Rational Design of Multifunctional Hydrogels for Wound Repair
Abstract
:1. Introduction
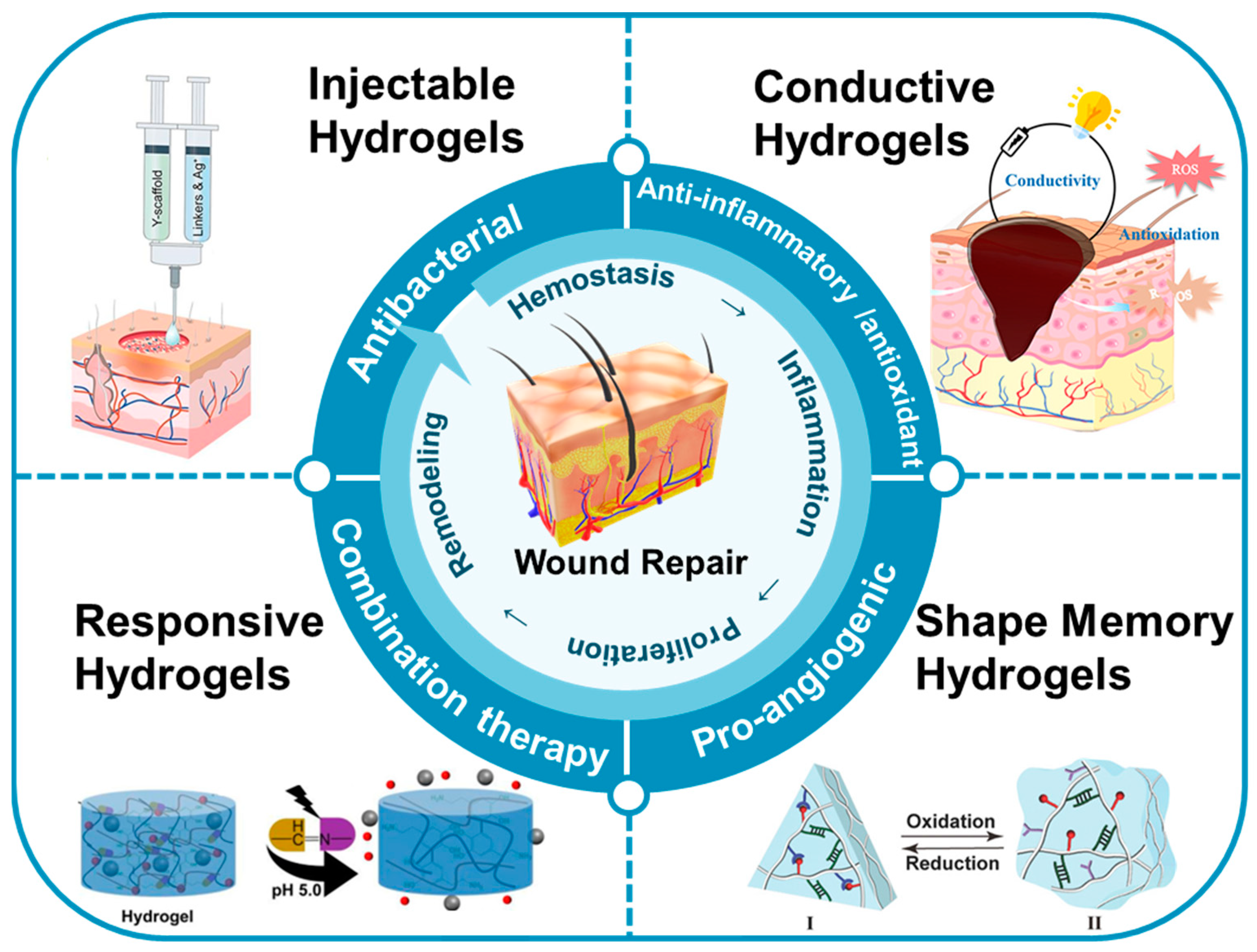
2. Types of Multifunctional Hydrogels
2.1. Injectable Hydrogels
2.2. Responsive Hydrogels
2.3. Conductive Hydrogels
2.4. Shape Memory Hydrogels
3. Applications in Wound Repair
3.1. Antibacterial
3.2. Anti-Inflammatory and Antioxidant
3.3. Pro-Angiogenic
3.4. Combination Therapy
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef] [PubMed]
- Mandla, S.; Davenport Huyer, L.; Radisic, M. Review: Multimodal bioactive material approaches for wound healing. APL Bioeng. 2018, 2, 021503. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.-T.; Drăgan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An Overview of Biopolymeric Electrospun Nanofibers Based on Polysaccharides for Wound Healing Management. Pharmaceutics 2020, 12, 938. [Google Scholar] [CrossRef]
- Korupalli, C.; Li, H.; Nguyen, N.; Mi, F.-L.; Chang, Y.; Lin, Y.-J.; Sung, H.-W. Conductive Materials for Healing Wounds: Their Incorporation in Electroactive Wound Dressings, Characterization, and Perspectives. Adv. Healthc. Mater. 2021, 10, 2001384. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2021, 14, 1. [Google Scholar] [CrossRef]
- Eming, S.A.; Murray, P.J.; Pearce, E.J. Metabolic orchestration of the wound healing response. Cell Metab. 2021, 33, 1726–1743. [Google Scholar] [CrossRef]
- Kloth, L.C. Discussion: Advanced Technologies to Improve Wound Healing: Electrical Stimulation, Vibration Therapy, and Ultrasound—What Is the Evidence? Plast. Reconstr. Surg. 2016, 138, 94S–104S. [Google Scholar] [CrossRef]
- Ashrafi, M.; Alonso-Rasgado, T.; Baguneid, M.; Bayat, A. The efficacy of electrical stimulation in lower extremity cutaneous wound healing: A systematic review. Exp. Dermatol. 2017, 26, 171–178. [Google Scholar] [CrossRef]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Phua, Q.H.; Han, H.A.; Soh, B.-S. Translational stem cell therapy: Vascularized skin grafts in skin repair and regeneration. J. Transl. Med. 2021, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Cook, K.A.; Martinez-Lozano, E.; Sheridan, R.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. Hydrogels for the management of second-degree burns: Currently available options and future promise. Burn. Trauma 2022, 10, tkac047. [Google Scholar] [CrossRef]
- Hu, C.; Yang, L.; Wang, Y. Recent advances in smart-responsive hydrogels for tissue repairing. MedComm–Biomater. Appl. 2022, 1, e23. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent trends on burn wound care: Hydrogel dressings and scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, 2100176. [Google Scholar] [CrossRef]
- Long, L.; Hu, C.; Liu, W.; Wu, C.; Lu, L.; Yang, L.; Wang, Y. Injectable multifunctional hyaluronic acid/methylcellulose hydrogels for chronic wounds repairing. Carbohydr. Polym. 2022, 289, 119456. [Google Scholar] [CrossRef]
- Loo, H.L.; Goh, B.H.; Lee, L.-H.; Chuah, L.H. Application of chitosan-based nanoparticles in skin wound healing. Asian J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Narayanan, K.B.; Uthappa, U.T.; Park, P.H.; Choi, I.; Han, S.S. Tissue Adhesive, Self-Healing, Biocompatible, Hemostasis, and Antibacterial Properties of Fungal-Derived Carboxymethyl Chitosan-Polydopamine Hydrogels. Pharmaceutics 2022, 14, 1028. