Effect of Sequential vs. Simultaneous Dual Growth Factor Release from Structured Heparin-Poly-Electrolyte Multilayer Coatings on Peri-Implant Bone Formation and Angiogenesis in Pig Mandibles
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Study
2.1.1. Titanium Specimen Fabrication
2.1.2. Multilayer Coating of Ti Discs
2.1.3. Variation in Film Architecture and Growth Factor Loading
- (a)
- One (PLL-Hep)20 multilayer system that was loaded with rhBMP2 (20-rhBMP2).
- (b)
- One (PLL-Hep)20 multilayer system that was loaded with rhVEGF165 (20-rhVEGF165).
- (c)
- Two subsequent (PLL-Hep)10 multilayer systems for dual growth factor loading using a two-step procedure: a (PLL-Hep)10 multilayer system was loaded with one growth factor, after which a second (PLL-Hep)10 multilayer system was added with subsequent loading of the second growth factor on top (10-rhBMP2-10-rhVEGF165).
- (d)
- This procedure was modified by changing the sequence of growth factor loading (10-rhVEGF165-10-rhBMP2).
- (e)
- One (PLL-Hep)20 multilayer system that was loaded with both growth factors together (20-rhBMP2 + rhVEGF165).
2.1.4. Growth Factor Loading/Samples for In Vitro Release
2.1.5. Release Experiments
2.2. In Vivo Study
2.2.1. Sample Size Calculation
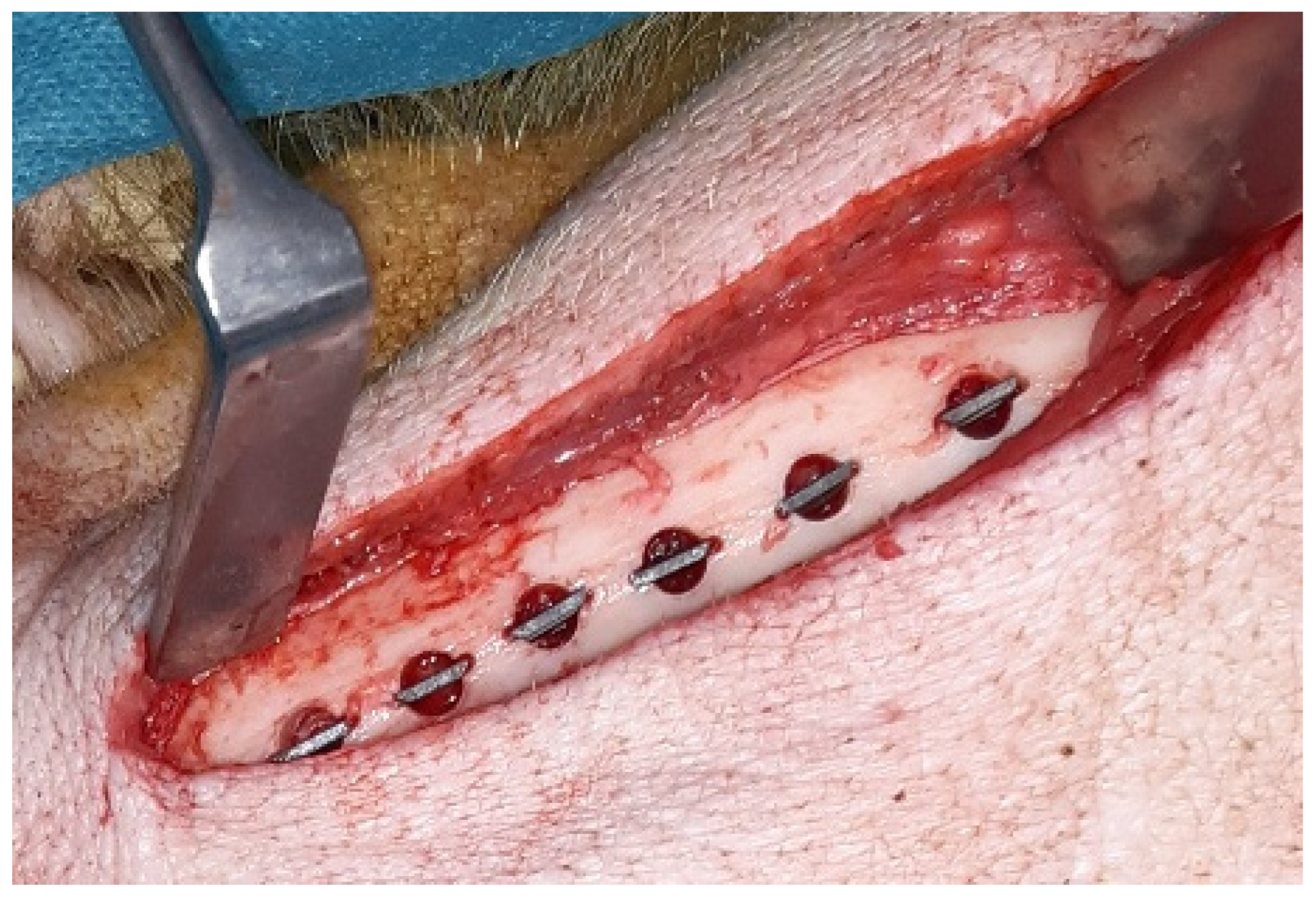
2.2.2. Surgical Procedures and Animal Care
2.2.3. Histologic Preparation and Morphometry
- (i).
