Polydopamine-Coated Copper-Doped Mesoporous Silica/Gelatin–Waterborne Polyurethane Composite: A Multifunctional GBR Membrane for Bone Defect Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Cu–MSNs
2.2. Synthesis of WPU Dispersions
2.3. Preparation of Gelatin–WPU Membranes and Cu–MSN Doped Gelatin–WPU Membranes
2.4. Preparation of PDA Coated Cu–MSN/GP
2.5. Material Characterization
2.5.1. Characterization of Cu–MSN/GP–PDA Membranes
2.5.2. Mechanical Property
2.5.3. Hydrophilic Property
2.5.4. In Vitro Degradation and Ions Release Property
2.6. In Vitro Biocompatibility Assay
2.7. Transwell Assay
2.8. Anti-Bacterial Test
2.9. In Vivo Assay
2.10. Micro-CT Analysis
2.11. Histological Examination
2.12. Statistical Analysis
3. Results and Discussion
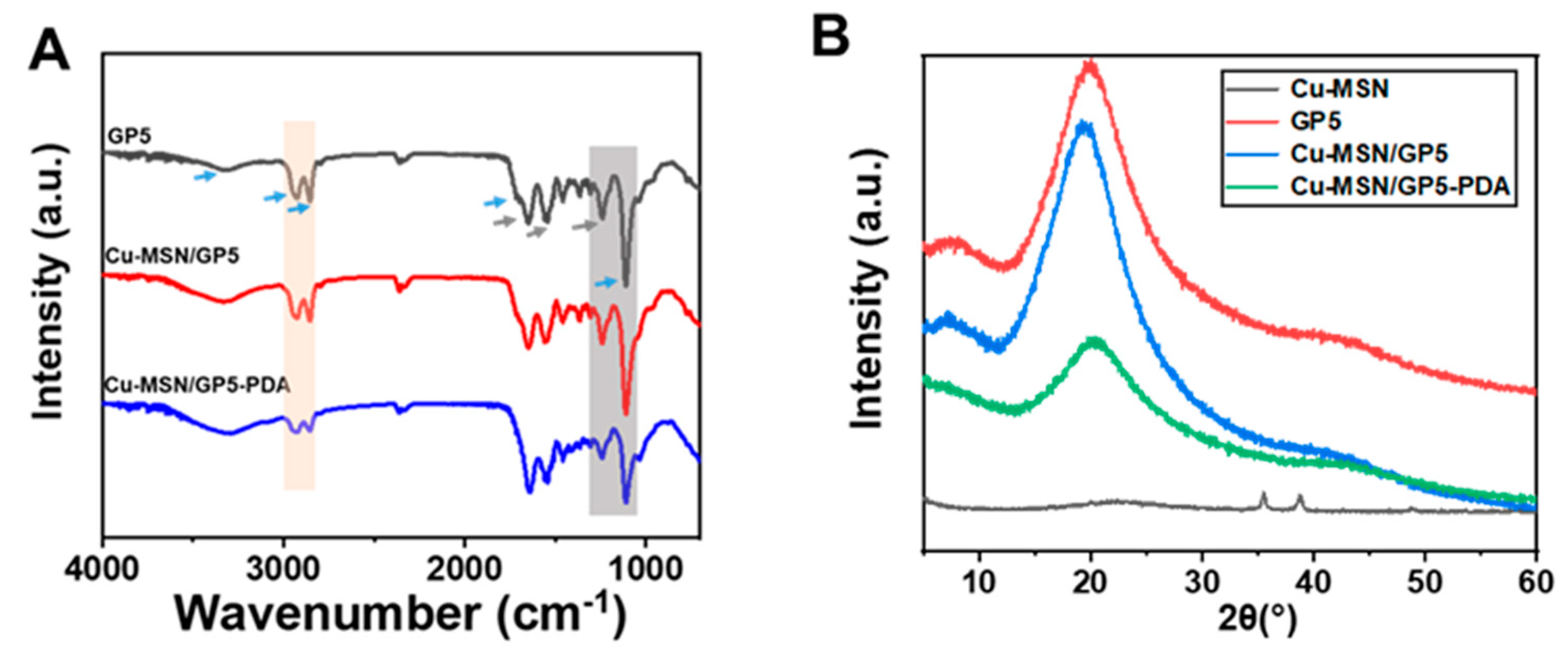
3.1. The Morphology and Characterization of Cu–MSN and Cu–MSN/GP–PDA Membranes
3.2. Mechanical Property
3.3. Hydrophilic Property
3.4. In Vitro Degradation and Ions Release Property
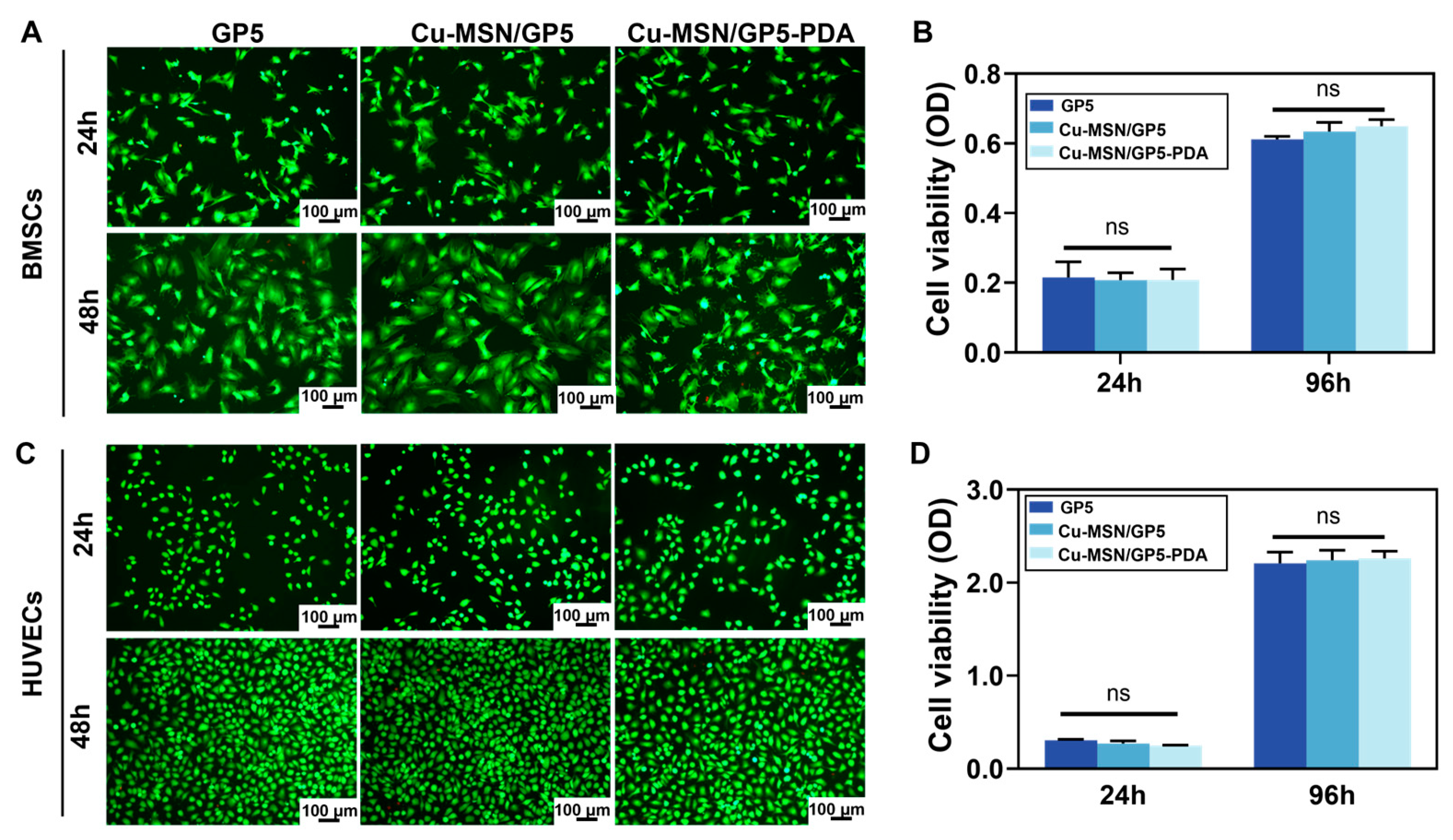
3.5. In Vitro Biocompatibility
3.6. HUVECs Migration and in Vivo Angiopoiesis
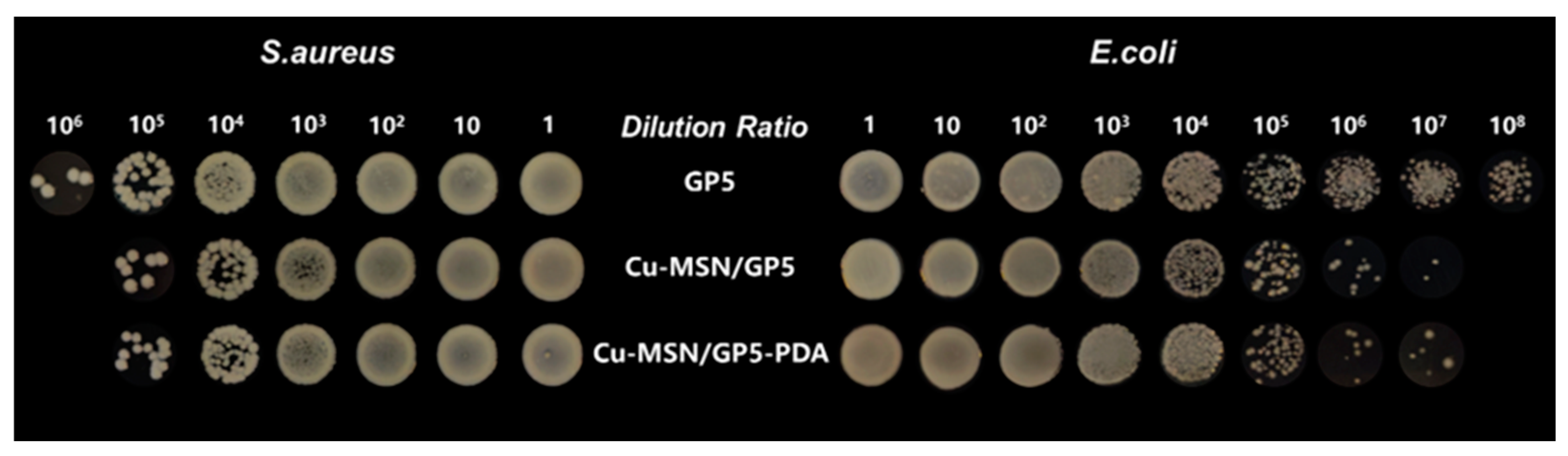
3.7. Anti-Bacterial Test
3.8. Regenerative Potential of Membranes in Bone Defect Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- He, Y.; Tian, M.; Li, X.; Hou, J.; Chen, S.; Yang, G.; Liu, X.; Zhou, S. A Hierarchical-Structured Mineralized Nanofiber Scaffold with Osteoimmunomodulatory and Osteoinductive Functions for Enhanced Alveolar Bone Regeneration. Adv. Healthc. Mater. 2022, 11, 2102236. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Healthc. Mater. 2020, 9, e2000707. [Google Scholar] [CrossRef]
- Major, R.; Kowalczyk, P.; Surmiak, M.; Łojszczyk, I.; Podgórski, R.; Trzaskowska, P.; Ciach, T.; Russmueller, G.; Kasperkiewicz, K.; Major, Ł.; et al. Patient specific implants for jawbone reconstruction after tumor resection. Colloids Surf. B Biointerfaces 2020, 193, 111056. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, X.; Li, X.; An, Q.; Zhang, Y.; Guo, C.; Zhao, Y.; Zhang, Y. Shear Flow-Assembled Janus Membrane with Bifunctional Osteogenic and Antibacterial Effects for Guided Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 3984–3993. [Google Scholar] [CrossRef]
