Effects of ECM Components on Periodontal Ligament Stem Cell Differentiation Under Conditions of Disruption of Wnt and TGF-β Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Decellularized Matrices
2.2. ECM Components
2.3. Signaling Pathway Inhibition
2.4. Preparation of Bioengineered Constructs in Collagen I Hydrogel
- (1)
- Col I + dECM + DMSO (DMSO);
- (2)
- Col I + dECM + DMSO + HA (DMSO+HA);
- (3)
- Col I + dECM + DMSO + Fn (DMSO+Fn);
- (4)
- Col I + dECM + DMSO + Lam (DMSO+Lam);
- (5)
- Col I + dECM + inhWnt (inhWnt);
- (6)
- Col I + dECM + inhTGF-β (inhTGFβ);
- (7)
- Col I + dECM + inhWnt + HA (inhWnt+HA);
- (8)
- Col I + dECM + inhWnt + Fn (inhWnt+Fn);
- (9)
- Col I + dECM + inhWnt + Lam (inhWnt+Lam);
- (10)
- Col I + dECM + inhTGFβ + HA (inhTGFβ+HA);
- (11)
- Col I + dECM + inhTGFβ + Fn (inhTGFβ+Fn);
- (12)
- Col I + dECM + inhTGFβ + Lam (inhTGFβ+Lam).
2.5. Histological and Immunohistochemical Examination
2.6. Semi-Quantitative and Quantitative Scoring of the Immunohistochemistry Study
2.7. ELISA Assay
2.8. Statistical Analysis
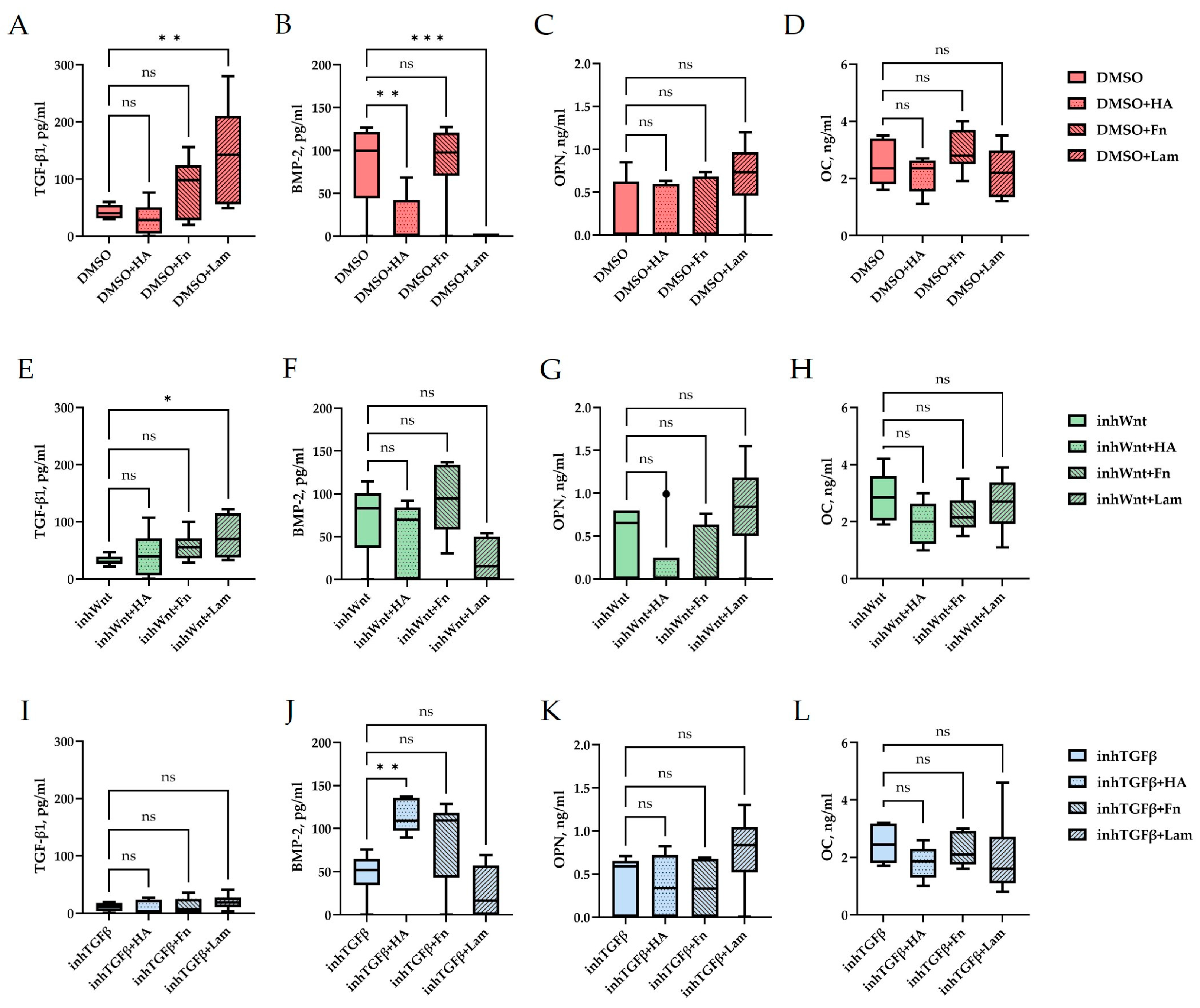
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TGF-β | Transforming growth factor beta |
| BMP | Bone morphogenetic protein |
| PDL | Periodontal ligament |
| PDLSCs | Periodontal ligament stem cells |
| dPDL | Decellularized periodontal ligament |
| dTM | Decellularized tooth matrix |
