Assessing the Effects of Surface-Stabilized Zero-Valent Iron Nanoparticles on Diverse Bacteria Species Using Complementary Statistical Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Nanoparticle Synthesis
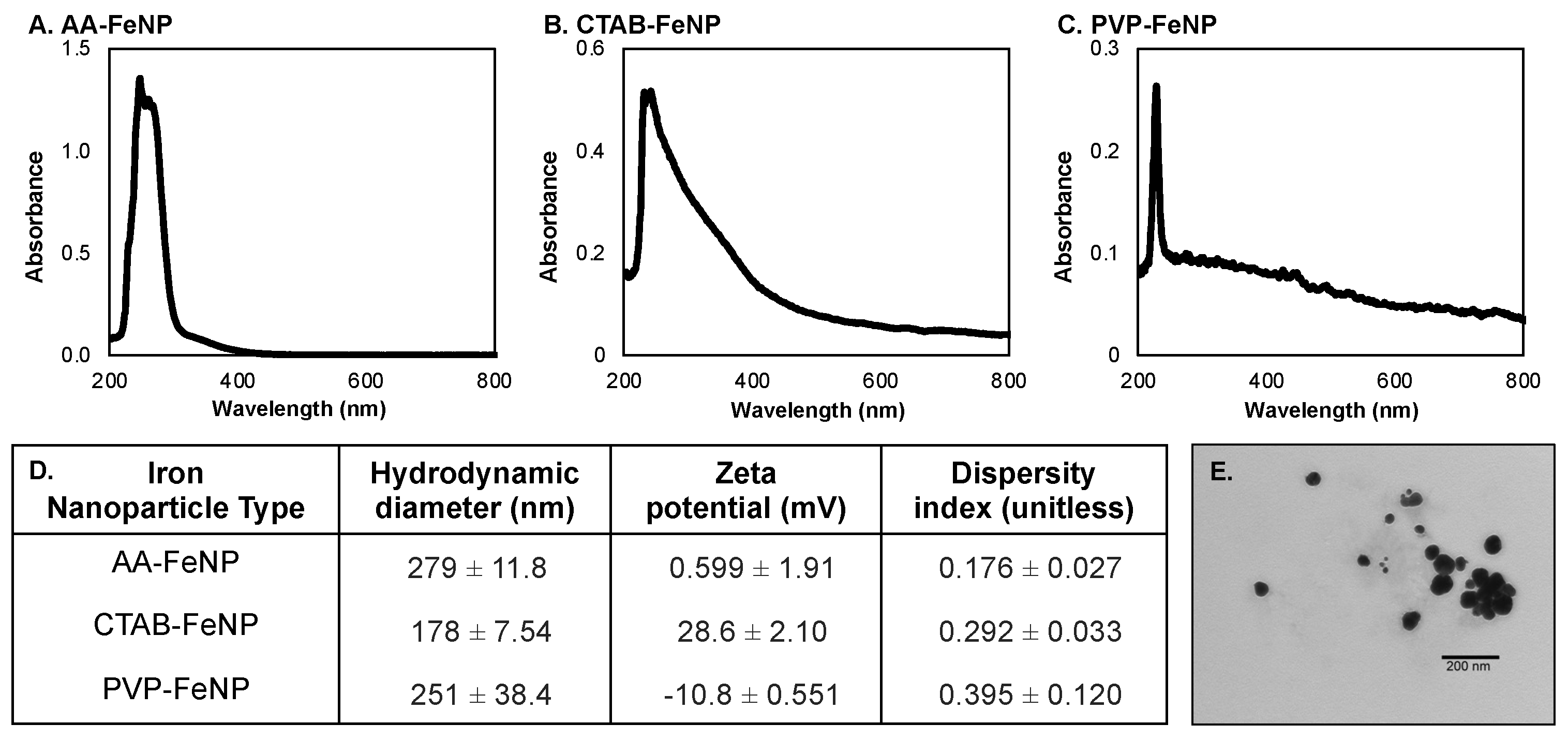
2.3. Nanoparticle Characterization
2.4. Bacterial Cultures
2.5. Antibacterial Disc Assay
2.6. Statistical Methods Overview
2.7. Generalized Linear Mixed Model Development
2.8. Ordinal Regression Model Development
3. Results and Discussion
3.1. Iron Nanoparticle Characterization
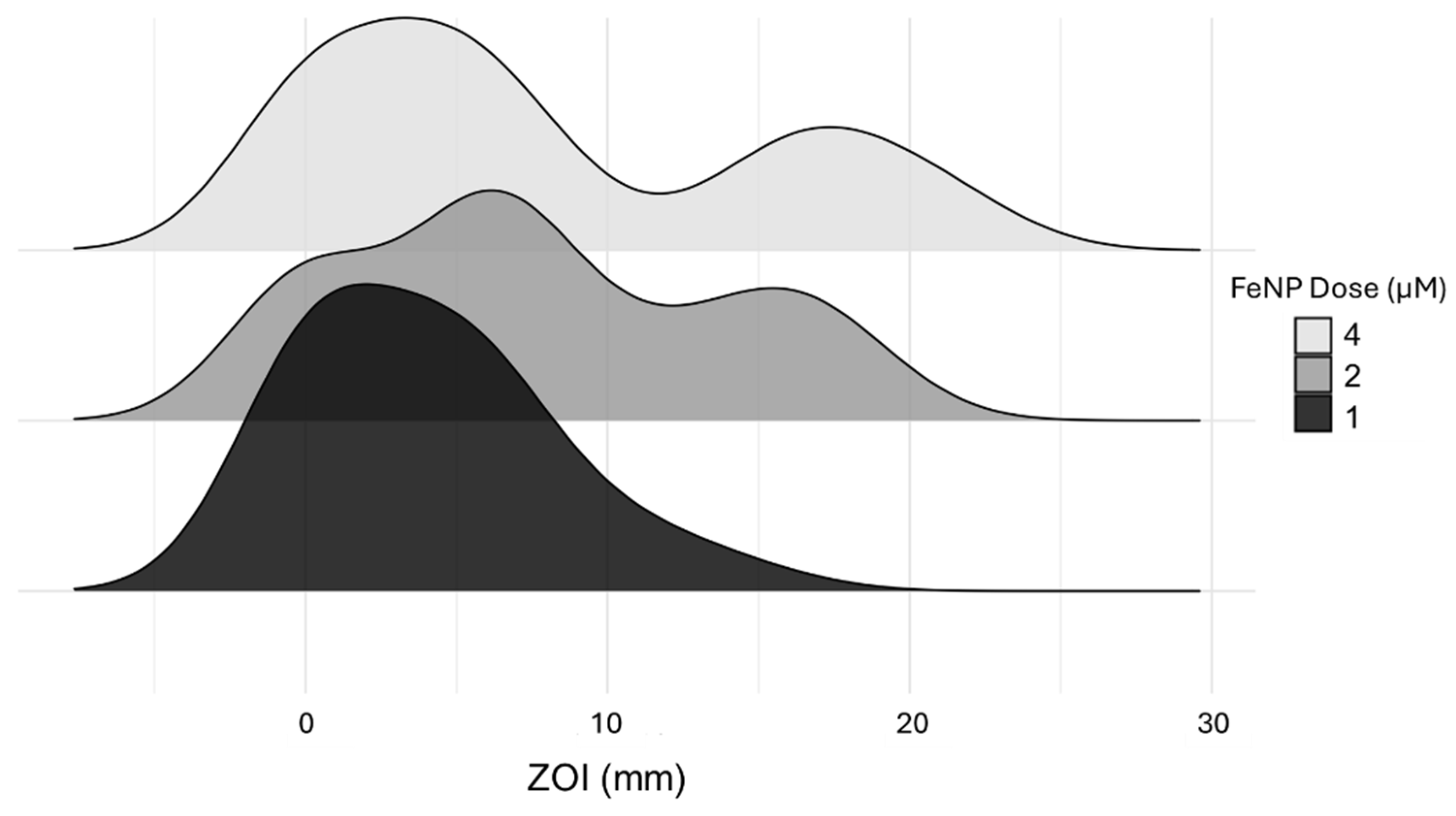
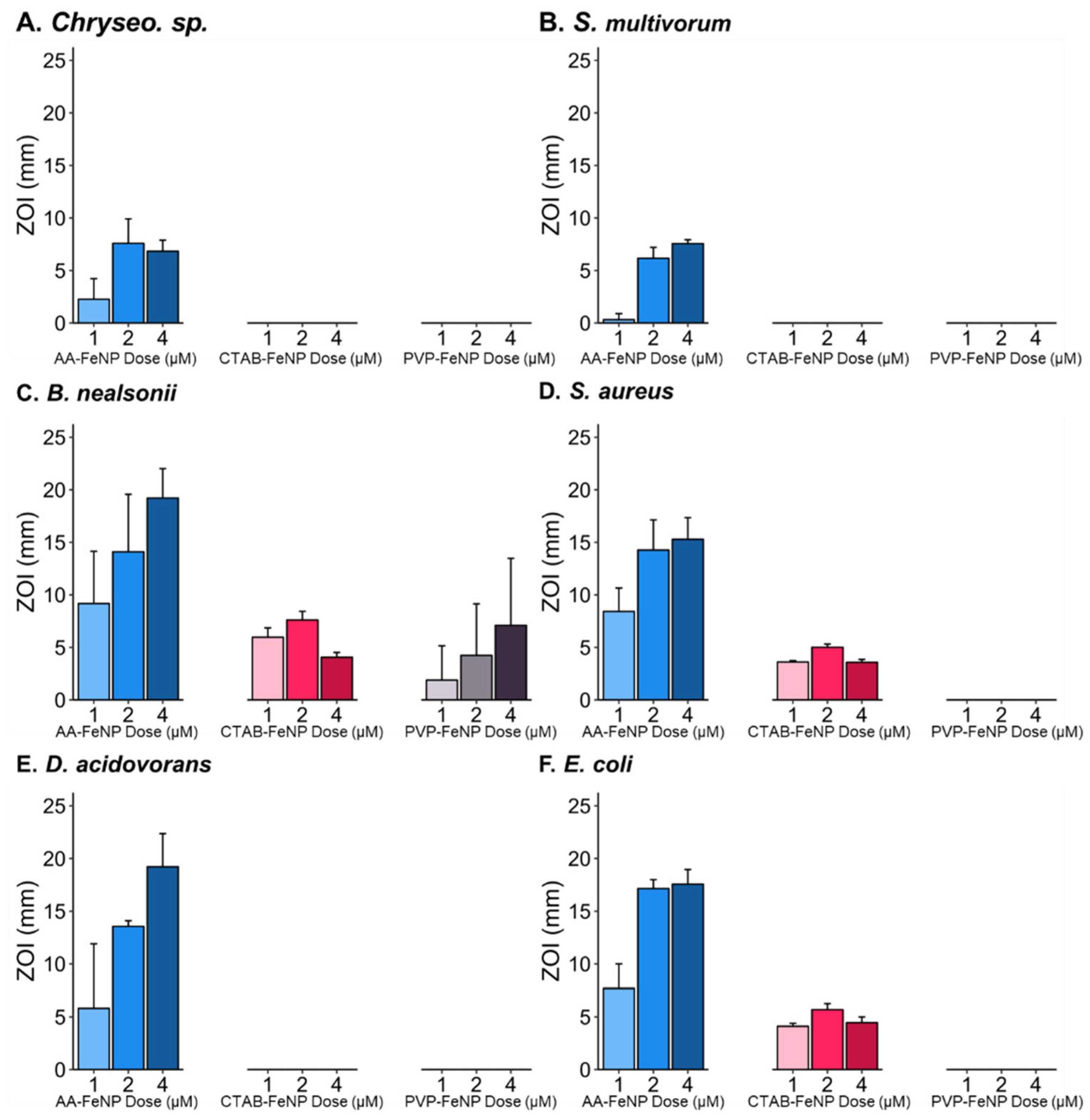
3.2. Coating and Dose Effect on Bacterial Inhibition
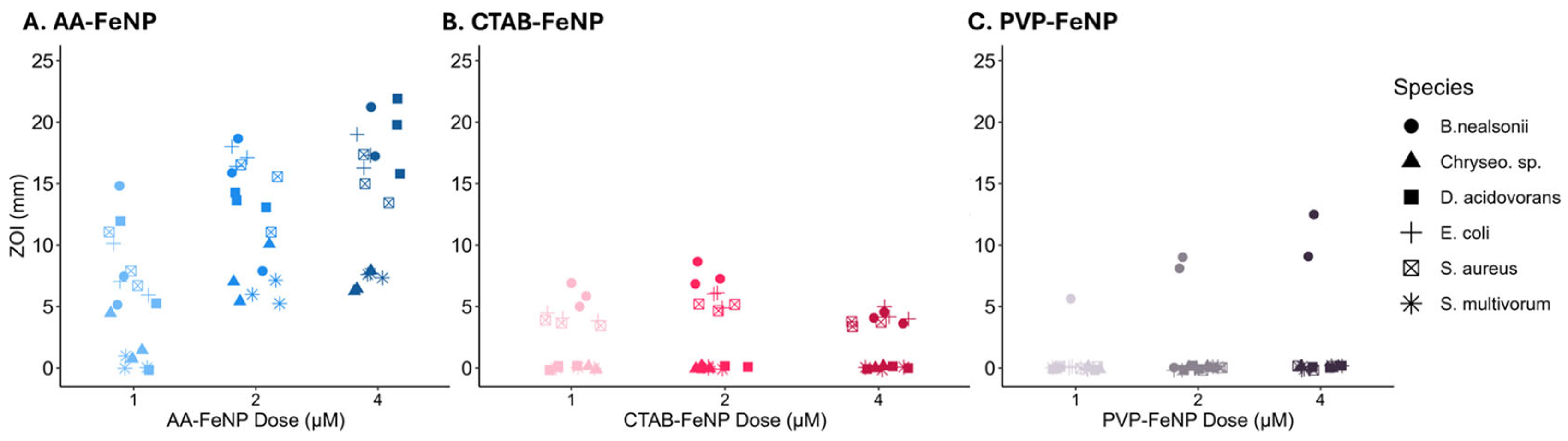
3.3. Dose Effects on the ZOI Varied Based on FeNP Surface Coating
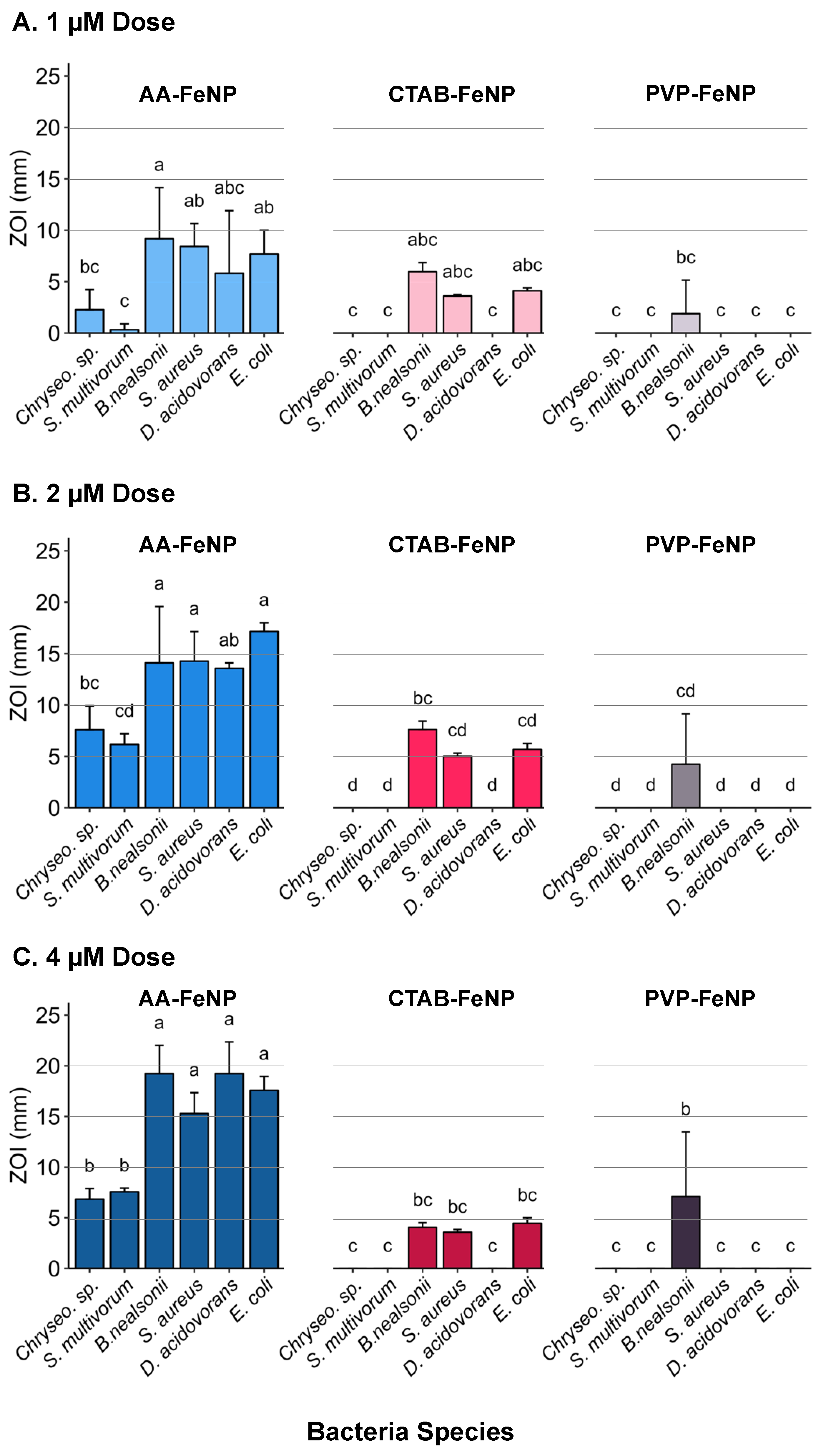
3.4. Intra-Species Analysis Revealed Differences in Response to FeNP Coating
3.5. Species Susceptibility to Coated Iron Nanoparticles
3.6. Statistical Data Analysis
3.7. Phylogenetic Considerations for Iron Nanoparticle Efficacy
3.8. Implications for Environmental and Biomedical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A.
Appendix A.1. Iron Nanoparticle Transmission Electron Microscopy
Appendix A.2. Response Variable Distribution
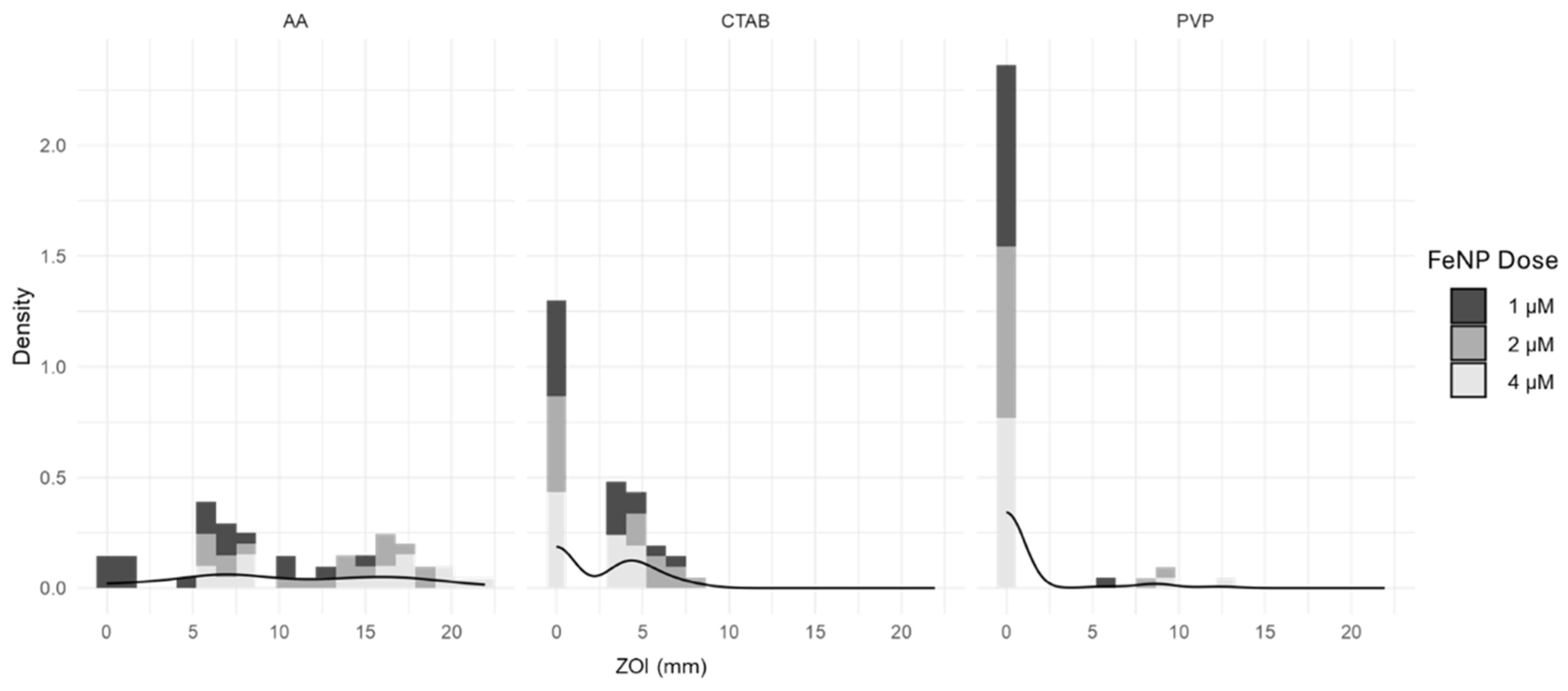
Appendix A.3. Ordinal Model Assumptions

References
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’neill, A.J.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Hńfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Kon, K.V.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Coping with antibiotic resistance: Combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev. Anti-Infect. Ther. 2011, 9, 1035–1052. [Google Scholar] [CrossRef]
- Ameh, T.; Zarzosa, K.; Dickinson, J.; Braswell, W.E.; Sayes, C.M. Nanoparticle surface stabilizing agents influence antibacterial action. Front. Microbiol. 2023, 14, 1119550. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Bharti, S. Harnessing the potential of bimetallic nanoparticles: Exploring a novel approach to address antimicrobial resistance. World J. Microbiol. Biotechnol. 2024, 40, 89. [Google Scholar] [CrossRef]
- Aazam, E.S.; Zaheer, Z. Growth of Ag-nanoparticles in an aqueous solution and their antimicrobial activities against Gram positive, Gram negative bacterial strains and Candida fungus. Bioprocess Biosyst. Eng. 2016, 39, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Huang, W.; Zhang, J.; Yu, Y.; Sun, T. Metal-Based Nanoparticles as Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2024, 7, 2529–2545. [Google Scholar] [CrossRef]
- McQuillan, J.S.; Groenaga Infante, H.; Stokes, E.; Shaw, A.M. Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology 2012, 6, 857–866. [Google Scholar] [CrossRef]
- Neihaya, H.; Zaman, H. Investigating the effect of biosynthesized silver nanoparticles as antibiofilm on bacterial clinical isolates. Microb. Pathog. 2018, 116, 200–208. [Google Scholar] [CrossRef]
- Modi, S.K.; Gaur, S.; Sengupta, M.; Singh, M.S. Mechanistic insights into nanoparticle surface-bacterial membrane interactions in overcoming antibiotic resistance. Front. Microbiol. 2023, 14, 1135579. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Willyard, C. Drug-resistant bacteria ranked. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Thimiri Govinda Raj, D.B.; Khan, N.A. Designer nanoparticle: Nanobiotechnology tool for cell biology. Nano Converg. 2016, 3, 22. [Google Scholar] [CrossRef]