- Li, S.; Zhao, J.; Xie, Y.; Tian, T.; Zhang, T.; Cai, X. Hard tissue stability after guided bone regeneration: A comparison between digital titanium mesh and resorbable membrane. Int. J. Oral Sci. 2021, 13, 37. [Google Scholar] [CrossRef]
- Ebrahimi, L.; Farzin, A.; Ghasemi, Y.; Alizadeh, A.; Goodarzi, A.; Basiri, A.; Zahiri, M.; Monabati, A.; Ai, J. Metformin-Loaded PCL/PVA Fibrous Scaffold Preseeded with Human Endometrial Stem Cells for Effective Guided Bone Regeneration Membranes. ACS Biomater. Sci. Eng. 2021, 7, 222–231. [Google Scholar] [CrossRef]
- Wu, S.; Luo, S.; Cen, Z.; Li, Q.; Li, L.; Li, W.; Huang, Z.; He, W.; Liang, G.; Wu, D.; et al. All-in-one porous membrane enables full protection in guided bone regeneration. Nat. Commun. 2024, 15, 119. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, W.; Yang, W.; Zhang, G.; Huang, C.; Han, J.; Narain, R.; Zeng, H. Dual-Protection Inorganic-Protein Coating on Mg-Based Biomaterials through Tooth-Enamel-Inspired Biomineralization. Adv. Mater. 2024, 36, e2313211. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, J.; Liu, C.; Liu, Y.; Si, M. Radiographic bone volume alteration after jaw cyst enucleation with or without simultaneous bone grafts: A prospective randomized study. Clin. Implant. Dent. Relat. Res. 2022, 24, 468–474. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, J.; Dai, B.; Liu, W.; Wang, J.; Li, Q.; Wang, J.; Zhao, L.; Ngai, T. A Bilayer Membrane Doped with Struvite Nanowires for Guided Bone Regeneration. Adv. Healthc. Mater. 2022, 11, e2201679. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.K.; Möhler, H.; Busch, F.; Mehl, A. Preclinical and clinical studies of a collagen membrane (Bio-Gide). Biomaterials 1997, 18, 535–538. [Google Scholar] [CrossRef]
- Zhang, K.-R.; Gao, H.-L.; Pan, X.-F.; Zhou, P.; Xing, X.; Xu, R.; Pan, Z.; Wang, S.; Zhu, Y.; Hu, B.; et al. Multifunctional Bilayer Nanocomposite Guided Bone Regeneration Membrane. Matter 2019, 1, 770–781. [Google Scholar] [CrossRef]