| ECM | Extracellular matrix |
| dECM | Decellularized ECM |
| Fn | Fibronectin |
| Lam | Laminin |
| HA | Hyaluronic acid |
| Col I | Collagen I hydrogel |
| inhWnt | Wnt/β-catenin inhibitor |
| inhTGFβ | TGF-β inhibitor |
| DMSO | Dimethylsulfoxide |
| ALP | Alkaline phosphatase |
| OPN | Osteopontin |
| OC | Osteocalcin |
| DSPP | Dentin sialophosphoprotein |
| DMP-1 | Dentin matrix acidic phosphoprotein 1 |
References
- Hathaway-Schrader, J.D.; Novince, C.M. Maintaining Homeostatic Control of Periodontal Bone Tissue. Periodontology 2000 2021, 86, 157–187. [Google Scholar] [CrossRef] [PubMed]
- Miguez, P.A.; Bash, E.; Musskopf, M.L.; Tuin, S.A.; Rivera-Concepcion, A.; Chapple, I.L.C.; Liu, J. Control of Tissue Homeostasis by the Extracellular Matrix: Synthetic Heparan Sulfate As a Promising Therapeutic for Periodontal Health and Bone Regeneration. Periodontology 2000 2024, 94, 510–531. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-C.; Bae, S.-H.; Lee, G.; Ryu, C.J.; Jang, Y.-J. Activation of β-Catenin by TGF-Β1 Promotes Ligament-Fibroblastic Differentiation and Inhibits Cementoblastic Differentiation of Human Periodontal Ligament Cells. Stem Cells 2020, 38, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The Intricate Anatomy of the Periodontal Ligament and Its Development: Lessons for Periodontal Regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef]