- Mahon, E.; Salvati, A.; Bombelli, F.B.; Lynch, I.; Dawson, K.A. Designing the nanoparticle–biomolecule interface for “targeting and therapeutic delivery”. J. Control. Release 2012, 161, 164–174. [Google Scholar] [CrossRef]
- Caldorera-Moore, M.; Guimard, N.; Shi, L.; Roy, K. Designer nanoparticles: Incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin. Drug Deliv. 2010, 7, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Love, S.A.; Maurer-Jones, M.A.; Thompson, J.W.; Lin, Y.-S.; Haynes, C.L. Assessing nanoparticle toxicity. Annu. Rev. Anal. Chem. 2012, 5, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Liu, X.; Hedrick, J.L.; Xie, Z.; Wang, S.; Lin, Q.-Y.; Hersam, M.C.; Dravid, V.P.; Mirkin, C.A. Polyelemental nanoparticle libraries. Science 2016, 352, 1565–1569. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging trends in nanomaterials for antibacterial applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Van Hengel, I.; Tierolf, M.; Valerio, V.; Minneboo, M.; Fluit, A.; Fratila-Apachitei, L.; Apachitei, I.; Zadpoor, A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B 2020, 8, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, E.; Li, J.; Qiu, Y.; Chen, R. Rapid ultrasonic-microwave assisted synthesis of spindle-like Ag/ZnO nanostructures and their enhanced visible-light photocatalytic and antibacterial activities. Catal. Today 2020, 339, 391–402. [Google Scholar] [CrossRef]
- Paterson, T.E.; Bari, A.; Bullock, A.J.; Turner, R.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C.; MacNeil, S.; Shepherd, J. Multifunctional copper-containing mesoporous glass nanoparticles as antibacterial and proangiogenic agents for chronic wounds. Front. Bioeng. Biotechnol. 2020, 8, 246. [Google Scholar] [CrossRef]
- Kumar, A.; Behl, T.; Chadha, S. Synthesis of physically crosslinked PVA/Chitosan loaded silver nanoparticles hydrogels with tunable mechanical properties and antibacterial effects. Int. J. Biol. Macromol. 2020, 149, 1262–1274. [Google Scholar] [CrossRef]
- Li, R.; Xu, Z.; Jiang, Q.; Zheng, Y.; Chen, Z.; Chen, X. Characterization and biological evaluation of a novel silver nanoparticle-loaded collagen-chitosan dressing. Regen. Biomater. 2020, 7, 371–380. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cho, H.; Ryu, S.-E.; Choi, M.-U. Effects of metal ions on the activity of protein tyrosine phosphatase VHR: Highly potent and reversible oxidative inactivation by Cu2+ ion. Arch. Biochem. Biophys. 2000, 382, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Han, D.; Han, Y.; Liang, Y.; Liu, X.; Li, Z.; Zhu, S.; Wu, S. Visible light responsive CuS/protonated g-C3N4 heterostructure for rapid sterilization. J. Hazard. Mater. 2020, 393, 122423. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Stevens, D.; Charlton-Sevcik, A.K.; Braswell, W.E.; Sayes, C.M. Evaluating the Antibacterial Potential of Distinct Size Populations of Stabilized Zinc Nanoparticles. ACS Appl. Mater. Interfaces 2024, 17, 322–332. [Google Scholar] [CrossRef]
- Diao, M.; Yao, M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009, 43, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.; Ortíz, L.; Rodríguez-Membibre, M.; Nande, M.; Lobo, M.; Martin, M. Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: A molecular approach. Chemosphere 2012, 86, 802–808. [Google Scholar] [CrossRef]