- Ma, K.; Liao, C.; Huang, L.; Liang, R.; Zhao, J.; Zheng, L.; Su, W. Electrospun PCL/MoS2 Nanofiber Membranes Combined with NIR-Triggered Photothermal Therapy to Accelerate Bone Regeneration. Small 2021, 17, e2104747. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhang, S.; Yang, G.; Li, Y.; Mao, Y.; Yang, L.; Chen, J.; Wang, J. Development of a rapid-shaping and user-friendly membrane with long-lasting space maintenance for guided bone regeneration. J. Mater. Chem. B 2024, 12, 1495–1511. [Google Scholar] [CrossRef]
- Wang, Y.J.; Jeng, U.S.; Hsu, S.H. Biodegradable Water-Based Polyurethane Shape Memory Elastomers for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 1397–1406. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; He, J.S.; Shu, Y.; Yang, H.C.; Liu, J.H.; Zhang, C.; Xiao, W.Q.; Liu, Z.N.; Liao, X.L. Silk fibroin/methacrylated gelatine/hydroxyapatite biomimetic nanofibrous membranes for guided bone regeneration. Int. J. Biol. Macromol. 2024, 263, 130380. [Google Scholar] [CrossRef]
- Shakeri, H.; Nazarpak, M.H.; Imani, R.; Tayebi, L. Poly (L-lactic acid)-based modified nanofibrous membrane with dual drug release capability for GBR application. Int. J. Biol. Macromol. 2023, 231, 123201. [Google Scholar] [CrossRef]
- Luz, E.; das Chagas, B.S.; de Almeida, N.T.; Borges, M.D.; Andrade, F.K.; Muniz, C.R.; Castro-Silva, I.I.; Teixeira, E.H.; Popat, K.; Rosa, M.D.; et al. Resorbable bacterial cellulose membranes with strontium release for guided bone regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 116, 111175. [Google Scholar] [CrossRef]
- Li, J.; Ding, J.; Zhou, T.; Li, B.; Wang, J.; Wang, H.; Fu, L. A novel functionally graded bilayer membrane with excellent barrier function and in vivo osteogenesis promotion for guided bone regeneration. Front. Pharmacol. 2024, 15, 1453036. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.X.; Xie, Y.J.; Wang, L.; Yang, L.S.; Yu, J.H.; Miyamoto, A.; Sun, F.H. Development of FGF-2-loaded electrospun waterborne polyurethane fibrous membranes for bone regeneration. Regen. Biomater. 2021, 8, rbaa046. [Google Scholar] [CrossRef]