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, H.; Wang, X.; Dong, S.; Guo, L.; Zhang, S.; Yang, X.; Liu, C.; Jiang, X.; Kan, M.; et al. Advances in preparation and application of antibacterial hydrogels. J. Nanobiotechnol. 2023, 21, 300. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Ma, Y.; Wang, M.; Pan, G. Nano-crosslinked dynamic hydrogels for biomedical applications. Mater. Today Bio 2023, 20, 100640. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Kim, S.W.; Kwon, D.Y.; Park, S.H.; Son, A.R.; Kim, J.H.; Kim, M.S. In vivo osteogenic differentiation of human turbinate mesenchymal stem cells in an injectable in situ-forming hydrogel. Biomaterials 2014, 35, 5337–5346. [Google Scholar] [CrossRef]
- Takei, T.; Yoshihara, R.; Danjo, S.; Fukuhara, Y.; Evans, C.; Tomimatsu, R.; Ohzuno, Y.; Yoshida, M. Hydrophobically-modified gelatin hydrogel as a carrier for charged hydrophilic drugs and hydrophobic drugs. Int. J. Biol. Macromol. 2020, 149, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Wang, X.; Park, S.; Jin, X.; Ma, P.X. Rapid Self-Integrating, Injectable Hydrogel for Tissue Complex Regeneration. Adv. Healthc. Mater. 2015, 4, 1491–1495. [Google Scholar] [CrossRef]
- Jia, Y.-G.; Zhu, X.X. Self-Healing Supramolecular Hydrogel Made of Polymers Bearing Cholic Acid and β-Cyclodextrin Pendants. Chem. Mater. 2015, 27, 387–393. [Google Scholar] [CrossRef]
- Cui, H.; Zhuang, X.; He, C.; Wei, Y.; Chen, X. High performance and reversible ionic polypeptide hydrogel based on charge-driven assembly for biomedical applications. Acta Biomater. 2015, 11, 183–190. [Google Scholar] [CrossRef]
- Lu, H.D.; Soranno, D.E.; Rodell, C.B.; Kim, I.L.; Burdick, J.A. Secondary Photocrosslinking of Injectable Shear-Thinning Dock-and-Lock Hydrogels. Adv. Healthc. Mater. 2013, 2, 1028–1036. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Espeel, P.; Du Prez, F.E. “Click”-Inspired Chemistry in Macromolecular Science: Matching Recent Progress and User Expectations. Macromolecules 2015, 48, 2–14. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Yang, F. Recent Progress in Developing Injectable Matrices for Enhancing Cell Delivery and Tissue Regeneration. Adv. Healthc. Mater. 2018, 7, 1701065. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Martin, J.R.; Werfel, T.A.; Shen, T.; Page, J.M.; Duvall, C.L. Cell Protective, ABC Triblock Polymer-Based Thermoresponsive Hydrogels with ROS-Triggered Degradation and Drug Release. J. Am. Chem. Soc. 2014, 136, 14896–14902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tokatlian, T.; Zhong, J.; Ng, Q.K.T.; Patterson, M.; Lowry, W.E.; Carmichael, S.T.; Segura, T. Physically Associated Synthetic Hydrogels with Long-Term Covalent Stabilization for Cell Culture and Stem Cell Transplantation. Adv. Mater. 2011, 23, 5098–5103. [Google Scholar] [CrossRef]
- Hu, C.; Liu, W.; Long, L.; Wang, Z.; Zhang, W.; He, S.; Lu, L.; Fan, H.; Yang, L.; Wang, Y. Regeneration of infarcted hearts by myocardial infarction-responsive injectable hydrogels with combined anti-apoptosis, anti-inflammatory and pro-angiogenesis properties. Biomaterials 2022, 290, 121849. [Google Scholar] [CrossRef]
- Yang, R.; Huang, J.; Zhang, W.; Xue, W.; Jiang, Y.; Li, S.; Wu, X.; Xu, H.; Ren, J.; Chi, B. Mechanoadaptive injectable hydrogel based on poly(γ-glutamic acid) and hyaluronic acid regulates fibroblast migration for wound healing. Carbohydr. Polym. 2021, 273, 118607. [Google Scholar] [CrossRef]