- Ru, L.; Pan, B.; Zheng, J. Signalling Pathways in the Osteogenic Differentiation of Periodontal Ligament Stem Cells. Open Life Sci. 2023, 18, 20220706. [Google Scholar] [CrossRef]
- Su, Q.; Huang, F.; Fang, X.; Lin, Q. The Effect of the Wnt Pathway on the Osteogenic Differentiation of Periodontal Ligament Stem Cells in Different Environments. PeerJ 2025, 13, e18770. [Google Scholar] [CrossRef]
- Willert, K.; Nusse, R. Wnt Proteins. Cold Spring Harb. Perspect. Biol. 2012, 4, a007864. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, X.; Perez, K.C.; Hyman, S.; Wang, L.; Pellegrini, G.; Salmon, B.; Bellido, T.; Helms, J.A. Aberrantly Elevated Wnt Signaling Is Responsible for Cementum Overgrowth and Dental Ankylosis. Bone 2019, 122, 176–183. [Google Scholar] [CrossRef]
- González-Quintanilla, D.; Abásolo, N.; Astudillo, P. Wnt Signaling in Periodontal Disease. Front. Dent. Med. 2021, 2, 763308. [Google Scholar] [CrossRef]
- Bao, J.; Yang, Y.; Xia, M.; Sun, W.; Chen, L. Wnt Signaling: An Attractive Target for Periodontitis Treatment. Biomed. Pharmacother. 2021, 133, 110935. [Google Scholar] [CrossRef]
- Massagué, J.; Sheppard, D. TGF-β Signaling in Health and Disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, H.; Jiang, Y.; Guan, Y.; Chen, S.; Wu, Z.; Zou, S.; Bonewald, L.F.; Duan, P. Canonical Wnt/b-catenin Signaling Has Positive Effects on Osteogenesis, but Can Have Negative Effects on Cementogenesis. J. Periodontol. 