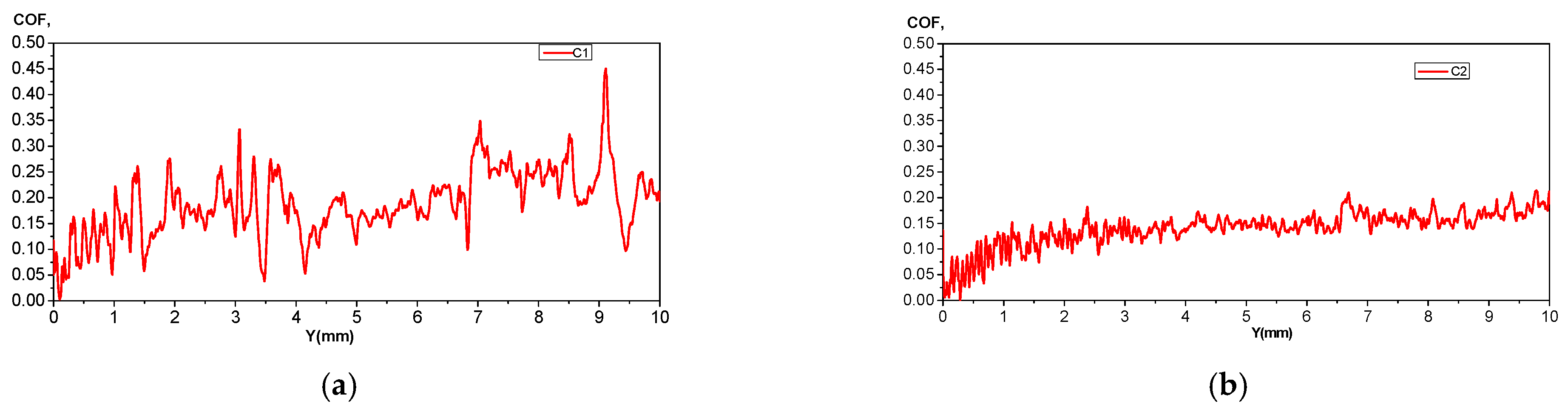
3.3. Electrochemical Corrosion Behavior
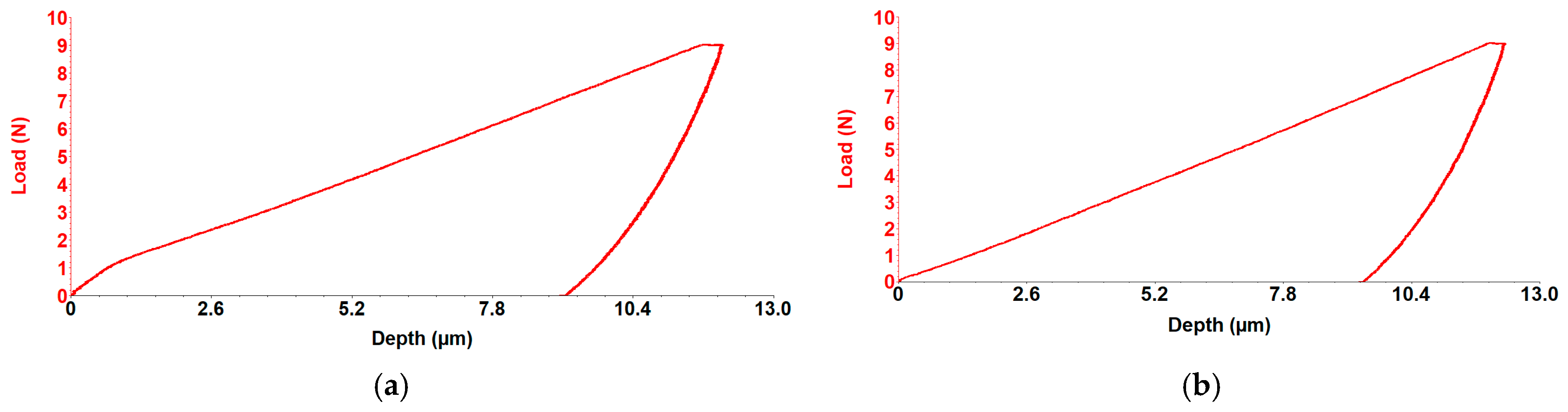
3.4. Mechanical Properties Testing. Determination of the Longitudinal Elastic Modulus Through Micro-Indentation Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, H.S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O–Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Atkinson, H.D.; Khan, S.; Lashgari, Y.; Ziegler, A. Hallux valgus correction utilising a modified short scarf osteotomy with a magnesium biodegradable or titanium compression screws—A comparative study of clinical outcomes. BMC Musculoskelet. Disord. 2019, 20, 334. [Google Scholar] [CrossRef]
- Sahu, M.R.; Kumar, T.S.S.; Chakkingal, U. A review on recent advancements in biodegradable Mg-Ca alloys. J. Magnes. Alloys 2022, 10, 2094–2117. [Google Scholar] [CrossRef]
- Holweg, P.; Berger, L.; Cihova, M.; Donohue, N.; Clement, B.; Schwarze, U.; Sommer, N.G.; Hohenberger, G.; van den Beucken, J.J.J.J.; Seibert, F.; et al. A lean magnesium–zinc–calcium alloy ZX00 used for bone fracture stabilization in a large growing-animal model. Acta Biomater. 2020, 113, 646–659. [Google Scholar] [CrossRef]
- Qu, X.; Yang, H.; Yu, Z.; Jia, B.; Qiao, H.; Zheng, Y.; Dai, K. Serum zinc levels and multiple health outcomes: Implications for zinc-based biomaterials. Bioact. Mater. 2020, 5, 410–422. [Google Scholar] [CrossRef]
- Xu, T.; Yang, Y.; Peng, X.; Song, J.; Pan, F. Overview of advancement and development trend on magnesium alloy. J. Magnes. Alloys 2019, 7, 536–544. [Google Scholar] [CrossRef]
- Koç, E.; Kannan, M.B.; Ünal, M.; Candan, E. Influence of zinc on the microstructure, mechanical properties and in vitro corrosion behavior of magnesium-zinc binary alloys. J. Alloys Compd. 2015, 648, 291–296. [Google Scholar] [CrossRef]
- Shishir, R.; Rahim, S.A.; Hanas, T. Effect of grain refinement on biodegradation and biomineralization of low calcium containing Mg–Ca alloy. Mater. Res. Express 2020, 7, 036501. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. 2012, C32, 2570–2577. [Google Scholar] [CrossRef]
