Therapeutic Potential of Nano-Sustained-Release Factors for Bone Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Attribute Requirements for Bone Scaffolds
2.2. Scaffold Material Types
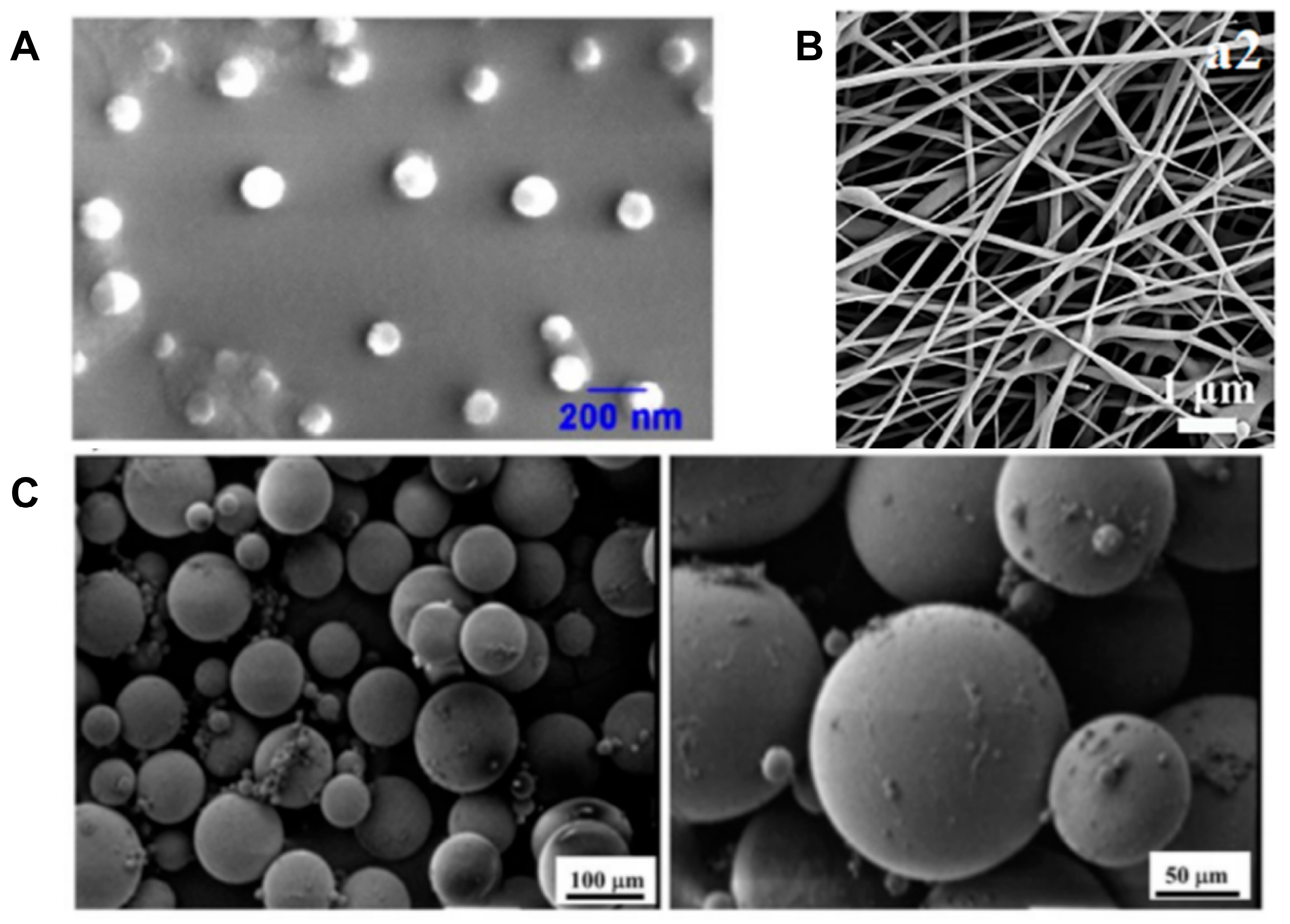
2.3. Preparation Techniques