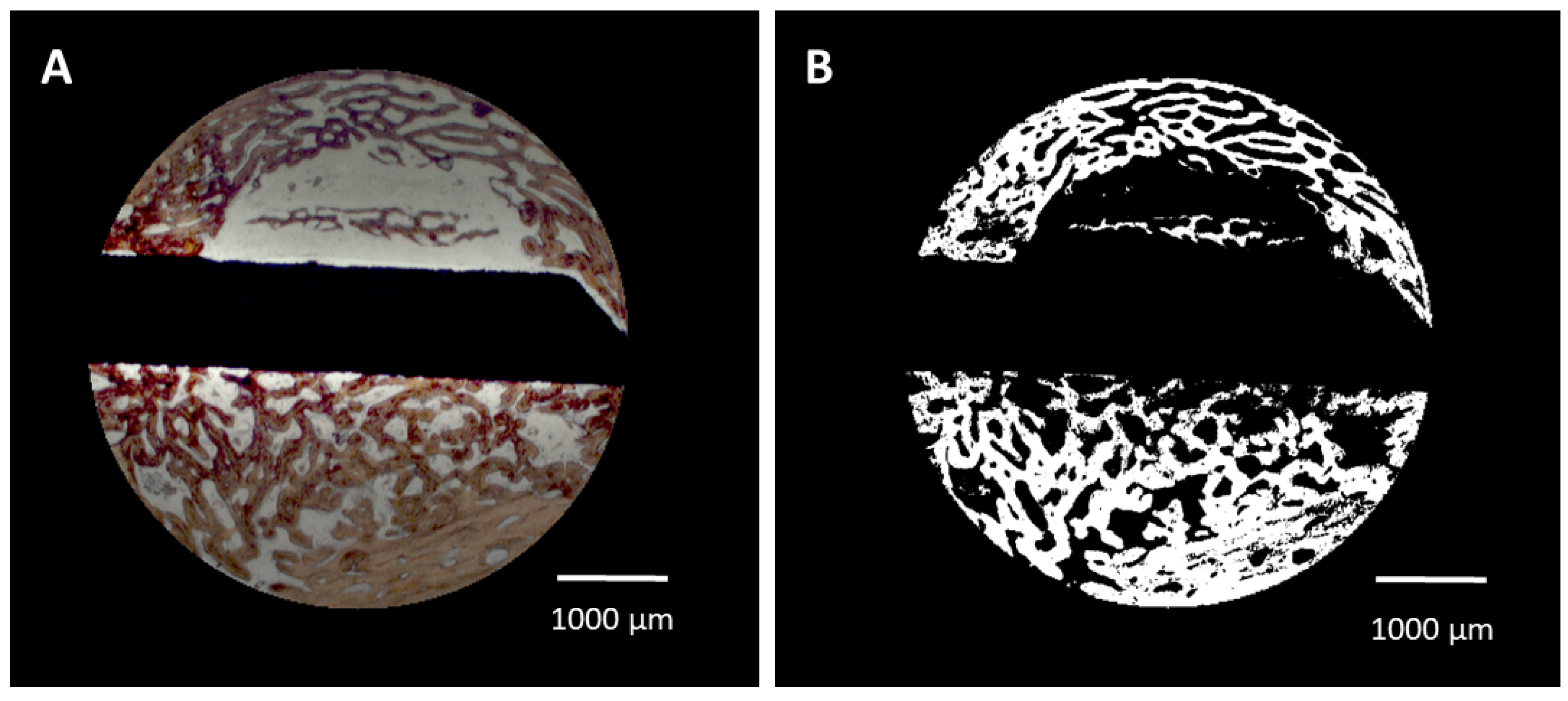
- Bone area/bone density. The algorithm automatically identified the color of the Alizarine Red-stained areas in the cross-section specimens and assessed the area occupied by bone both in absolute values (bone formation (BF)) and in relation to each section area (bone density (BD)) by pixel counting. Pixels were converted in mm2 using the calculated pixel size of 17.43 μm2/pixel (Figure 2A,B). Bone density was only evaluated for the trephine defects as a whole. To account for variations in the appearance of the color of Alizarine Red in the difference and in cases in which the newly formed bone covered the entire trephine defect, parameters were manually adjusted.