- Hsueh, Y.-H.; Tsai, P.-H.; Lin, K.-S.; Ke, W.-J.; Chiang, C.-L. Antimicrobial effects of zero-valent iron nanoparticles on gram-positive Bacillus strains and gram-negative Escherichia coli strains. J. Nanobiotechnol. 2017, 15, 77. [Google Scholar] [CrossRef]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.-A.; Chanéac, C.; Waite, D.T.; Masion, A.; Woicik, J.C.; Wiesner, M.R.; Bottero, J.-Y. Relation between the Redox State of Iron-Based Nanoparticles and Their Cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef]
- Kotchaplai, P.; Khan, E.; Vangnai, A.S. Membrane Alterations in Pseudomonas putida F1 Exposed to Nanoscale Zerovalent Iron: Effects of Short-Term and Repetitive nZVI Exposure. Environ. Sci. Technol. 2017, 51, 7804–7813. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Sun, H.; Wang, S.; Liao, X.; Zhang, L. Membrane disruption boosts iron overload and endogenous oxidative stress to inactivate Escherichia coli by nanoscale zero-valent iron. J. Hazard. Mater. 2022, 435, 128951. [Google Scholar] [CrossRef] [PubMed]
- Sacca, M.L.; Fajardo, C.; Martinez-Gomariz, M.; Costa, G.; Nande, M.; Martin, M. Molecular stress responses to nano-sized zero-valent iron (nZVI) particles in the soil bacterium Pseudomonas stutzeri. PLoS ONE 2014, 9, e89677. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal Effect of Zero-Valent Iron Nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef]
- Amin, R.M.; Mahmoud, R.K.; Gadelhak, Y.; Abo El-Ela, F.I. Gamma irradiated green synthesized zero valent iron nanoparticles as promising antibacterial agents and heavy metal nano-adsorbents. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100461. [Google Scholar] [CrossRef]
- Chau, T.P.; Brindhadevi, K.; Krishnan, R.; Alyousef, M.A.; Almoallim, H.S.; Whangchai, N.; Pikulkaew, S. A novel synthesis, analysis and evaluation of Musa coccinea based zero valent iron nanoparticles for antimicrobial and antioxidant. Environ. Res. 2022, 209, 112770. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Phulpoto, I.A.; Yu, Z.; Hu, B.; Wang, Y.; Ndayisenga, F.; Li, J.; Liang, H.; Qazi, M.A. Production and characterization of surfactin-like biosurfactant produced by novel strain Bacillus nealsonii S2MT and it’s potential for oil contaminated soil remediation. Microb. Cell Factories 2020, 19, 1–12. [Google Scholar] [CrossRef]
- Chen, F.-L.; Wang, G.-C.; Teng, S.-O.; Ou, T.-Y.; Yu, F.-L.; Lee, W.-S. Clinical and epidemiological features of Chryseobacterium indologenes infections: Analysis of 215 cases. J. Microbiol. Immunol. Infect. 2013, 46, 425–432. [Google Scholar] [CrossRef]
- Kämpfer, P.; McInroy, J.A.; Glaeser, S.P. Chryseobacterium zeae sp. nov., Chryseobacterium arachidis sp. nov., and Chryseobacterium geocarposphaerae sp. nov. isolated from the rhizosphere environment. Antonie Van Leeuwenhoek 2014, 105, 491–500. [Google Scholar] [CrossRef]
- Montero-Calasanz, M.d.C.; Göker, M.; Rohde, M.; Spröer, C.; Schumann, P.; Busse, H.-J.; Schmid, M.; Tindall, B.J.; Klenk, H.-P.; Camacho, M. Chryseobacterium hispalense sp. nov., a plant-growth-promoting bacterium isolated from a rainwater pond in an olive plant nursery, and emended descriptions of Chryseobacterium defluvii, Chryseobacterium indologenes, Chryseobacterium wanjuense and Chryseobacterium gregarium. Int. J. Syst. Evol. Microbiol. 2013, 63, 4386–4395. [Google Scholar]
- Bhat, S.V.; Maughan, H.; Cameron, A.D.; Yost, C.K. Phylogenomic analysis of the genus Delftia reveals distinct major lineages with ecological specializations. Microb. Genom. 2022, 8, 000864. [Google Scholar] [CrossRef]