2.4. Sustained-Release Factors
2.4.1. Bone Morphogenetic Proteins
2.4.2. Small-Molecule Drugs
2.4.3. Anti-Osteoporosis Drug
2.4.4. Detection Method of Drug Release
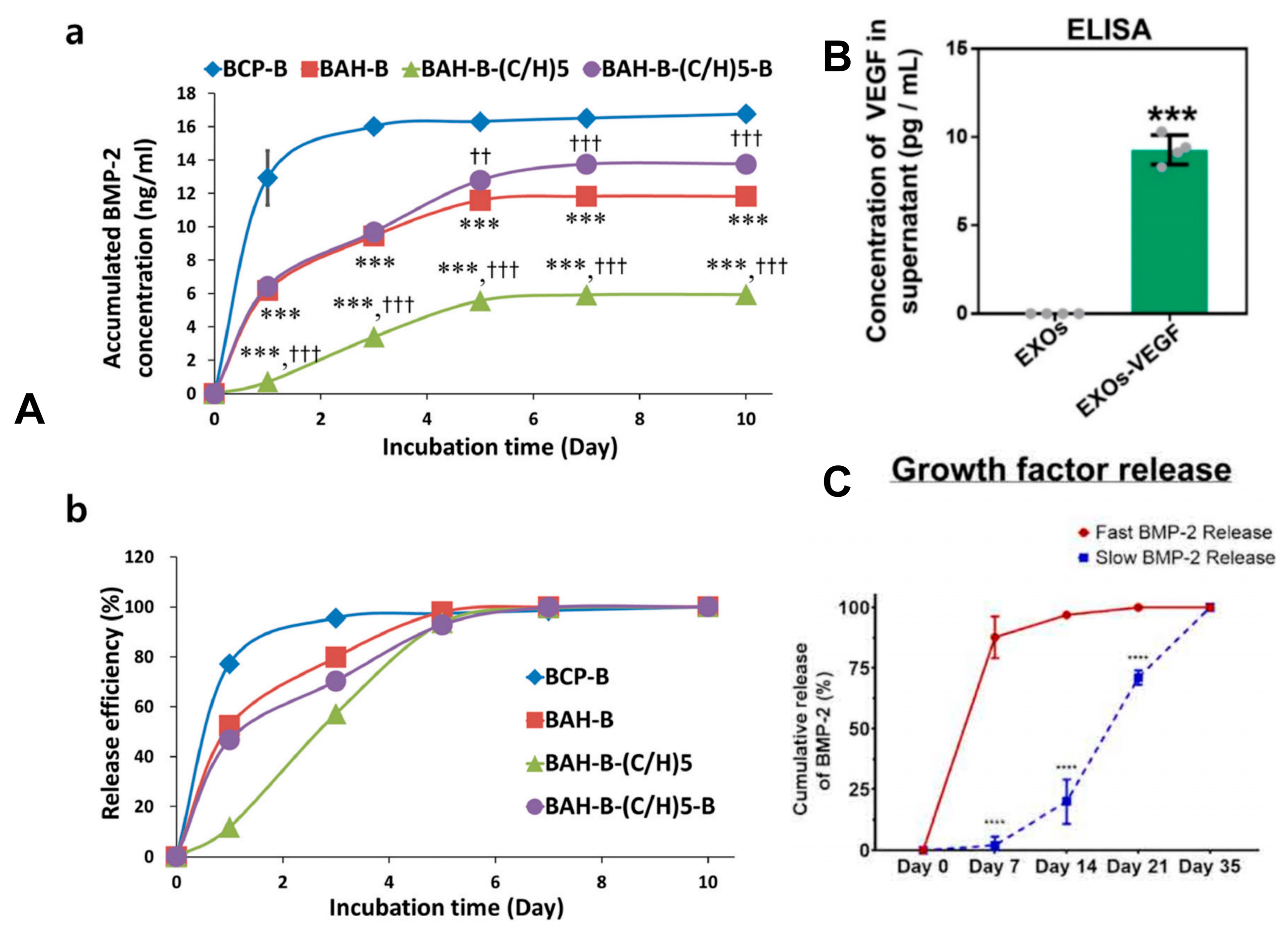
2.5. Sustained-Release Mechanisms and Controlled-Release Strategies
2.5.1. Liposomes
2.5.2. Polymer Nanoparticles (Such as PLGA, PCL, etc.)
2.5.3. Mesoporous Materials
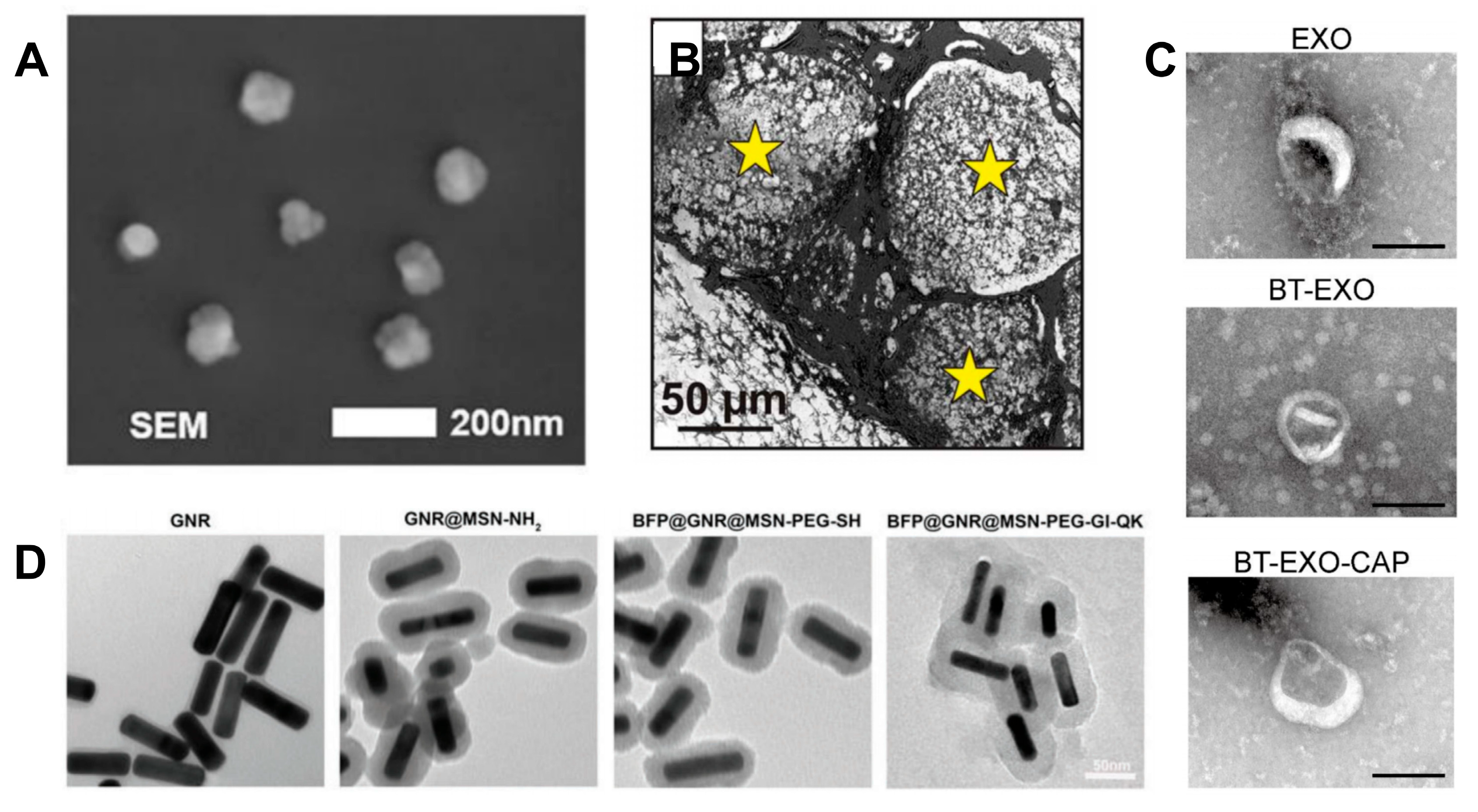
2.5.4. Exosomes
2.5.5. Strategies for Growth Factor Binding and Release Mechanisms
3. Clinical Application Scenarios of Bone Scaffolds
4. Clinical Translation and Challenges
- (Medtronic)’s collagen sponge scaffold, which is loaded with recombinant human BMP-2 (rhBMP-2), is used for spinal fusion and tibial fractures. It has a remarkable curative effect (a fusion rate of 95% vs. 85% for autologous bone grafting), but it requires a high dose of BMP-2 (4.2–12 mg), and is prone to side effects such as heterotopic ossification (with an incidence rate of 10–30%) and neuroinflammation [121];
- (Stryker)’s BMP-7 and collagen complex is generally used for the treatment of non-union of bones. However, due to high costs and insufficient market competitiveness, it has gradually withdrawn from the mainstream market [122];
- The following lists some nano-sustained-release bone scaffold systems that have started clinical trials:
- 3.1.
- One study plans to enroll forty patients requiring the extraction of one hopeless tooth, followed by alveolar bone regeneration (ABR) and the placement of an endosteal implant. After exodontia, participants will be randomized and divided into two groups: in the experimental group, the socket will be grafted with rhBMP-2-BBM granules; in the control group, the sockets will be grafted with Bio-Oss granules and covered with porcine collagen barrier membrane;
- 3.2.
- One trial concerned a process in which the surface of a 3D-printed titanium-alloy scaffold is coated with a biphasic sustained-release layer of VEGF/BMP-2, which is used for the repair of comminuted fractures. The bone density recovery rate reached 90% of the normal bone structure 12 months after surgery.
- The following lists some scaffolds that have not yet undergone clinical trials:
- 4.1.
- Gene-activated scaffold (GAM): PEI-modified mesoporous silica nanoparticles are used to deliver the Runx2 gene. In animal experiments, the volume of new bone has been shown to be three times higher than that of traditional scaffolds. The initiation of a Phase I clinical trial is planned for 2025;
- 4.2.