- Huang, D.; Yang, D.; Li, K.D.; Wang, J.R.; Zheng, X.H.; Long, J.; Liu, L. A multifunctional collagen-base bilayer membrane integrated with a bimetallic/polydopamine network for enhanced guided bone regeneration. J. Mater. Chem. B 2024, 12, 7171–7190. [Google Scholar] [CrossRef]
- Zeng, J.; Gu, C.; Geng, X.; Lin, K.; Xie, Y.; Chen, X. Combined photothermal and sonodynamic therapy using a 2D black phosphorus nanosheets loaded coating for efficient bacterial inhibition and bone-implant integration. Biomaterials 2023, 297, 122122. [Google Scholar] [CrossRef]
- Chen, X.; Huang, N.; Wang, D.; Zhang, M.; Deng, X.; Guo, F.; Yi, B.; Yuan, C.; Zhou, Q. Sulfated Chitosan-Modified CuS Nanocluster: A Versatile Nanoformulation for Simultaneous Antibacterial and Bone Regenerative Therapy in Periodontitis. ACS Nano 2024, 18, 14312–14326. [Google Scholar] [CrossRef]
- Qiu, X.; Feng, C.; Wang, W.; Wu, G.; Hu, Y.; Li, S.; Gao, X.; Chen, X.; Ji, Q. Copper-deposited diatom-biosilica enhanced osteogenic potential in periodontal ligament stem cells and rat cranium. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 1286–1298. [Google Scholar] [CrossRef]
- Shi, M.; Chen, Z.; Farnaghi, S.; Friis, T.; Mao, X.; Xiao, Y.; Wu, C. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016, 30, 334–344. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.C.; Lee, K.H. Polyurethane Anionomer Dispersion from Erher-type Polyols And Isophorone Diisocyanate. J. Macromol. Sci.-Pure Appl. Chem. 1994, A31, 1241–1257. [Google Scholar] [CrossRef]
- Hia, E.M.; Jang, S.R.; Maharjan, B.; Park, J.; Park, C.H.; Kim, C.S. Construction of a PEGDA/chitosan hydrogel incorporating mineralized copper-doped mesoporous silica nanospheres for accelerated bone regeneration. Int. J. Biol. Macromol. 2024, 262, 130218. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, C.C.; Yan, L.Z.; Lei, H.Q.; Peng, C.X.; Liu, S.; Fan, L.H.; Chu, Y.Y. Antibacterial and osteoconductive polycaprolactone/polylactic acid/nano-hydroxyapatite/Cu@ZIF-8 GBR membrane with asymmetric porous structure. Int. J. Biol. Macromol. 2023, 224, 1040–1051. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; He, Y.; Zhao, X.; Lin, J.; Feng, Y.; Chen, J.; Luo, F.; Li, Z.; Li, J.; et al. A bioinspired Janus polyurethane membrane for potential periodontal tissue regeneration. J. Mater. Chem. B 2022, 10, 2602–2616. [Google Scholar] [CrossRef]