- Højgaard, S.M.M.; Rezahosseini, O.; Knudsen, J.D.; Fuglebjerg, N.J.U.; Skov, M.; Nielsen, S.D.; Harboe, Z.B. Characteristics and outcomes of patients with Delftia acidovorans infections: A retrospective cohort study. Microbiol. Spectr. 2022, 10, e00326-22. [Google Scholar] [CrossRef] [PubMed]
- Suchan, D.M.; Bergsveinson, J.; Manzon, L.; Pierce, A.; Kryachko, Y.; Korber, D.; Tan, Y.; Tambalo, D.D.; Khan, N.H.; Whiting, M. Transcriptomics reveal core activities of the plant growth-promoting bacterium Delftia acidovorans RAY209 during interaction with canola and soybean roots. Microb. Genom. 2020, 6, e000462. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhao, K.; Yang, S.; Chen, S.; Li, C.; Han, X.; Li, J.; Hu, K.; Liu, S.; Ma, M. Insights into antibiotic and heavy metal resistance interactions in Escherichia coli isolated from livestock manure and fertilized soil. J. Environ. Manag. 2024, 351, 119935. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Chen, Q.; White, J.F. Evaluation of colonization and mutualistic endophytic symbiosis of Escherichia coli with tomato and Bermuda grass seedlings. PeerJ 2022, 10, e13879. [Google Scholar] [CrossRef]
- Wight, J.; Byrne, A.S.; Tahlan, K.; Lang, A.S. Anthropogenic contamination sources drive differences in antimicrobial-resistant Escherichia coli in three urban lakes. Appl. Environ. Microbiol. 2024, 90, e01809-23. [Google Scholar] [CrossRef]
- An, X.; Tian, C.; Xu, J.; Dong, F.; Liu, X.; Wu, X.; Zheng, Y. Characterization of hexaconazole-degrading strain Sphingobacterium multivorum and analysis of transcriptome for biodegradation mechanism. Sci. Total Environ. 2020, 722, 137171. [Google Scholar] [CrossRef]
- Barahona, F.; Slim, J. Sphingobacterium multivorum: Case report and literature review. New Microbes New Infect. 2015, 7, 33–36. [Google Scholar] [CrossRef]
- Hassan, I.A.; Mohamedelhassan, E.E.; Yanful, E.K.; Yuan, Z.-C. Mitigation of soil contaminated with diesel fuel using bioelectrokinetics. J. Environ. Sci. Health Part A 2019, 54, 416–426. [Google Scholar] [CrossRef]
- Tien, C.J.; Huang, H.J.; Chen, C.S. Accessing the carbofuran degradation ability of cultures from natural river biofilms in different environments. CLEAN–Soil Air Water 2017, 45, 1600380. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.J.; Bacigalupe, R.; Harrison, E.M.; Weinert, L.A.; Lycett, S.; Vrieling, M.; Robb, K.; Hoskisson, P.A.; Holden, M.T.; Feil, E.J. Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nat. Ecol. Evol. 2018, 2, 1468–1478. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chen, D.-H. Synthesis of high-concentration Cu nanoparticles in aqueous CTAB solutions. J. Colloid Interface Sci. 2004, 273, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-C.; Ehrman, S.H. Synthesis of Iron Nanoparticles via Chemical Reduction with Palladium Ion Seeds. Langmuir 2007, 23, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Hosmer Jr, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Skrondal, A.; Rabe-Hesketh, S. Generalized Latent Variable Modeling: Multilevel, Longitudinal, and Structural Equation Models; Chapman and Hall/CRC: London, UK, 2004. [Google Scholar]
- Feng, C.X. A comparison of zero-inflated and hurdle models for modeling zero-inflated count data. J. Stat. Distrib. Appl. 2021, 8, 8. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Abdelfatah, A.M.; Fawzy, M.; Eltaweil, A.S.; El-Khouly, M.E. Green synthesis of nano-zero-valent iron using ricinus communis seeds extract: Characterization and application in the treatment of methylene blue-polluted water. ACS Omega 2021, 6, 25397–25411. [Google Scholar] [CrossRef]