- Yan, Y.; Chu, X.; Luo, X.; Xu, X.; Zhang, Y.; Dai, Y.; Li, D.; Chen, L.; Xiao, T.; Yu, K. A homogenous microstructural Mg-based matrix model for orthopedic application with generating uniform and smooth corrosion product layer in Ringer’s solution: Study on biodegradable behavior of Mg-Zn alloys prepared by powder metallurgy as a case. J. Magnes. Alloys 2021, 9, 225–240. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, B.; Zhao, R.; Yang, X.; Xiao, Z.; Aurora, A.; Iulia, B.A.; Zhu, X.; Iulian, A.V.; Zhang, X. Evaluation on the corrosion resistance, antibacterial property and osteogenic activity of biodegradable Mg-Ca and Mg-Ca-Zn-Ag alloys. J. Magnes. Alloys 2022, 10, 3380–3396. [Google Scholar] [CrossRef]
- Cărăuşu, E.M.; Checheriță, L.E.; Stamatin, O.; Albu, A. Study of Serum and Saliva Biochemical Levels for Copper, Zinc and Copper-Zinc Imbalance in Patients with Oral Cancer and Oral Potentially Malignant Disorders and their Prostetical and DSSS (Disfunctional Syndrome of Stomatognathic System) Treatment. Rev. Chim. 2016, 67, 1832–1836, Accession WOS: 000385266600037. [Google Scholar]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Emerging Magnesium-Based Biomaterials for Orthopedic Implantation. Emerg. Mater. Res. 2019, 8, 305–319. [Google Scholar] [CrossRef]
- Sharma, S.K.; Saxena, K.K.; Malik, V.; Mohammed, K.A.; Prakash, C.; Buddhi, D.; Dixit, S. Significance of Alloying Elements on the Mechanical Characteristics of Mg-Based Materials for Biomedical Applications. Crystals 2022, 12, 138. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Sun, M.; Ding, W. Recent developments and applications on high-performance cast magnesium rare-earth alloys. J. Magnes. Alloys 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Venkateswarlu, B.; Sunil, B.R.; Kumar, R.S. Magnesium based alloys and composites: Revolutionized biodegradable temporary implants and strategies to enhance their performance. Materialia 2023, 20, 101680. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Bahmani, A.; Nayebi, B.; Zonoozi, S.B.; Wang, L.; Shokouhimehr, M. Mechanochemical characteristics of Ca-added Mg-based alloys: A multimodality approach. Mater. Charact. 2020, 167, 110475. [Google Scholar] [CrossRef]
