Long-Term Safety Evaluation of Fluorescent Gold Nanoclusters Conjugated with α-Lipoic Acid: Insights from a Six-Month In Vivo Study
Abstract
1. Introduction
2. Results
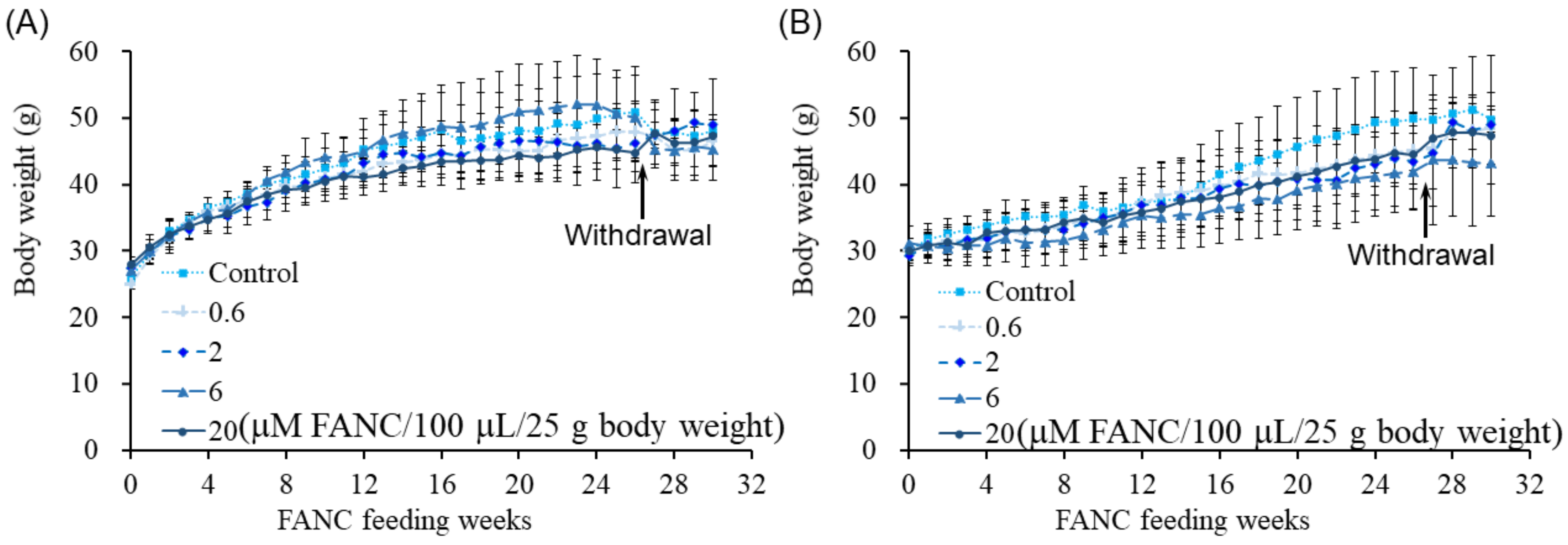
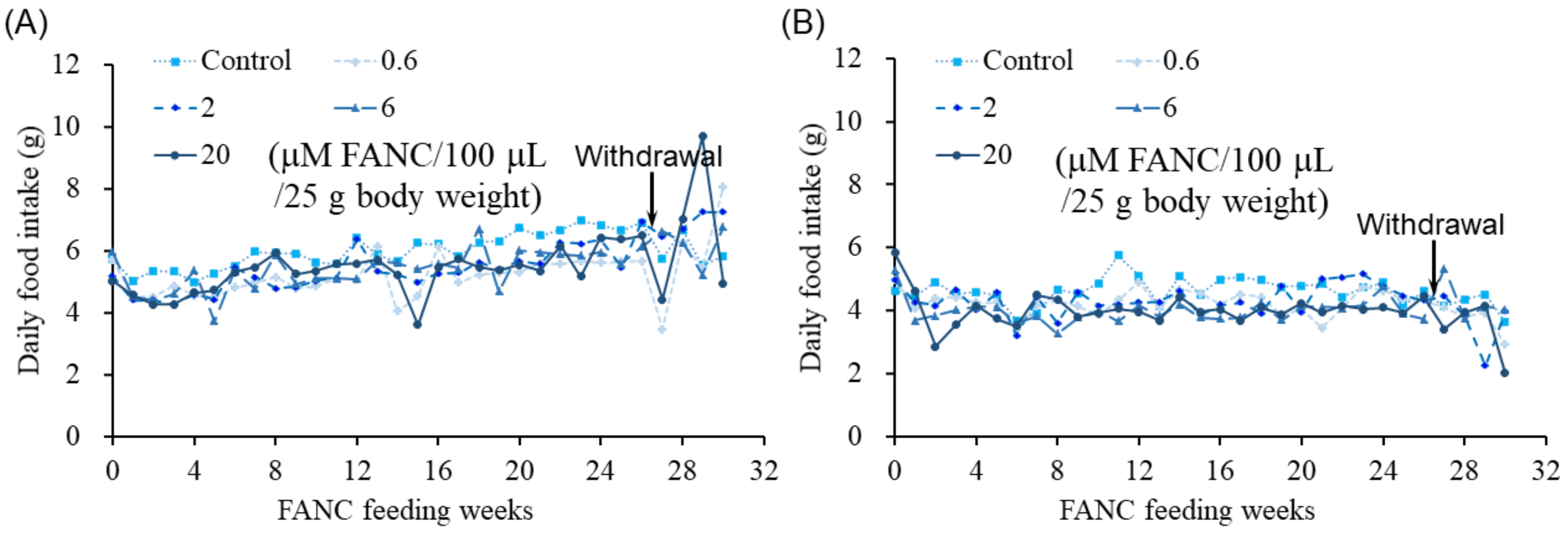
2.1. Behavior, Body Weight, and Food Intake
2.2. Hematological Parameters
2.3. Biochemical Parameters
2.4. Organ Weight
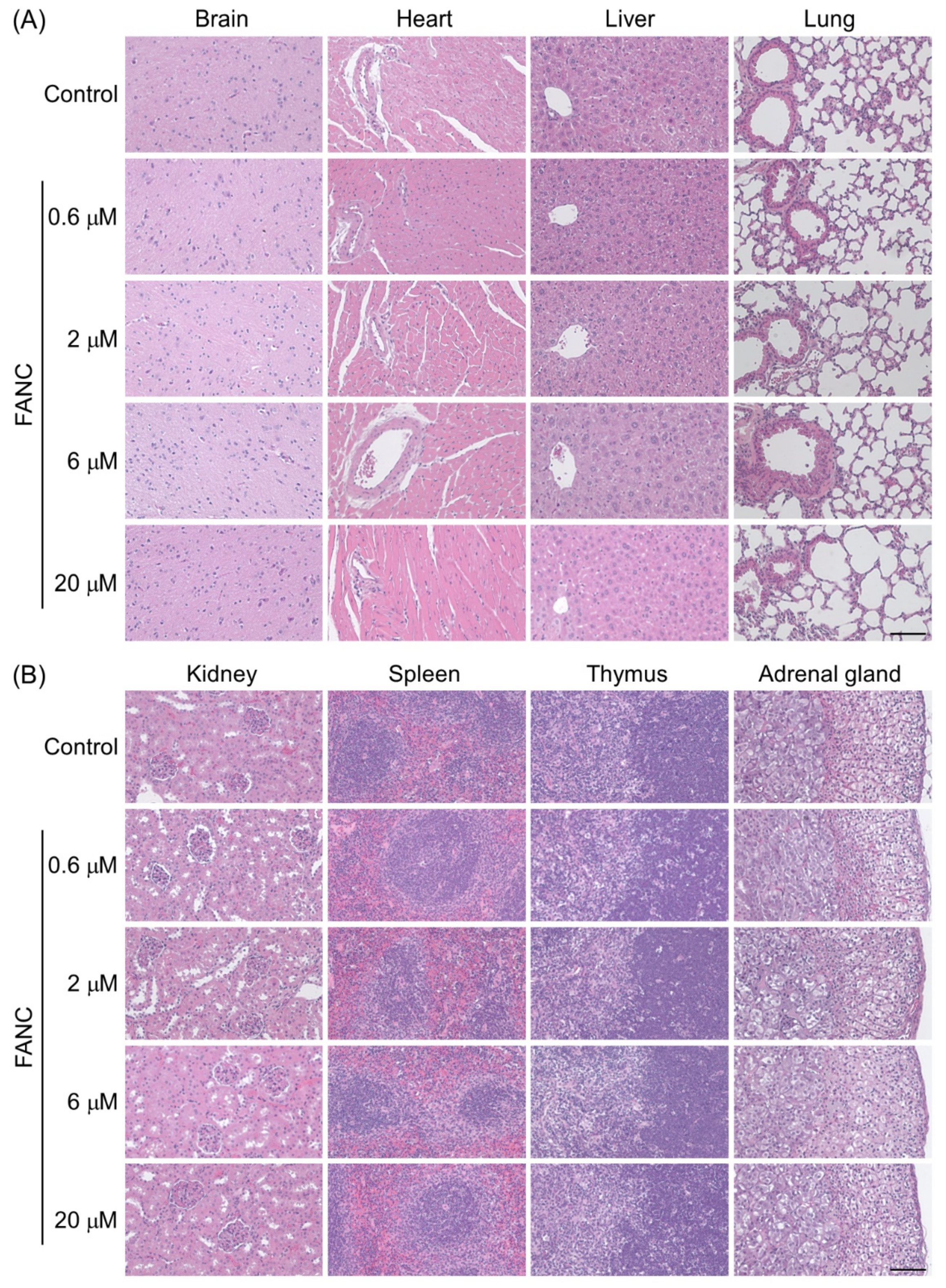
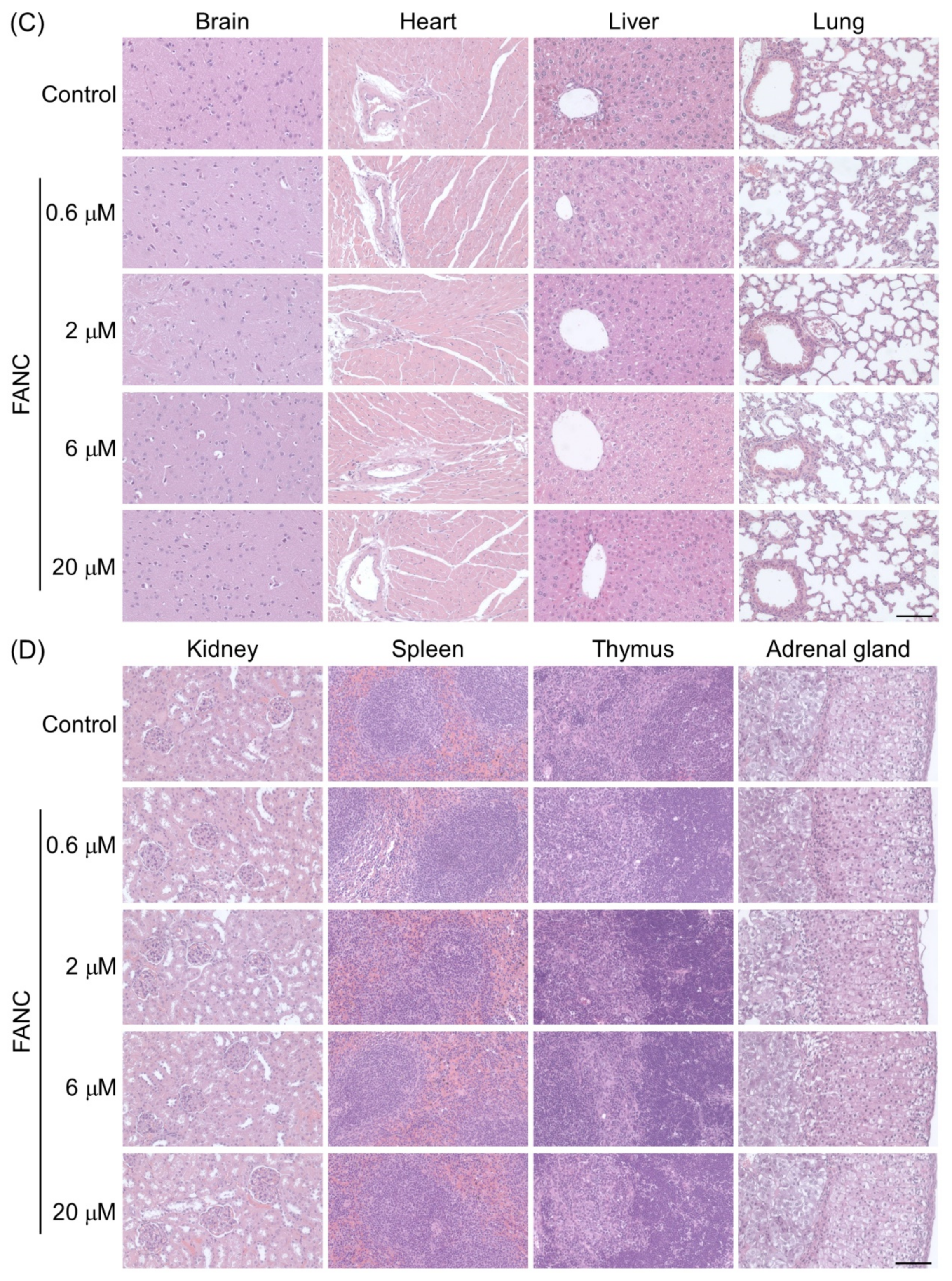
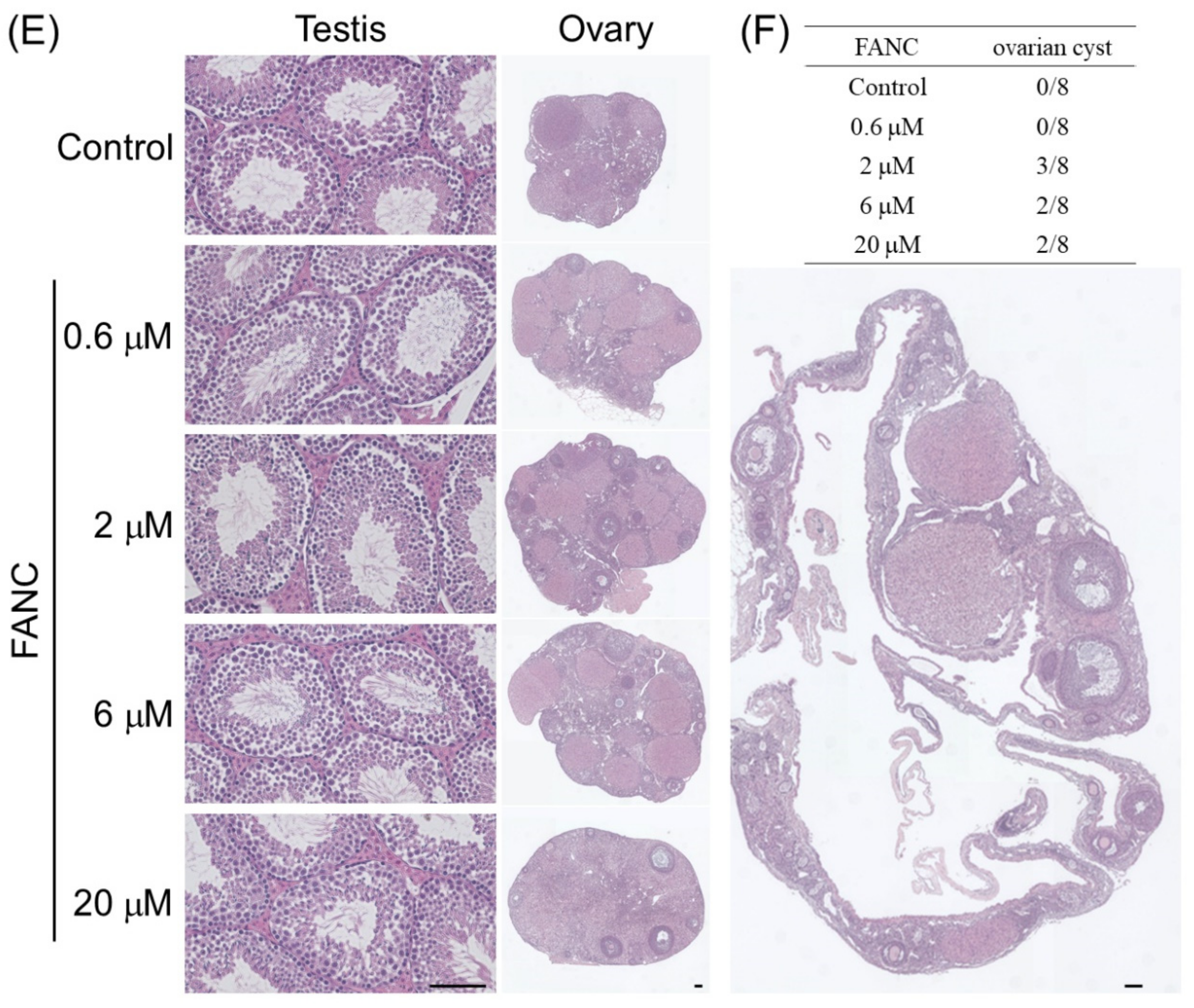
2.5. Histopathological Changes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Synthesis of FANCs via a One-Pot Synthetic Strategy
5.2. Animals and Ethical Statements
5.3. Chronic Toxicity Test
5.4. Hematological Analysis
5.5. Serum Biochemical Analysis
5.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FANCs | Fluorescent gold nanoclusters conjugated with α-lipoic acid |
| AuNCs | Gold nanoclusters |
| DHLA | Dihydrolipoic acid |
| ICR | Institute of Cancer Research |
| RBC | Red blood cell |
| HGB | Hemoglobin |
| HCT | Hematocrit |
| MCV | Mean corpuscular volume |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| RDW | Red blood cell distribution width |
| WBC | White blood cell |
| LYM% | Lymphocyte percentage |
| LYM# | Lymphocyte count |
| PLT | Platelet |
| CRE | Creatinine |
| BUN | Blood urea nitrogen |
| GPT | Glutamic–pyruvic transaminase |
| GOT | Glutamic–oxaloacetic transaminase |
| LDH | Lactate dehydrogenase |
| NOAEL | No-observed-adverse-effect level |
References
