Bone Regeneration in Defects Created on Rat Calvaria Grafted with Porcine Xenograft and Synthetic Hydroxyapatite Reinforced with Titanium Particles—A Microscopic and Histological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Groups
2.3. Biomaterials
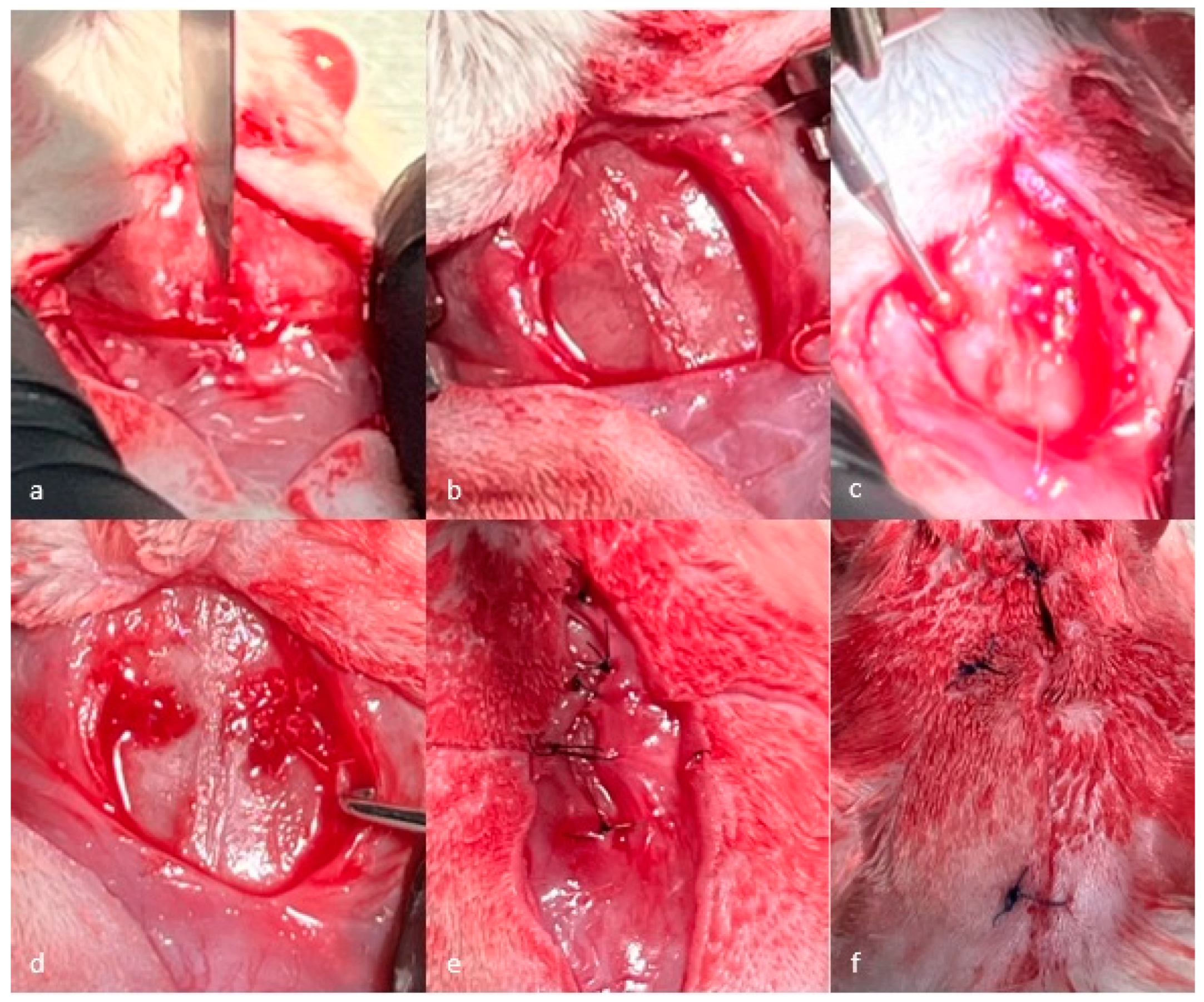
2.4. Surgical Procedure
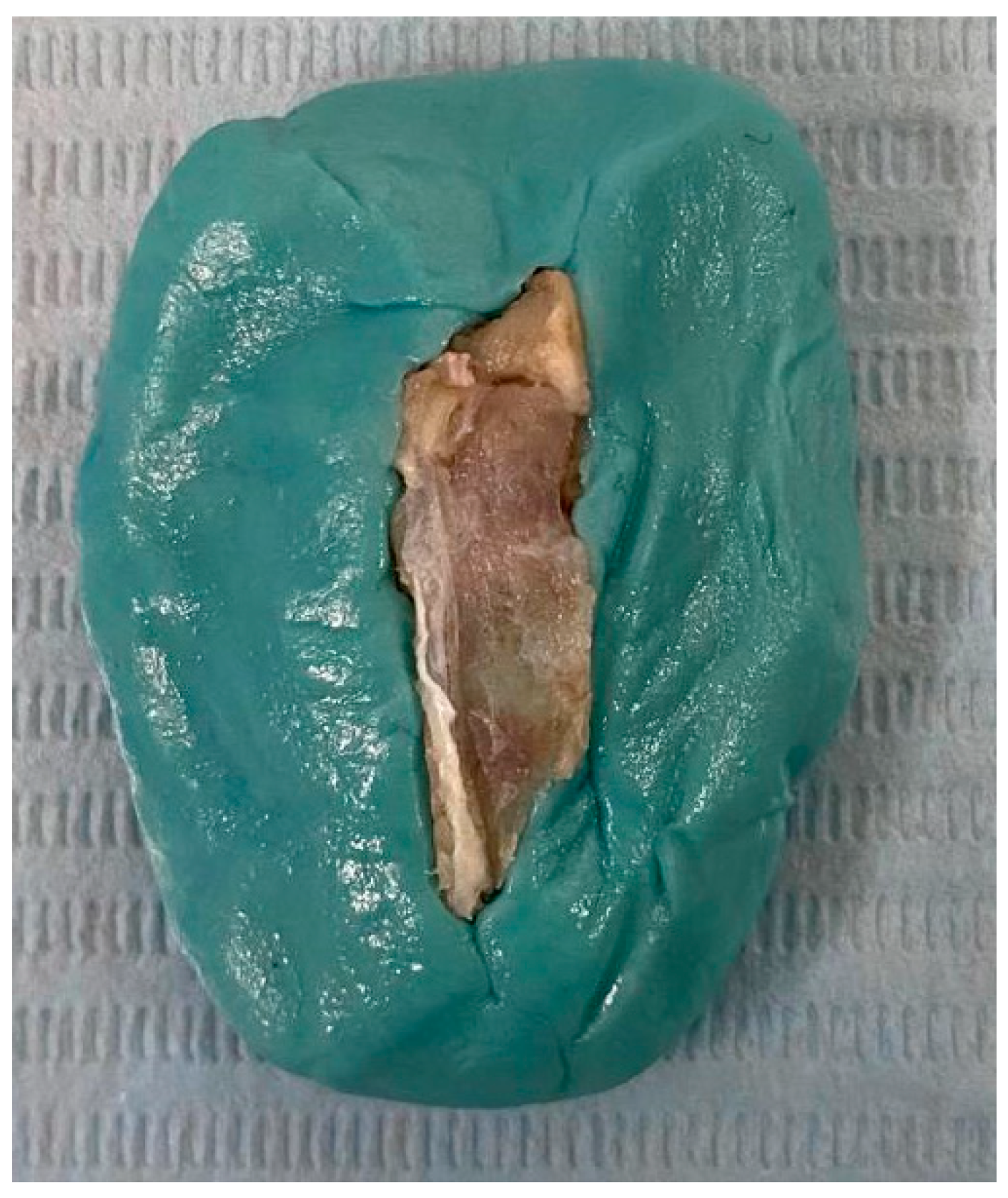
2.5. Bone Sample Preparation
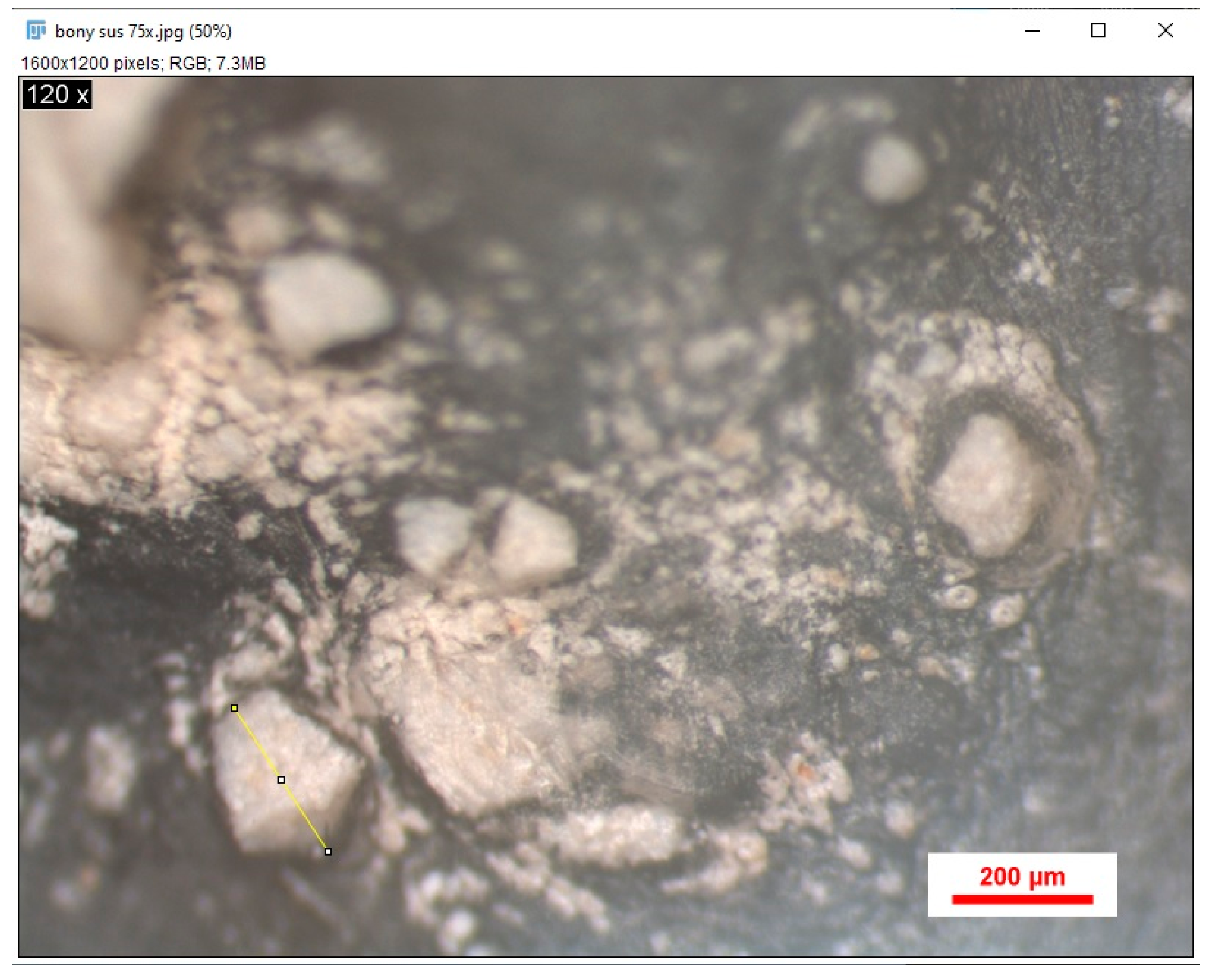
2.6. Microscopic Analysis
2.7. Analysis of Stereomicroscopic Images Using Image J
2.8. Histologic Analysis
2.9. Analysis of Histologic Images Using Image J
2.10. Statistical Analysis
3. Results
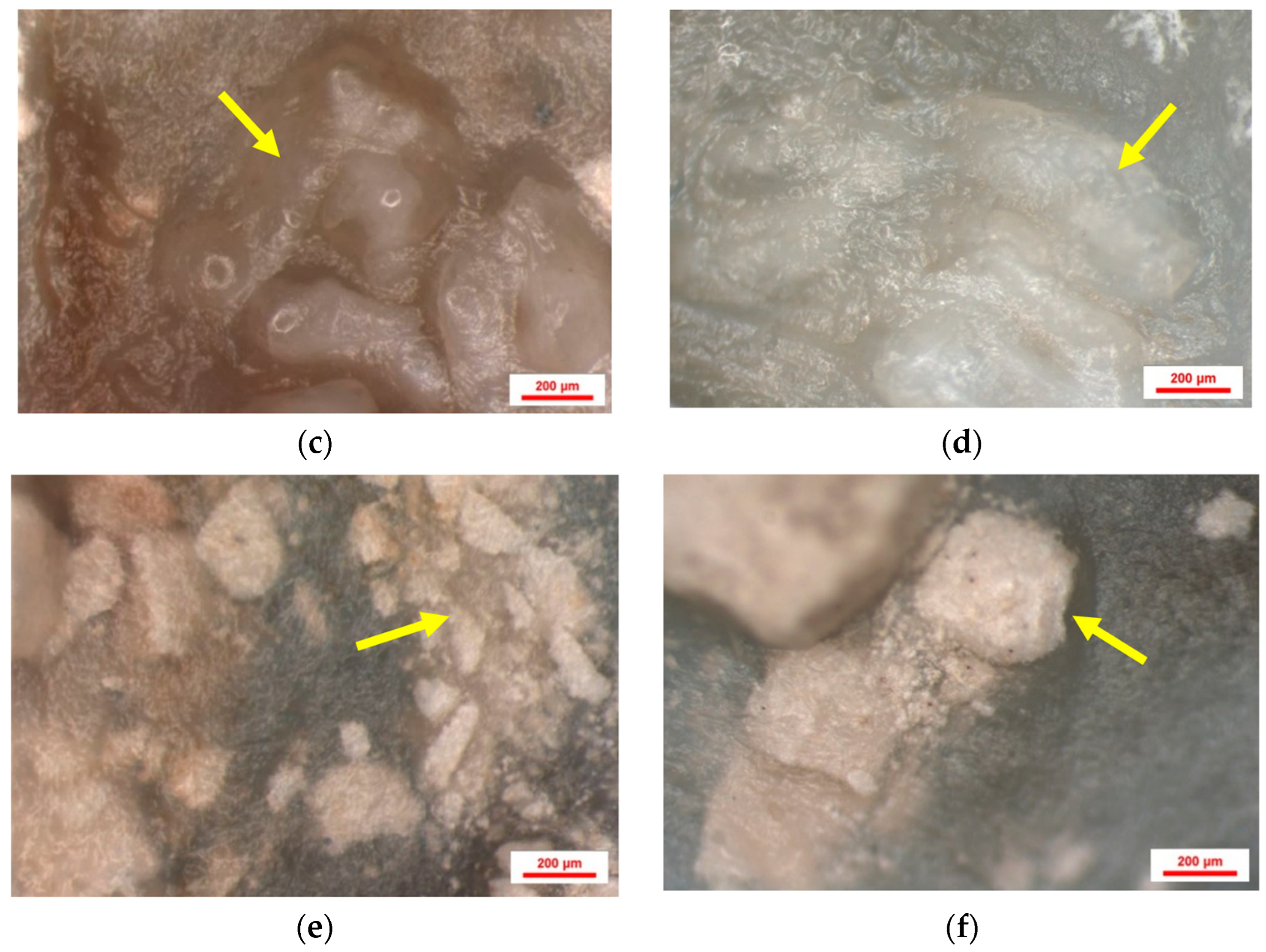
3.1. Results of Stereomicroscopic Analysis of Samples
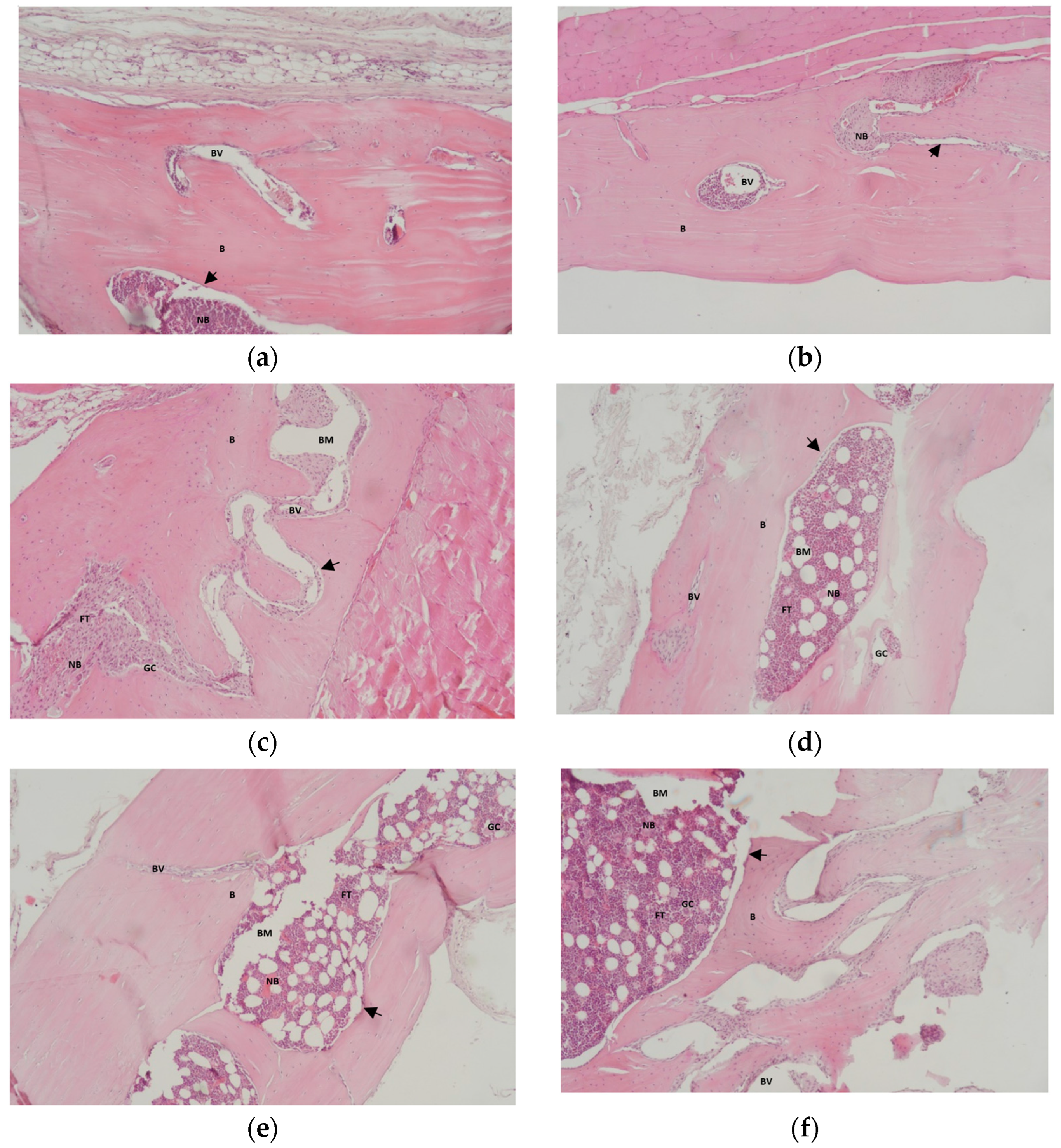
3.2. Results of Histological Analysis of Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSS | two-stage sintering |
| NIH | National Institutes of Health |
| EDTA | ethylenediaminetetraacetic acid |
| HE | hematoxylin–eosin |
| BS | bone surface |
| TS | total surface |
| SD | standard deviation |
| B | native bone |
| NB | newly formed bone |
| BM | biomaterial |
| BV | blood vessel |
References
- Mikael, P.E.; Golebiowska, A.A.; Xin, X.; Rowe, D.W.; Nukavarapu, S.P. Evaluation of an Engineered Hybrid Matrix for Bone Regeneration via Endochondral Ossification. Ann. Biomed. Eng. 2020, 48, 992–1005. [Google Scholar] [CrossRef]
- Frigério, P.B.; Quirino, L.C.; Gabrielli, M.A.C.; Carvalho, P.H.A.; Garcia Júnior, I.R.; Pereira-Filho, V.A. Evaluation of Bone Repair Using a New Biphasic Synthetic Bioceramic (Plenum® Osshp) in Critical Calvaria Defect in Rats. Biology 2023, 12, 1417. [Google Scholar] [CrossRef] [PubMed]
- Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Smith, T.L.; Ornelles, D.A.; Deora, R.; Parks, G.D.; Van Dyke, M.E.; Whitlock, P.W. A porcine xenograft-derived bone scaffold is a biocompatible bone graft substitute: An assessment of cytocompatibility and the alpha-Gal epitope. Xenotransplantation 2019, 26, e12534. [Google Scholar] [CrossRef] [PubMed]
- Shamsoddin, E.; Houshmand, B.; Golabgiran, M. Biomaterial selection for bone augmentation in implant dentistry: A systematic review. J. Adv. Pharm. Technol. Res. 2019, 10, 46–50. [Google Scholar]
- Sánchez-Labrador, L.; Molinero-Mourelle, P.; Pérez-González, F.; Saez-Alcaide, L.M.; Brinkmann, J.C.; Martínez, J.L.; Martínez-González, J.M. Clinical performance of alveolar ridge augmentation with xenogeneic bone block grafts versus autogenous bone block grafts. A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 293–302. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimabukuro, M.