- Morgada, M.E.; Levy, I.K.; Salomone, V.; Farías, S.S.; Lopez, G.; Litter, M.I. Arsenic (V) removal with nanoparticulate zerovalent iron: Effect of UV light and humic acids. Catal. Today 2009, 143, 261–268. [Google Scholar] [CrossRef]
- Singh, A.; Hou, W.-C.; Lin, T.-F. Combined impact of silver nanoparticles and chlorine on the cell integrity and toxin release of Microcystis aeruginosa. Chemosphere 2021, 272, 129825. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Bhatia, P. Comparison study of iron and iron-oxide nanoparticles for thermoplasmonic applications. Mater. Today Commun. 2023, 35, 106008. [Google Scholar] [CrossRef]
- Hussain, M.H.; Abu Bakar, N.F.; Mustapa, A.N.; Low, K.-F.; Othman, N.H.; Adam, F. Synthesis of various size gold nanoparticles by chemical reduction method with different solvent polarity. Nanoscale Res. Lett. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.; Jain, T.K. Ascorbic acid-mediated synthesis and characterisation of iron oxide/gold core–shell nanoparticles. J. Exp. Nanosci. 2016, 11, 370–382. [Google Scholar] [CrossRef]
- Elfeky, S.A.; Mahmoud, S.E.; Youssef, A.F. Applications of CTAB modified magnetic nanoparticles for removal of chromium (VI) from contaminated water. J. Adv. Res. 2017, 8, 435–443. [Google Scholar] [CrossRef]
- Sui, Z.; Chen, X.; Wang, L.; Xu, L.; Zhuang, W.; Chai, Y.; Yang, C. Capping effect of CTAB on positively charged Ag nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2006, 33, 308–314. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Bélteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Kónya, Z.; Kiricsi, M. Polyvinyl-pyrrolidone-coated silver nanoparticles—The colloidal, chemical, and biological consequences of steric stabilization under biorelevant conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef]
- Esfandiari, N.; Khosrovan, S.; Mirjalili, M. A study on disinfection and adhesion behaviour between bacteria and photocatalytic nanostructures by extended DLVO. Environ. Technol. 2024, 45, 6106–6120. [Google Scholar] [CrossRef]
- Ammendolia, M.G.; De Berardis, B. Nanoparticle impact on the bacterial adaptation: Focus on nano-titania. Nanomaterials 2022, 12, 3616. [Google Scholar] [CrossRef]
- Gaminda, K.A.P.; Thomas, I.B.K.; Lakmauri, P.; Abeysinghe, T.; Jayasinghe, C.; Senthilnithy, R. Green synthesis of iron nanoparticles using Syzygium aromaticum extracts and their applications: Nitrate removal, malachite green degradation and antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100925. [Google Scholar] [CrossRef]
- Abdelraheem, W.M.; Refaie, M.M.; Yousef, R.K.M.; Abd El Fatah, A.S.; Mousa, Y.M.; Rashwan, R. Assessment of antibacterial and anti-biofilm effects of vitamin C against Pseudomonas aeruginosa clinical isolates. Front. Microbiol. 2022, 13, 847449. [Google Scholar] [CrossRef] [PubMed]
- Hassuna, N.A.; Rabie, E.; Mahd, W.; Refaie, M.M.; Yousef, R.K.M.; Abdelraheem, W.M. Antibacterial effect of vitamin C against uropathogenic E. coli in vitro and in vivo. BMC Microbiol. 2023, 23, 112. [Google Scholar] [CrossRef]
- Venkateswaran, K.; Kempf, M.; Chen, F.; Satomi, M.; Nicholson, W.; Kern, R. Bacillus nealsonii sp. nov., isolated from a spacecraft-assembly facility, whose spores are γ-radiation resistant. Int. J. Syst. Evol. Microbiol. 2003, 53, 165–172. [Google Scholar] [CrossRef]
