Intramuscular Reactivity of the Modified Graphene Oxides and Their Bio-Reactivity in Aging Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Synthesis of GO, PEG-GO, PHBV-GO and PPP-GO
2.3. GO Nanoparticles Characterization
2.4. GO Nanoparticles Muscle Implantation and Sample Collection
2.5. Histological and Immunofluorescence Staining
2.6. Flow Cytometric Analysis
2.7. In Vitro Test
2.8. Statistical Analysis
2.9. Single Sample Gene Set Enrichment Analysis (ssGSEA)
3. Results and Discussion
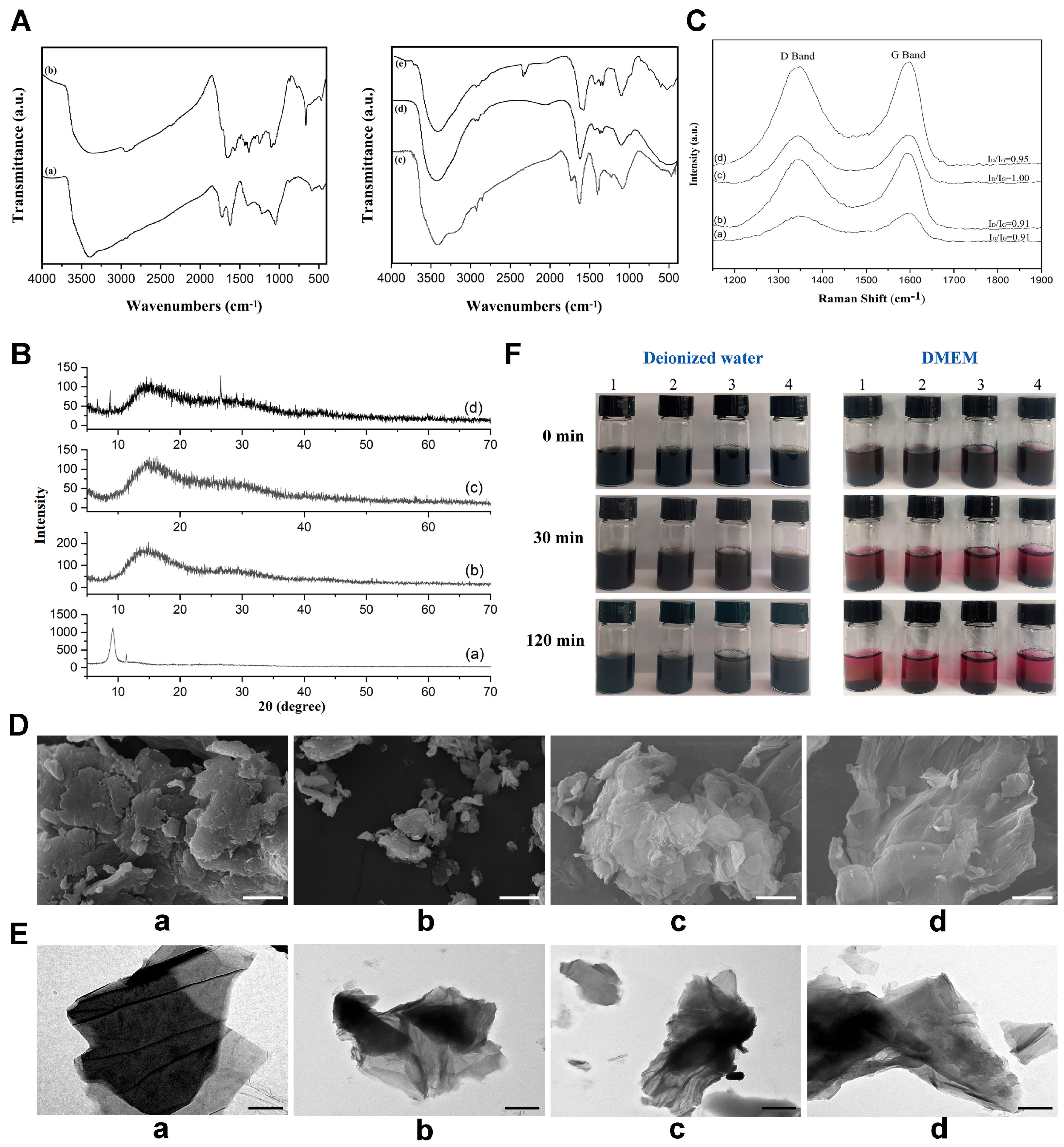
3.1. Structure and Characterization of the GO Nanoparticles
3.2. Myotoxicity Analysis of the Modified GOs Exposing to Skeletal Muscle
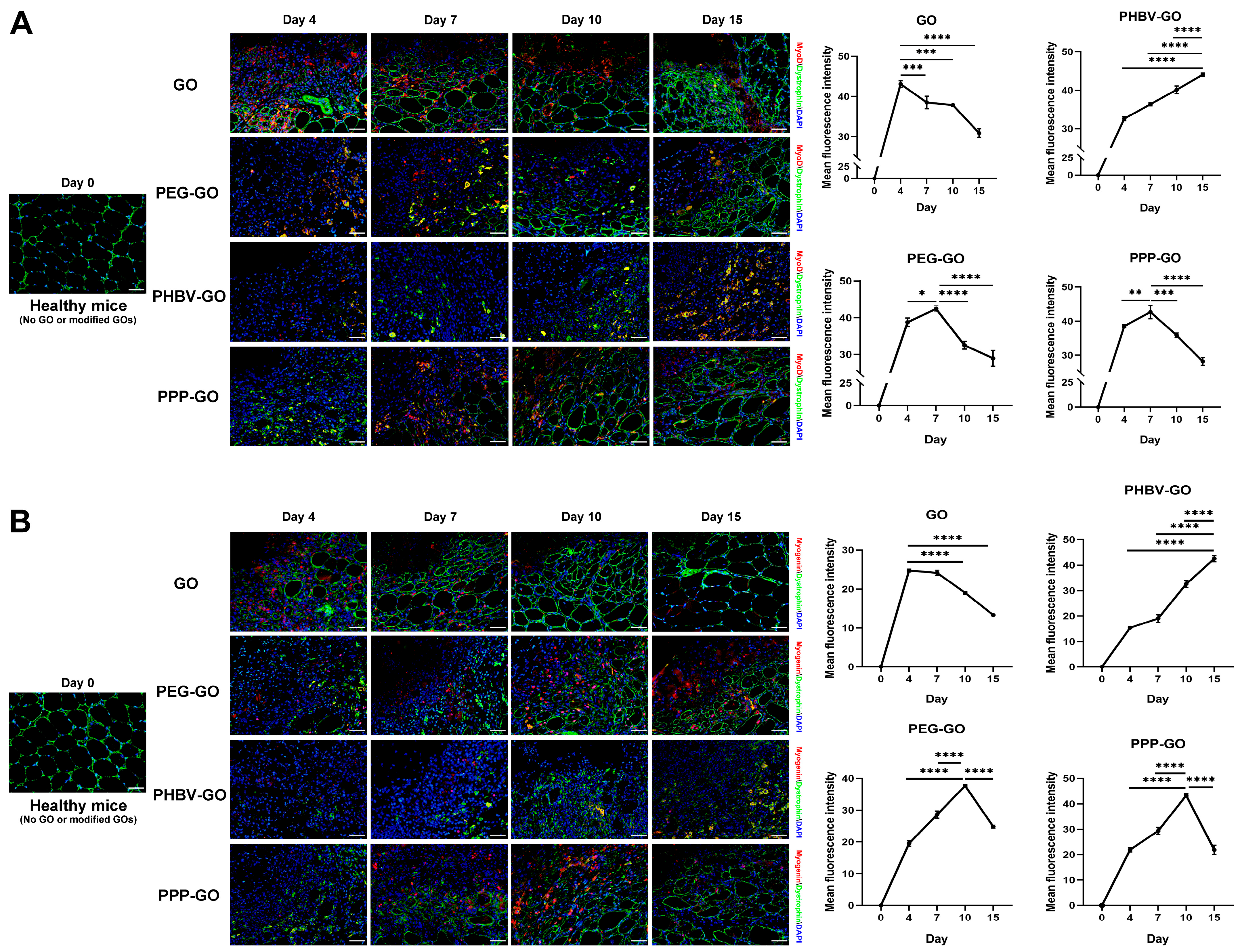
3.3. Myogenesis Comparison of Muscle Exposing to the Modified GOs
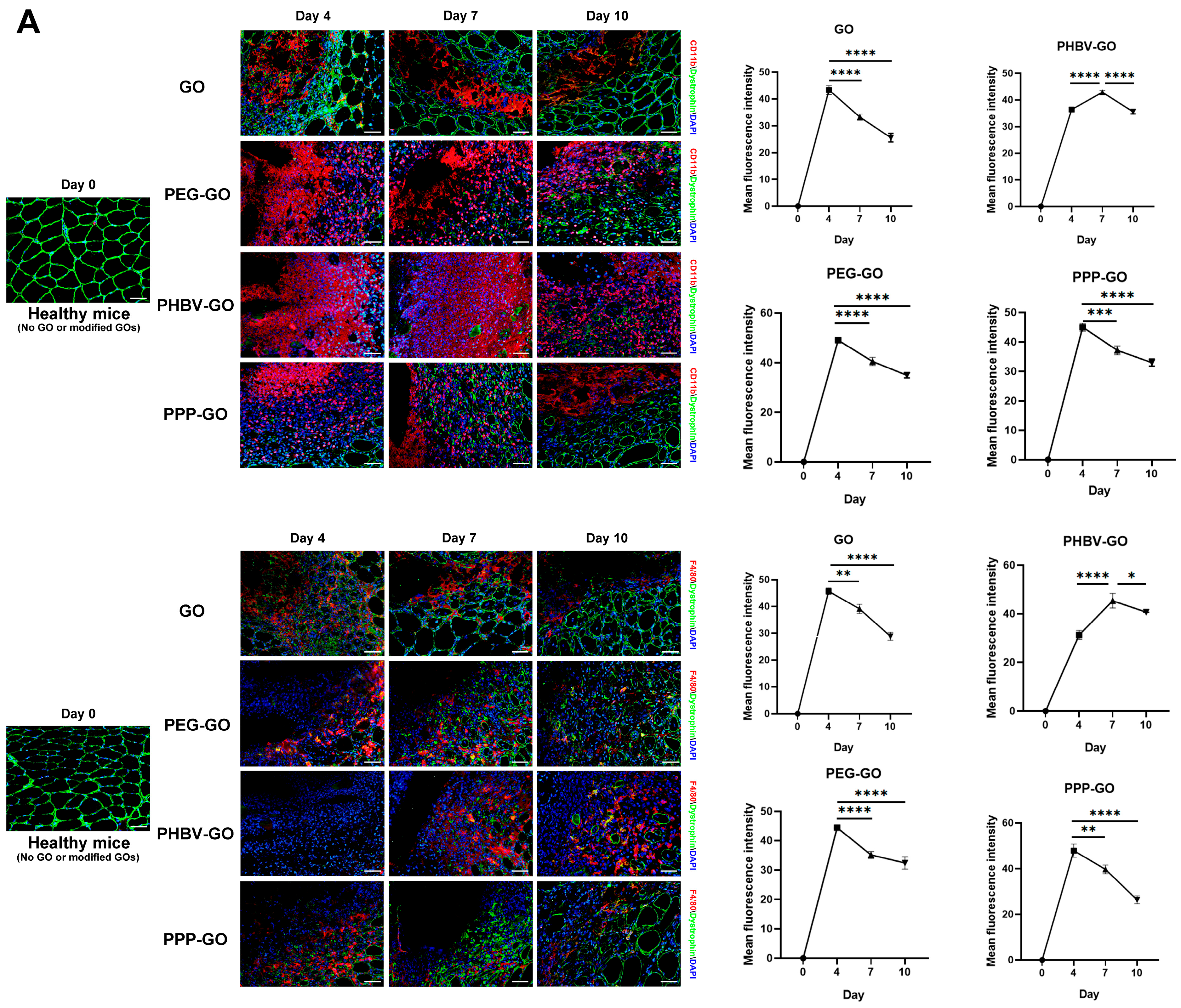
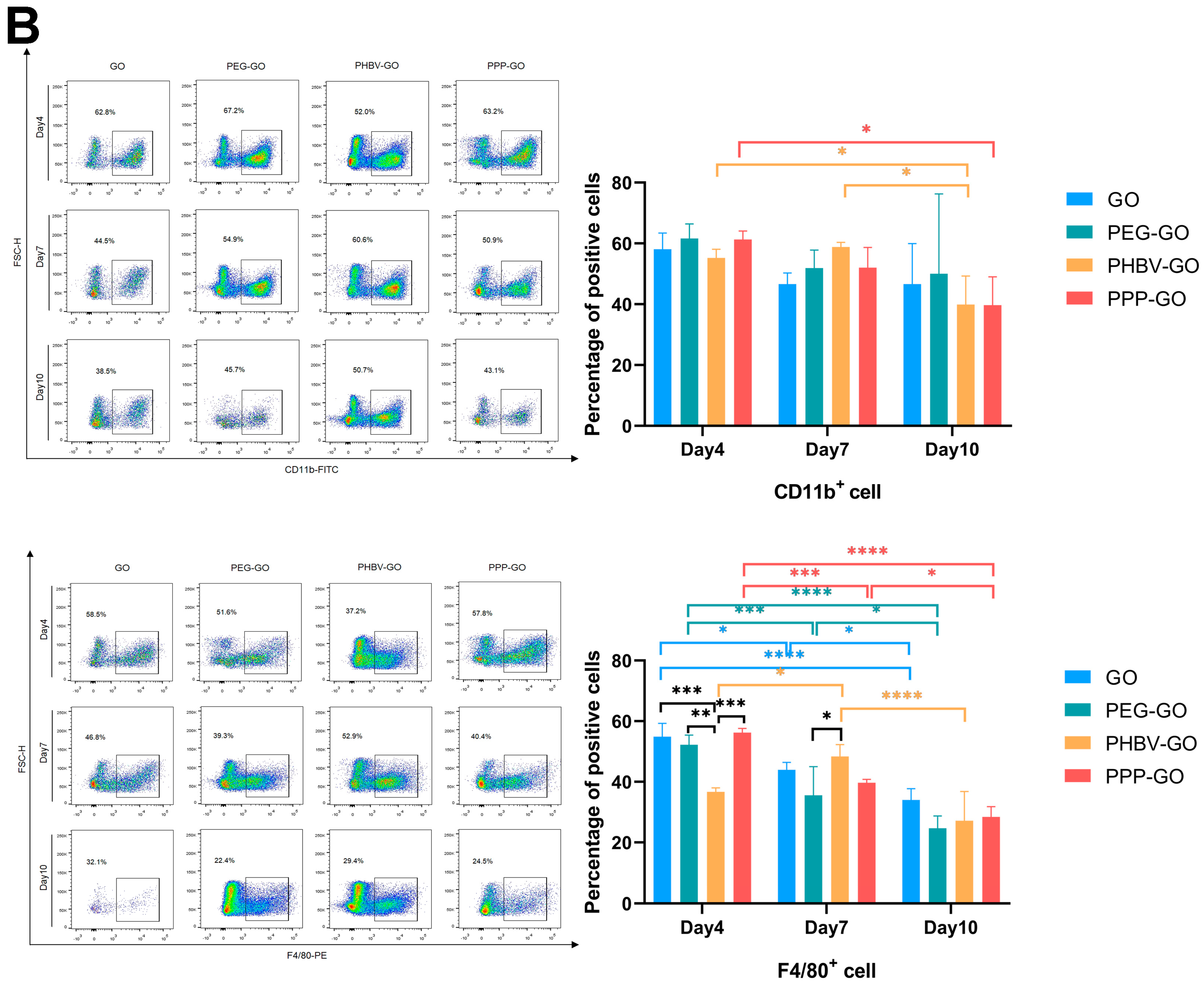
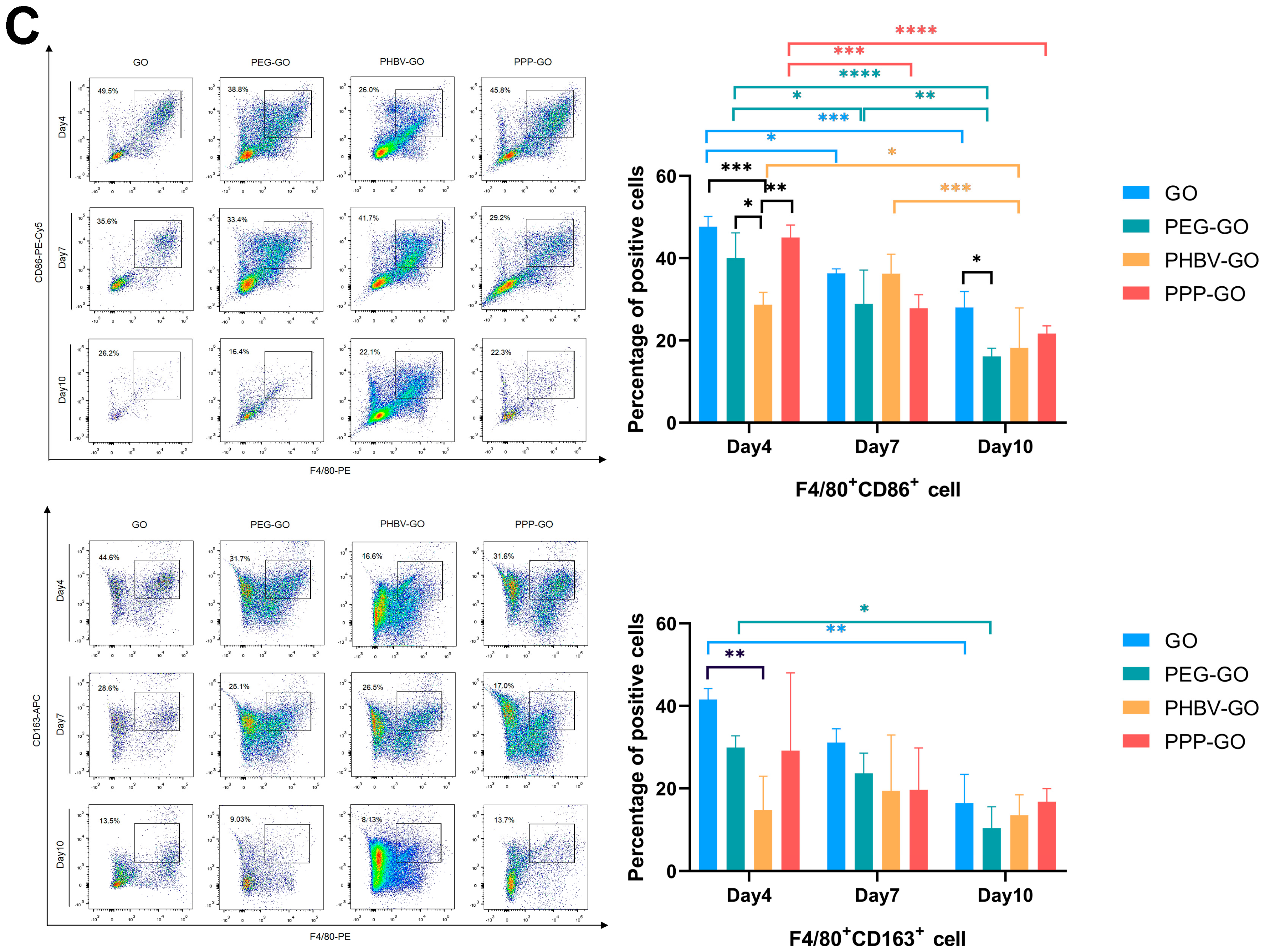
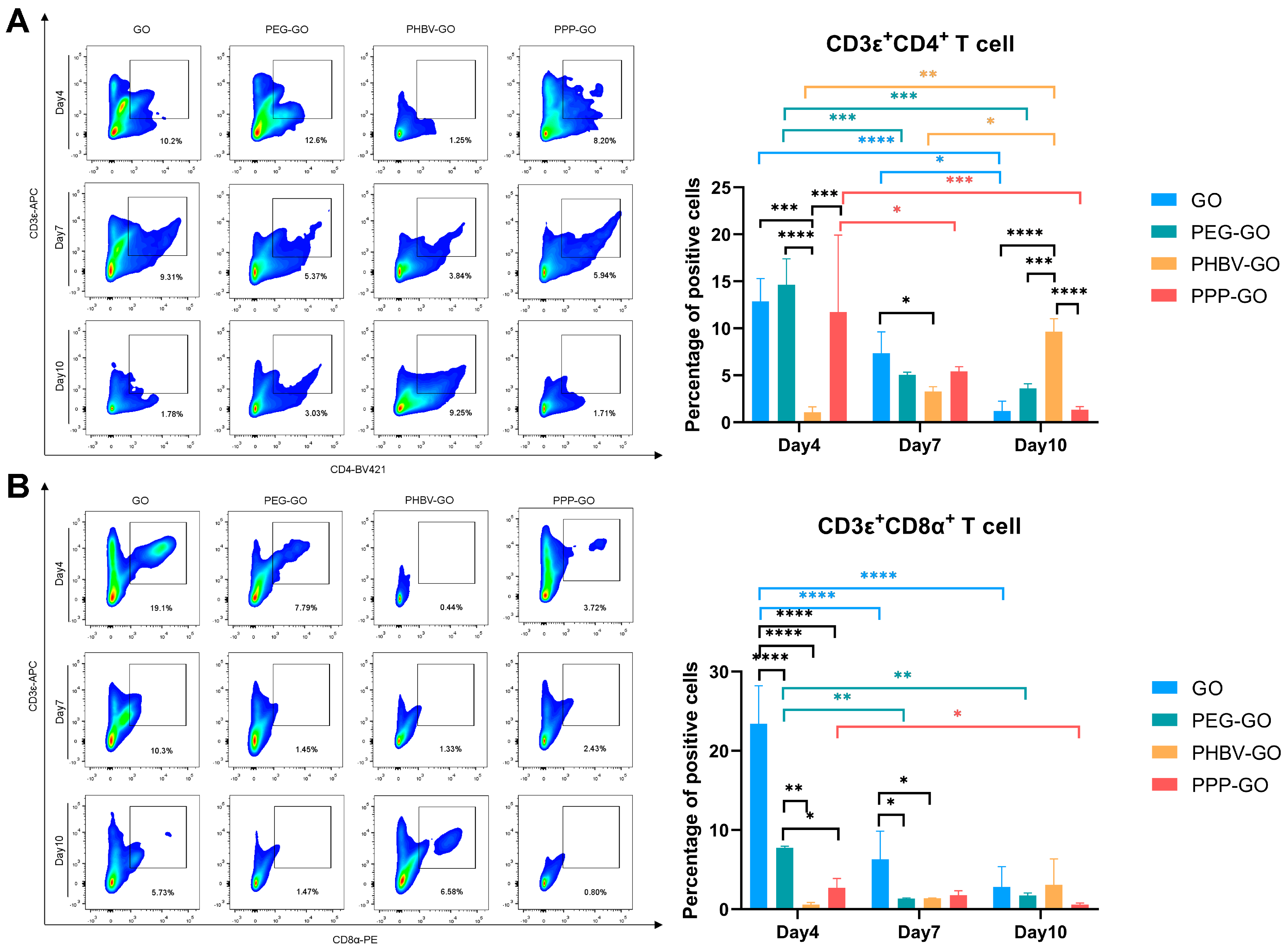
3.4. Comparison of Intramuscular Inflammation Induced by the Modified GOs
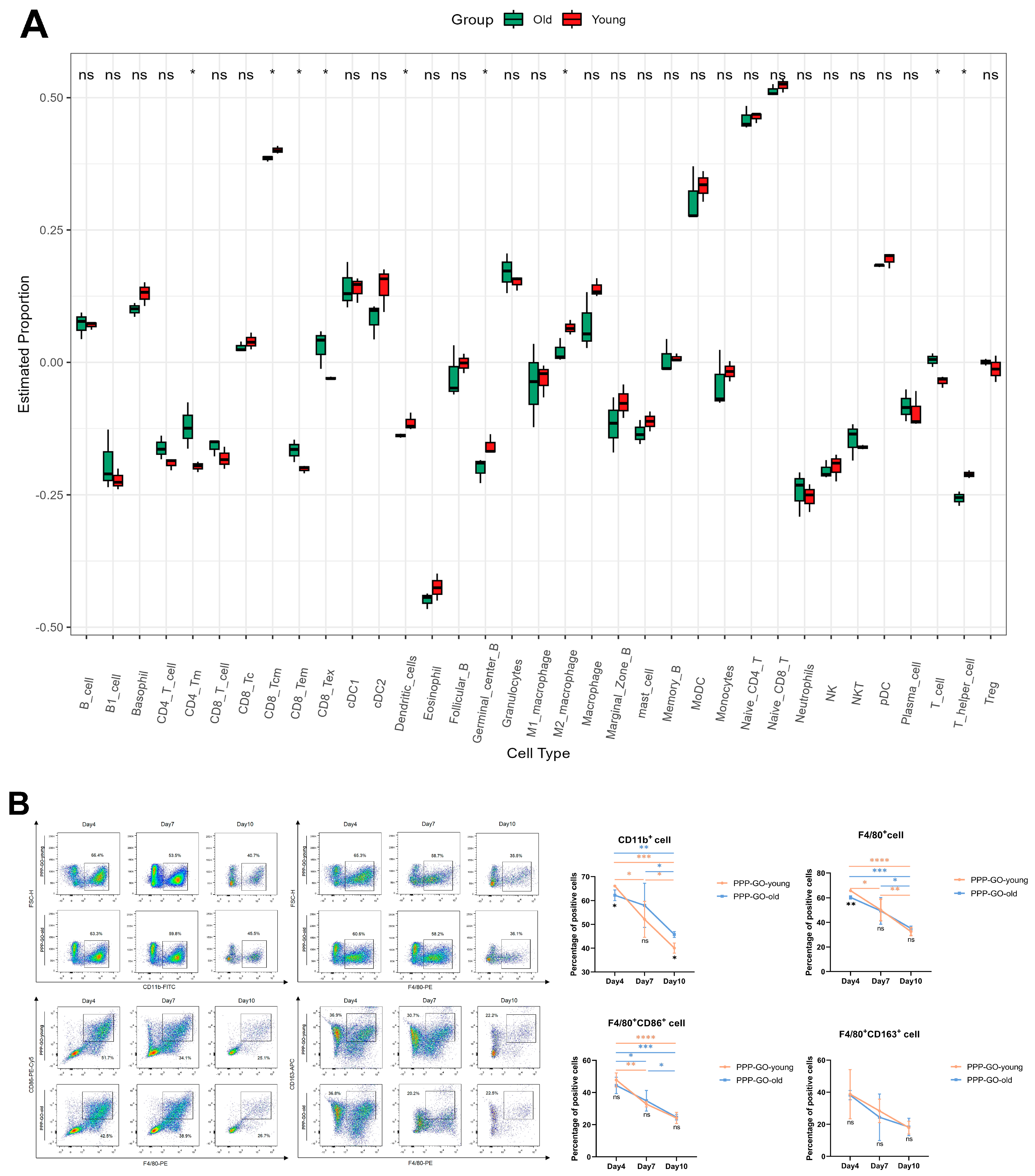
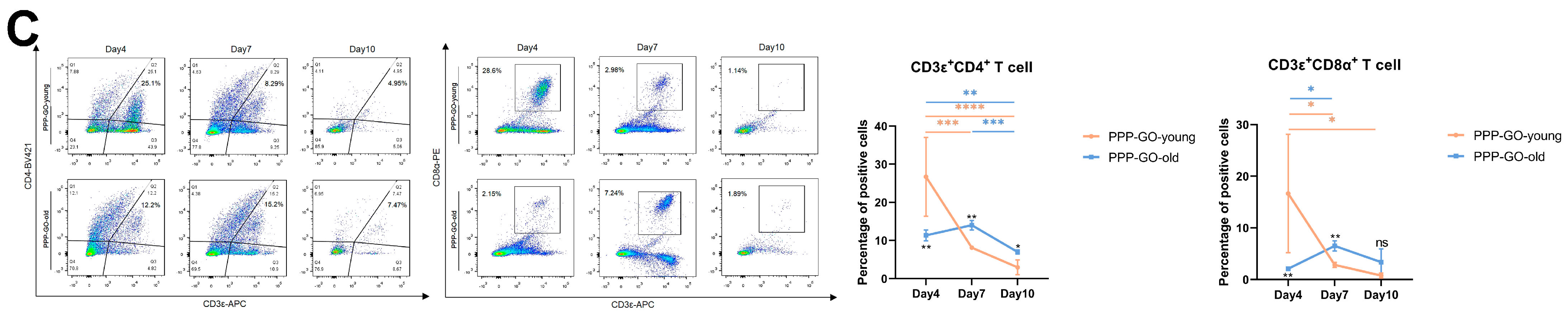
3.5. Aging Related Features of PPP-GO Induced Myo-Reactivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Peng, B.; Feng, P.; Yu, L.; Lai, R.; Min, A. In Situ Synthesis of Hydroxyapatite Nanorods on Graphene Oxide Nanosheets and Their Reinforcement in Biopolymer Scaffold. J. Adv. Res. 2022, 35, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-H.; Pi, J.; Jin, H.; Cai, J.