- Lee, T.H.; Oh, J.Y.; Hong, S.P.; Lee, J.M.; Roh, S.M.; Kim, S.H.; Park, H.B. ZIF-8 particle size effects on reverse osmosis performance of polyamide thin-film nanocomposite membranes: Importance of particle deposition. J. Membr. Sci. 2019, 570, 23–33. [Google Scholar] [CrossRef]
- Li, X.G.; Wu, C.Y.; Wu, J.H.; Sun, R.J.; Hou, B.; Liu, C.; Chen, M.J. Molecular Investigation of the Self-Assembly Mechanism Underlying Polydopamine Coatings: The Synergistic Effect of Typical Building Blocks Acting on Interfacial Adhesion. Acs Appl. Mater. Interfaces 2024, 16, 51699–51714. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, G.; Zhan, W.; Wang, J.; Wang, C.; Yue, Q.; Huang, Z.; Wang, Y. Hyaluronidase-responsive hydrogel loaded with magnetic nanoparticles combined with external magnetic stimulation for spinal cord injury repair. Mater. Today Bio 2025, 30, 101378. [Google Scholar] [CrossRef]
- Bahmani, S.; Khajavi, R.; Ehsani, M.; Rahimi, M.K.; Kalaee, M.R. A development of a gelatin and sodium carboxymethyl cellulose hydrogel system for dual-release transdermal delivery of lidocaine hydrochloride. Int. J. Biol. Macromol. 2025, 284 Pt 2, 138034. [Google Scholar] [CrossRef]
- Hou, Y.; Jin, M.; Liu, Y.; Jiang, N.; Zhang, L.; Zhu, S. Biomimetic construction of a lubricious hydrogel with robust mechanics via polymer chains interpenetration and entanglement for TMJ disc replacement. Chem. Eng. J. 2023, 460, 141731. [Google Scholar] [CrossRef]
- He, M.L.; Hou, Y.; Zhu, C.; He, M.M.; Jiang, Y.L.; Feng, G.J.; Liu, L.M.; Li, Y.B.; Chen, C.; Zhang, L. 3D-Printing Biodegradable PU/PAAM/Gel Hydrogel Scaffold with High Flexibility and Self-Adaptibility to Irregular Defects for Nonload-Bearing Bone Regeneration. Bioconjugate Chem. 2021, 32, 1915–1925. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, N.; Sun, D.; Wang, Y.; Chen, X.; Zhu, S.; Zhang, L. A fast UV-curable PU-PAAm hydrogel with mechanical flexibility and self-adhesion for wound healing. RSC Adv. 2020, 10, 4907–4915. [Google Scholar] [CrossRef]