- Intelligent response system: pH-sensitive PLGA microspheres are loaded with vancomycin, which can achieve on-demand drug release in an infected bone defect model, with a bacterial clearance rate of >99%.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar]
- Kim, M.J.; Park, J.H.; Seok, J.M.; Jung, J.; Hwang, T.S.; Lee, H.C.; Lee, J.H.; Park, S.A.; Byun, J.H.; Oh, S.H. BMP-2-immobilized PCL 3D printing scaffold with a leaf-stacked structure as a physically and biologically activated bone graft. Biofabrication 2024, 16, 025014. [Google Scholar]
- Huang, L.; Zhang, S.; Bian, M.; Xiang, X.; Xiao, L.; Wang, J.; Lu, S.; Chen, W.; Zhang, C.; Mo, G.; et al. Injectable, anti-collapse, adhesive, plastic and bioactive bone graft substitute promotes bone regeneration by moderating oxidative stress in osteoporotic bone defect. Acta Biomater. 2024, 180, 82–103. [Google Scholar]
- Xie, X.; Cai, J.; Li, D.; Chen, Y.; Wang, C.; Hou, G.; Steinberg, T.; Rolauffs, B.; El-Newehy, M.; El-Hamshary, H.; et al. Multiphasic bone-ligament-bone integrated scaffold enhances ligamentization and graft-bone integration after anterior cruciate ligament reconstruction. Bioact. Mater. 2024, 31, 178–191. [Google Scholar]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar]
- Buck, D.W., 2nd; Dumanian, G.A. Bone biology and physiology: Part I. The fundamentals. Plast. Reconstr. Surg. 2012, 129, 1314–1320. [Google Scholar]
- Olsen, B.R.; Reginato, A.M.; Wang, W. Bone development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar]
- Ding, P.; Gao, C.; Gao, Y.; Liu, D.; Li, H.; Xu, J.; Chen, X.; Huang, Y.; Zhang, C.; Zheng, M.; et al. Osteocytes regulate senescence of bone and bone marrow. eLife 2022, 11, e81480. [Google Scholar]
- Petre, D.G.; Leeuwenburgh, S.C.G. The Use of Fibers in Bone Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 141–159. [Google Scholar]
- Wysolmerski, J.J. Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone 2013, 54, 230–236. [Google Scholar] [PubMed]
- Cipriani, C.; Colangelo, L.; Santori, R.; Renella, M.; Mastrantonio, M.; Minisola, S.; Pepe, J. The Interplay Between Bone and Glucose Metabolism. Front. Endocrinol. 2020, 11, 122. [Google Scholar]
- Willems, W.F.; Kremer, T.; Friedrich, P.; Bishop, A.T. Surgical revascularization in structural orthotopic bone allograft increases bone remodeling. Clin. Orthop. Relat. Res. 2014, 472, 2870–2877. [Google Scholar] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. S2), S18–S22. [Google Scholar]
- Shahdad, S.; Gamble, E.; Matani, J.; Zhang, L.; Gambôa, A. Randomized clinical trial comparing PEG-based synthetic to porcine-derived collagen membrane in the preservation of alveolar bone following tooth extraction in anterior maxilla. Clin. Oral Implant. Res. 2020, 31, 1010–1024. [Google Scholar]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar]
- Zdeblick, T.A.; Cooke, M.E.; Kunz, D.N.; Wilson, D.; McCabe, R.P. Anterior cervical discectomy and fusion using a porous hydroxyapatite bone graft substitute. Spine 1994, 19, 2348–2357. [Google Scholar]
- Chung, J.J.; Yoo, J.; Sum, B.S.T.; Li, S.; Lee, S.; Kim, T.H.; Li, Z.; Stevens, M.M.; Georgiou, T.K.; Jung, Y.; et al. 3D Printed Porous Methacrylate/Silica Hybrid Scaffold for Bone Substitution. Adv. Healthc. Mater. 2021, 10, e2100117. [Google Scholar]
- Mohammadi, M.; Mousavi Shaegh, S.A.; Alibolandi, M.; Ebrahimzadeh, M.H.; Tamayol, A.; Jaafari, M.R.; Ramezani, M. Micro and nanotechnologies for bone regeneration: Recent advances and emerging designs. J. Control. Release Off. J. Control. Release Soc. 2018, 274, 35–55. [Google Scholar]
- Cui, S.H.; Yan, Y.; Lu, A.; Dou, Y.; Li, Z.W.; Zhu, Z.H.; Du, M.Z.; Zhu, Y.F.; Chen, X.; Wang, X.; et al. Nanomedicines Promote Cartilage Regeneration in Osteoarthritis by Synergistically Enhancing Chondrogenesis of Mesenchymal Stem Cells and Regulating Inflammatory Environment. ACS Nano 2024, 18, 8125–8142. [Google Scholar]
- Urist, M.R.; Iwata, H.; Ceccotti, P.L.; Dorfman, R.L.; Boyd, S.D.; McDowell, R.M.; Chien, C. Bone morphogenesis in implants of insoluble bone gelatin. Proc. Natl. Acad. Sci. USA 1973, 70, 3511–3515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, P.B.; Wang, Z.L.; Lyu, Z.W.; Wu, H. Tissue-engineered composite scaffold of poly(lactide-co-glycolide) and hydroxyapatite nanoparticles seeded with autologous mesenchymal stem cells for bone regeneration. J. Zhejiang Univ. Sci. B 2017, 18, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Rajalakshmanan, E.; Wang, C.K.; Chen, C.H.; Fu, Y.C.; Tsai, T.L.; Chang, J.K.; Ho, M.L. PLGA-linked alendronate enhances bone repair in diaphysis defect model. J. Tissue Eng. Regen. Med. 2017, 11, 2603–2612. [Google Scholar] [PubMed]
- Zheng, C.; Zhang, M. 3D-printed PCL/β-TCP/CS composite artificial bone and histocompatibility study. J. Orthop. Surg. Res. 2023, 18, 981. [Google Scholar] [CrossRef]
- Li, P.; Ruan, L.; Jiang, G.; Sun, Y.; Wang, R.; Gao, X.; Yunusov, K.E.; Aharodnikau, U.E.; Solomevich, S.O. Design of 3D polycaprolactone/ε-polylysine-modified chitosan fibrous scaffolds with incorporation of bioactive factors for accelerating wound healing. Acta Biomater. 2022, 152, 197–209. [Google Scholar]
- Oley, M.H.; Oley, M.C.; Langi, F.; Flapper, W.; Islam, A.A.; Hatta, M.; Laidding, S.R.; Limarga, N.; Faruk, M. Serum BMP-2 and osteocalcin levels, and CT Hounsfield unit post hyperbaric oxygen therapy in patients with cleft lip and palate post alveolar bone graft: A case study. Heliyon 2023, 9, e19955. [Google Scholar]
- Song, S.; Zhang, G.; Chen, X.; Zheng, J.; Liu, X.; Wang, Y.; Chen, Z.; Wang, Y.; Song, Y.; Zhou, Q. HIF-1α increases the osteogenic capacity of ADSCs by coupling angiogenesis and osteogenesis via the HIF-1α/VEGF/AKT/mTOR signaling pathway. J. Nanobiotechnol. 2023, 21, 257. [Google Scholar]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112466. [Google Scholar]
- Wu, Z.; Bai, J.; Ge, G.; Wang, T.; Feng, S.; Ma, Q.; Liang, X.; Li, W.; Zhang, W.; Xu, Y.; et al. Regulating Macrophage Polarization in High Glucose Microenvironment Using Lithium-Modified Bioglass-Hydrogel for Diabetic Bone Regeneration. Adv. Healthc. Mater. 2022, 11, e2200298. [Google Scholar] [CrossRef]