- Gao, F.; Ma, X.; Wang, F.; Zhou, F.; Ye, J.; Yang, D.; Li, M.; Wang, P. Injectable multifunctional DNA hydrogel for accelerated wound healing. Chem. Eng. J. 2023, 470, 144347. [Google Scholar] [CrossRef]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005941. [Google Scholar] [CrossRef]
- Belal, K.; Stoffelbach, F.; Lyskawa, J.; Fumagalli, M.; Hourdet, D.; Marcellan, A.; Smet, L.D.; de la Rosa, V.R.; Cooke, G.; Hoogenboom, R.; et al. Recognition-Mediated Hydrogel Swelling Controlled by Interaction with a Negative Thermoresponsive LCST Polymer. Angew. Chem. Int. Ed. 2016, 55, 13974–13978. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Silva, E.D.; Babo, P.S.; Costa-Almeida, R.; Domingues, R.M.A.; Mendes, B.B.; Paz, E.; Freitas, P.; Rodrigues, M.T.; Granja, P.L.; Gomes, M.E. Multifunctional magnetic-responsive hydrogels to engineer tendon-to-bone interface. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Y.; Zhang, Y.; Ren, J.; Yu, X.; Cao, X. Switchable Supramolecular Configurations of Al3+/LysTPY Coordination Polymers in a Hydrogel Network Controlled by Ultrasound and Heat. ACS Appl. Mater. Interfaces 2021, 13, 40079–40087. [Google Scholar] [CrossRef]
- Son, H.; Byun, E.; Yoon, Y.J.; Nam, J.; Song, S.H.; Yoon, C. Untethered Actuation of Hybrid Hydrogel Gripper via Ultrasound. ACS Macro Lett. 2020, 9, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, P.; Gaspar, V.M.; Mano, J.F. Stimuli-responsive nanocarriers for delivery of bone therapeutics–Barriers and progresses. J. Control. Release 2018, 273, 51–67. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, J.; Yang, D.; Shao, J.; Wang, W.; Zhang, Q.; Dong, X. Recent advances in pH-responsive nanomaterials for anti-infective therapy. J. Mater. Chem. B 2020, 8, 10700–10711. [Google Scholar] [CrossRef]
- Messersmith, P.B.; He, L.; Fullenkamp, D.E. pH responsive self-healing hydrogels formed by boronate-catechol complexation. Chem. Commun. 2017, 47, 7497–7499. [Google Scholar]
- Martin, J.R.; Duvall, C.L. Chapter Nine-Oxidation State as a Bioresponsive Trigger. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 225–250. [Google Scholar]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Gao, N.; You, H. Recent Applications of Point-of-Care Devices for Glucose Detection on the Basis of Stimuli-Responsive Volume Phase Transition of Hydrogel. BioChip J. 2021, 15, 23–41. [Google Scholar] [CrossRef]
- Sharifzadeh, G.; Hosseinkhani, H. Biomolecule-Responsive Hydrogels in Medicine. Adv. Healthc. Mater. 2017, 6, 1700801. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, X.; Ma, X.; Hu, Y.; Zhang, Y.; Li, S.; Wang, J.; Zhao, Y.; Chai, R. Controllable growth of spiral ganglion neurons by magnetic colloidal nanochains. Nano Today 2022, 44, 101507. [Google Scholar] [CrossRef]
- Najafipour, A.; Gharieh, A.; Fassihi, A.; Sadeghi-Aliabadi, H.; Mahdavian, A.R. MTX-Loaded Dual Thermoresponsive and pH-Responsive Magnetic Hydrogel Nanocomposite Particles for Combined Controlled Drug Delivery and Hyperthermia Therapy of Cancer. Mol. Pharm. 2021, 18, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Long, L.; Hu, C.; Kong, Q.; Wang, Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control. Release 2022, 341, 147–165. [Google Scholar] [CrossRef]
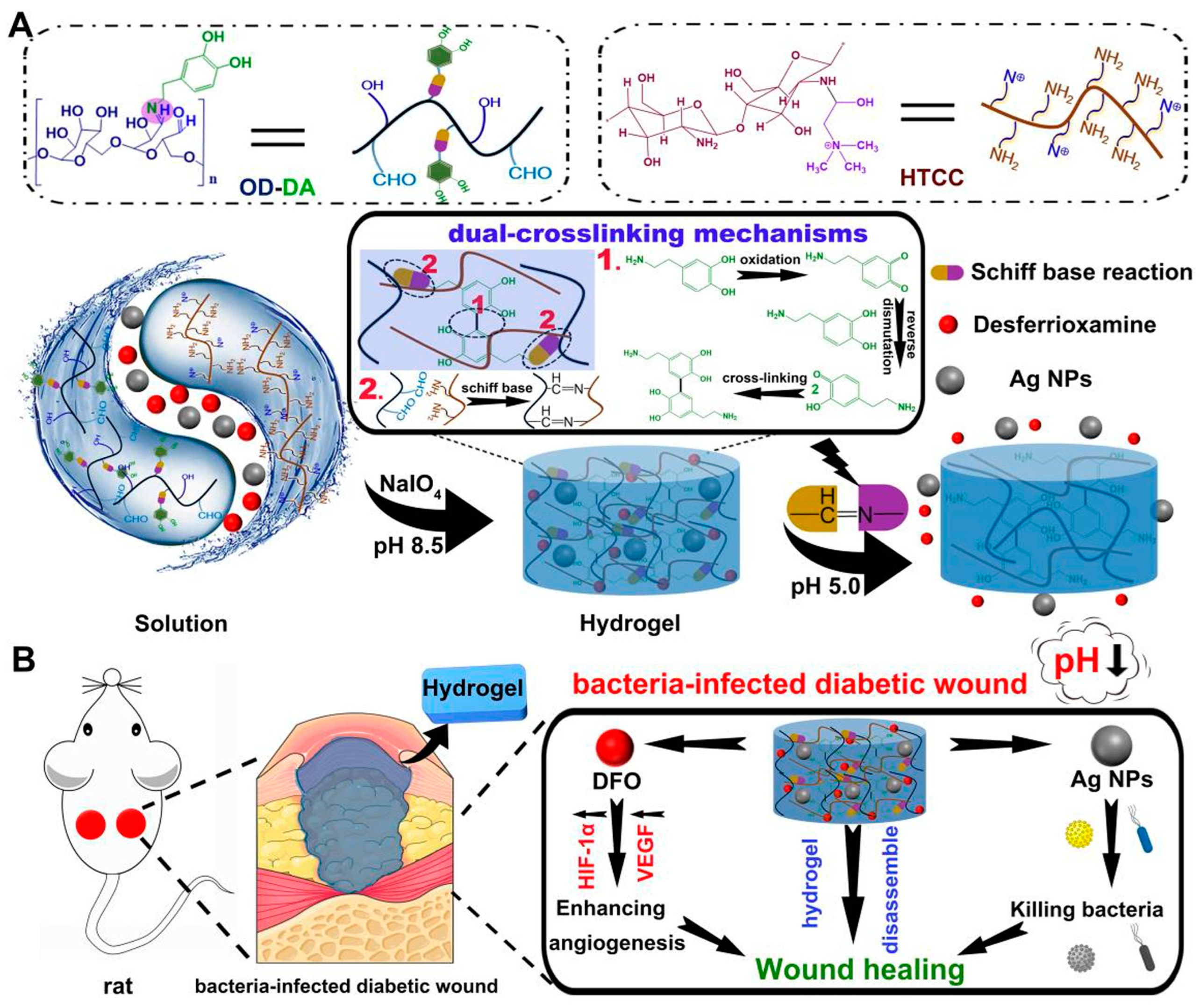
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Pan, G.; Chen, J.; Guo, B. Multiple Stimuli-Responsive Nanozyme-Based Cryogels with Controlled NO Release as Self-Adaptive Wound Dressing for Infected Wound Healing. Adv. Funct. Mater. 2023, 33, 2214089. [Google Scholar] [CrossRef]