-Y. Functional Graphene Oxide as Cancer-Targeted Drug Delivery System to Selectively Induce Oesophageal Cancer Cell Apoptosis. Artif. Cells Nanomed. Biotechnol. 2018, 46, S297–S307. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Singh, D.P.; Kumar, R.; Matsuda, A. Graphene Oxide for Drug Delivery and Cancer Therapy. In Nanostructured Polymer Composites for Biomedical Applications; Swain, S.K., Jawaid, M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–488, Chapter 22; ISBN 978-0-12-816771-7. [Google Scholar]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity Effects of Graphene and Single-Wall Carbon Nanotubes in Neural Phaeochromocytoma-Derived PC12 Cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, S.; Wang, M.; Li, Y.; Shi, P.; Huang, X. Delivery of Paclitaxel Using PEGylated Graphene Oxide as a Nanocarrier. ACS Appl. Mater. Interfaces 2015, 7, 1355–1363. [Google Scholar] [CrossRef]
- Bian, J.; Lin, H.L.; Wang, G.; Zhou, Q.; Wang, Z.J.; Zhou, X.; Lu, Y.; Zhao, X.W. Morphological, Mechanical and Thermal Properties of Chemically Bonded Graphene Oxide Nanocomposites with Biodegradable Poly(3-Hydroxybutyrate) by Solution Intercalation. Polym. Polym. Compos. 2016, 24, 133–141. [Google Scholar] [CrossRef]
- Jing, X.; Qiu, Z. Crystallization Kinetics and Thermal Property of Biodegradable Poly(3-Hydroxybutyrate)/Graphene Oxide Nanocomposites. J. Nanosci. Nanotechnol. 2012, 12, 7314–7321. [Google Scholar] [CrossRef]