- Mosquera Rodríguez, F.S.; Quintero Vélez, A.; Córdoba Urrutia, E.; Ramírez-Malule, H.; Mina Hernandez, J.H. Study of the Degradation of a TPS/PCL/Fique Biocomposite Material in Soil, Compost, and Water. Polymers 2023, 15, 3952. [Google Scholar] [CrossRef]
- Bao, H.; Tian, Y.; Wang, H.; Ye, T.; Wang, S.; Zhao, J.; Qiu, Y.; Li, J.; Pan, C.; Ma, G.; et al. Exosome-loaded degradable polymeric microcapsules for the treatment of vitreoretinal diseases. Nat. Biomed. Eng. 2024, 8, 1436–1452. [Google Scholar] [CrossRef] [PubMed]
- John, J.V.; Choksi, M.; Chen, S.; Boda, S.K.; Su, Y.; McCarthy, A.; Teusink, M.J.; Reinhardt, R.A.; Xie, J. Tethering peptides onto biomimetic and injectable nanofiber microspheres to direct cellular response. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102081. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Alfarhan, M.F.; Mazher, J.; Bayan, Y.; Cooper, P.R.; Dias, G.J.; Adanir, N.; Ratnayake, J. Extraction of Hydroxyapatite from Camel Bone for Bone Tissue Engineering Application. Molecules 2022, 27, 7946. [Google Scholar] [CrossRef]
- Granel, H.; Bossard, C.; Nucke, L.; Wauquier, F.; Rochefort, G.Y.; Guicheux, J.; Jallot, E.; Lao, J.; Wittrant, Y. Optimized Bioactive Glass: The Quest for the Bony Graft. Adv. Healthc. Mater. 2019, 8, e1801542. [Google Scholar] [CrossRef]
- Xing, F.; Chi, Z.; Yang, R.; Xu, D.; Cui, J.; Huang, Y.; Zhou, C.; Liu, C. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration. Int. J. Biol. Macromol. 2021, 184, 170–180. [Google Scholar]
- Che, Z.; Sheng, X.; Sun, Q.; Wu, Y.; Song, K.; Chen, A.; Chen, J.; Chen, Q.; Cai, M. Deferoxamine functionalized alginate-based collagen composite material enhances the integration of metal implant and bone interface. Carbohydr. Polym. 2025, 349 Pt A, 122944. [Google Scholar]
- Cawthray, J.F.; Creagh, A.L.; Haynes, C.A.; Orvig, C. Ion exchange in hydroxyapatite with lanthanides. Inorg. Chem. 2015, 54, 1440–1445. [Google Scholar] [CrossRef]
- Sihn, Y.; Yang, H.M.; Park, C.W.; Yoon, I.H.; Kim, I. Post-substitution of magnesium at Ca(I) of nano-hydroxyapatite surface for highly efficient and selective removal of radioactive (90)Sr from groundwater. Chemosphere 2022, 295, 133874. [Google Scholar] [CrossRef]
- Wingender, B.; Azuma, M.; Krywka, C.; Zaslansky, P.; Boyle, J.; Deymier, A. Carbonate substitution significantly affects the structure and mechanics of carbonated apatites. Acta Biomater. 2021, 122, 377–386. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W.; Betancourt, T. Synthesis, bioactivity and zeta potential investigations of chlorine and fluorine substituted hydroxyapatite. Mater. Sci. Engineering. C Mater. Biol. Appl. 2016, 59, 78–85. [Google Scholar]
- Cho, J.S.; Yoo, D.S.; Chung, Y.C.; Rhee, S.H. Enhanced bioactivity and osteoconductivity of hydroxyapatite through chloride substitution. J. Biomed. Mater. Res. Part A 2014, 102, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Z.; Liu, Q.; Yang, P.; Wang, P.; Wei, S.; Liu, A.; Zhao, Z. Potential Load-Bearing Bone Substitution Material: Carbon-Fiber-Reinforced Magnesium-Doped Hydroxyapatite Composites with Excellent Mechanical Performance and Tailored Biological Properties. ACS Biomater. Sci. Eng. 2022, 8, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, D.; Almora-Barrios, N.; de Leeuw, N.H.; Gervais, C.; Bonhomme, C.; Mauri, F.; Chrzanowski, W.; Knowles, J.C.; Newport, R.J.; Wong, A.; et al. Magnesium incorporation into hydroxyapatite. Biomaterials 2011, 32, 1826–1837. [Google Scholar] [CrossRef]
- Jamilludin, M.A.; Dinatha, I.K.H.; Supii, A.I.; Partini, J.; Kusindarta, D.L.; Yusuf, Y. Functionalized cellulose nanofibrils in carbonate-substituted hydroxyapatite nanorod-based scaffold from long-spined sea urchin (Diadema setosum) shells reinforced with polyvinyl alcohol for alveolar bone tissue engineering. RSC Adv. 2023, 13, 32444–32456. [Google Scholar]
- Swain, S.; Bowen, C.; Rautray, T. Dual response of osteoblast activity and antibacterial properties of polarized strontium substituted hydroxyapatite-Barium strontium titanate composites with controlled strontium substitution. J. Biomed. Mater. Res. Part A 2021, 109, 2027–2035. [Google Scholar]
- Kitsugi, T.; Yamamuro, T.; Nakamura, T.; Oka, M. Transmission electron microscopy observations at the interface of bone and four types of calcium phosphate ceramics with different calcium/phosphorus molar ratios. Biomaterials 1995, 16, 1101–1107. [Google Scholar]
- Liu, Y.; Cui, H.; Zhuang, X.; Wei, Y.; Chen, X. Electrospinning of aniline pentamer-graft-gelatin/PLLA nanofibers for bone tissue engineering. Acta Biomater. 2014, 10, 5074–5080. [Google Scholar]
- Laratta, J.L.; Vivace, B.J.; López-Peña, M.; Guzón, F.M.; Gonzalez-Cantalpeidra, A.; Jorge-Mora, A.; Villar-Liste, R.M.; Pino-Lopez, L.; Lukyanchuk, A.; Taghizadeh, E.A.; et al. 3D-printed titanium cages without bone graft outperform PEEK cages with autograft in an animal model. Spine J. Off. J. N. Am. Spine Soc. 2022, 22, 1016–1027. [Google Scholar]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar]
- Huang, S.; Kang, X.; Cheng, Z.; Ma, P.; Jia, Y.; Lin, J. Electrospinning preparation and drug delivery properties of Eu3+/Tb3+ doped mesoporous bioactive glass nanofibers. J. Colloid Interface Sci. 2012, 387, 285–291. [Google Scholar]
- Zhang, F.; Zuo, B.; Fan, Z.; Xie, Z.; Lu, Q.; Zhang, X.; Kaplan, D.L. Mechanisms and control of silk-based electrospinning. Biomacromolecules 2012, 13, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Kalalinia, F.; Taherzadeh, Z.; Jirofti, N.; Amiri, N.; Foroghinia, N.; Beheshti, M.; Bazzaz, B.S.F.; Hashemi, M.; Shahroodi, A.; Pishavar, E.; et al. Evaluation of wound healing efficiency of vancomycin-loaded electrospun chitosan/poly ethylene oxide nanofibers in full thickness wound model of rat. Int. J. Biol. Macromol. 2021, 177, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Feng, Z.; He, W.; Li, C.; Han, S.; Li, Z.; Guo, R. Novel 3D-printing bilayer GelMA-based hydrogel containing BP,β-TCP and exosomes for cartilage-bone integrated repair. Biofabrication 2023, 16, 7946. [Google Scholar] [CrossRef] [PubMed]