- Thakral, G.; LaFontaine, J.; Najafi, B.; Talal, T.K.; Kim, P.; Lavery, L.A. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 2013, 4, 22081. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, H.; Makin, I.R.S.; Skiba, J.B.; Izadjoo, M.J. Measurement of microelectric potentials in a bioelectrically-active wound care device in the presence of bacteria. J. Wound Care 2014, 24, 23–33. [Google Scholar] [CrossRef]
- Torkaman, G. Electrical Stimulation of Wound Healing: A Review of Animal Experimental Evidence. Adv. Wound Care 2013, 3, 202–218. [Google Scholar] [CrossRef]
- Lala, D.; Spaulding, S.J.; Burke, S.M.; Houghton, P.E. Electrical stimulation therapy for the treatment of pressure ulcers in individuals with spinal cord injury: A systematic review and meta-analysis. Int. Wound J. 2016, 13, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
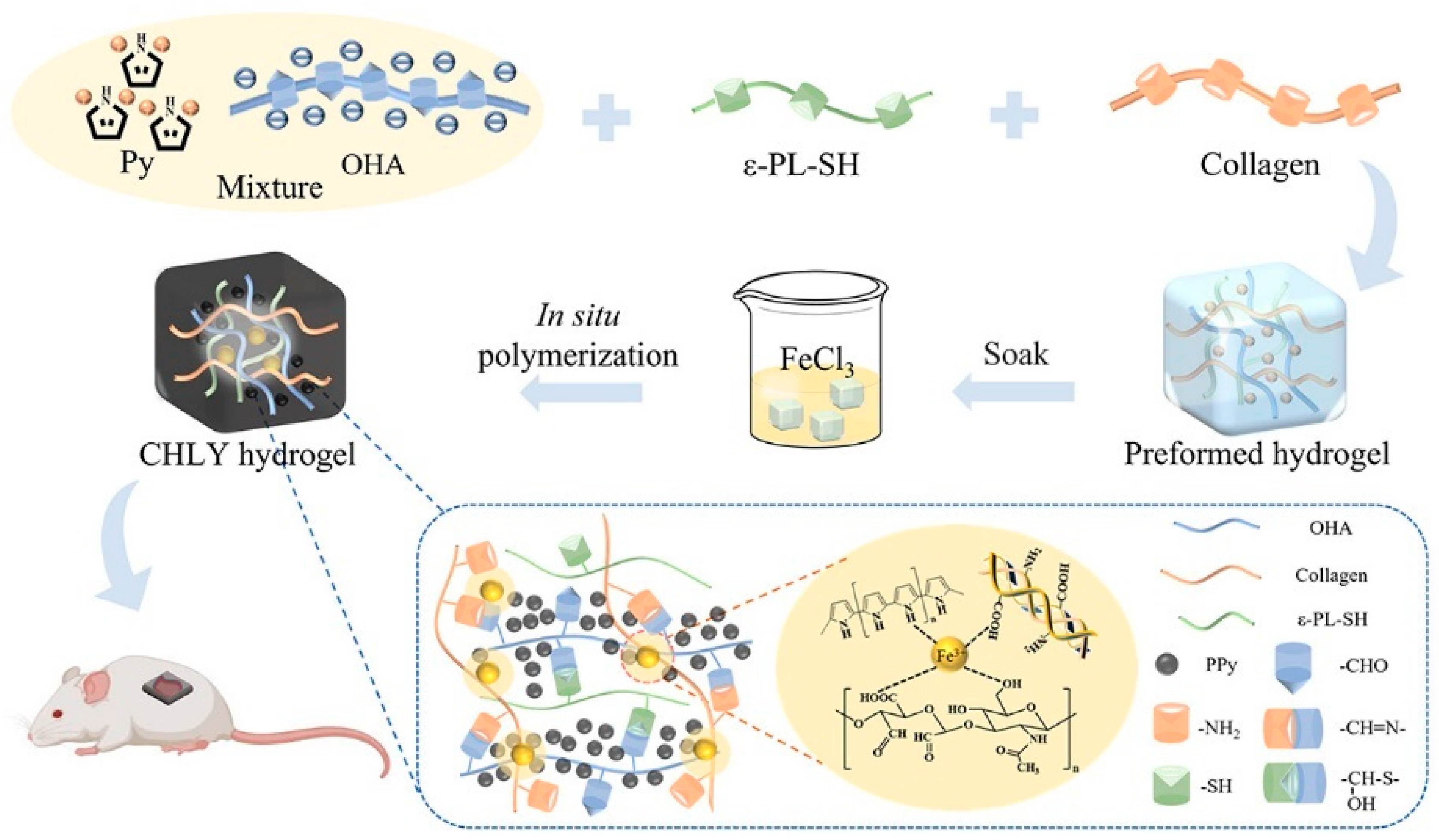
- Lei, H.; Fan, D. Conductive, adaptive, multifunctional hydrogel combined with electrical stimulation for deep wound repair. Chem. Eng. J. 2021, 421, 129578. [Google Scholar] [CrossRef]
- Gao, C.; Song, S.; Lv, Y.; Huang, J.; Zhang, Z. Recent Development of Conductive Hydrogels for Tissue Engineering: Review and Perspective. Macromol. Biosci. 2022, 22, 2200051. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef]
- Guan, L.; Liu, H.; Ren, X.; Wang, T.; Zhu, W.; Zhao, Y.; Feng, Y.; Shen, C.; Zvyagin, A.V.; Fang, L.; et al. Balloon Inspired Conductive Hydrogel Strain Sensor for Reducing Radiation Damage in Peritumoral Organs during Brachytherapy. Adv. Funct. Mater. 2022, 32, 2112281. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Song, S.; Yang, K.; Liu, H.; Yang, Z.; Wang, J.; Yang, B.; Lin, Q. Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv. Funct. Mater. 2019, 29, 1901474. [Google Scholar] [CrossRef]
- Zhao, Y.; Ohm, Y.; Liao, J.; Luo, Y.; Cheng, H.-Y.; Won, P.; Roberts, P.; Carneiro, M.R.; Islam, M.F.; Ahn, J.H.; et al. A self-healing electrically conductive organogel composite. Nat. Electron. 2023, 6, 206–215. [Google Scholar] [CrossRef]
- Li, G.; Huang, K.; Deng, J.; Guo, M.; Cai, M.; Zhang, Y.; Guo, C.F. Highly Conducting and Stretchable Double-Network Hydrogel for Soft Bioelectronics. Adv. Mater. 2022, 34, 2200261. [Google Scholar] [CrossRef]
- Lin, X.; Yang, X.; Li, P.; Xu, Z.; Zhao, L.; Mu, C.; Li, D.; Ge, L. Antibacterial Conductive Collagen-Based Hydrogels for Accelerated Full-Thickness Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 22817–22829. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, M.; Sun, Y.; Zuo, B. Self-Healing, Wet-Adhesion silk fibroin conductive hydrogel as a wearable strain sensor for underwater applications. Chem. Eng. J. 2022, 446, 136931. [Google Scholar] [CrossRef]
- Qiao, L.; Liang, Y.; Chen, J.; Huang, Y.; Alsareii, S.A.; Alamri, A.M.; Harraz, F.A.; Guo, B. Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing. Bioact. Mater. 2023, 30, 129–141. [Google Scholar] [CrossRef]
- Löwenberg, C.; Balk, M.; Wischke, C.; Behl, M.; Lendlein, A. Shape-Memory Hydrogels: Evolution of Structural Principles To Enable Shape Switching of Hydrophilic Polymer Networks. Acc. Chem. Res. 2017, 50, 723–732. [Google Scholar] [CrossRef]
- Lu, W.; Le, X.; Zhang, J.; Huang, Y.; Chen, T. Supramolecular shape memory hydrogels: A new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef]