- Chen, X.; Cui, H.; Li, H.; Wang, J.; Fu, P.; Yin, J.; Tang, S.; Ke, Y. Functionalization of Graphene Oxide with Amphiphilic Block Copolymer to Enhance Antibacterial Activity. Colloids Surf. B Biointerfaces 2024, 234, 113690. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. BioMed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef] [PubMed]
- Duch, M.C.; Budinger, G.R.S.; Liang, Y.T.; Soberanes, S.; Urich, D.; Chiarella, S.E.; Campochiaro, L.A.; Gonzalez, A.; Chandel, N.S.; Hersam, M.C.; et al. Minimizing Oxidation and Stable Nanoscale Dispersion Improves the Biocompatibility of Graphene in the Lung. Nano Lett. 2011, 11, 5201–5207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and Biocompatibility Studies of Graphene Oxide in Mice after Intravenous Administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In Vivo Biodistribution and Toxicology of Functionalized Nano-Graphene Oxide in Mice after Oral and Intraperitoneal Administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Mu, L. Biotransformation of Graphene Oxide Nanosheets in Blood Plasma Affects Their Interactions with Cells. Environ. Sci. Nano 2017, 4, 1569–1578. [Google Scholar] [CrossRef]
- Liu, X.T.; Mu, X.Y.; Wu, X.L.; Meng, L.X.; Guan, W.B.; Ma, Y.Q.; Sun, H.; Wang, C.J.; Li, X.F. Toxicity of Multi-Walled Carbon Nanotubes, Graphene Oxide, and Reduced Graphene Oxide to Zebrafish Embryos. Biomed. Environ. Sci. 2014, 27, 676–683. [Google Scholar] [CrossRef]