- Khodaei, T.; Nourmohammadi, J.; Ghaee, A.; Khodaii, Z. An antibacterial and self-healing hydrogel from aldehyde-carrageenan for wound healing applications. Carbohydr. Polym. 2023, 302, 120371. [Google Scholar] [CrossRef]
- Liang, S.; Ziyu, Z.; Fulong, W.; Maojuan, B.; Xiaoyan, D.; Lingyun, W. Activation of persulfate by mesoporous silica spheres-doping CuO for bisphenol A removal. Environ. Res. 2022, 205, 112529. [Google Scholar] [CrossRef]
- Miao, Y.G.; He, H.; Li, Z.H. Strain hardening behaviors and mechanisms of polyurethane under various strain rate loading. Polym. Eng. Sci. 2020, 60, 1083–41092. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J.; Zo, S.M.; Kummara, M.R.; Kim, C.M.; Kumar, A.; Bae, H.J.; Sood, A.; Han, S.S. Development of Scalable Elastic Gelatin Hydrogel Films Crosslinked with Waterborne Polyurethane for Enhanced Mechanical Properties and Strain Recovery. Gels 2025, 11, 49. [Google Scholar] [CrossRef]
- Yu, S.; Shi, J.; Liu, Y.T.; Si, J.W.; Yuan, Y.; Liu, C.S. A mechanically robust and flexible PEGylated poly(glycerol sebacate)/β-TCP nanoparticle composite membrane for guided bone regeneration. J. Mater. Chem. B 2019, 7, 3279–3290. [Google Scholar] [CrossRef]
- Hemmati, S.; Zangeneh, M.M.; Zangeneh, A. CuCl2 anchored on polydopamine coated-magnetic nanoparticles (Fe3O4@PDA/Cu(II)): Preparation, characterization and evaluation of its cytotoxicity, antioxidant, antibacterial, and antifungal properties. Polyhedron 2020, 177, 114327. [Google Scholar] [CrossRef]
- Hong, B.; Xian, G.; Li, H. Effects of water or alkali solution immersion on the water uptake and physicomechanical properties of polyurethane. Polym. Eng. Sci. 2018, 58, 2276–2287. [Google Scholar]
- Zhang, T.; Yang, J.; Lu, Y.; Wang, Y.; Wang, X.; Li, Y.; Li, W.; Wang, Y. Synergistic Functions of the Janus Fibrous Membrane for Enhanced Bone Repair. ACS Appl. Mater. Interfaces 2025, 17, 14873–14887. [Google Scholar] [CrossRef]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical qualification of collagen membranes used in dentistry. Ann. Ist. Super. Sanita 2015, 51, 229–235. [Google Scholar] [CrossRef]