- Korde, J.M.; Kandasubramanian, B. Naturally biomimicked smart shape memory hydrogels for biomedical functions. Chem. Eng. J. 2020, 379, 122430. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Wen, J.; Liu, J.; Zhang, D.; Li, J.; Chu, H. Ultra-Stretchable, Variable Modulus, Shape Memory Multi-Purpose Low Hysteresis Hydrogel Derived from Solvent-Induced Dynamic Micelle Sea-Island Structure. Adv. Funct. Mater. 2021, 31, 2011259. [Google Scholar] [CrossRef]
- Xiang, T.; Guo, Q.; Jia, L.; Yin, T.; Huang, W.; Zhang, X.; Zhou, S. Multifunctional Hydrogels for the Healing of Diabetic Wounds. Adv. Healthc. Mater. 2023, 2301885. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Wang, T.; Sun, W.; Tong, Z. NIR-Triggered Rapid Shape Memory PAM–GO–Gelatin Hydrogels with High Mechanical Strength. ACS Appl. Mater. Interfaces 2016, 8, 12384–12392. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Z.; Gao, H.; Fan, C.; Ma, G.; Zhang, D.; Xiao, M.; Zhang, B.; Yang, Y.; Cui, C.; et al. Stiffness Self-Tuned Shape Memory Hydrogels for Embolization of Aneurysms. Adv. Funct. Mater. 2020, 30, 1910197. [Google Scholar] [CrossRef]
- Costa, D.C.S.; Costa, P.D.C.; Gomes, M.C.; Chandrakar, A.; Wieringa, P.A.; Moroni, L.; Mano, J.F. Universal Strategy for Designing Shape Memory Hydrogels. ACS Mater. Lett. 2022, 4, 701–706. [Google Scholar] [CrossRef]
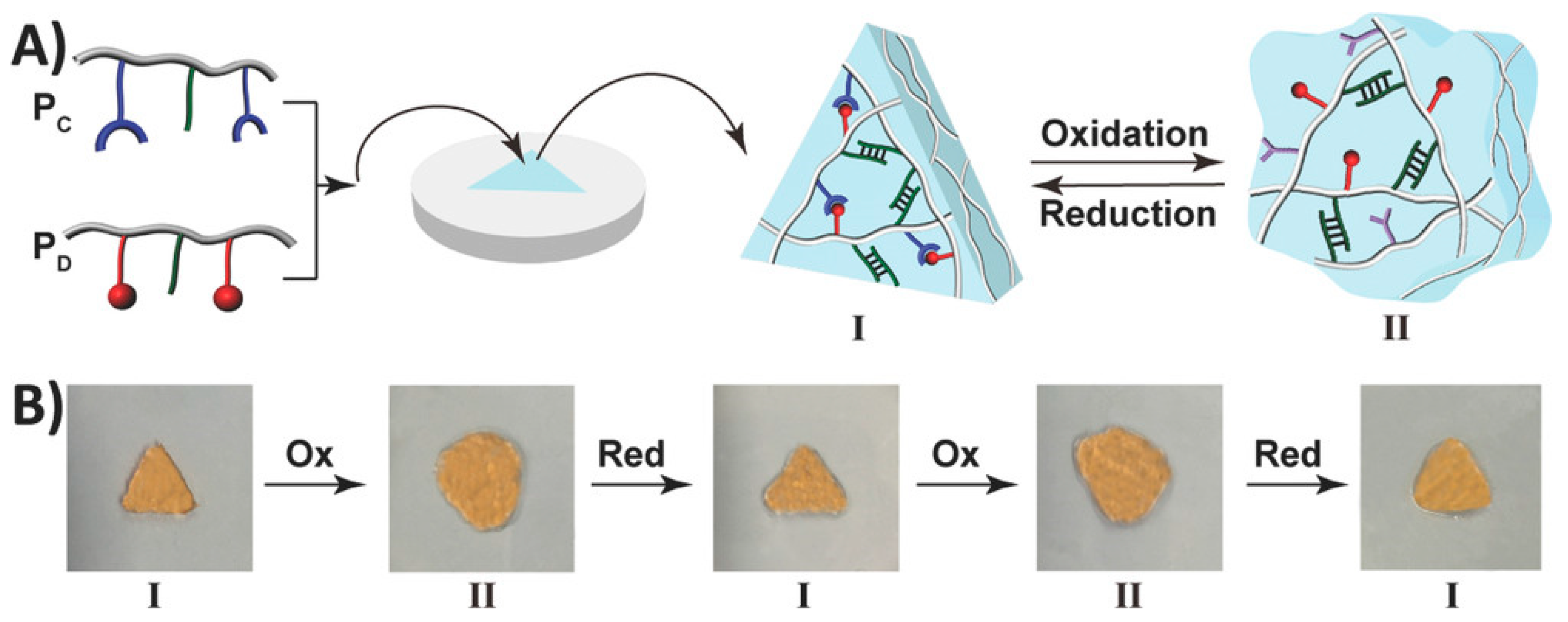
- Wang, C.; Fadeev, M.; Vázquez-González, M.; Willner, I. Stimuli-Responsive Donor–Acceptor and DNA-Crosslinked Hydrogels: Application as Shape-Memory and Self-Healing Materials. Adv. Funct. Mater. 2018, 28, 1803111. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Wang, Y.; Ashammakhi, N.; Alem, H.; Erdem, A.; Chang, Q.; Xu, K.; Liu, Y.; Luo, G.; et al. An Alkaline Based Method for Generating Crystalline, Strong, and Shape Memory Polyvinyl Alcohol Biomaterials. Adv. Sci. 2020, 7, 1902740. [Google Scholar] [CrossRef]
- Lu, H.; Tu, C.; Zhou, T.; Zhang, W.; Zhan, Y.; Ding, J.; Wu, X.; Yang, Z.; Cao, W.; Deng, L.; et al. A ROS-scavenging hydrogel loaded with bacterial quorum sensing inhibitor hyperbranched poly-L-lysine promotes the wound scar-free healing of infected skin in vivo. Chem. Eng. J. 2022, 436, 135130. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Yan, Z.; Wang, T.; Chen, Z.; Song, H.; Zheng, Y. Microneedle Patches with Antimicrobial and Immunomodulating Properties for Infected Wound Healing. Adv. Sci. 2023, 10, 2300576. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Seidi, F.; Wang, Y.; Zheng, L.; Jin, Y.; Xiao, H. Injectable chitosan hydrogels tailored with antibacterial and antioxidant dual functions for regenerative wound healing. Carbohydr. Polym. 2022, 298, 120103. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, G.; Huang, J.; Wu, J. Novel Glucose-Responsive Antioxidant Hybrid Hydrogel for Enhanced Diabetic Wound Repair. ACS Appl. Mater. Interfaces 2022, 14, 7680–7689. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Y.; Xie, C.; Lei, B. Multiple Coordination-Derived Bioactive Hydrogel with Proangiogenic Hemostatic Capacity for Wound Repair. Adv. Healthc. Mater. 2022, 11, 2200722. [Google Scholar] [CrossRef]