2022, 93, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, X.; Shen, Y.; Meng, L. Cell–Scaffold Interactions in Tissue Engineering for Oral and Craniofacial Reconstruction. Bioact. Mater. 2023, 23, 16–44. [Google Scholar] [CrossRef]
- Agarwal, G.; Agiwal, S.; Srivastava, A. Hyaluronic Acid Containing Scaffolds Ameliorate Stem Cell Function for Tissue Repair and Regeneration. Int. J. Biol. Macromol. 2020, 165, 388–401. [Google Scholar] [CrossRef]
- Wang, S.; Niu, Y.; Jia, P.; Liao, Z.; Guo, W.; Chaves, R.C.; Tran-Ba, K.-H.; He, L.; Bai, H.; Sia, S.; et al. Alkaline Activation of Endogenous Latent TGFβ1 by an Injectable Hydrogel Directs Cell Homing for In Situ Complex Tissue Regeneration. Bioact. Mater. 2022, 15, 316–329. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Kuznetsova, A.V.; Popova, O.P.; Danilova, T.I.; Latyshev, A.V.; Yanushevich, O.O. Influence of Extracellular Matrix Components on the Differentiation of Periodontal Ligament Stem Cells in Collagen I Hydrogel. Cells 2023, 12, 2335. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Danilova, T.I.; Kuznetsova, A.V.; Popova, O.P.; Yanushevich, O.O. Decellularized Matrix Induced Spontaneous Odontogenic and Osteogenic Differentiation in Periodontal Cells. Biomolecules 2023, 13, 122. [Google Scholar] [CrossRef]