- Aihemaiti, P.; Jia, R.; Aiyiti, W.; Jiang, H.; Kasimu, A. Study on 3D printing process of continuous polyglycolic acid fiber-reinforced polylactic acid degradable composites. Int. J. Bioprinting 2023, 9, 734. [Google Scholar] [CrossRef]
- Cao, B.; Lin, J.; Tan, J.; Li, J.; Ran, Z.; Deng, L.; Hao, Y. 3D-printed vascularized biofunctional scaffold for bone regeneration. Int. J. Bioprinting 2023, 9, 702. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhang, Q.; Fan, Y.; Zhang, H.; Ahmad, K.; Hou, H. Distribution, Typical Structure and Self-Assembly Properties of Collagen from Fish Skin and Bone. Molecules 2023, 28, 6529. [Google Scholar] [CrossRef]
- Feng, K.; Liu, J.; Gong, L.; Ye, T.; Chen, Z.; Wang, Y.; Li, Q.; Xie, X. Engineered MSC-sEVs as a Versatile Nanoplatform for Enhanced Osteoarthritis Treatment via Targeted Elimination of Senescent Chondrocytes and Maintenance of Cartilage Matrix Metabolic Homeostasis. Adv. Sci. (Weinh. Baden Wurtt. Ger.) 2025, 12, e2413759. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Gao, R.; Liu, X.; Feng, Z.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. Biomimetic glycopeptide hydrogel coated PCL/nHA scaffold for enhanced cranial bone regeneration via macrophage M2 polarization-induced osteo-immunomodulation. Biomaterials 2022, 285, 121538. [Google Scholar] [CrossRef]
- Zhong, W.; Li, J.; Hu, C.; Quan, Z.; Jiang, D. Enhancement of the bone-implant interface by applying a plasma-sprayed titanium coating on nanohydroxyapatite/polyamide66 implants in a rabbit model. Sci. Rep. 2021, 11, 19971. [Google Scholar] [CrossRef]
- Howard, M.T.; Wang, S.; Berger, A.G.; Martin, J.R.; Jalili-Firoozinezhad, S.; Padera, R.F.; Hammond, P.T. Sustained release of BMP-2 using self-assembled layer-by-layer film-coated implants enhances bone regeneration over burst release. Biomaterials 2022, 288, 121721. [Google Scholar] [CrossRef]
- Han, S.; Paeng, K.W.; Park, S.; Jung, U.W.; Cha, J.K.; Hong, J. Programmed BMP-2 release from biphasic calcium phosphates for optimal bone regeneration. Biomaterials 2021, 272, 120785. [Google Scholar] [CrossRef] [PubMed]
- Schwarting, T.; Benölken, M.; Ruchholtz, S.; Frink, M.; Lechler, P. Bone morphogenetic protein-7 enhances bone-tendon integration in a murine in vitro co-culture model. Int. Orthop. 2015, 39, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Vanhatupa, S.; Ojansivu, M.; Autio, R.; Juntunen, M.; Miettinen, S. Bone Morphogenetic Protein-2 Induces Donor-Dependent Osteogenic and Adipogenic Differentiation in Human Adipose Stem Cells. Stem Cells Transl. Med. 2015, 4, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Luther, G.A.; Harris, M.B.; Farokhzad, O.C.; Mahmoudi, M. Nanomedicine for safe healing of bone trauma: Opportunities and challenges. Biomaterials 2017, 146, 168–182. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, G.P.; Zhang, Z.P.; Xia, J.J.; Yan, J.L.; Pan, S.H. BMP-2/PLGA delayed-release microspheres composite graft, selection of bone particulate diameters, and prevention of aseptic inflammation for bone tissue engineering. Ann. Biomed. Eng. 2010, 38, 632–639. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397–409. [Google Scholar] [CrossRef]
- Geng, Y.; Duan, H.; Xu, L.; Witman, N.; Yan, B.; Yu, Z.; Wang, H.; Tan, Y.; Lin, L.; Li, D.; et al. BMP-2 and VEGF-A modRNAs in collagen scaffold synergistically drive bone repair through osteogenic and angiogenic pathways. Commun. Biol. 2021, 4, 82. [Google Scholar] [CrossRef]
- Freeman, F.E.; Pitacco, P.; van Dommelen, L.H.A.; Nulty, J.; Browe, D.C.; Shin, J.Y.; Alsberg, E.; Kelly, D.J. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci. Adv. 2020, 6, eabb5093. [Google Scholar] [CrossRef]
- Pei, F.; Ma, L.; Jing, J.; Feng, J.; Yuan, Y.; Guo, T.; Han, X.; Ho, T.V.; Lei, J.; He, J.; et al. Sensory nerve niche regulates mesenchymal stem cell homeostasis via FGF/mTOR/autophagy axis. Nat. Commun. 2023, 14, 344. [Google Scholar] [CrossRef]
- Bidossi, A.; Bottagisio, M.; Logoluso, N.; De Vecchi, E. In Vitro Evaluation of Gentamicin or Vancomycin Containing Bone Graft Substitute in the Prevention of Orthopedic Implant-Related Infections. Int. J. Mol. Sci. 2020, 21, 9250. [Google Scholar] [CrossRef]
- Sawin, P.D.; Dickman, C.A.; Crawford, N.R.; Melton, M.S.; Bichard, W.D.; Sonntag, V.K. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J. Neurosurg. 2001, 94 (Suppl. S1), 76–81. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- He, K.; Jiang, H.; Li, W.; Toutounchi, S.; Huang, Y.; Wu, J.; Ma, X.; Baehr, W.; Pignolo, R.J.; Ling, K.; et al. Primary cilia mediate skeletogenic BMP and Hedgehog signaling in heterotopic ossification. Sci. Transl. Med. 2024, 16, eabn3486. [Google Scholar] [CrossRef]
- Evans, C.H. Gene delivery to bone. Adv. Drug Deliv. Rev. 2012, 64, 1331–1340. [Google Scholar]
- Lü, K.; Zeng, D.; Zhang, Y.; Xia, L.; Xu, L.; Kaplan, D.L.; Jiang, X.; Zhang, F. BMP-2 gene modified canine bMSCs promote ectopic bone formation mediated by a nonviral PEI derivative. Ann. Biomed. Eng. 2011, 39, 1829–1839. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Z.; Koh, J.T.; Jin, T.; Franceschi, R.T. Combinatorial gene therapy for bone regeneration: Cooperative interactions between adenovirus vectors expressing bone morphogenetic proteins 2, 4, and 7. J. Cell. Biochem. 2005, 95, 1–16. [Google Scholar] [CrossRef]
- Mora-Raimundo, P.; Lozano, D.; Manzano, M.; Vallet-Regí, M. Nanoparticles to Knockdown Osteoporosis-Related Gene and Promote Osteogenic Marker Expression for Osteoporosis Treatment. ACS Nano 2019, 13, 5451–5464. [Google Scholar] [CrossRef]
- Đorđević, S.; Medel, M.; Hillaert, J.; Masiá, E.; Conejos-Sánchez, I.; Vicent, M.J. Critical Design Strategies Supporting Optimized Drug Release from Polymer-Drug Conjugates. Small (Weinh. Der Bergstr. Ger.) 2024, 20, e2303157. [Google Scholar] [CrossRef]