- Lei, X.-X.; Zou, C.-Y.; Hu, J.-J.; Jiang, Y.-L.; Zhang, X.-Z.; Zhao, L.-M.; He, T.; Zhang, Q.-Y.; Li, Y.-X.; Li-Ling, J.; et al. Click-crosslinked in-situ hydrogel improves the therapeutic effect in wound infections through antibacterial, antioxidant and anti-inflammatory activities. Chem. Eng. J. 2023, 461, 142092. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, T.; Jiang, J.; He, Y.; Xiang, T.; Zhou, S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact. Mater. 2023, 20, 561–573. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, H.; Cai, J.; Wang, M.; Zhou, X.; Ren, L. Poly(pentahydropyrimidine)-Based Hybrid Hydrogel with Synergistic Antibacterial and Pro-Angiogenic Ability for the Therapy of Diabetic Foot Ulcers. Adv. Funct. Mater. 2023, 2303147. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Tu, C.; Lu, H.; Zhou, T.; Zhang, W.; Deng, L.; Cao, W.; Yang, Z.; Wang, Z.; Wu, X.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef]
- Li, Y.; Yu, P.; Wen, J.; Sun, H.; Wang, D.; Liu, J.; Li, J.; Chu, H. Nanozyme-Based Stretchable Hydrogel of Low Hysteresis with Antibacterial and Antioxidant Dual Functions for Closely Fitting and Wound Healing in Movable Parts. Adv. Funct. Mater. 2022, 32, 2110720. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y.; Cai, G.; Fu, S.; Yang, L.; Ma, Y.; Dong, Z. Reactive incorporation of Ag into porous TiO2 coating and its influence on its microstructure, in vitro antibacterial efficacy and cytocompatibility. Prog. Nat. Sci. Mater. Int. 2021, 31, 215–229. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Ma, W.; Chen, X.; Fu, L.; Zhu, J.; Fan, M.; Chen, J.; Yang, C.; Yang, G.; Wu, L.; Mao, G.; et al. Ultra-efficient Antibacterial System Based on Photodynamic Therapy and CO Gas Therapy for Synergistic Antibacterial and Ablation Biofilms. ACS Appl. Mater. Interfaces 2020, 12, 22479–22491. [Google Scholar] [CrossRef]
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The role of the light source in antimicrobial photodynamic therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Li, H.; Niu, X.; Zhang, D.; Wang, K. Antimicrobial Hydrogels: Potential Materials for Medical Application. Small 2023, 2304047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Long, L.; He, S.; Wang, Z.; Liu, Y.; Yang, L.; Chen, N.; Hu, C.; Wang, Y. Responsive multifunctional hydrogels emulating the chronic wounds healing cascade for skin repair. J. Control. Release 2023, 354, 821–834. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Hu, C.; Liu, W.; Long, L.; Wang, Z.; Yuan, Y.; Zhang, W.; He, S.; Wang, J.; Yang, L.; Lu, L.; et al. Microenvironment-responsive multifunctional hydrogels with spatiotemporal sequential release of tailored recombinant human collagen type III for the rapid repair of infected chronic diabetic wounds. J. Mater. Chem. B 2021, 9, 9684–9699. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, F.; Kong, Q.; Lu, Y.; Zhang, B.; Wu, C.; Luo, R.; Wang, Y. Synergistic Chemical and Photodynamic Antimicrobial Therapy for Enhanced Wound Healing Mediated by Multifunctional Light-Responsive Nanoparticles. Biomacromolecules 2019, 20, 4581–4592. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Zhao, X.; Pei, D.; Yang, Y.; Xu, K.; Yu, J.; Zhang, Y.; Zhang, Q.; He, G.; Zhang, Y.; Li, A.; et al. Green Tea Derivative Driven Smart Hydrogels with Desired Functions for Chronic Diabetic Wound Treatment. Adv. Funct. Mater. 2021, 31, 2009442. [Google Scholar] [CrossRef]
- Wang, P.; Huang, S.; Hu, Z.; Yang, W.; Lan, Y.; Zhu, J.; Hancharou, A.; Guo, R.; Tang, B. In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing. Acta Biomater. 2019, 100, 191–201. [Google Scholar] [CrossRef]