- Kim, M.; Eom, H.-J.; Choi, I.; Hong, J.; Choi, J. Graphene Oxide-Induced Neurotoxicity on Neurotransmitters, AFD Neurons and Locomotive Behavior in Caenorhabditis Elegans. Neurotoxicology 2020, 77, 30–39. [Google Scholar] [CrossRef]
- Kessler, P.D.; Podsakoff, G.M.; Chen, X.; McQuiston, S.A.; Colosi, P.C.; Matelis, L.A.; Kurtzman, G.J.; Byrne, B.J. Gene Delivery to Skeletal Muscle Results in Sustained Expression and Systemic Delivery of a Therapeutic Protein. Proc. Natl. Acad. Sci. USA 1996, 93, 14082–14087. [Google Scholar]
- Wiendl, H.; Hohlfeld, R.; Kieseier, B.C. Immunobiology of Muscle: Advances in Understanding an Immunological Microenvironment. Trends Immunol. 2005, 26, 373–380. [Google Scholar] [CrossRef]
- Shi, L.; Hu, W.; He, Y.; Ke, Y.; Wu, G.; Xiao, M.; Huang, L.; Tan, S. Preparation and Characterization of Poly (Ethylene Glycol)-Block-Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)-Block-Poly (Ethylene Glycol) Triblock Copolymers. Macromol. Res. 2020, 28, 310–318. [Google Scholar] [CrossRef]
- Zhong, Q.; Long, H.; Hu, W.; Shi, L.; Zan, F.; Xiao, M.; Tan, S.; Ke, Y.; Wu, G.; Chen, H. Dual-Function Antibacterial Micelle via Self-Assembling Block Copolymers with Various Antibacterial Nanoparticles. ACS Omega 2020, 5, 8523–8533. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Feng, J.; Ye, Y.; Xiao, M.; Wu, G.; Ke, Y. Synthetic Routes of the Reduced Graphene Oxide. Chem. Pap. 2020, 74, 3767–3783. [Google Scholar] [CrossRef]