- Li, J.; Wei, G.; Liu, G.; Du, Y.; Zhang, R.; Wang, A.; Liu, B.; Cui, W.; Jia, P.; Xu, Y. Regulating Type H Vessel Formation and Bone Metabolism via Bone-Targeting Oral Micro/Nano-Hydrogel Microspheres to Prevent Bone Loss. Adv. Sci. (Weinh. Baden-Wurtt. Ger.) 2023, 10, e2207381. [Google Scholar] [CrossRef]
- Lee, C.S.; Hsu, G.C.; Sono, T.; Lee, M.; James, A.W. Development of a Biomaterial Scaffold Integrated with Osteoinductive Oxysterol Liposomes to Enhance Hedgehog Signaling and Bone Repair. Mol. Pharm. 2021, 18, 1677–1689. [Google Scholar] [CrossRef]
- Yang, N.; Sun, Q.; Wang, Y.; Mei, D.; Wang, X.; Zhang, J.; Liu, D.; Huo, R.; Tian, Y.; Su, Y.; et al. Endosomal disruption by co-encapsulating gentamicin in lipid nanoparticles for efficient siRNA delivery and cancer therapy. Asian J. Pharmceutical Sci. 2024, 12, 101011. [Google Scholar]
- Wang, D.Y.; Yang, G.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Liposomes with Water as a pH-Responsive Functionality for Targeting of Acidic Tumor and Infection Sites. Angew. Chem. (Int. Ed. Engl.) 2021, 60, 17714–17719. [Google Scholar] [PubMed]
- Feng, S.; Wu, Z.X.; Zhao, Z.; Liu, J.; Sun, K.; Guo, C.; Wang, H.; Wu, Z. Engineering of Bone- and CD44-Dual-Targeting Redox-Sensitive Liposomes for the Treatment of Orthotopic Osteosarcoma. ACS Appl. Mater. Interfaces 2019, 11, 7357–7368. [Google Scholar] [PubMed]
- Subbiah, R.; Hwang, M.P.; Van, S.Y.; Do, S.H.; Park, H.; Lee, K.; Kim, S.H.; Yun, K.; Park, K. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue. Adv. Healthc. Mater. 2015, 4, 1982–1992. [Google Scholar]
- Maiti, S.; Manna, S.; Shen, J.; Esser-Kahn, A.P.; Du, W. Mitigation of Hydrophobicity-Induced Immunotoxicity by Sugar Poly(orthoesters). J. Am. Chem. Soc. 2019, 141, 4510–4514. [Google Scholar]
- Yi, M.; Nie, Y.; Zhang, C.; Shen, B. Application of Mesoporous Silica Nanoparticle-Chitosan-Loaded BMP-2 in the Repair of Bone Defect in Chronic Osteomyelitis. J. Immunol. Res. 2022, 2022, 4450196. [Google Scholar]
- Zhang, H.; Yuan, S.; Zheng, B.; Wu, P.; He, X.; Zhao, Y.; Zhong, Z.; Zhang, X.; Guan, J.; Wang, H.; et al. Lubricating and Dual-Responsive Injectable Hydrogels Formulated From ZIF-8 Facilitate Osteoarthritis Treatment by Remodeling the Microenvironment. Small (Weinh. Der Bergstr. Ger.) 2025, 21, e2407885. [Google Scholar]
- Chen, Y.; Jin, X.; Lu, J.; Li, S.; Li, C.; Yu, C.; Jiang, G.; Ji, X.; Yao, M.; Xiang, Z.; et al. Enzyme-Photodynamic Adaptive Bionic Periosteum for Bone Revitalization. Adv. Funct. Mater. 2024, 34, 2314120. [Google Scholar]
- Talaat, S.; Hashem, A.A.; Abu-Seida, A.; Abdel Wahed, A.; Abdel Aziz, T.M. Regenerative potential of mesoporous silica nanoparticles scaffold on dental pulp and root maturation in immature dog’s teeth: A histologic and radiographic study. BMC Oral Health 2024, 24, 817. [Google Scholar]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, L.; Zhang, J.; Chiang, C.L.; Pan, J.; Wang, X.; Kwak, K.J.; Li, H.; Zhao, R.; Rima, X.Y.; et al. Exosomal mRNAs for Angiogenic-Osteogenic Coupled Bone Repair. Adv. Sci. (Weinh. Baden Wurtt. Ger.) 2023, 10, e2302622. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Z.; Yu, N.; Zhang, L.; Li, H.; Chen, Y.; Gong, F.; Lin, W.; He, X.; Wang, S.; et al. Bone-targeting exosome nanoparticles activate Keap1/Nrf2/GPX4 signaling pathway to induce ferroptosis in osteosarcoma cells. J. Nanobiotechnol. 2023, 21, 355. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Liao, B.; Peng, S.; Fang, P.; Bao, N.; Zhang, L. Synovial fibroblast-miR-214-3p-derived exosomes inhibit inflammation and degeneration of cartilage tissues of osteoarthritis rats. Mol. Cell. Biochem. 2023, 478, 637–649. [Google Scholar] [CrossRef]
- Xue, B.; Cao, W.; Zhao, H.; Zhang, B.; Liu, J.; Zhang, H.; Qi, H.; Zhou, Q. Nanocrystal hydroxyapatite carrying traditional Chinese medicine for osteogenic differentiation. Colloids Surf. B Biointerfaces 2024, 244, 114186. [Google Scholar] [CrossRef]
- Choudhury, S.; Madhu Krishna, M.; Sen, D.; Ghosh, S.; Basak, P.; Das, A. 3D Porous Polymer Scaffold-Conjugated KGF-Mimetic Peptide Promotes Functional Skin Regeneration in Chronic Diabetic Wounds. ACS Appl. Mater. Interfaces 2024, 16, 37418–37434. [Google Scholar] [CrossRef]
- Shi, Y.; Gu, J.; Zhang, C.; Mi, R.; Ke, Z.; Xie, M.; Jin, W.; Shao, C.; He, Y.; Shi, J.; et al. A Janus Microsphere Delivery System Orchestrates Immunomodulation and Osteoinduction by Fine-tuning Release Profiles. Small (Weinh. Der Bergstr. Ger.) 2024, 20, e2403835. [Google Scholar] [CrossRef]
- Yang, W.; Zou, Q.; Wang, C.; Ren, Y.; Zhang, R.; Lin, M.; Huang, Z.; Huangfu, M.; Lin, L.; Li, W.; et al. Enhancing Bone Regeneration and Osteogenic Quality by n-HA Internalized Osteoblasts Synergized with ON Protein: Mechanistic Insights. ACS Appl. Mater. Interfaces 2024, 16, 68967–68982. [Google Scholar] [CrossRef]
- Grottkau, B.E.; Hui, Z.; Ran, C.; Pang, Y. Fabricating vascularized, anatomically accurate bone grafts using 3D bioprinted sectional bone modules, in-situ angiogenesis, BMP-2 controlled release, and bioassembly. Biofabrication 2024, 16, 045008. [Google Scholar] [CrossRef]
- Polak, D.; Falcoff, D.; Chackartchi, T.; Asher, R.; Assad, R. Sustainability and Release Pattern of Growth Factors from Bone Grafts Prepared with Platelet-Rich Fibrin. Int. J. Oral Maxillofac. Implant. 2024, 39, 473. [Google Scholar]
- Wu, Y.; Liao, Q.; Wu, L.; Luo, Y.; Zhang, W.; Guan, M.; Pan, H.; Tong, L.; Chu, P.K.; Wang, H. ZnL(2)-BPs Integrated Bone Scaffold under Sequential Photothermal Mediation: A Win-Win Strategy Delivering Antibacterial Therapy and Fostering Osteogenesis Thereafter. ACS Nano 2021, 15, 17854–17869. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhuang, Z.M.; Xu, X.; Han, B.; Song, G.Y.; Xu, T.M. Mechanical force increases tooth movement and promotes remodeling of alveolar bone defects augmented with bovine bone mineral. Prog. Orthod. 2024, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Guo, B.; Ma, P.X. Cytocompatible injectable carboxymethyl chitosan/N-isopropylacrylamide hydrogels for localized drug delivery. Carbohydr. Polym. 2014, 103, 110–118. [Google Scholar] [CrossRef]