- Huang, X.; Ye, Y.; Zhang, J.; Zhang, X.; Ma, H.; Zhang, Y.; Fu, X.; Tang, J.; Jiang, N.; Han, Y.; et al. Reactive Oxygen Species Scavenging Functional Hydrogel Delivers Procyanidins for the Treatment of Traumatic Brain Injury in Mice. ACS Appl. Mater. Interfaces 2022, 14, 33756–33767. [Google Scholar] [CrossRef]
- Soares, R.D.F.; Campos, M.G.N.; Ribeiro, G.P.; Salles, B.C.C.; Cardoso, N.S.; Ribeiro, J.R.; Souza, R.M.; Leme, K.C.; Soares, C.B.; de Oliveira, C.M.; et al. Development of a chitosan hydrogel containing flavonoids extracted from Passiflora edulis leaves and the evaluation of its antioxidant and wound healing properties for the treatment of skin lesions in diabetic mice. J. Biomed. Mater. Res. Part A 2020, 108, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, J.; Gao, L. Nanozyme-based medicine for enzymatic therapy: Progress and challenges. Biomed. Mater. 2021, 16, 042002. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Kim, J. ROS-Scavenging Therapeutic Hydrogels for Modulation of the Inflammatory Response. ACS Appl. Mater. Interfaces 2022, 14, 23002–23021. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, B.; Xiang, F.; Tan, J.; Chen, Z.; Zhang, X.; Wu, C.; Mao, Z.; Luo, G.; Chen, X.; et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020, 11, 2788. [Google Scholar] [CrossRef]
- Wu, H.; Li, F.; Shao, W.; Gao, J.; Ling, D. Promoting Angiogenesis in Oxidative Diabetic Wound Microenvironment Using a Nanozyme-Reinforced Self-Protecting Hydrogel. ACS Cent. Sci. 2019, 5, 477–485. [Google Scholar] [CrossRef]
- Song, H.H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, L.; Sigen, A.; Gao, Y.; Zhou, D.; Greiser, U.; Creagh-Flynn, J.; Zhang, H.; Dong, Y.; Cutlar, L.; et al. Injectable hyperbranched poly(β-amino ester) hydrogels with on-demand degradation profiles to match wound healing processes. Chem. Sci. 2018, 9, 2179–2187. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, Y.; Tao, J.; Baumgarten, K.M.; Sun, H. Hypoxia-Mimicking Nanofibrous Scaffolds Promote Endogenous Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 32450–32459. [Google Scholar] [CrossRef]
- Chen, H.; Guo, L.; Wicks, J.; Ling, C.; Zhao, X.; Yan, Y.; Qi, J.; Cui, W.; Deng, L. Quickly promoting angiogenesis by using a DFO-loaded photo-crosslinked gelatin hydrogel for diabetic skin regeneration. J. Mater. Chem. B 2016, 4, 3770–3781. [Google Scholar] [CrossRef]
- He, J.; Li, Z.; Wang, J.; Li, T.; Chen, J.; Duan, X.; Guo, B. Photothermal antibacterial antioxidant conductive self-healing hydrogel with nitric oxide release accelerates diabetic wound healing. Compos. Part B Eng. 2023, 266, 110985. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Z.; Zhang, Y.; Wang, E.; Ma, B.; Xu, Q.; Ma, L.; Zhang, M.; Pei, G.; Chang, J. A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials 2021, 264, 120414. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Zhou, L.; Pi, W.; Cheng, S.; Gu, Z.; Zhang, K.; Min, T.; Zhang, W.; Du, H.; Zhang, P.; Wen, Y. Multifunctional DNA Hydrogels with Hydrocolloid-Cotton Structure for Regeneration of Diabetic Infectious Wounds. Adv. Funct. Mater. 2021, 31, 2106167. [Google Scholar] [CrossRef]
- Sun, X.; Ma, Z.; Zhao, X.; Jin, W.; Zhang, C.; Ma, J.; Qiang, L.; Wang, W.; Deng, Q.; Yang, H.; et al. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact. Mater. 2021, 6, 757–769. [Google Scholar] [CrossRef]
- Alizadehgiashi, M.; Nemr, C.R.; Chekini, M.; Pinto Ramos, D.; Mittal, N.; Ahmed, S.U.; Khuu, N.; Kelley, S.O.; Kumacheva, E. Multifunctional 3D-Printed Wound Dressings. ACS Nano 2021, 15, 12375–12387. [Google Scholar] [CrossRef]