- Miao, Y.-R.; Xia, M.; Luo, M.; Luo, T.; Yang, M.; Guo, A.-Y. ImmuCellAI-Mouse: A Tool for Comprehensive Prediction of Mouse Immune Cell Abundance and Immune Microenvironment Depiction. Bioinformatics 2022, 38, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Zhang, M.; Yao, B.; Li, Y.; Huang, L.; Li, C.; Shi, G. Water-Enhanced Oxidation of Graphite to Graphene Oxide with Controlled Species of Oxygenated Groups. Chem. Sci. 2016, 7, 1874–1881. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Baraket, M.; Walton, S.G.; Wei, Z.; Lock, E.H.; Robinson, J.T.; Sheehan, P. Reduction of Graphene Oxide by Electron Beam Generated Plasmas Produced in Methane/Argon Mixtures. Carbon 2010, 48, 3382–3390. [Google Scholar] [CrossRef]
- Pinto, A.M.; Moreira, S.; Gonçalves, I.C.; Gama, F.M.; Mendes, A.M.; Magalhães, F.D. Biocompatibility of Poly (Lactic Acid) with Incorporated Graphene-Based Materials. Colloids Surf. B Biointerfaces 2013, 104, 229–238. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Jiang, J.; Wang, Y.; Jiang, H.; Zhang, J.; Nie, X.; Liu, B. Systematic Assessment of the Toxicity and Potential Mechanism of Graphene Derivatives In Vitro and In Vivo. Toxicol. Sci. 2019, 167, 269–281. [Google Scholar] [CrossRef]