- Delavar, H.; Mostahsan, A.J.; Ibrahim, H. Corrosion and corrosion-fatigue behavior of magnesium metal matrix composites for bio-implant applications: A review. J. Magnes. Alloys 2023, 11, 1125–1161. [Google Scholar] [CrossRef]
- Balasubramani, N.; StJohn, D.; Dargusch, M.; Wang, G. Ultrasonic Processing for Structure Refinement: An Overview of Mechanisms and Application of the Interdependence Theory. Materials 2019, 12, 3187. [Google Scholar] [CrossRef] [PubMed]
- Diaconu-Popa, D.; Vitalariu, A.; Cioloca, C.A.; Aungurencei, A.; Luca, O.E.; Tatarciuc, M. Researches on the Influence of Thermal Treatment on the Mechanical Properties of Titanium Dental Prostheses. Rev. Chim. 2017, 68, 2382–2384. [Google Scholar] [CrossRef]
- Horky, J.; Ghaffar, A.; Werbach, K.; Mingler, B.; Pogatscher, S.; Schäublin, R.; Setman, D.; Uggowitzer, P.J.; Löffler, J.F.; Zehetbauer, M.J. Exceptional Strengthening of Biodegradable Mg-Zn-Ca Alloys through High Pressure Torsion and Subsequent Heat Treatment. Materials 2019, 12, 2460. [Google Scholar] [CrossRef]
- Zerankeshi, M.M.; Alizadeh, R.; Gerashi, E.; Asadollahi, M.; Langdon, T.G. Effects of heat treatment on the corrosion behavior and mechanical properties of biodegradable Mg alloys. J. Magnes. Alloys 2022, 10, 1737–1785. [Google Scholar] [CrossRef]
- Diaconu-Popa, D.; Tatarciuc, M.; Brăescu, R.; Vițalariu, A. Possibilities of improving the mechanical parameters of the dental alloys. Rom. J. Med. Dent. Educ. 2019, 8, 35–42. [Google Scholar]
- Cimpoesu, R.; Lutcanu, M.; Cazac, A.M.; Adomnitei, I.; Bejinariu, C.; Andrusca, L.; Prelipceanu, M.; Cioca, L.I.; Chicet, D.L.; Radu, A.M.; et al. Electrochemical Corrosion Resistance of Al2O3-YSZ Coatings on Steel Substrates. Appl. Sci. 2024, 14, 10877. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Baltatu, M.S.; Cimpoesu, R.; Ioanid, N. Microstructural and Electrochemical Influence of Zn in MgCaZn Biodegradable Alloys. Materials 2023, 16, 2487. [Google Scholar] [CrossRef]