- de Souza, J.A.C.; Junior, C.R.; Garlet, G.P.; Nogueira, A.V.B.; Cirelli, J.A. Modulation of Host Cell Signaling Pathways As a Therapeutic Approach in Periodontal Disease. J. Appl. Oral. Sci. 2012, 20, 128–138. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Zeng, A.; Li, H.; Liu, J.; Wu, M. The Progress of Decellularized Scaffold in Stomatology. Tissue Eng. Regen. Med. 2022, 19, 451–461. [Google Scholar] [CrossRef]
- Lumelsky, N. Creating a Pro-Regenerative Tissue Microenvironment: Local Control Is the Key. Front. Bioeng. Biotechnol. 2021, 9, 712685. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, S.; Chen, W.; Li, Y.-P. The Roles and Regulatory Mechanisms of TGF-β and BMP Signaling in Bone and Cartilage Development, Homeostasis and Disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef]
- Purwaningrum, M.; Giachelli, C.M.; Osathanon, T.; Rattanapuchpong, S.; Sawangmake, C. Dissecting Specific Wnt Components Governing Osteogenic Differentiation Potential by Human Periodontal Ligament Stem Cells Through Interleukin-6. Sci. Rep. 2023, 13, 9055. [Google Scholar] [CrossRef]
- Savithri, N.; Sangeetha, S.; Mary, J.; Gayathri, P. R-Spondins and Wnt Signalling Pathway: A Periodontal Perspective. J. Clin. Diagn. Res. 2023, 17, ZE19–ZE22. [Google Scholar] [CrossRef]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elçin, A.E.; Elçin, Y.M. Clinical Applications of Decellularized Extracellular Matrices for Tissue Engineering and Regenerative Medicine. Biomed. Mater. 2016, 11, 022003. [Google Scholar] [CrossRef]