- Jo, H.; Sim, M.; Kim, S.; Yang, S.; Yoo, Y.; Park, J.-H.; Yoon, T.H.; Kim, M.-G.; Lee, J.Y. Electrically Conductive Graphene/Polyacrylamide Hydrogels Produced by Mild Chemical Reduction for Enhanced Myoblast Growth and Differentiation. Acta Biomater. 2017, 48, 100–109. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Bhadra, D.; Moroni, L.; Pramanik, K. Myoblast Differentiation of Human Mesenchymal Stem Cells on Graphene Oxide and Electrospun Graphene Oxide-Polymer Composite Fibrous Meshes: Importance of Graphene Oxide Conductivity and Dielectric Constant on Their Biocompatibility. Biofabrication 2015, 7, 015009. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Park, C.B. Myoblast Differentiation on Graphene Oxide. Biomaterials 2013, 34, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the Myogenic Regulatory Factors Myf5, MyoD, Myogenin and MRF4 in Skeletal Muscle, Satellite Cells and Regenerative Myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Sharma, A.; Kumar, P.; Kumar, A.; Bharadwaj, A.; Saini, M.; Kardon, G.; Mathew, S.J. Myosin Heavy Chain-Embryonic Regulates Skeletal Muscle Differentiation during Mammalian Development. Development 2020, 147, dev184507. [Google Scholar] [CrossRef]
- Tidball, J.G. Inflammatory Processes in Muscle Injury and Repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R345–R353. [Google Scholar] [CrossRef]
- Han, J.; Kim, Y.S.; Lim, M.-Y.; Kim, H.Y.; Kong, S.; Kang, M.; Choo, Y.W.; Jun, J.H.; Ryu, S.; Jeong, H.-Y.; et al. Dual Roles of Graphene Oxide to Attenuate Inflammation and Elicit Timely Polarization of Macrophage Phenotypes for Cardiac Repair. ACS Nano 2018, 12, 1959–1977. [Google Scholar] [CrossRef]