- Oliveira, B.B.; Ferreira, D.; Fernandes, A.R.; Baptista, P.V. Engineering gold nanoparticles for molecular diagnostics and biosensing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1836. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Prostate-specific membrane antigen targeted gold nanoparticles for prostate cancer radiotherapy: Does size matter for targeted particles? Chem. Sci. 2019, 10, 8119–8128. [Google Scholar] [CrossRef] [PubMed]
- van de Looij, S.M.; Hebels, E.R.; Viola, M.; Hembury, M.; Oliveira, S.; Vermonden, T. Gold Nanoclusters: Imaging, Therapy, and Theranostic Roles in Biomedical Applications. Bioconjug. Chem. 2022, 33, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Tang, S.; Chen, M.; Zheng, N. Amphiphilic modification and asymmetric silica encapsulation of hydrophobic Au-Fe3O4 dumbbell nanoparticles. Chem. Commun. 2014, 50, 174–176. [Google Scholar] [CrossRef]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.J.; Menichetti, S.; Rotello, V.M. Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Reed, L.J. A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes. J. Biol. Chem. 2001, 276, 38329–38336. [Google Scholar] [CrossRef]
- Perham, R.N.; Jones, D.D.; Chauhan, H.J.; Howard, M.J. Substrate channelling in 2-oxo acid dehydrogenase multienzyme complexes. Biochem. Soc. Trans. 2002, 30, 47–51. [Google Scholar] [CrossRef]
- Bilska, A.; Wlodek, L. Lipoic acid—The drug of the future? Pharmacol. Rep. 2005, 57, 570–577. [Google Scholar]