- Cui, S.-J.; Fu, Y.; Yu, M.; Zhang, L.; Zhao, W.-Y.; Zhang, T.; Qiu, L.-X.; Gu, Y.; Zhou, Y.-H.; Liu, Y. Functional Periodontal Regeneration Using Biomineralized Extracellular Matrix/Stem Cell Microspheroids. Chem. Eng. J. 2022, 431, 133220. [Google Scholar] [CrossRef]
- de Gorter, D.J.; van Dinther, M.; Korchynskyi, O.; ten Dijke, P. Biphasic Effects of Transforming Growth Factor β on Bone Morphogenetic Protein–Induced Osteoblast Differentiation. J. Bone Miner. Res. 2011, 26, 1178–1187. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kanatani, N.; Rokutanda, S.; Yoshida, C.; Toyosawa, S.; Nakamura, R.; Takada, S.; Komori, T. Inhibition of the Terminal Differentiation of Odontoblasts and Their Transdifferentiation Into Osteoblasts in Runx2 Transgenic Mice. Arch. Histol. Cytol. 2008, 71, 131–146. [Google Scholar] [CrossRef]
- Givant-Horwitz, V.; Davidson, B.; Reich, R. Laminin-Induced Signaling in Tumor Cells. Cancer Lett. 2005, 223, 1–10. [Google Scholar] [CrossRef]
- Ou, J.; Deng, J.; Wei, X.; Xie, G.; Zhou, R.; Yu, L.; Liang, H. Fibronectin Extra Domain A (EDA) Sustains CD133+/CD44+ Subpopulation of Colorectal Cancer Cells. Stem Cell Res. 2013, 11, 820–833. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Wu, J.; Song, C.; Tian, Z.; Jiang, B. Hyaluronan Degradation by HYAL2 Is Essential for Odontoblastic Differentiation and Migration of Mouse Dental Papilla Cells. Matrix Biol. 2024, 129, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Higuchi, C.; Kunugiza, Y.; Yoshida, K.; Sakai, T.; Yoshikawa, H.; Nakata, K. Hyaluronan Inhibits BMP-induced Osteoblast Differentiation. FEBS Lett. 2015, 589, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-B.; Wang, N.-X.; Xu, Y.; Yu, C.-Y.; Liu, R.-M.; Luo, Y.; Xiao, J.-H. Hyaluronic Acid Ameliorates the Proliferative Ability of Human Amniotic Epithelial Cells Through Activation of TGF-β/BMP Signaling. PeerJ 2020, 8, e10104. [Google Scholar] [CrossRef] [PubMed]
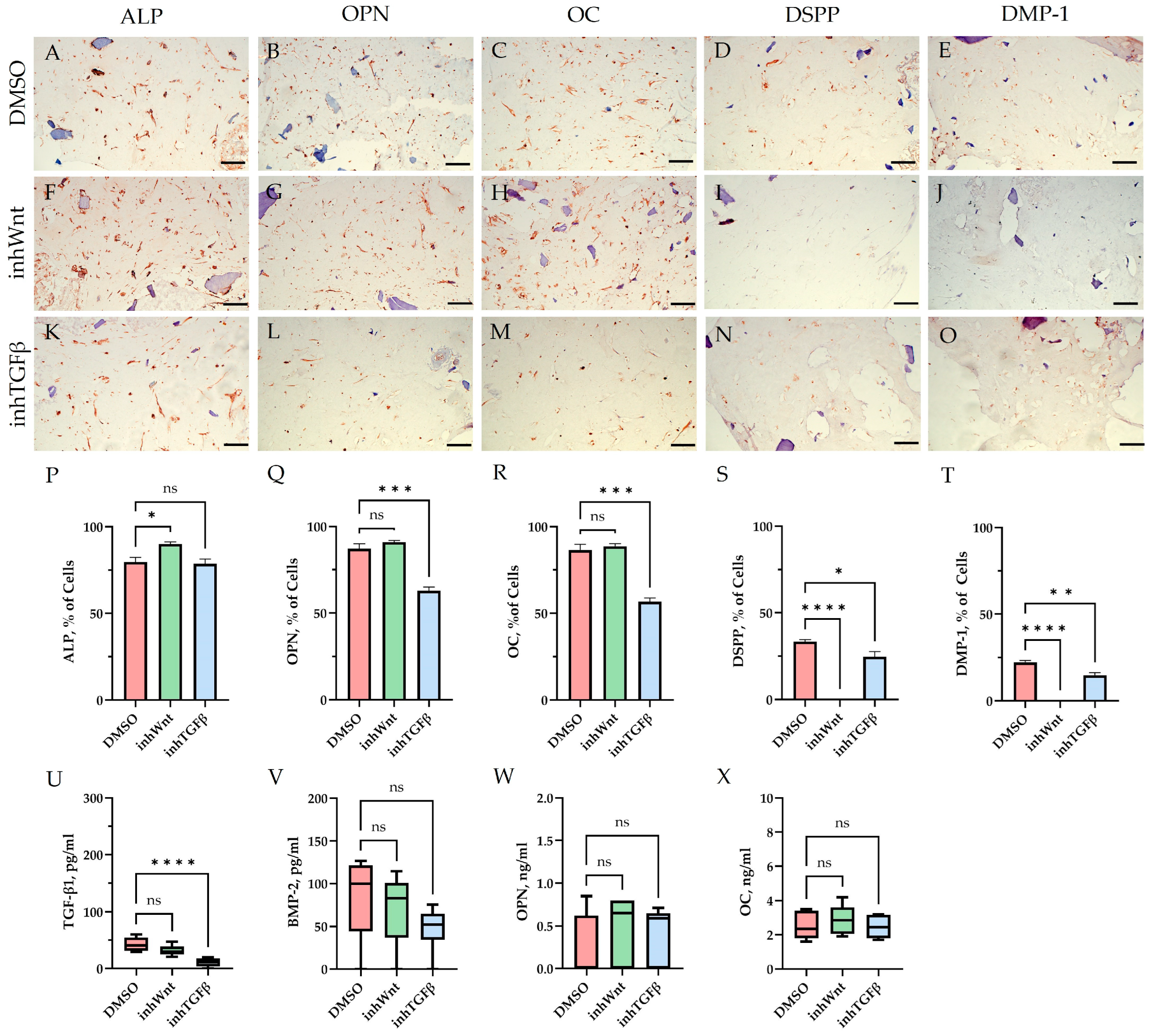
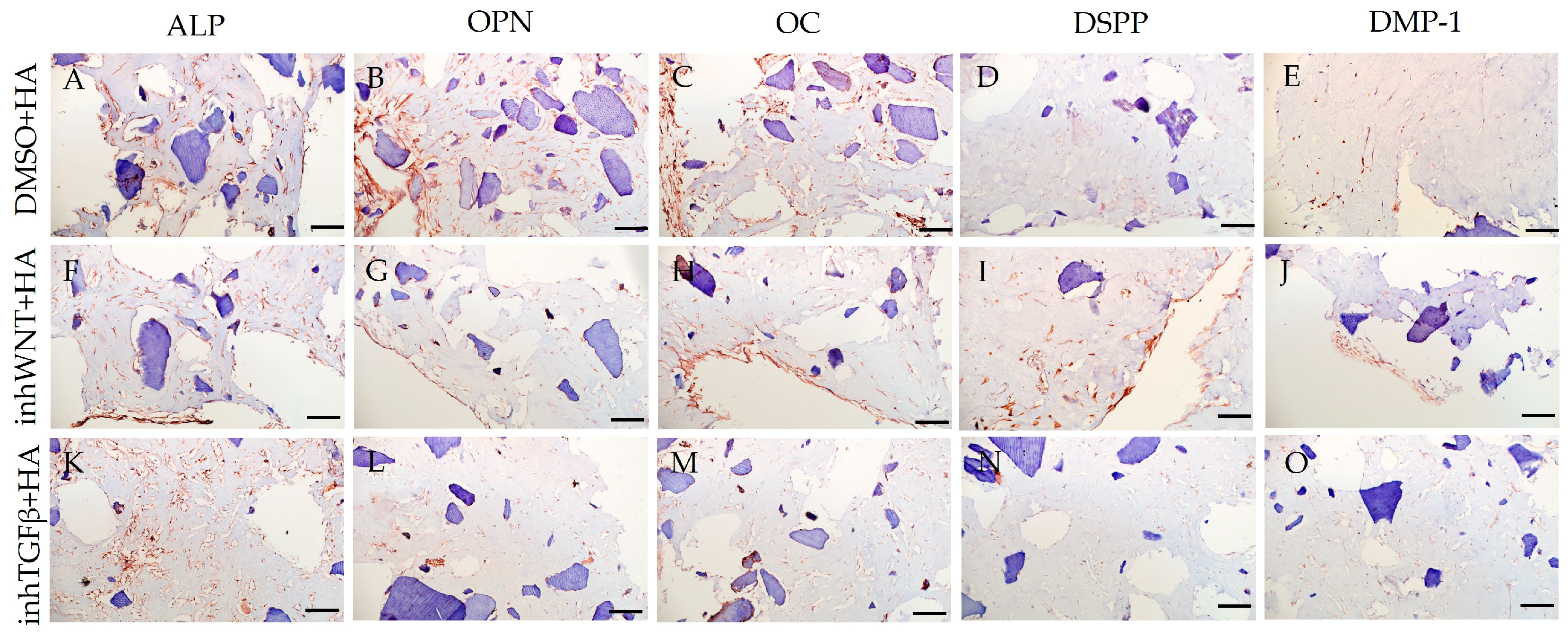
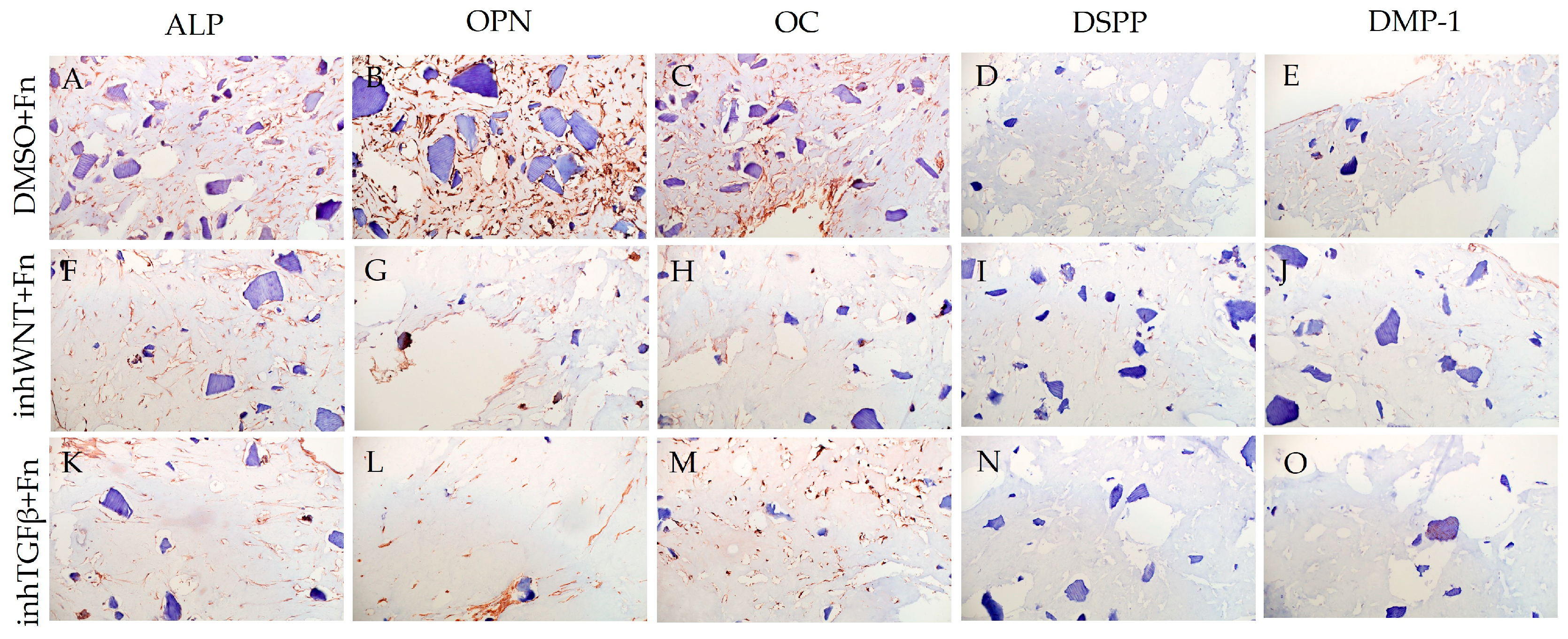
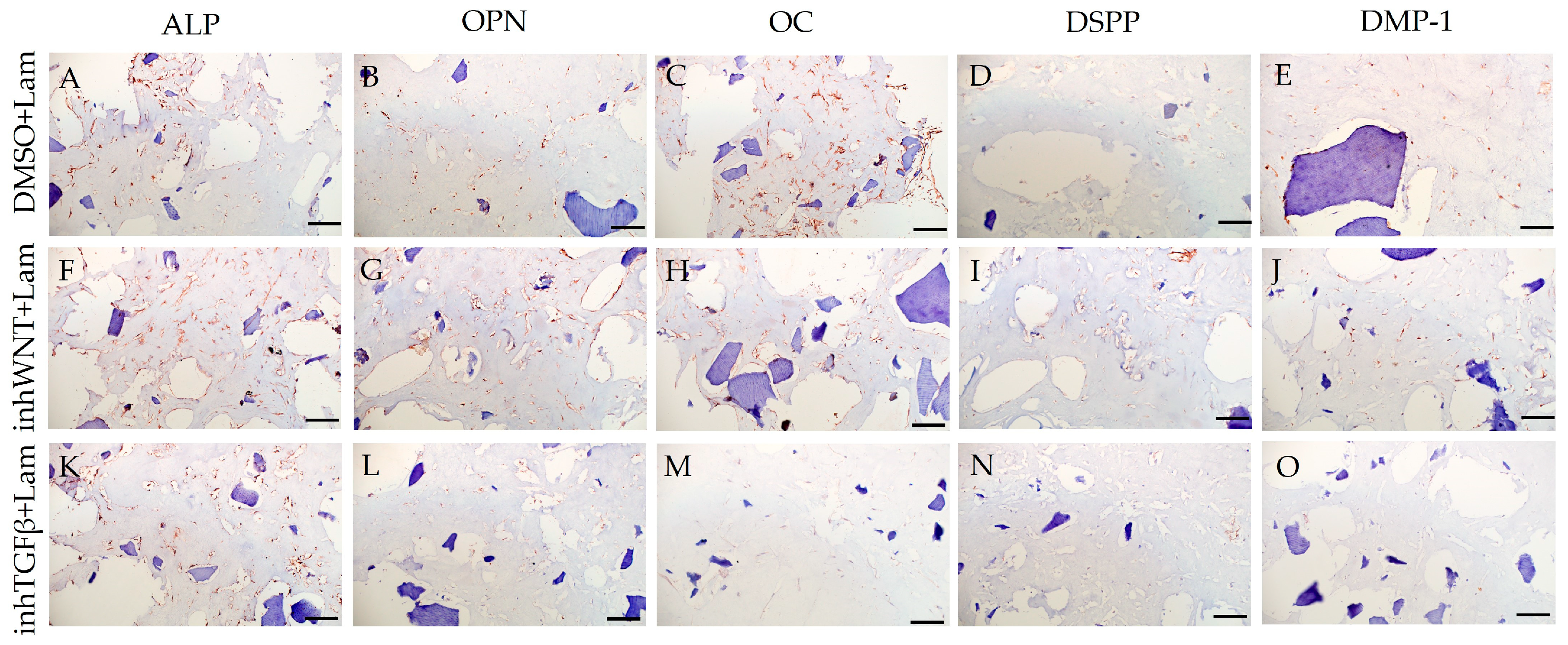

| Marker | DMSO | DMSO +HA | DMSO +Fn | DMSO +Lam | inhWnt | inhWnt +HA | inhWnt +Fn | inhWnt +Lam | inhTGFβ | inhTGFβ +HA | inhTGFβ +Fn | inhTGFβ +Lam |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALP | +++ | ++ | +++ | ++ | ++ | + /++ | + | +/++ | + | + | + | + |
| OPN | ++ | ++ | +++ | ++ | ++ | + | ++ | +/++ | + | + | + | + |
| OC | ++ | ++ | +++ | ++ | ++ | + | +/++ | +/++ | + | + | ++ | + |
| DSPP | + | + | + | + | − | + | + | + | +/+− | + | − | − |