- Clegg, J.R.; Wagner, A.M.; Shin, S.R.; Hassan, S.; Khademhosseini, A.; Peppas, N.A. Modular fabrication of intelligent material-tissue interfaces for bioinspired and biomimetic devices. Prog. Mater. Sci. 2019, 106, 100589. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Yang, F.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Smart wound dressing for advanced wound management: Real-time monitoring and on-demand treatment. Mater. Des. 2023, 229, 111917. [Google Scholar] [CrossRef]
- Qi, M.; Yang, R.; Wang, Z.; Liu, Y.; Zhang, Q.; He, B.; Li, K.; Yang, Q.; Wei, L.; Pan, C.; et al. Bioinspired Self-healing Soft Electronics. Adv. Funct. Mater. 2023, 33, 2214479. [Google Scholar] [CrossRef]

| Hydrogels | Functional Elements | Therapeutic Strategies | References | ||
|---|---|---|---|---|---|
| Antibacterial (Bacterial Species) | Anti-Inflammatory and Antioxidant (Evaluating Test) | Pro-Angiogenic (Effect Cargos) | |||
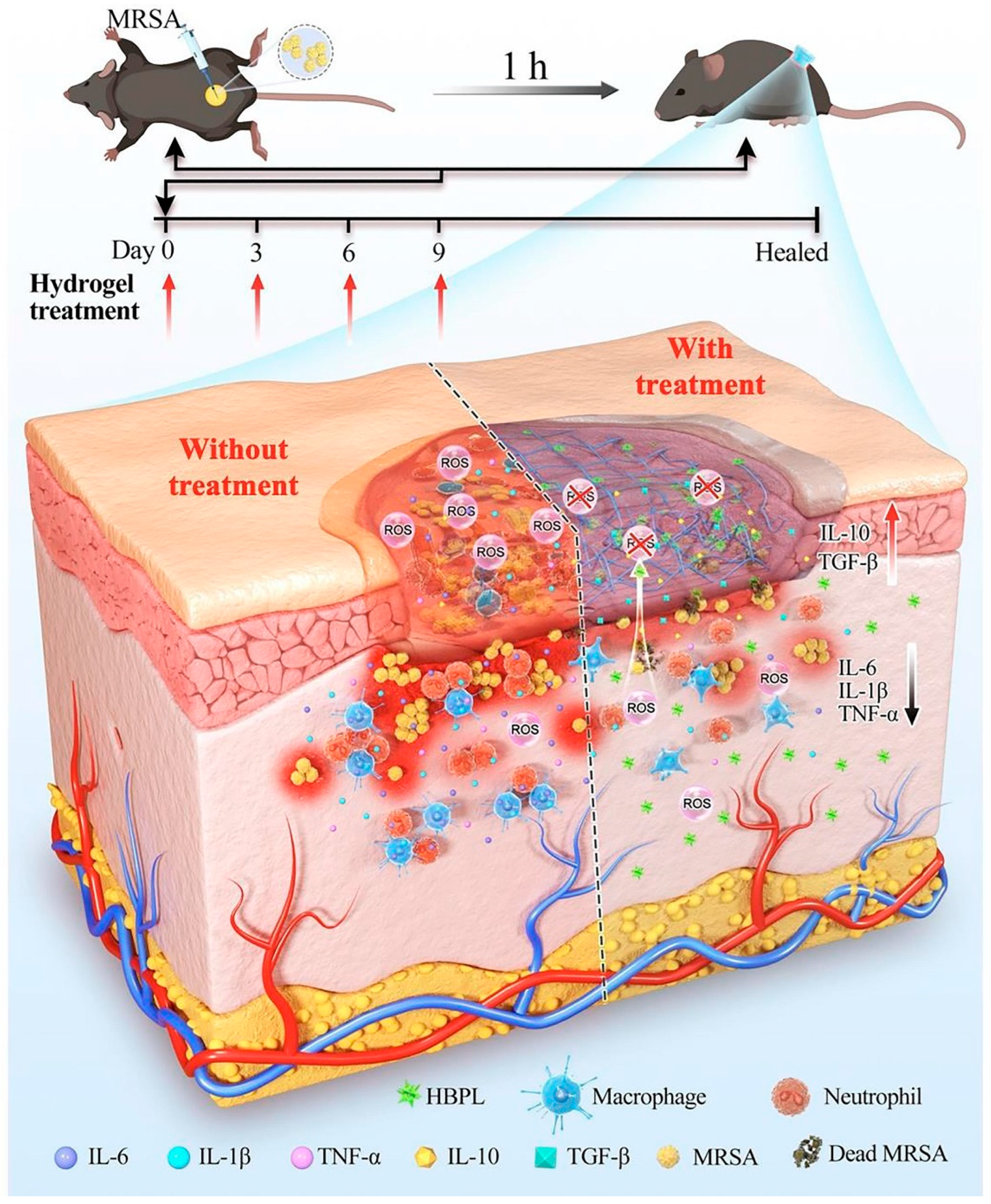
| PPG hydrogel | HBPL | + MRSA | [86] | ||
| QCS/OD/TOB/PPY@PDA | TOB | + Pseudomonas aeruginosa (PA), Staphylococcus aureus (S. aureus) | [87] | ||
| PFG/M microneedle | polydopamine (PDA)-loaded iron oxide | + Escherichia coli (E. coli), S. aureus | [88] | ||
| quaternized chitosan hydrogel | quaternized chitosan and EGCG | + 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical and reactive oxygen species assay | [89] | ||
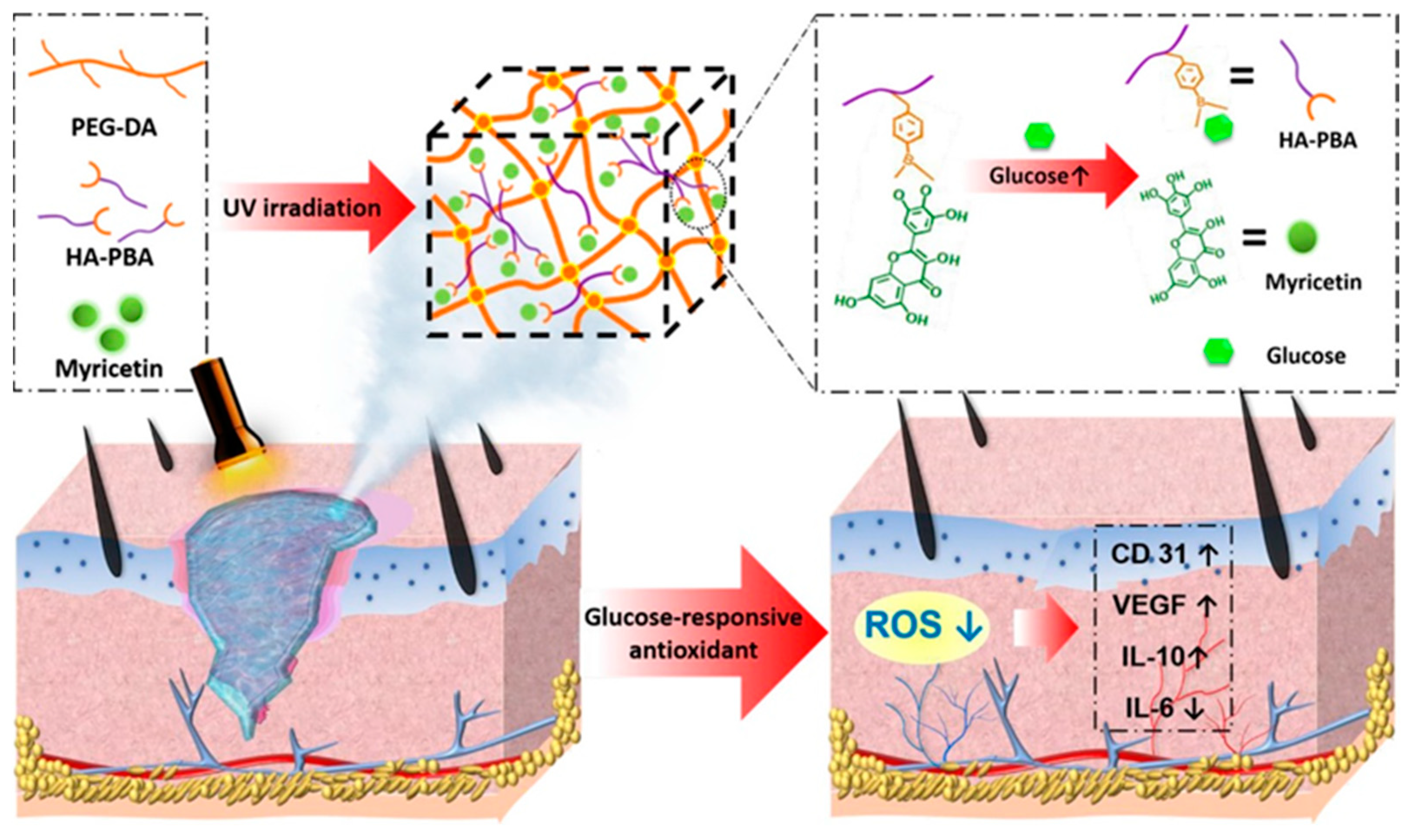
| PEG-DA/HA-PBA hydrogel | myricetin | + DPPH, 2′,7′-dichlorodihydrofluoresceindiacetate (DCFH-DA), interleukin-6 (IL-6), IL-10 | [90] | ||
| SGPA hydrogel | poly(citrate-ethylene glycol-alendronate) (PCA) and Gd3+ | + c | + PCA | [91] | |
| maleimide-based oxidized sodium alginate and sulfhydryl carboxymethyl chitosan hydrogel | sodium alginate and sulfhydryl carboxymethyl chitosan | + E. coli, S. aureus | + DHE probe | [92] | |
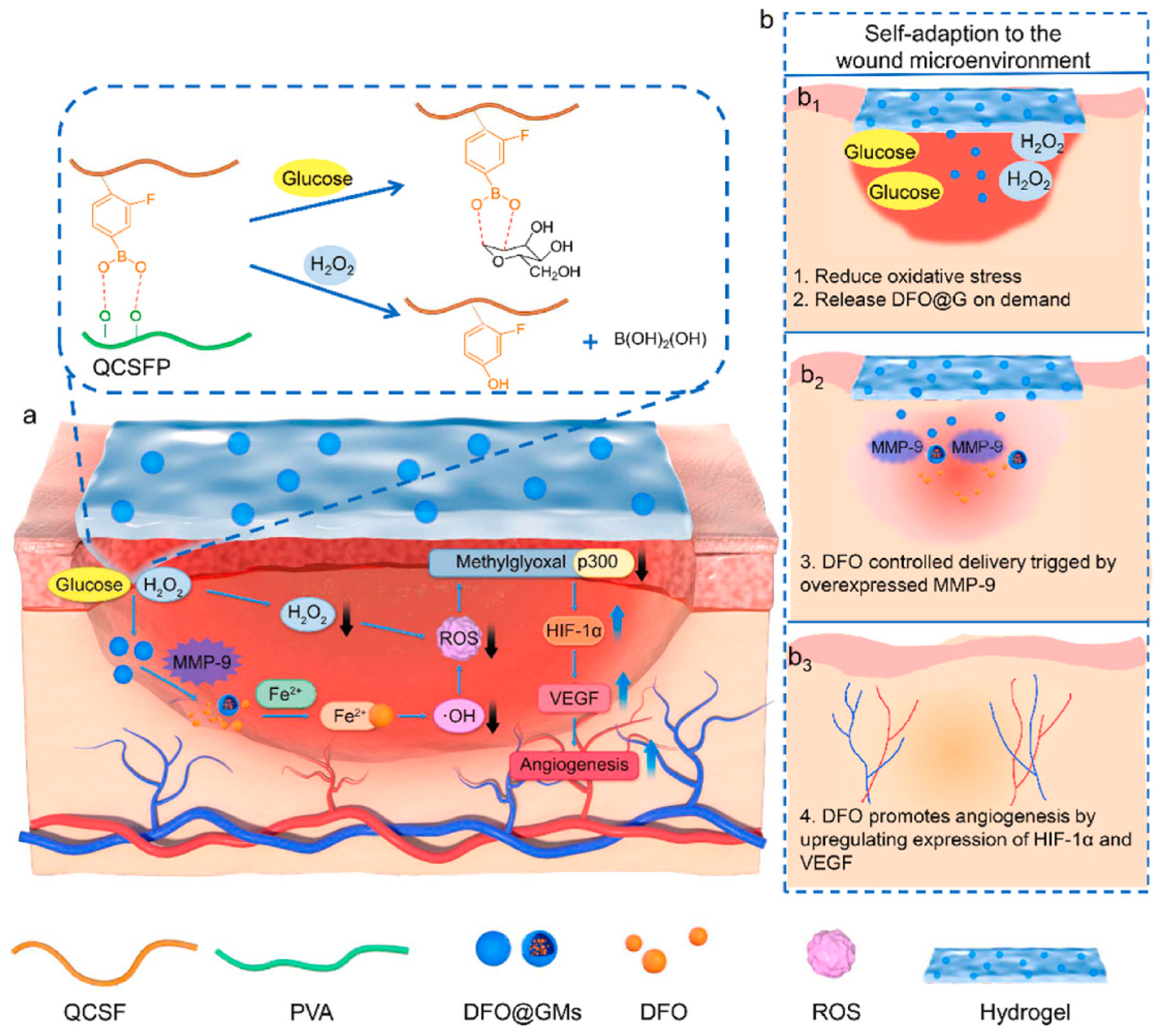
| DFO@G-QCSFP hydrogel | DFO | + DFO | [93] | ||
| PEGS-PBA-BA/CS-DA-LAG hydrogel | metformin and graphene oxide | + DPPH | + metformin (Met) | [94] | |
| Fe\PPHP15 hybrid hydrogel | Fe2+ and Fe3+ | + E. coli, S. aureus | + Fe2+\Fe3+ | [95] | |
| Ag-SH-PEG hydrogel | Ag+ and DFO | + S. aureus | + DFO | [96] | |
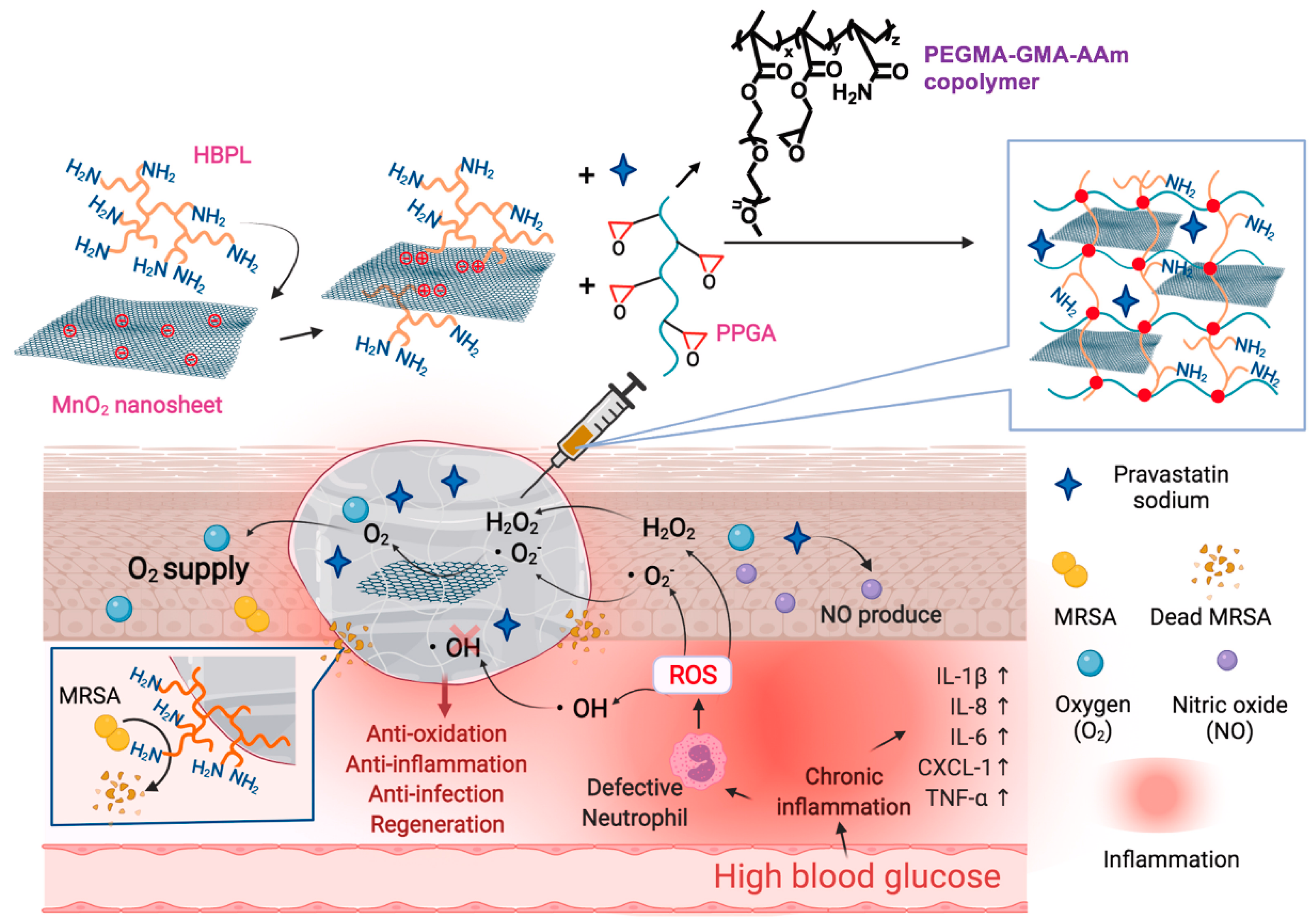
| HMP hydrogel | HBPL and NO | + MRSA | + DPPH, superoxide anion free radical (∙O2−), hydroxyl radical (∙OH) | [97] | |
| MP composite hydrogel | molybdenum disulfide-polydopamine nanozyme | + E. coli, S. aureus | + ·OH scavenging efficiency, DCFH-DA | [98] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Wu, B.; Yuan, P.; Liu, Y.; Hu, C. Rational Design of Multifunctional Hydrogels for Wound Repair. J. Funct. Biomater. 2023, 14, 553. https://doi.org/10.3390/jfb14110553
Cao J, Wu B, Yuan P, Liu Y, Hu C. Rational Design of Multifunctional Hydrogels for Wound Repair. Journal of Functional Biomaterials. 2023; 14(11):553. https://doi.org/10.3390/jfb14110553
Chicago/Turabian StyleCao, Juan, Bo Wu, Ping Yuan, Yeqi Liu, and Cheng Hu. 2023. "Rational Design of Multifunctional Hydrogels for Wound Repair" Journal of Functional Biomaterials 14, no. 11: 553. https://doi.org/10.3390/jfb14110553
APA StyleCao, J., Wu, B., Yuan, P., Liu, Y., & Hu, C. (2023). Rational Design of Multifunctional Hydrogels for Wound Repair. Journal of Functional Biomaterials, 14(11), 553. https://doi.org/10.3390/jfb14110553