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Structurally optimized honeycomb scaffolds with outstanding ability for vertical bone augmentation. J. Adv. Res. 2022, 41, 101–112. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Susin, C.; Lee, J.; Fiorini, T.; Koo, K.T.; Schüpbach, P.; Finger Stadler, A.; Wikesjö, U.M. Screening of Hydroxyapatite Biomaterials for Alveolar Augmentation Using a Rat Calvaria Critical-Size Defect Model: Bone Formation/Maturation and Biomaterials Resolution. Biomolecules 2022, 12, 1677. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Geng, Z.; Su, J. The horizon of bone organoid: A perspective on construction and application. Bioact. Mater. 2022, 18, 15–25. [Google Scholar] [CrossRef]
- Naini, A.Y.; Kobravi, S.; Jafari, A.; Lotfalizadeh, M.; Lotfalizadeh, N.; Farhadi, S. Comparing the effects of Bone+B® xenograft and InterOss® xenograft bone material on rabbit calvaria bone defect regeneration. Clin. Exp. Dent. Res. 2024, 10, e875. [Google Scholar] [CrossRef]
- Fishman, J.A. Risks of Infectious Disease in Xenotransplantation. N. Engl. J. Med. 2022, 387, 2258–2267. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes—comparison with human bone. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef]
- Meza-Mauricio, J.; Furquim, C.P.; Dos Reis, L.D.; Maximiano, M.M.; Mendoza-Azpur, G.; Muniz, F.W.; Rasperini, G.; Faveri, M. How efficacious is the combination of substitute bone graft with autogenous bone graft in comparison with substitute bone graft alone in the horizontal bone gain? A systematic review and meta-analysis. J. Clin. Exp. Dent. 2022, 14, e678–e688. [Google Scholar] [CrossRef]
- Falacho, R.I.; Palma, P.J.; Marques, J.A.; Figueiredo, M.H.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules 2021, 26, 1339. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Junior, J.M.; Montagner, P.G.; Carrijo, R.C.; Martinez, E.F. Physical characterization of biphasic bioceramic materials with different granulation sizes and their influence on bone repair and inflammation in rat calvaria. Sci. Rep. 2021, 11, 4484. [Google Scholar] [CrossRef]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced Biomaterials and Techniques for Oral Tissue Engineering and Regeneration—A Review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef]
- Fang, C.H.; Lin, Y.W.; Lin, F.H.; Sun, J.S.; Chao, Y.H.; Lin, H.Y.; Chang, Z.C. Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 6002. [Google Scholar] [CrossRef] [PubMed]