- Shanaida, M.; Lysiuk, R.; Mykhailenko, O.; Hudz, N.; Abdulsalam, A.; Gontova, T.; Oleshchuk, O.; Ivankiv, Y.; Shanaida, V.; Lytkin, D.; et al. Alpha-lipoic Acid: An Antioxidant with Anti-aging Properties for Disease Therapy. Curr. Med. Chem. 2025, 32, 23–54. [Google Scholar] [CrossRef]
- Lin, C.A.; Yang, T.Y.; Lee, C.H.; Huang, S.H.; Sperling, R.A.; Zanella, M.; Li, J.K.; Shen, J.L.; Wang, H.H.; Yeh, H.I.; et al. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N.; Wu, Y.J.; Su, C.H.; Wang, B.J.; Yang, S.H.; Lee, H.I.; Chou, Y.H.; Tien, T.Y.; Lin, C.F.; Chan, W.H.; et al. Fluorescent gold nanoclusters possess multiple actions against atherosclerosis. Redox Biol. 2024, 78, 103427. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.C.; Su, K.H.; Kou, Y.R.; Shyue, S.K.; Ching, L.C.; Yu, Y.B.; Wu, Y.L.; Pan, C.C.; Lee, T.S. alpha-Lipoic acid ameliorates foam cell formation via liver X receptor alpha-dependent upregulation of ATP-binding cassette transporters A1 and G1. Free Radic. Biol. Med. 2011, 50, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; An, L.P.; Li, Y.F.; An, R.; Bian, Z.; Liu, W.Z.; Song, Q.H.; Li, A.Y. Alpha-lipoic acid ameliorates H2O2-induced human vein endothelial cells injury via suppression of inflammation and oxidative stress. Biosci. Biotechnol. Biochem. 2020, 84, 2253–2263. [Google Scholar] [CrossRef]
- Liu, A.; Ye, B. Application of gold nanoparticles in biomedical researches and diagnosis. Clin. Lab. 2013, 59, 23–36. [Google Scholar] [CrossRef]