- Parker, H.; Gravagnuolo, A.M.; Vranic, S.; Crica, L.E.; Newman, L.; Carnell, O.; Bussy, C.; Dookie, R.S.; Prestat, E.; Haigh, S.J.; et al. Graphene Oxide Modulates Dendritic Cell Ability to Promote T Cell Activation and Cytokine Production. Nanoscale 2022, 14, 17297–17314. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Lin, K.-J.; Wang, P.-Y.; Lin, C.-W.; Yang, H.-W.; Ma, C.-C.M.; Lu, Y.-J.; Jan, T.-R. Polyethylene Glycol-Coated Graphene Oxide Attenuates Antigen-Specific IgE Production and Enhanced Antigen-Induced T-Cell Reactivity in Ovalbumin-Sensitized BALB/c Mice. Int. J. Nanomed. 2014, 9, 4257–4266. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Xiong, S.; Luo, J.; Wang, Q.; Li, Z.; Li, J.; Liu, Q.; Gao, L.; Fang, S.; Li, Y.; Pan, H.; et al. Targeted Graphene Oxide for Drug Delivery as a Therapeutic Nanoplatform against Parkinson’s Disease. Biomater. Sci. 2021, 9, 1705–1715. [Google Scholar] [CrossRef]
- Saravanan, S.; Sareen, N.; Abu-El-Rub, E.; Ashour, H.; Sequiera, G.L.; Ammar, H.I.; Gopinath, V.; Shamaa, A.A.; Sayed, S.S.E.; Moudgil, M.; et al. Graphene Oxide-Gold Nanosheets Containing Chitosan Scaffold Improves Ventricular Contractility and Function After Implantation into Infarcted Heart. Sci. Rep. 2018, 8, 15069. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; McKay, L.K.; Bareja, A.; Li, Y.; Khodabukus, A.; Bursac, N.; Taylor, G.A.; Baht, G.S.; White, J.P. Meteorin-like Is an Injectable Peptide That Can Enhance Regeneration in Aged Muscle through Immune-Driven Fibro/Adipogenic Progenitor Signaling. Nat. Commun. 2022, 13, 7613. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, X.; Wang, J.; Hu, J.; Li, Y.; Wang, Q.; Wang, H.; Huang, J.; Ke, Y.; Liao, H. Intramuscular Reactivity of the Modified Graphene Oxides and Their Bio-Reactivity in Aging Muscle. J. Funct. Biomater. 2025, 16, 115. https://doi.org/10.3390/jfb16040115
Jian X, Wang J, Hu J, Li Y, Wang Q, Wang H, Huang J, Ke Y, Liao H. Intramuscular Reactivity of the Modified Graphene Oxides and Their Bio-Reactivity in Aging Muscle. Journal of Functional Biomaterials. 2025; 16(4):115. https://doi.org/10.3390/jfb16040115
Chicago/Turabian StyleJian, Xiaoting, Jiayin Wang, Jijie Hu, Yangyang Li, Qisen Wang, Han Wang, Jingwen Huang, Yu Ke, and Hua Liao. 2025. "Intramuscular Reactivity of the Modified Graphene Oxides and Their Bio-Reactivity in Aging Muscle" Journal of Functional Biomaterials 16, no. 4: 115. https://doi.org/10.3390/jfb16040115
APA StyleJian, X., Wang, J., Hu, J., Li, Y., Wang, Q., Wang, H., Huang, J., Ke, Y., & Liao, H. (2025). Intramuscular Reactivity of the Modified Graphene Oxides and Their Bio-Reactivity in Aging Muscle. Journal of Functional Biomaterials, 16(4), 115. https://doi.org/10.3390/jfb16040115