- Hudecki, A.; Łyko-Morawska, D.; Kasprzycka, A.; Kazek-Kęsik, A.; Likus, W.; Hybiak, J.; Jankowska, K.; Kolano-Burian, A.; Włodarczyk, P.; Wolany, W.; et al. Comparison of Physicochemical, Mechanical, and (Micro-) Biological Properties of Sintered Scaffolds Based on Natural- and Synthetic Hydroxyapatite Supplemented with Selected Dopants. Int. J. Mol. Sci. 2022, 23, 4692. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Gayathri, V.S.; Sowmya, S.V.; Augustine, D.; Alamoudi, A.; Zidane, B.; Hassan Mohammad Albar, N.; Bhandi, S. Nanohydroxyapatite in dentistry: A comprehensive review. Saudi Dent. J. 2023, 35, 741–752. [Google Scholar] [CrossRef]
- Mucalo, M.R. Special Issue: Novel Advances and Approaches in Biomedical Materials Based on Calcium Phosphates. Materials 2019, 12, 405. [Google Scholar] [CrossRef]
- Siswanto, S.; Hikmawati, D.; Kulsum, U.; Rudyardjo, D.I.; Apsari, R.; Aminatun, A. Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration. Open Chem. 2020, 18, 584–590. [Google Scholar] [CrossRef]
- Mahfuri, A.S.; Shehada, A.; Darwich, K.; Saima, R. Radiological Comparative Study Between Conventional and Nano Hydroxyapatite With Platelet-Rich Fibrin (PRF) Membranes for Their Effects on Alveolar Bone Density. Cureus 2022, 14, e32381. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.C.; Stan, G.E.; Maidaniuc, A.; Miculescu, M.; Antoniac, I.V.; Ciocoiu, R.C.; Voicu, Ș.I.; Mitran, V.; Cîmpean, A.; Miculescu, F. Naturally-Derived Biphasic Calcium Phosphates through Increased Phosphorus-Based Reagent Amounts for Biomedical Applications. Materials 2019, 12, 381. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Shanbhag, S.; Suliman, S.; Mohamed-Ahmed, S.; Kampleitner, C.; Hassan, M.N.; Heimel, P.; Dobsak, T.; Tangl, S.; Bolstad, A.I.; Mustafa, K. Bone regeneration in rat calvarial defects using dissociated or spheroid mesenchymal stromal cells in scaffold-hydrogel constructs. Stem Cell Res. Ther. 2021, 12, 575. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Sindhu, A.; Kumar, V.; Kumar, R.; Lamberti, L.; Pruncu, C.I.; Thakur, R. Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials 2020, 10, 2019. [Google Scholar] [CrossRef] [PubMed]
- Uwazumi, S.; Nakagawa, M.; Morinaga, K.; Hashimoto, Y.; Honda, Y.; Baba, S. Utility of Stereo Microscopy in the Evaluation of Organic-Inorganic Composite Material. Nano Biomed. 2023, 15, 88–96. [Google Scholar]
- Varela, A.; Jolette, J. Bone Toolbox: Biomarkers, Imaging Tools, Biomechanics, and Histomorphometry. Toxicol. Pathol. 2018, 46, 511–529. [Google Scholar] [CrossRef]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef]
- Palma, P.; Matos, S.; Ramos, J.; Guerra, F.; Figueiredo, M.; Kauser, J. New formulations for space provision and bone regeneration. Biodental Eng. 2010, 1, 71–76. [Google Scholar]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Roca-Millan, E.; Jané-Salas, E.; Marí-Roig, A.; Jiménez-Guerra, Á.; Ortiz-García, I.; Velasco-Ortega, E.; López-López, J.; Monsalve-Guil, L. The Application of Beta-Tricalcium Phosphate in Implant Dentistry: A Systematic Evaluation of Clinical Studies. Materials 2022, 15, 655. [Google Scholar] [CrossRef]