- Ding, H.; Chen, Z. Nanotheranostic Application of Fluorescent Protein-Gold Nanocluster Hybrid Materials: A Mini-review. Nanotheranostics 2021, 5, 461–471. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Cao, Y.; Chai, O.J.H.; Xie, J. Ligand Design in Ligand-Protected Gold Nanoclusters. Small 2021, 17, e2004381. [Google Scholar] [CrossRef]
- Santhoshkumar, S.; Madhu, M.; Tseng, W.B.; Tseng, W.L. Gold nanocluster-based fluorescent sensors for in vitro and in vivo ratiometric imaging of biomolecules. Phys. Chem. Chem. Phys. 2023, 25, 21787–21801. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Guo, Y.; Kawasaki, H. A Review on Gold Nanoclusters for Cancer Phototherapy. ACS Appl. Bio Mater. 2023, 6, 4504–4517. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial Gold Nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.K.; Jittiwat, J.; Manikandan, J.; Ong, C.N.; Yu, L.E.; Ong, W.Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials 2010, 31, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Lin, C.A.; Lee, C.H.; Lin, Y.C.; Tseng, Y.M.; Hsieh, C.L.; Chen, C.H.; Tsai, C.H.; Hsieh, C.T.; Shen, J.L.; et al. Fluorescent gold nanoclusters as a biocompatible marker for in vitro and in vivo tracking of endothelial cells. ACS Nano 2011, 5, 4337–4344. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Lin, C.J.; Tseng, Y.M.; Lee, H.I.; Lee, Y.N.; Yeh, H.I.; Yang, P.S.; Peng, H.Y.; Wu, Y.J. Dihydrolipoic acid-coated gold nanocluster bioactivity against senescence and inflammation through the mitochondria-mediated JNK/AP-1 pathway. Nanomedicine 2021, 36, 102427. [Google Scholar] [CrossRef]