- Panaghie, C.; Zegan, G.; Sodor, A.; Cimpoesu, N.; Lohan, N.M.; Istrate, B.; Roman, A.M.; Ioanid, N. Analysis of Degradation Products of Biodegradable ZnMgY Alloy. Materials 2023, 16, 3092. [Google Scholar] [CrossRef]
- Huan, Z.G.; Leeflang, M.A.; Zhou, J.; Fratila-Apachitei, L.E.; Duszczyk, J. In vitro degradation behavior and cytocompatibility of Mg–Zn–Zr alloys. J. Mater. Sci. Mater. Med. 2010, 21, 2623–2635. [Google Scholar] [CrossRef]
- Lei, T.; Ouyanga, C.; Tanga, W.; Li, L.-F.; Zhou, L.-S. Preparation of MgO coatings on magnesium alloys for corrosion protection. Surf. Coat. Technol. 2010, 204, 3798–3803. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, Y.; Bühring, J.; Tian, L.; Koblenzer, M.; Schröder, K.-U.; Li, F.; Van Dessel, J.; Politis, C.; Jahr, H.; et al. Biocompatibility and osteogenic capacity of additively manufactured biodegradable porous WE43 scaffolds: An in vivo study in a canine model. Biomater. Adv. 2024, 164, 213984. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Husain, M.M.; Prasad, N. Microstructural and mechanical properties evaluation of Calcium and zinc-modified WE43-based nanocomposites through stir casting for biodegradable applications. Ceram. Int. 2024, 50, 30284–30305. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Naveenkumar, R.; Radhika, N.; Ismail, S.O.; Mohanavel, V. Recent advances in magnesium alloys for biomedical applications: A review. Mater. Today Commun. 2025, 42, 111239. [Google Scholar] [CrossRef]


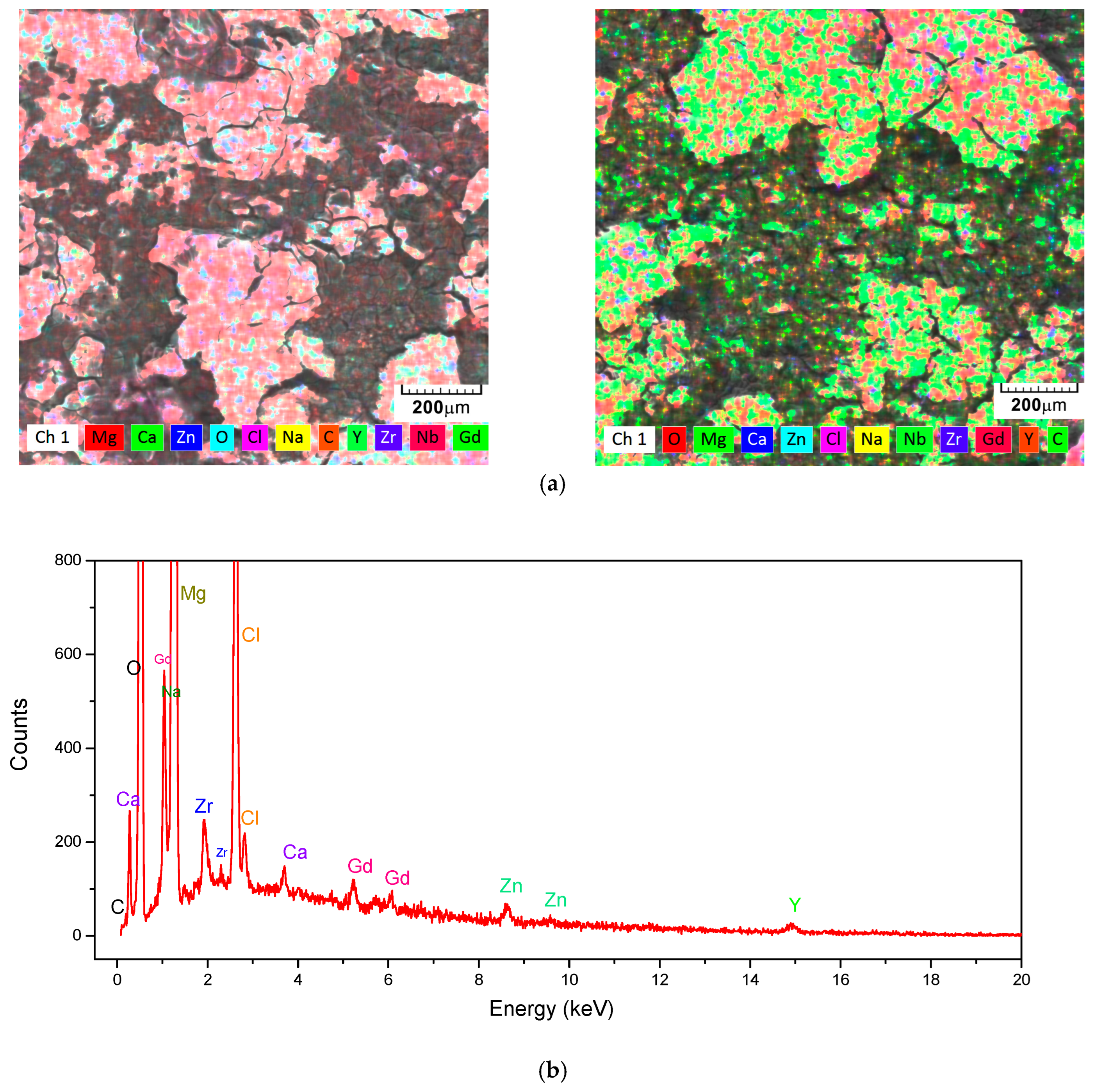

| System | Mg | Ca | Zn | Y | Zr | Nd | Dy | Gd |
|---|---|---|---|---|---|---|---|---|
| C1 (spark) | 94.7 | 0.16 | 1.42 | 1.19 | 0.6 | 0.46 | 0.4 | 0.17 |
| C1 (XRF) | 92.68 | 0.13 | 3.4 | - | 0.38 | 0.1 | - | - |
| C2 (spark) | 92 | 0.39 | 2.78 | 2.10 | 0.6 | 0.69 | 0.4 | 0.28 |
| C2 (xrf) | 90.81 | 0.42 | 3.79 | - | 1.3 | 0.1 | - | - |
| System | Corrosion Process Parameters | |||||
|---|---|---|---|---|---|---|
| E(I = 0) (mV) | icorr (mA/cm2) | Rp (ohm/cm2) | vcorr (mm/year) | βc (mV/dec) | βa (mV/dec) | |
| C1 | −1662.7 | 0.14 | 327.4 | 3.39 | −185.0 | 461.6 |
| C2 | −1762.5 | 0.16 | 122.3 | 3.79 | −146.1 | 122.8 |