- Russmueller, G.; Winkler, L.; Lieber, R.; Seemann, R.; Pirklbauer, K.; Perisanidis, C.; Kapeller, B.; Spassova, E.; Halwax, E.; Poeschl, W.P.; et al. In Vitro effects of particulate bone substitute materials on the resorption activity of human osteoclasts. Eur. Cell Mater. 2017, 34, 291–306. [Google Scholar] [CrossRef]
- Olăreț, E.; Stancu, I.C.; Iovu, H.; Serafim, A. Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications. Materials 2021, 14, 6763. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Jia, L.; Duan, K.; Li, Y.; Ma, Y.; Yan, J.; Duan, X.; Wu, G. Preparation and properties of a new artificial bone composite material. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2023, 37, 488–494. [Google Scholar] [PubMed]
- Marinescu, C.; Sofronia, A.; Anghel, E.M.; Baies, R.; Constantin, D.; Seciu, A.M.; Tanasescu, S. Microstructure, stability and biocompatibility of hydroxyapatite–titania nanocomposites formed by two step sintering process. Arab. J. Chem. 2019, 12, 857–867. [Google Scholar] [CrossRef]
- Drăghici, M.A.; Mitruţ, I.; Sălan, A.I.; Mărăşescu, P.C.; Caracaş, R.E.; Camen, A.; Ciocan, L.T.; Gîngu, O.; Manolea, H.O. Osseointegration evaluation of an experimental bone graft material based on hydroxyapatite, reinforced with titanium-based particles. Rom. J. Morphol. Embryol. 2023, 64, 49–55. [Google Scholar] [CrossRef]
- Rădoi, M.A.; Osiac, E.; Lascu, L.C.; Gîngu, O.; Mitruț, I.; Sălan, A.I.; Manolea, H.O. A digital approach for the analysis of the optical coherence tomography evaluation of hydroxyapatite–based bone graft materials. Rom. J. Oral Rehabil. 2023, 15, 42–53. [Google Scholar]
- Caracaş, R.E.; Osiac, E.; Ciocan, L.T.; Mihăiescu, D.E.; Buteică, S.A.; Mitruţ, I.; Manolea, H.O. An optical coherence tomography evaluation of two synthetic bone augmentation materials in calvaria and mandibular lab rats cavities. Rom. J. Oral Rehabil. 2021, 13, 27–35. [Google Scholar]
- Gingu, O.; Benga, G.; Olei, A.; Lupu, N.; Rotaru, P.; Tanasescu, S.; Sima, G. Wear behaviour of ceramic biocomposites based on hydroxiapatite nanopowders. Proc. Inst. Mech. Eng. Part E. J. Process Mech. Eng. 2011, 225, 62–71. [Google Scholar] [CrossRef]
- Dominguez, V.M.; Agnew, A.M. The use of ROI overlays and a semi-automated method for measuring cortical area in ImageJ for histological analysis. Am. J. Phys. Anthropol. 2019, 168, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Jang, K.; Lee, J.H.; Oh, S.H.; Ham, B.D.; Chung, S.M.; Lee, J.K.; Ku, J.K. Bone graft materials for current implant dentistry. J. Dent. Implant. Res. 2020, 39, 1–10. [Google Scholar] [CrossRef]