- Mathes, A.; Engelhardt, H. Nonlinear and asymmetric open channel characteristics of an ion-selective porin in planar membranes. Biophys. J. 1998, 75, 1255–1262. [Google Scholar] [CrossRef]
- Zachariae, U.; Kluhspies, T.; De, S.; Engelhardt, H.; Zeth, K. High resolution crystal structures and molecular dynamics studies reveal substrate binding in the porin Omp32. J. Biol. Chem. 2006, 281, 7413–7420. [Google Scholar] [CrossRef] [PubMed]
- Pajerski, W.; Ochonska, D.; Brzychczy-Wloch, M.; Indyka, P.; Jarosz, M.; Golda-Cepa, M.; Sojka, Z.; Kotarba, A. Attachment efficiency of gold nanoparticles by Gram-positive and Gram-negative bacterial strains governed by surface charges. J. Nanopart. Res. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Wenck, C.; Leopoldt, D.; Habib, M.; Hegermann, J.; Stiesch, M.; Doll-Nikutta, K.; Heisterkamp, A.; Torres-Mapa, M.L. Colorimetric detection of oral bacteria using functionalized gold nanoparticles as a plasmonic biosensor array. Nanoscale Adv. 2024, 6, 1447–1459. [Google Scholar] [CrossRef]
- Bankier, C.; Matharu, R.; Cheong, Y.; Ren, G.; Cloutman-Green, E.; Ciric, L. Synergistic antibacterial effects of metallic nanoparticle combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef]
- Sinha, R.; Karan, R.; Sinha, A.; Khare, S. Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour. Technol. 2011, 102, 1516–1520. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.; Li, Z.; Fan, Y.; Liu, S. An ultra-small fluorescence zero-valent iron nanoclusters selectively kill gram-positive bacteria by promoting reactive oxygen species generation. Colloids Surf. B Biointerfaces 2023, 227, 113343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zheng, W.; Tran, K.; Kamilar, E.; Bariwal, J.; Ma, H.; Liang, H. Hydrophilic nanoparticles that kill bacteria while sparing mammalian cells reveal the antibiotic role of nanostructures. Nat. Commun. 2022, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wiese, A.; Münstermann, M.; Gutsmann, T.; Lindner, B.; Kawahara, K.; Zähringer, U.; Seydel, U. Molecular Mechanisms of Polymyxin B-Membrane Interactions: Direct Correlation Between Surface Charge Density and Self-Promoted Transport. J. Membr. Biol. 1998, 162, 127–138. [Google Scholar] [CrossRef]
- Ndaba, B.; Roopnarain, A.; Haripriya, R.; Maaza, M. Biosynthesized metallic nanoparticles as fertilizers: An emerging precision agriculture strategy. J. Integr. Agric. 2022, 21, 1225–1242. [Google Scholar] [CrossRef]
- Li, X.-Q.; Elliott, D.W.; Zhang, W.-X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects. In Particulate Systems in Nano and Biotechnologies; CRC Press: Boca Raton, FL, USA, 2008; pp. 309–330. [Google Scholar]
- Basak, S.; Bhattacharyya, P.; Pudake, R.N.; Lokhande, P.E.; Rednam, U.; Chakrabarti, S. Metal-organic framework as nanocarriers for agricultural applications: A review. Front. Nanotechnol. 2024, 6, 1385981. [Google Scholar] [CrossRef]
- Marco-Brown, J.L.; Valiente, R.; Ramos, C.P.; Fernandez, M.A.; Candal, R. Stable nZVI-based nanocomposites for adsorption and reduction processes: The case of U (VI) removal. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100563. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Zhang, W.-X. Nanoscale Iron Particles for Environmental Remediation: An Overview. J. Nanoparticle Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Stephano-Hornedo, J.L.; Torres-Gutiérrez, O.; Toledano-Magaña, Y.; Gradilla-Martínez, I.; Pestryakov, A.; Sánchez-González, A.; García-Ramos, J.C.; Bogdanchikova, N. Argovit™ silver nanoparticles to fight Huanglongbing disease in Mexican limes (Citrus aurantifolia Swingle). RSC Adv. 2020, 10, 6146–6155. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Elliott, D.W.; Zhang, W.-x. Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: Materials and Engineering Aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Qin, F.; Dong, H.; Wang, W.; Luo, H.; Qin, D.; Zhang, C.; Tan, H. Nano zero valent iron in the 21st century: A data-driven visualization and analysis of research topics and trends. J. Clean. Prod. 2023, 415, 137812. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Handa, N.; Kaur, H.; Ohri, P.; Bhardwaj, R.; Yousaf, B.; Rinklebe, J.; Ahmad, P. Enthralling the impact of engineered nanoparticles on soil microbiome: A concentric approach towards environmental risks and cogitation. Ecotoxicol. Environ. Saf. 2021, 222, 112459. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, H.; Fernández-Luqueño, F.; Huerta-Lwanga, E.; Mendoza-Vega, J.; Álvarez-Solís José, D. Effect of engineered nanoparticles on soil biota: Do they improve the soil quality and crop production or jeopardize them? Land Degrad. Dev. 2020, 31, 2213–2230. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a key player in sustainable agriculture and human health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Chen, P.-J.; Liao, V.H.-C. Nanoscale zerovalent iron (nZVI) at environmentally relevant concentrations induced multigenerational reproductive toxicity in Caenorhabditis elegans. Chemosphere 2016, 150, 615–623. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C. Recent trends in preparation and biomedical applications of iron oxide nanoparticles. J. Nanobiotechnol. 2024, 22, 24. [Google Scholar] [CrossRef]
- Laffon, B.; Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V. Cellular and molecular toxicity of iron oxide nanoparticles. Cell. Mol. Toxicol. Nanopart. 2018, 1048, 199–213. [Google Scholar]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef] [PubMed]
- Joris, F.; Valdepérez, D.; Pelaz, B.; Soenen, S.J.; Manshian, B.B.; Parak, W.J.; De Smedt, S.C.; Raemdonck, K. The impact of species and cell type on the nanosafety profile of iron oxide nanoparticles in neural cells. J. Nanobiotechnol. 2016, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.S.; Adireddy, S.; Jaiswal, A.; Mandava, S.; Lee, B.R.; Chrisey, D.B. In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2015, 16, 24417–24450. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Harrell, F. Violation of Proportional Odds Is Not Fatal; Creative Commons: Mountain View, CA, USA, 2020; Volume 2024. [Google Scholar]

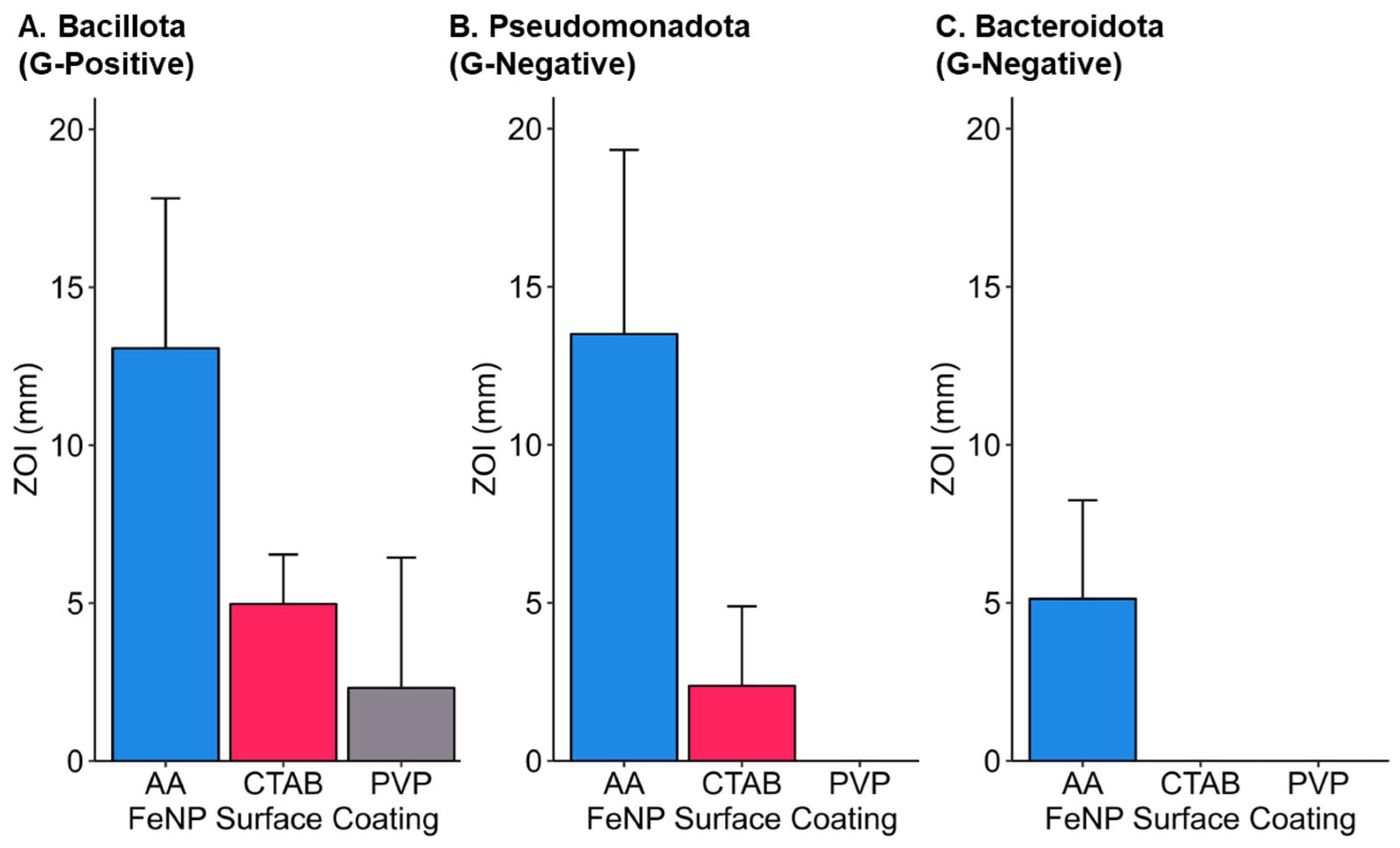
| Bacteria | Phylum | Class | Cell Wall and Morphology | Environmental Compartment | References |
|---|---|---|---|---|---|
| Bacillus nealsonii | Bacillota | Bacilli | Gram-positive rods | Sediment | [47] |
| Chryseobacterium sp. | Bacteroidota | Flavobacteriia | Gram-negative rods | Human tissue, Soil, Water, Plants | [48,49,50] |
| Delftia acidovorans | Pseudomonadota | Betaproteobacteria | Gram-negative rods | Human tissue, Soil, Water, Plants | [51,52,53] |
| Escherichia coli | Pseudomonadota | Gammaproteobacteria | Gram-negative rods | Human tissue, Soil, Water, Plants | [54,55,56] |
| Sphingobacterium multivorum | Bacteroidota | Sphingobacteriia | Gram-negative rods | Human tissue, Soil, Water | [57,58,59,60] |