- Wang, R.; Zha, X.; Chen, J.; Fu, R.; Fu, Y.; Xiang, J.; Yang, W.; Zhao, L. Hierarchical Composite Scaffold with Deferoxamine Delivery System to Promote Bone Regeneration via Optimizing Angiogenesis. Adv. Healthc. Mater. 2024, 13, e2304232. [Google Scholar] [CrossRef]
- Pang, K.M.; Um, I.W.; Kim, Y.K.; Woo, J.M.; Kim, S.M.; Lee, J.H. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin. Oral Implant. Res. 2017, 28, 809–815. [Google Scholar] [CrossRef]
- Demidov, V.V.; Clark, M.A.; Streeter, S.S.; Sottosanti, J.S.; Gitajn, I.L.; Elliott, J.T. High-energy open-fracture model with initial experience of fluorescence-guided bone perfusion assessment. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2023, 41, 1040–1048. [Google Scholar] [CrossRef]
- Ouyang, L.; Li, J.; Dong, Y.; Li, J.; Jin, F.; Luo, Y.; Wang, R.; Wang, S. Comparison of clinical efficacy between autologous partially demineralized dentin matrix and deproteinized bovine bone mineral for bone augmentation in orthodontic patients with alveolar bone deficiency: A randomized controlled clinical trial. BMC Oral Health 2024, 24, 984. [Google Scholar] [CrossRef]
- Jackson, M.; Kummer, M.; Auer, J.; Hagen, R.; Fuerst, A. Treatment of type 2 and 4 olecranon fractures with locking compression plate osteosynthesis in horses: A prospective study (2002–2008). Vet. Comp. Orthop. Traumatol. 2011, 24, 57–61. [Google Scholar]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Ryu, J.I.; Yang, B.E.; Yi, S.M.; Choi, H.G.; On, S.W.; Hong, S.J.; Lim, H.K.; Byun, S.H. Bone Regeneration of a 3D-Printed Alloplastic and Particulate Xenogenic Graft with rhBMP-2. Int. J. Mol. Sci. 2021, 22, 12518. [Google Scholar] [CrossRef]
- Horner, E.A.; Kirkham, J.; Wood, D.; Curran, S.; Smith, M.; Thomson, B.; Yang, X.B. Long bone defect models for tissue engineering applications: Criteria for choice. Tissue Eng. Part B Rev. 2010, 16, 263–271. [Google Scholar] [PubMed]
- Liu, F.; Cheng, X.; Xiao, L.; Wang, Q.; Yan, K.; Su, Z.; Wang, L.; Ma, C.; Wang, Y. Inside-outside Ag nanoparticles-loaded polylactic acid electrospun fiber for long-term antibacterial and bone regeneration. Int. J. Biol. Macromol. 2021, 167, 1338–1348. [Google Scholar] [PubMed]
- Jamil, K.; Zacharin, M.; Foster, B.; Donald, G.; Hassall, T.; Siafarikas, A.; Johnson, M.; Tham, E.; Whitewood, C.; Gebski, V.; et al. Protocol for a randomised control trial of bisphosphonate (zoledronic acid) treatment in childhood femoral head avascular necrosis due to Perthes disease. BMJ Paediatr. Open 2017, 1, e000084. [Google Scholar] [PubMed]
- Li, C.J.; Park, J.H.; Jin, G.S.; Mandakhbayar, N.; Yeo, D.; Lee, J.H.; Lee, J.H.; Kim, H.S.; Kim, H.W. Strontium/Silicon/Calcium-Releasing Hierarchically Structured 3D-Printed Scaffolds Accelerate Osteochondral Defect Repair. Adv. Healthc. Mater. 2024, 13, e2400154. [Google Scholar]
- Lu, Y.; Li, M.; Long, Z.; Di, Y.; Guo, S.; Li, J.; Liu, D.; Gao, P.; Chen, G.; Lu, X.; et al. Collagen/β-TCP composite as a bone-graft substitute for posterior spinal fusion in rabbit model: A comparison study. Biomed. Mater. (Bristol Engl.) 2019, 14, 045009. [Google Scholar]
- Liu, C.; Wang, C.; Liu, Y.; Huang, J.; Xu, W.; Li, J.; Wang, Y.; Xu, Y.; Zhu, L.; Xu, H. Selenium nanoparticles/carboxymethyl chitosan/alginate antioxidant hydrogel for treating steroid-induced osteonecrosis of the femoral head. Int. J. Pharm. 2024, 653, 123929. [Google Scholar]
- Wang, G.; Li, Y.; Sun, T.; Wang, C.; Qiao, L.; Wang, Y.; Dong, K.; Yuan, T.; Chen, J.; Chen, G.; et al. BMSC affinity peptide-functionalized β-tricalcium phosphate scaffolds promoting repair of osteonecrosis of the femoral head. J. Orthop. Surg. Res. 2019, 14, 204. [Google Scholar]
- Cohen, D.J.; Lohmann, C.H.; Scott, K.M.; Olson, L.C.; Boyan, B.D.; Schwartz, Z. Osseointegration and Remodeling of Mineralized Bone Graft Are Negatively Impacted by Prior Treatment with Bisphosphonates. J. Bone Jt. Surg. Am. Vol. 2022, 104, 1750–1759. [Google Scholar]
- Hassanein, A.H.; Arany, P.R.; Couto, R.A.; Clune, J.E.; Glowacki, J.; Rogers, G.F.; Mulliken, J.B.; Greene, A.K. Cranial particulate bone graft ossifies calvarial defects by osteogenesis. Plast. Reconstr. Surg. 2012, 129, 796e–802e. [Google Scholar]
- Sun, H.; Liu, W.; Zhou, G.; Zhang, W.; Cui, L.; Cao, Y. Tissue engineering of cartilage, tendon and bone. Front. Med. 2011, 5, 61–69. [Google Scholar]
- Singh, K.; Dumonski, M.; Stanley, T.; Ponnappan, R.; Phillips, F.M. Repeat use of human recombinant bone morphogenetic protein-2 for second level lumbar arthrodesis. Spine 2011, 36, 192–196. [Google Scholar] [CrossRef]
- Poynton, A.R.; Lane, J.M. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine 2002, 27 (Suppl. S16), S40–S48. [Google Scholar] [CrossRef]
| Electrospinning | 3D Printing | Self-Assembly | Nanocoating | |
|---|---|---|---|---|
| Applicable Materials | Synthetic polymers (PLA, PCL), natural polymers (collagen, chitosan) | Thermoplastics (PLGA, PEG), ceramics (hydroxyapatite), metal composites | Peptides, liposomes, amphiphilic polymers, DNA nanostructures | Titanium alloys, bioceramics, polymer substrates; coating materials (PLGA, chitosan, bioactive glass) |
| Applicable Factors | Antibiotics (gentamicin), growth factors (BMP-2, VEGF), small-molecule drugs | Cells (stem cells), macromolecular proteins (collagen), drugs (bisphosphonates) | Genes (siRNA), enzymes, hydrophobic drugs (paclitaxel) | Antimicrobials (Ag nanoparticles), osteogenic factors (Ca2+/PO43− ions), anti-inflammatory drugs (dexamethasone) |
| Loading Capacity | High loading (intrafiber encapsulation), uneven distribution | Moderate loading (porosity-controlled release), high spatial specificity | Low loading (molecular-level encapsulation), uniform distribution | Low-medium loading (surface adsorption/covalent bonding), tunable release kinetics |
| Release Mechanism | Fiber network delays diffusion + material degradation synergy | Macropore architecture regulation + material gradient design | Intermolecular forces (H-bonding/hydrophobic interactions) forming nanocapsules | Surface coating chemisorption/physisorption + ion-exchange release |