- Kolerman, R.; Qahaz, N.; Barnea, E.; Mijiritsky, E.; Chaushu, L.; Tal, H.; Nissan, J. Allograft and Collagen Membrane Augmentation Procedures Preserve the Bone Level around Implants after Immediate Placement and Restoration. Int. J. Environ. Res. Public Health 2020, 17, 1133. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Ravi, P.; Kundu, S.; Tappa, K. Emerging Biomedical and Clinical Applications of 3D-Printed Poly(Lactic Acid)-Based Devices and Delivery Systems. Bioengineering 2024, 11, 705. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.; Ding, X.; Zhao, Y.; Qian, X.; Shang, L. Biomimetic mineralized organic–inorganic hybrid scaffolds from microfluidic 3D printing for bone repair. Adv. Funct. Mater. 2025, 35, 2410927. [Google Scholar] [CrossRef]
- Takauti, C.A.; Futema, F.; Brito Junior, R.B.; Abrahão, A.C.; Costa, C.; Queiroz, C.S. Assessment of bone healing in rabbit calvaria grafted with three different biomaterials. Braz. Dent. J. 2014, 25, 379–384. [Google Scholar] [CrossRef][Green Version]
- Musson, D.S.; Gao, R.; Watson, M.; Lin, J.M.; Park, Y.E.; Tuari, D.; Callon, K.E.; Zhu, M.; Dalbeth, N.; Naot, D.; et al. Bovine bone particulates containing bone anabolic factors as a potential xenogenic bone graft substitute. J. Orthop. Surg. Res. 2019, 14, 60. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed]
- Bielenstein, J.; Radenković, M.; Najman, S.; Liu, L.; Ren, Y.; Cai, B.; Beuer, F.; Rimashevskiy, D.; Schnettler, R.; Alkildani, S.; et al. In Vivo Analysis of the Regeneration Capacity and Immune Response to Xenogeneic and Synthetic Bone Substitute Materials. Int. J. Mol. Sci. 2022, 23, 10636. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.B.; Kim, H.J.; Ahn, J.J.; Bae, H.Y.; Kim, H.J.; Huh, J.B. Comparison of Bone Regeneration between Porcine-Derived and Bovine-Derived Xenografts in Rat Calvarial Defects: A Non-Inferiority Study. Materials 2019, 12, 3412. [Google Scholar] [CrossRef]
- Luca, R.E.; Giuliani, A.; Mănescu, A.; Heredea, R.; Hoinoiu, B.; Constantin, G.D.; Duma, V.F.; Todea, C.D. Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology. Int. J. Mol. Sci. 2020, 21, 778. [Google Scholar] [CrossRef] [PubMed]
- Waletzko-Hellwig, J.; Sass, J.O.; Bader, R.; Frerich, B.; Dau, M. Evaluation of Integrity of Allogeneic Bone Processed with High Hydrostatic Pressure: A Pilot Animal Study. Biomater. Res. 2024, 28, 0067. [Google Scholar] [CrossRef]