- Xiao, L.; Wei, F.; Zhou, Y.; Anderson, G.J.; Frazer, D.M.; Lim, Y.C.; Liu, T.; Xiao, Y. Dihydrolipoic Acid-Gold Nanoclusters Regulate Microglial Polarization and Have the Potential to Alter Neurogenesis. Nano Lett. 2020, 20, 478–495. [Google Scholar] [CrossRef]
- Tam, J.M.; Tam, J.O.; Murthy, A.; Ingram, D.R.; Ma, L.L.; Travis, K.; Johnston, K.P.; Sokolov, K.V. Controlled assembly of biodegradable plasmonic nanoclusters for near-infrared imaging and therapeutic applications. ACS Nano 2010, 4, 2178–2184. [Google Scholar] [CrossRef]
- Chen, Y.F.; Hsu, C.C.; Chung, C.H. Acute and Subacute Toxicity of Fluorescent Gold Nanoclusters Conjugated with alpha-Lipoic Acid. Nanomaterials 2022, 12, 3868. [Google Scholar] [CrossRef]
- Miller, M.A. Gender-based differences in the toxicity of pharmaceuticals—The Food and Drug Administration’s perspective. Int. J. Toxicol. 2001, 20, 149–152. [Google Scholar] [CrossRef]
- Mennecozzi, M.; Landesmann, B.; Palosaari, T.; Harris, G.; Whelan, M. Sex differences in liver toxicity-do female and male human primary hepatocytes react differently to toxicants in vitro? PLoS ONE 2015, 10, e0122786. [Google Scholar] [CrossRef]
- Sun, P.P.; Lai, C.S.; Hung, C.J.; Dhaiveegan, P.; Tsai, M.L.; Chiu, C.L.; Fang, J.M. Subchronic oral toxicity evaluation of gold nanoparticles in male and female mice. Heliyon 2021, 7, e06577. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Lentz, G.M.; Valea, F.A.; Lobo, R.A. Comprehensive Gynecology, 8th ed.; eBook; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Brown, J.; Farquhar, C. Clomiphene and other antioestrogens for ovulation induction in polycystic ovarian syndrome. Cochrane Database Syst. Rev. 2016, 12, CD002249. [Google Scholar] [CrossRef]
- Cole, L.A.; Butler, S.A. Human Gonadotropins; eBook; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Cibas, E.S.; Ducatman, B.S. Cytology-Diagnostic Principles and Clinical Correlates; eBook; Saunders: Philadelphia, PA, USA, 2014. [Google Scholar]
- Monroe, G.R.; van Eerde, A.M.; Tessadori, F.; Duran, K.J.; Savelberg, S.M.C.; van Alfen, J.C.; Terhal, P.A.; van der Crabben, S.N.; Lichtenbelt, K.D.; Fuchs, S.A.; et al. Identification of human D lactate dehydrogenase deficiency. Nat. Commun. 2019, 10, 1477. [Google Scholar] [CrossRef]



| μM FANC/100 μL/25 g Body Weight | |||||
|---|---|---|---|---|---|
| Male | 0 | 0.6 | 2 | 6 | 20 |
| RBC | 6.1 ± 0.8 | 6.4 ± 0.5 | 6.9 ± 0.2 | 6.2 ± 0.4 | 6.3 ± 0.4 |
| HGB | 11 ± 1 | 11 ± 1 | 12 ± 1 | 12 ± 1 | 11 ± 1 |
| HCT | 35 ± 5 | 36 ± 2 | 38 ± 2 | 34 ± 1 | 35 ± 3 |
| MCV | 57 ± 1 | 56 ± 1 | 55 ± 3 | 55 ± 2 | 56 ± 2 |
| MCH | 18 ± 2 | 18 ± 1 | 17 ± 2 | 19 ± 1 | 18 ± 2 |
| MCHC | 32 ± 4 | 32 ± 2 | 31 ± 2 | 34 ± 1 | 31 ± 3 |
| RDW | 14 ± 2 | 12 ± 1 | 15 ± 5 | 16 ± 1 | 15 ± 1 |
| WBC | 5.4 ± 1.2 | 5.8 ± 3.9 | 4.1 ± 2.6 | 5.7 ± 4.5 | 4.8 ± 1.4 |
| LYM% | 73 ± 11 | 80 ± 4 | 63 ± 17 | 61 ± 16 | 78 ± 23 |