| Sample/ Chemical Elements | O% | Mg% | Cl% | Na% | Y% | Zn% | Gd% | Ca% | Zr% | C% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | wt | at | |
| C1(0.1%) | 57.3 | 64.3 | 30.2 | 22.3 | 2.3 | 1.2 | 1.6 | 1.3 | 0.9 | 0.2 | 0.25 | 0.07 | 0.11 | 0.01 | 0.1 | 0.05 | 0.1 | 0.02 | 7.1 | 10.6 |
| C2(0.5%) | 56.7 | 66.6 | 29.9 | 22.4 | 3.2 | 1.7 | 0.9 | 0.7 | 1.3 | 0.3 | 0.3 | 0.1 | 0.4 | 0.1 | 0.5 | 0.2 | 0.30 | 0.1 | 6.6 | 10 |
| EDS error | 5 | 2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 10 | ||||||||||
| Avg. C1 Alloy | Avg. C2 Alloy | |
|---|---|---|
| Stiffness (N/µm) | 2.2 (±0.1) | 2.52 (±0.1) |
| Depth (µm) | 13.35 (±0.6) | 11.07 (±1.1) |
| Young’s (Gpa) | 14.85 (±1.1) | 18.60 (±1.7) |
| Hardness (Gpa) | 0.57 (±0.02) | 0.67 (±0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivănescu, M.C.; Munteanu, C.; Cimpoeșu, R.; Istrate, B.; Lupu, F.C.; Benchea, M.; Șindilar, E.V.; Vlasa, A.; Stamatin, O.; Zegan, G. The Influence of Ca on Mechanical Properties of the Mg–Ca–Zn–RE–Zr Alloy for Orthopedic Applications. J. Funct. Biomater. 2025, 16, 170. https://doi.org/10.3390/jfb16050170
Ivănescu MC, Munteanu C, Cimpoeșu R, Istrate B, Lupu FC, Benchea M, Șindilar EV, Vlasa A, Stamatin O, Zegan G. The Influence of Ca on Mechanical Properties of the Mg–Ca–Zn–RE–Zr Alloy for Orthopedic Applications. Journal of Functional Biomaterials. 2025; 16(5):170. https://doi.org/10.3390/jfb16050170
Chicago/Turabian StyleIvănescu, Mircea Cătălin, Corneliu Munteanu, Ramona Cimpoeșu, Bogdan Istrate, Fabian Cezar Lupu, Marcelin Benchea, Eusebiu Viorel Șindilar, Alexandru Vlasa, Ovidiu Stamatin, and Georgeta Zegan. 2025. "The Influence of Ca on Mechanical Properties of the Mg–Ca–Zn–RE–Zr Alloy for Orthopedic Applications" Journal of Functional Biomaterials 16, no. 5: 170. https://doi.org/10.3390/jfb16050170
APA StyleIvănescu, M. C., Munteanu, C., Cimpoeșu, R., Istrate, B., Lupu, F. C., Benchea, M., Șindilar, E. V., Vlasa, A., Stamatin, O., & Zegan, G. (2025). The Influence of Ca on Mechanical Properties of the Mg–Ca–Zn–RE–Zr Alloy for Orthopedic Applications. Journal of Functional Biomaterials, 16(5), 170. https://doi.org/10.3390/jfb16050170