- Al Maruf, D.S.A.; Cheng, K.; Xin, H.; Cheung, V.K.Y.; Foley, M.; Wise, I.K.; Lewin, W.; Froggatt, C.; Wykes, J.; Parthasarathi, K.; et al. A Comparison of In Vivo Bone Tissue Generation Using Calcium Phosphate Bone Substitutes in a Novel 3D Printed Four-Chamber Periosteal Bioreactor. Bioengineering 2023, 10, 1233. [Google Scholar] [CrossRef]
- Fu, R.; Liu, C.; Yan, Y.; Li, Q.; Huang, R.L. Bone defect reconstruction via endochondral ossification: A developmental engineering strategy. J. Tissue Eng. 2021, 12, 20417314211004211. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, Z.; Xiong, H.; Yan, Y.; Chen, X.; Shao, L. A bioactive glass functional hydrogel enhances bone augmentation via synergistic angiogenesis, self-swelling and osteogenesis. Bioact. Mater. 2022, 22, 201–210. [Google Scholar] [CrossRef]
- Zaki, J.; Yusuf, N.; El-Khadem, A.; Scholten, R.J.P.M.; Jenniskens, K. Efficacy of bone-substitute materials use in immediate dental implant placement: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2021, 23, 506–519. [Google Scholar] [CrossRef]
- Park, S.A.; Shin, J.W.; Yang, Y.I.; Kim, Y.K.; Park, K.D.; Lee, J.W.; Jo, I.H.; Kim, Y.J. In Vitro study of osteogenic differentiation of bone marrow stromal cells on heat-treated porcine trabecular bone blocks. Biomaterials 2004, 25, 527–535. [Google Scholar] [CrossRef]
- Amini, Z.; Lari, R. A systematic review of decellularized allograft and xenograft-derived scaffolds in bone tissue regeneration. Tissue Cell 2021, 69, 101494. [Google Scholar] [CrossRef] [PubMed]
- Romasco, T.; Tumedei, M.; Inchingolo, F.; Pignatelli, P.; Montesani, L.; Iezzi, G.; Petrini, M.; Piattelli, A.; Di Pietro, N. A Narrative Review on the Effectiveness of Bone Regeneration Procedures with OsteoBiol® Collagenated Porcine Grafts: The Translational Research Experience over 20 Years. J. Funct. Biomater. 2022, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Correia, F.; Pozza, D.H.; Gouveia, S.; Felino, A.C.; Faria-Almeida, R. Advantages of Porcine Xenograft over Autograft in Sinus Lift: A Randomised Clinical Trial. Materials 2021, 14, 3439. [Google Scholar] [CrossRef]
- Salamanca, E.; Hsu, C.C.; Yao, W.L.; Choy, C.S.; Pan, Y.H.; Teng, N.C.; Chang, W.J. Porcine Collagen-Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo. Polymers 2020, 12, 93. [Google Scholar] [CrossRef]
- Choi, J.W.; Hwang, S.S.; Yun, P.Y.; Kim, Y.K. Horizontal ridge augmentation with porcine bone-derived grafting material: A long-term retrospective clinical study with more than 5 years of follow-up. J. Korean Assoc. Oral Maxillofac. Surg. 2023, 49, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, S.; Kato-Kogoe, N.; Omori, M.; Yamamoto, K.; Taguchi, S.; Fujita, H.; Imagawa, N.; Sunano, A.; Inoue, K.; Ito, Y.; et al. New Bone Formation Process Using Bio-Oss and Collagen Membrane for Rat Calvarial Bone Defect: Histological Observation. Implant. Dent. 2018, 27, 158–164. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Wang, W.; Ge, G.; Xu, N.; Zheng, D.; Jiang, S.; Zhao, G.; Xu, Y.; Wang, Y.; et al. Harmine Alleviates Titanium Particle-Induced Inflammatory Bone Destruction by Immunomodulatory Effect on the Macrophage Polarization and Subsequent Osteogenic Differentiation. Front. Immunol. 2021, 12, 657687. [Google Scholar] [CrossRef]
- Bhat, S.; Uthappa, U.T.; Altalhi, T.; Jung, H.Y.; Kurkuri, M.D. Functionalized Porous Hydroxyapatite Scaffolds for Tissue Engineering Applications: A Focused Review. ACS Biomater. Sci. Eng. 2022, 8, 4039–4076. [Google Scholar] [CrossRef]
- Suzuki, O. Octacalcium phosphate (OCP)-based bone substitute materials. Jpn. Dent. Sci. Rev. 2013, 49, 58–71. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Prathigudupu, R.S.; Jairaj, A.; Khader, M.A.; Rajeev, K.; Khader, A.A. Role of Synthetic Hydroxyapatite-In Socket Preservation: A Systematic Review and Meta-analysis. J. Contemp. Dent. Pract. 2019, 20, 987–993. [Google Scholar]
- Kaneko, A.; Marukawa, E.; Harada, H. Hydroxyapatite Nanoparticles as Injectable Bone Substitute Material in a Vertical Bone Augmentation Model. In Vivo 2020, 34, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.R.G.; Astarita, C.; D’Aquino, R.; Pelegrine, A.A. Evaluation of Bone Regeneration in Rat Calvaria Using Bone Autologous Micrografts and Xenografts: Histological and Histomorphometric Analysis. Materials 2020, 13, 4284. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K. Biomaterials for Clinical Applications; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Khaddour, A.S.; Ghiță, R.E.; Ionescu, M.; Rîcă, R.G.; Mercuț, V.; Manolea, H.O.; Camen, A.; Drăghici, E.C.; Radu, A.; Popescu, S.M. Healing of Extraction Sites after Alveolar Ridge Preservation Using Advanced Platelet-Rich Fibrin: A Retrospective Study. Bioengineering 2024, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Trajculeski, S.; Veleska-Stevkovska, D.; Trajculeski, M. Advanced Platelet-rich Fibrin versus Sticky Bone in Socket Preservation-Clinical and X-ray Assessment: Case Report. South East Eur. J. Immunol. 2023, 6, 12–17. [Google Scholar] [CrossRef]






| Sample | Measurements | P (2M–4M) | |||
|---|---|---|---|---|---|
| 2 Months | 4 Months | ||||
| Mean | Standard Deviation | Mean | Standard Deviation | ||
| Positive Control Group (Porcine Xenograft) | 0.416 | 0.063 | 0.331 | 0.037 | 0.057 * |
| Study Group (Synthetic Hydroxyapatite Reinforced with Titanium Particles) | 0.364 | 0.060 | 0.238 | 0.042 | 0.026 *,# |