| LYM# | 3.8 ± 0.7 | 4.6 ± 3.1 | 2.7 ± 1.8 | 3.2 ± 1.8 | 3.1 ± 1.5 |
| PLT | 835 ± 198 | 817 ± 374 | 729 ± 115 | 760 ± 168 | 800 ± 152 |
| Female | 0 | 0.6 | 2 | 6 | 20 |
| RBC | 6.6 ± 0.7 | 6.8 ± 0.6 | 6.7 ± 0.5 | 6.9 ± 0.2 | 7.1 ± 0.3 |
| HGB | 11 ± 1 | 11 ± 1 | 11 ± 1 | 11 ± 1 | 11 ± 1 |
| HCT | 36 ± 4 | 35 ± 3 | 36 ± 3 | 36 ± 2 | 37 ± 2 |
| MCV | 54 ± 1 | 52 ± 1 | 54 ± 1 | 53 ± 3 | 52 ± 2 |
| MCH | 17 ± 1 | 16 ± 1 | 17 ± 0 | 16 ± 1 | 16 ± 1 |
| MCHC | 31 ± 1 | 30 ± 1 | 31 ± 0 | 30 ± 1 | 30 ± 1 |
| RDW | 29 ± 1 | 28 ± 1 | 26 ± 4 | 28 ± 1 | 28 ± 1 |
| WBC | 2.2 ± 1.2 | 2.4 ± 1.4 | 2.5 ± 0.8 | 3.1 ± 0.7 | 2.7 ± 0.8 |
| LYM% | 92 ± 9 | 77 ± 9 | 85 ± 12 | 82 ± 8 | 81 ± 14 |
| LYM# | 2.4 ± 1.0 | 3.7 ± 2.3 | 2.1 ± 0.5 | 2.5 ± 0.6 | 2.2 ± 0.7 |
| PLT | 1051 ± 300 | 1067 ± 141 | 975 ± 143 | 1002 ± 137 | 1065 ± 145 |
| Male | GOT | GPT | LDH | BUN | CRE |
| (μM) | (U/L) | (U/L) | (U/L) | (mg/dL) | (mg/dL) |
| 0 | 49 ± 8 | 23 ± 9 | 263 ± 57 | 25 ± 6 | 0.29 ± 0.15 |
| 0.6 | 43 ± 7 | 26 ± 7 | 199 ± 64 | 23 ± 5 | 0.15 ± 0.08 |
| 2 | 54 ± 17 | 23 ± 7 | 222 ± 74 | 24 ± 2 | 0.23 ± 0.14 |
| 6 | 45 ± 6 | 19 ± 4 | 224 ± 29 | 25 ± 8 | 0.20 ± 0.10 |
| 20 | 60 ± 5 | 23 ± 5 | 268 ± 126 | 24 ± 5 | 0.17 ± 0.06 |
| Female | GOT | GPT | LDH | BUN | CRE |
| (μM) | (U/L) | (U/L) | (U/L) | (mg/dL) | (mg/dL) |
| 0 | 56 ± 11 | 22 ± 14 | 347 ± 29 | 24 ± 4 | 0.12 ± 0.03 |
| 0.6 | 60 ± 29 | 13 ± 6 | 447 ± 339 | 28 ± 4 | 0.19 ± 0.17 |
| 2 | 62 ± 25 | 11 ± 1 | 283 ± 112 | 25 ± 1 | 0.13 ± 0.04 |
| 6 | 60 ± 18 | 19 ± 11 | 369 ± 164 | 26 ± 4 | 0.17 ± 0.03 |