| DMP-1 | + | + | + | + | − | + | + | + | +/+− | + | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, A.V.; Popova, O.P.; Danilova, T.I.; Latyshev, A.V.; Yanushevich, O.O.; Ivanov, A.A. Effects of ECM Components on Periodontal Ligament Stem Cell Differentiation Under Conditions of Disruption of Wnt and TGF-β Signaling Pathways. J. Funct. Biomater. 2025, 16, 94. https://doi.org/10.3390/jfb16030094
Kuznetsova AV, Popova OP, Danilova TI, Latyshev AV, Yanushevich OO, Ivanov AA. Effects of ECM Components on Periodontal Ligament Stem Cell Differentiation Under Conditions of Disruption of Wnt and TGF-β Signaling Pathways. Journal of Functional Biomaterials. 2025; 16(3):94. https://doi.org/10.3390/jfb16030094
Chicago/Turabian StyleKuznetsova, Alla V., Olga P. Popova, Tamara I. Danilova, Andrey V. Latyshev, Oleg O. Yanushevich, and Alexey A. Ivanov. 2025. "Effects of ECM Components on Periodontal Ligament Stem Cell Differentiation Under Conditions of Disruption of Wnt and TGF-β Signaling Pathways" Journal of Functional Biomaterials 16, no. 3: 94. https://doi.org/10.3390/jfb16030094
APA StyleKuznetsova, A. V., Popova, O. P., Danilova, T. I., Latyshev, A. V., Yanushevich, O. O., & Ivanov, A. A. (2025). Effects of ECM Components on Periodontal Ligament Stem Cell Differentiation Under Conditions of Disruption of Wnt and TGF-β Signaling Pathways. Journal of Functional Biomaterials, 16(3), 94. https://doi.org/10.3390/jfb16030094