| 0.288 ** | 0.039 **,# | ||||
| Sample | Bone Surface | Mean Thickness of the Trabeculae | Mean Diameter of the Osteocytes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 Months | 4 Months | p | 2 Months | 4 Months | p | 2 Months | 4 Months | p | |
| Negative Control Group | 102.436 ± 125.012 | 122.037 ± 127.383 | 0.221 * | 8.63 ± 46.388 | 18.716 ± 92.024 | 0.120 * | 15.502 ± 69.553 | 17.606 ± 66.883 | 0.472 * |
| Positive Control Group (Porcine Xenograft) | 109.494 ± 126.222 | 112.129 ± 126.570 | 0.047 *,# | 6.910 ± 44.401 | 6.674 ± 36.082 | 0.763 * | 14.376 ± 10.317 | 13.917 ± 9.368 | 0.387 * |
| Study Group (Synthetic Hydroxyapatite Reinforced with Titanium Particles) | 45.372 ± 97.372 | 77.642 ± 117.348 | 0.006 *,## | 12.143 ± 38.125 | 10.582 ± 38.597 | 0.255 * | 13.895 ± 13.520 | 12.663 ± 8.386 | 0.059 * |
| 0.019 **,# | 0.037 **,# | 0.128 **,# | 0.024 **,# | 0.685 ** | 0.048 **,# | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaddour, A.S.; Drăghici, E.C.; Ionescu, M.; Andrei, C.E.; Ghiţă, R.E.; Mercuţ, R.; Gîngu, O.; Sima, G.; Toma Tumbar, L.; Popescu, S.M. Bone Regeneration in Defects Created on Rat Calvaria Grafted with Porcine Xenograft and Synthetic Hydroxyapatite Reinforced with Titanium Particles—A Microscopic and Histological Study. J. Funct. Biomater. 2025, 16, 146. https://doi.org/10.3390/jfb16040146
Khaddour AS, Drăghici EC, Ionescu M, Andrei CE, Ghiţă RE, Mercuţ R, Gîngu O, Sima G, Toma Tumbar L, Popescu SM. Bone Regeneration in Defects Created on Rat Calvaria Grafted with Porcine Xenograft and Synthetic Hydroxyapatite Reinforced with Titanium Particles—A Microscopic and Histological Study. Journal of Functional Biomaterials. 2025; 16(4):146. https://doi.org/10.3390/jfb16040146
Chicago/Turabian StyleKhaddour, Antonia Samia, Emma Cristina Drăghici, Mihaela Ionescu, Cristina Elena Andrei, Răzvan Eugen Ghiţă, Răzvan Mercuţ, Oana Gîngu, Gabriela Sima, Lavinia Toma Tumbar, and Sanda Mihaela Popescu. 2025. "Bone Regeneration in Defects Created on Rat Calvaria Grafted with Porcine Xenograft and Synthetic Hydroxyapatite Reinforced with Titanium Particles—A Microscopic and Histological Study" Journal of Functional Biomaterials 16, no. 4: 146. https://doi.org/10.3390/jfb16040146
APA StyleKhaddour, A. S., Drăghici, E. C., Ionescu, M., Andrei, C. E., Ghiţă, R. E., Mercuţ, R., Gîngu, O., Sima, G., Toma Tumbar, L., & Popescu, S. M. (2025). Bone Regeneration in Defects Created on Rat Calvaria Grafted with Porcine Xenograft and Synthetic Hydroxyapatite Reinforced with Titanium Particles—A Microscopic and Histological Study. Journal of Functional Biomaterials, 16(4), 146. https://doi.org/10.3390/jfb16040146