| 20 | 37 ± 5 * | 10 ± 3 | 222 ± 57 * | 22 ± 2 | 0.14 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-W.; Lee, Y.-N.; Yeh, H.-I.; Wu, Y.-J.; Chan, W.-H.; Wang, S.-W.; Lin, C.-F.; Lin, C.-H.; Chen, Y.-F.; Chung, C.-H. Long-Term Safety Evaluation of Fluorescent Gold Nanoclusters Conjugated with α-Lipoic Acid: Insights from a Six-Month In Vivo Study. J. Funct. Biomater. 2025, 16, 89. https://doi.org/10.3390/jfb16030089
Lai Y-W, Lee Y-N, Yeh H-I, Wu Y-J, Chan W-H, Wang S-W, Lin C-F, Lin C-H, Chen Y-F, Chung C-H. Long-Term Safety Evaluation of Fluorescent Gold Nanoclusters Conjugated with α-Lipoic Acid: Insights from a Six-Month In Vivo Study. Journal of Functional Biomaterials. 2025; 16(3):89. https://doi.org/10.3390/jfb16030089
Chicago/Turabian StyleLai, Yu-Wei, Yi-Nan Lee, Hung-I Yeh, Yih-Jer Wu, Wen-Hsiung Chan, Shih-Wei Wang, Chao-Feng Lin, Chun-Hsuan Lin, Yun-Fang Chen, and Ching-Hu Chung. 2025. "Long-Term Safety Evaluation of Fluorescent Gold Nanoclusters Conjugated with α-Lipoic Acid: Insights from a Six-Month In Vivo Study" Journal of Functional Biomaterials 16, no. 3: 89. https://doi.org/10.3390/jfb16030089
APA StyleLai, Y.-W., Lee, Y.-N., Yeh, H.-I., Wu, Y.-J., Chan, W.-H., Wang, S.-W., Lin, C.-F., Lin, C.-H., Chen, Y.-F., & Chung, C.-H. (2025). Long-Term Safety Evaluation of Fluorescent Gold Nanoclusters Conjugated with α-Lipoic Acid: Insights from a Six-Month In Vivo Study. Journal of Functional Biomaterials, 16(3), 89. https://doi.org/10.3390/jfb16030089