| Mechanical Properties | High flexibility (5–50 MPa), low compressive strength | High strength (50–200 MPa), trabecular bone-mimicking capability | Poor mechanical strength (<5 MPa), requires reinforcement | Substrate-dependent (titanium >300 MPa), coatings enhance surface bioactivity |
| Degradation Control | Tunable via polymer blending (3–12 months) | Material-dependent (PLGA: 6 months; ceramics: non-degradable) | Dynamic responsive degradation (pH/enzyme-triggered) | Independent coating degradation (1–-6 months), decoupled from substrate |
| Clinical Potential | Soft tissue-bone interface repair (enthesis regeneration) | Personalized large-segment defect reconstruction (craniofacial surgery) | Targeted drug delivery (post-tumor resection filling) | Anti-infection/osseointegration in joint implants (hip revision surgery) |
| Current Challenges | Limited 3D structural complexity; dense fibers impede cell infiltration | Trade-off between high-resolution printing and bioactivity; insufficient vascularization | Scalability issues; poor in vivo stability | Long-term delamination risks; microcracks due to interfacial stress |
| Future Directions | Multi-nozzle heterogeneous fiber composites + microfluidic electrospinning | 4D-printed shape-memory scaffolds + integrated vascular networks | Biomimetic mineralization for mechanical enhancement + stimuli-responsive assembly | Atomic Layer Deposition (ALD) gradient coatings + self-healing coatings |
| Summery: 1. Electrospinning and 3D printing suit high-dose sustained release, while self-assembly and nanocoating show promise for targeted delivery. 2. 3D printing allows customization for cortical or trabecular bone requirements; other techniques require composite reinforcement. 3. Electrospinning and nanocoating lead in translation, whereas self-assembly remains preclinical | ||||
| Status of Approval | Future Therapeutic Potential | Key Technologies/Mechanisms | Main Challenges | |
|---|---|---|---|---|
| collagen sponge scaffold | FDA approval (for spinal fusion and tibia fractures) | High fusion rate (95% vs. 85% autologous bone graft) but dose optimization to reduce side effects | Collagen sponge scaffold loaded rhBMP-2 and promoted osteogenesis through sustained release of growth factors | High dose BMP-2 (4.2–12 mg) causes heterotopic ossification (10–30%), neuroinflammation and other side effects |
| BMP-7 and collagen complex | Has obtained FDA/CE certification, and gradually withdrew from the mainstream market due to the lack of market competitiveness | Suitable for nonunion therapy and need to reduce costs to improve clinical accessibility | BMP-7 is complexed with collagen, which stimulates bone regeneration | The cost is high, and the advantage of the efficacy compared with autologous bone transplantation is not significant |
| rhBMP-2-BBM granules | Clinical phase II trial (alveolar bone regeneration test in 40 patients) | May replace the traditional Bio-Oss bone powder and shorten the implant implantation cycle | Bioactive particles loaded with rhBMP-2, promoting a local increase in Bdensity | Small sample test, long-term stability to be verified |
| VEGF/BMP-2 coated 3D printed titanium-alloy scaffold | Phase I/II clinical trial (fracture repair) | With personalized bone defect repair, the recovery rate of BMD reached 90% in 12 months | The biphasic slow-release layer (VE GF/BMP-2) on the surface of the titanium alloy stent jointly promotes angiogenesis and osteogenesis | Long-term coating stability and large-scale production feasibility are yet to be solved |
| Gene-activation scaffold (GAM) | Clinical Phase I is scheduled to start in 2025 | In animal experiments, the volume of new bone reached 3 times that of traditional stent, which is suitable for the regeneration of large bone defects | PEI modified mesoporous silica nanoparticles to deliver the Runx 2 gene and enhance the osteogenic differentiation of stem cells | Gene-editing safety is controversial, and the large-scale production process is complex |
| Intelligent-responsive PLGA microspheres | Preclinical study (infectious bone defect model) | PH Triggered drug release, bacterial clearance rate > 99%, promising to replace systemic antibiotic therapy | PH Sensitive PLGA microsphere load vancomycin, and the infectious microenvironment triggers targeted drug release | The metabolic path in vivo is unknown and the release kinetics need to be optimized to match the bone repair cycle |
| Summery: 1. 3D printing combined with growth factor release (such as VEGF/BMP-2 coated titanium-alloy scaffold) has become the mainstream direction of personalized treatment. 2. GAM drives bone repair, from structural replacement to functional regeneration, spanning. 3. Marketed products face side effects (such as heterotopic ossification of InFuse®), while new technologies (such as intelligent response systems) need to solve large-scale production and long-term safety verification. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Zhang, M.; Qu, Y.; Xing, B.; Wang, B.; Liu, Y.; Zhang, P. Therapeutic Potential of Nano-Sustained-Release Factors for Bone Scaffolds. J. Funct. Biomater. 2025, 16, 136. https://doi.org/10.3390/jfb16040136
Jiang H, Zhang M, Qu Y, Xing B, Wang B, Liu Y, Zhang P. Therapeutic Potential of Nano-Sustained-Release Factors for Bone Scaffolds. Journal of Functional Biomaterials. 2025; 16(4):136. https://doi.org/10.3390/jfb16040136
Chicago/Turabian StyleJiang, Haoran, Meng Zhang, Yang Qu, Bohan Xing, Bojiang Wang, Yanqun Liu, and Peixun Zhang. 2025. "Therapeutic Potential of Nano-Sustained-Release Factors for Bone Scaffolds" Journal of Functional Biomaterials 16, no. 4: 136. https://doi.org/10.3390/jfb16040136
APA StyleJiang, H., Zhang, M., Qu, Y., Xing, B., Wang, B., Liu, Y., & Zhang, P. (2025). Therapeutic Potential of Nano-Sustained-Release Factors for Bone Scaffolds. Journal of Functional Biomaterials, 16(4), 136. https://doi.org/10.3390/jfb16040136