- Dal-Fabbro, R.; Anselmi, C.; Swanson, W.B.; Medeiros Cardoso, L.; Toledo, P.T.A.; Daghrery, A.; Kaigler, D.; Abel, A.; Becker, M.L.; Soliman, S.; et al. Amino Acid-Based Poly(ester urea) Biodegradable Membrane for Guided Bone Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 53419–53434. [Google Scholar] [CrossRef]
- Wang, D.X.; Zhou, X.C.; Cao, H.; Zhang, H.W.; Wang, D.P.; Guo, J.M.; Wang, J.F. Barrier membranes for periodontal guided bone regeneration: A potential therapeutic strategy. Front. Mater. 2023, 10, 1220420. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhao, Y.F.; Zha, X.J.; Zhang, Z.M.; Zhang, L.L.; Wu, Y.K.; Ren, R.Y.; Zhao, Z.H.; Yang, W.; Zhao, L.X. A Janus, robust, biodegradable bacterial cellulose/Ti3C2Tx MXene bilayer membranes for guided bone regeneration. Biomater. Adv. 2024, 161, 213892. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Hou, Y.; Kang, F. Polydopamine-Coated Copper-Doped Mesoporous Silica/Gelatin–Waterborne Polyurethane Composite: A Multifunctional GBR Membrane for Bone Defect Repair. J. Funct. Biomater. 2025, 16, 122. https://doi.org/10.3390/jfb16040122
Jin M, Hou Y, Kang F. Polydopamine-Coated Copper-Doped Mesoporous Silica/Gelatin–Waterborne Polyurethane Composite: A Multifunctional GBR Membrane for Bone Defect Repair. Journal of Functional Biomaterials. 2025; 16(4):122. https://doi.org/10.3390/jfb16040122
Chicago/Turabian StyleJin, Mengmeng, Yi Hou, and Feiwu Kang. 2025. "Polydopamine-Coated Copper-Doped Mesoporous Silica/Gelatin–Waterborne Polyurethane Composite: A Multifunctional GBR Membrane for Bone Defect Repair" Journal of Functional Biomaterials 16, no. 4: 122. https://doi.org/10.3390/jfb16040122
APA StyleJin, M., Hou, Y., & Kang, F. (2025). Polydopamine-Coated Copper-Doped Mesoporous Silica/Gelatin–Waterborne Polyurethane Composite: A Multifunctional GBR Membrane for Bone Defect Repair. Journal of Functional Biomaterials, 16(4), 122. https://doi.org/10.3390/jfb16040122