| Staphylococcus aureus | Bacillota | Bacilli | Gram-positive coccus | Human tissue, Livestock | [61,62] |
| Symbol | Variable Explanation |
|---|---|
| Intercept term; Estimated logged mean inhibition for Coating = CTAB on Species = S. multivorum using Dose = 1 µM | |
| Coefficient for each covariate; Estimated multiplicative factor for log mean inhibition | |
| Indicator variable for Coating = AA, | |
| Indicator variable for Species = Chryseobacterium sp., | |
| Indicator variable for Species = S. aureus, | |
| Indicator variable for Species = D. acidovorans, | |
| Indicator variable for Species = E. coli, | |
| Indicator variable for Species = B. nealsonii, | |
| Indicator variable for Dose = 2 µM, | |
| Indicator variable for Dose = 4 µM, |
| Coating | Odds Ratio | p-Value |
|---|---|---|
| PVP-FeNP | 1 | NA |
| CTAB-FeNP | 10.0 | <0.001 |
| AA-FeNP | 167 | <0.001 |
| Model | Coefficient | Estimate | p-Value |
|---|---|---|---|
| Gamma | Intercept | 1.6079 | 0.0015 |
| Coating = AA | 1.6944 | <0.001 | |
| Species = Chryseo. sp. | 1.0607 | 0.668 | |
| Species = S. aureus | 2.4879 | <0.001 | |
| Species = D. acidovorans | 2.7729 | <0.001 | |
| Species = E. coli | 2.7901 | <0.001 | |
| Species = B. nealsonii | 3.1417 | <0.001 | |
| Dose = 2 | 1.3422 | 0.0208 | |
| Dose = 4 | 0.8969 | 0.3933 | |
| Coating = AA × Dose = 2 | 1.5756 | 0.0044 | |
| Coating = AA × Dose = 4 | 2.7055 | <0.001 | |
| ZI | Intercept | 0.3896 | <0.001 |
| Dispersion | Intercept | 13.7187 | NA |
| Variable | Estimate (Exponentiated) | p-Value |
|---|---|---|
| Coating = AA | 160.4317 | <0.0001 |
| Species = Chryseo. sp. | 1.8263 | <0.0001 |
| Species = S. aureus | 883.8004 | <0.0001 |
| Species = D. acidovorans | 81.9498 | 0.1204 |
| Species = E. coli | 3051.8002 | 0.0007 |
| Species = B. nealsonii | 7912.0398 | <0.0001 |
| Dose = 2 | 7.6041 | 0.0064 |
| Dose = 4 | 0.5471 | 0.3816 |
| Coating = AA × Dose = 2 | 43.2076 | 0.0002 |
| Coating = AA × Dose = 4 | 2628.5285 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnathan, B.J.; Stevens, D.; Shikha, S.; Slater, C.; Byford, N.; Sturdivant, R.X.; Zarzosa, K.; Braswell, W.E.; Sayes, C.M. Assessing the Effects of Surface-Stabilized Zero-Valent Iron Nanoparticles on Diverse Bacteria Species Using Complementary Statistical Models. J. Funct. Biomater. 2025, 16, 113. https://doi.org/10.3390/jfb16030113
Carnathan BJ, Stevens D, Shikha S, Slater C, Byford N, Sturdivant RX, Zarzosa K, Braswell WE, Sayes CM. Assessing the Effects of Surface-Stabilized Zero-Valent Iron Nanoparticles on Diverse Bacteria Species Using Complementary Statistical Models. Journal of Functional Biomaterials. 2025; 16(3):113. https://doi.org/10.3390/jfb16030113
Chicago/Turabian StyleCarnathan, Brittany J., Dinny Stevens, Swarna Shikha, Carson Slater, Nathen Byford, Rodney X. Sturdivant, Kuzy Zarzosa, W. Evan Braswell, and Christie M. Sayes. 2025. "Assessing the Effects of Surface-Stabilized Zero-Valent Iron Nanoparticles on Diverse Bacteria Species Using Complementary Statistical Models" Journal of Functional Biomaterials 16, no. 3: 113. https://doi.org/10.3390/jfb16030113
APA StyleCarnathan, B. J., Stevens, D., Shikha, S., Slater, C., Byford, N., Sturdivant, R. X., Zarzosa, K., Braswell, W. E., & Sayes, C. M. (2025). Assessing the Effects of Surface-Stabilized Zero-Valent Iron Nanoparticles on Diverse Bacteria Species Using Complementary Statistical Models. Journal of Functional Biomaterials, 16(3), 113. https://doi.org/10.3390/jfb16030113