- (ii).
- Bone–implant contact (BIC). The algorithm identified the surface area occupied by bone by image analysis routines and calculated the bone–implant contact (BIC) as percentage of occupied surface area. In brief, the surface of the identified cross-section of the implant was enlarged by 1 pixel (approx. 4.18 μm) and limited to the trephine defect size. The resulting mask was multiplied with the bone mask and the ratio of those pixels to the entire surface of the implant was calculated.
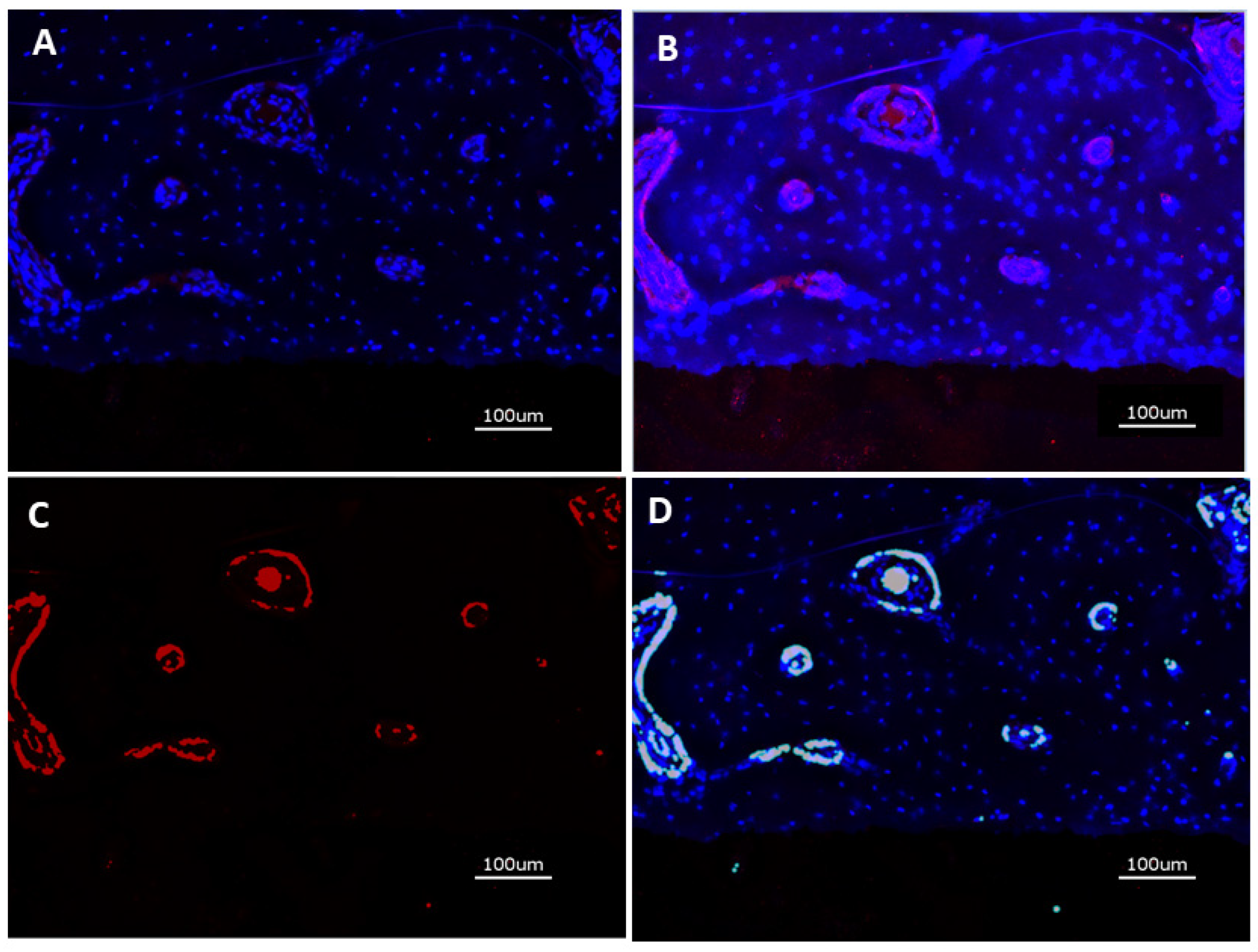
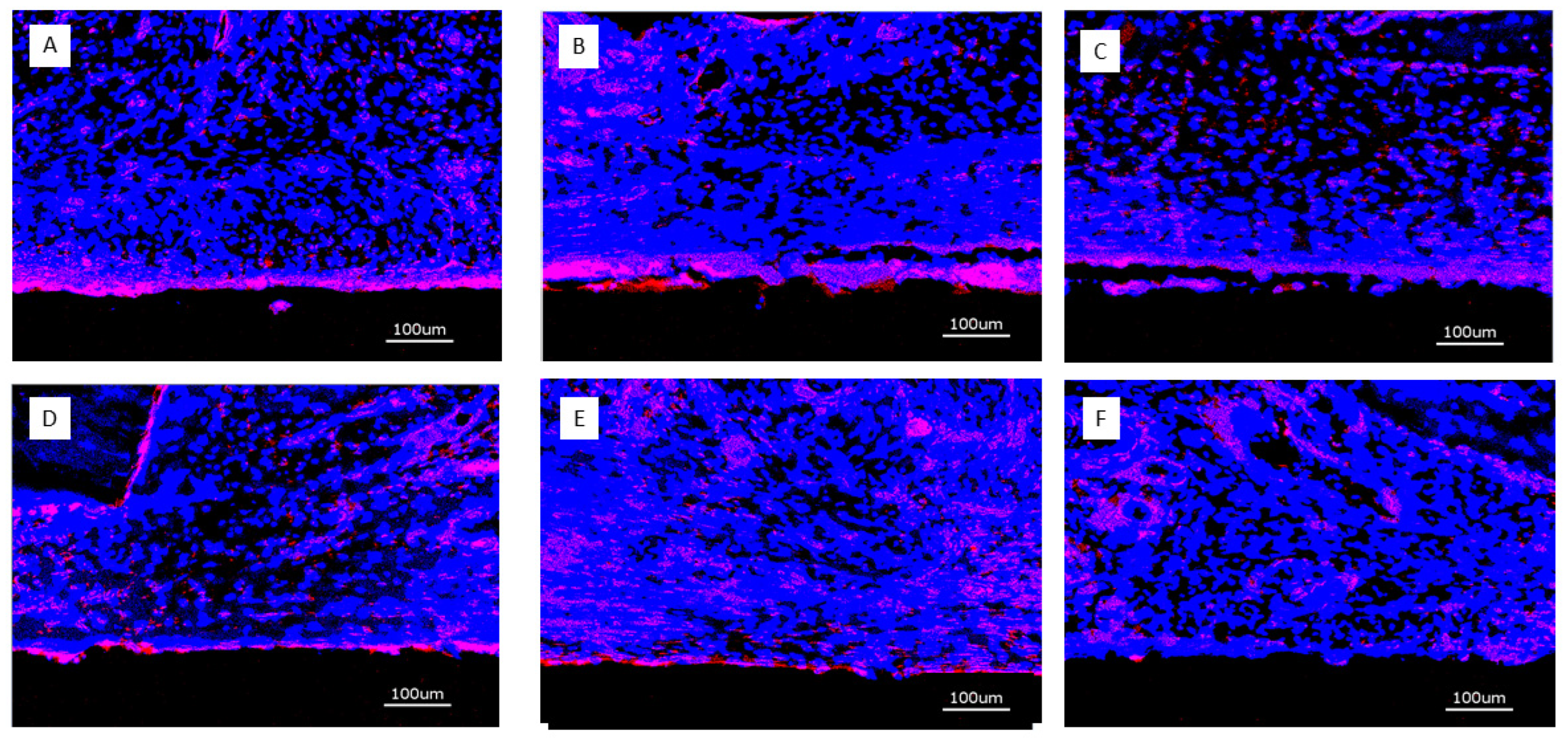
2.2.4. Immunohistochemical Preparation and Evaluation of Immunofluorescence
2.2.5. Statistics
3. Results
3.1. In Vitro Experiments
3.1.1. Growth Factor Loading
3.1.2. Growth Factor Release
3.2. In Vivo Experiments
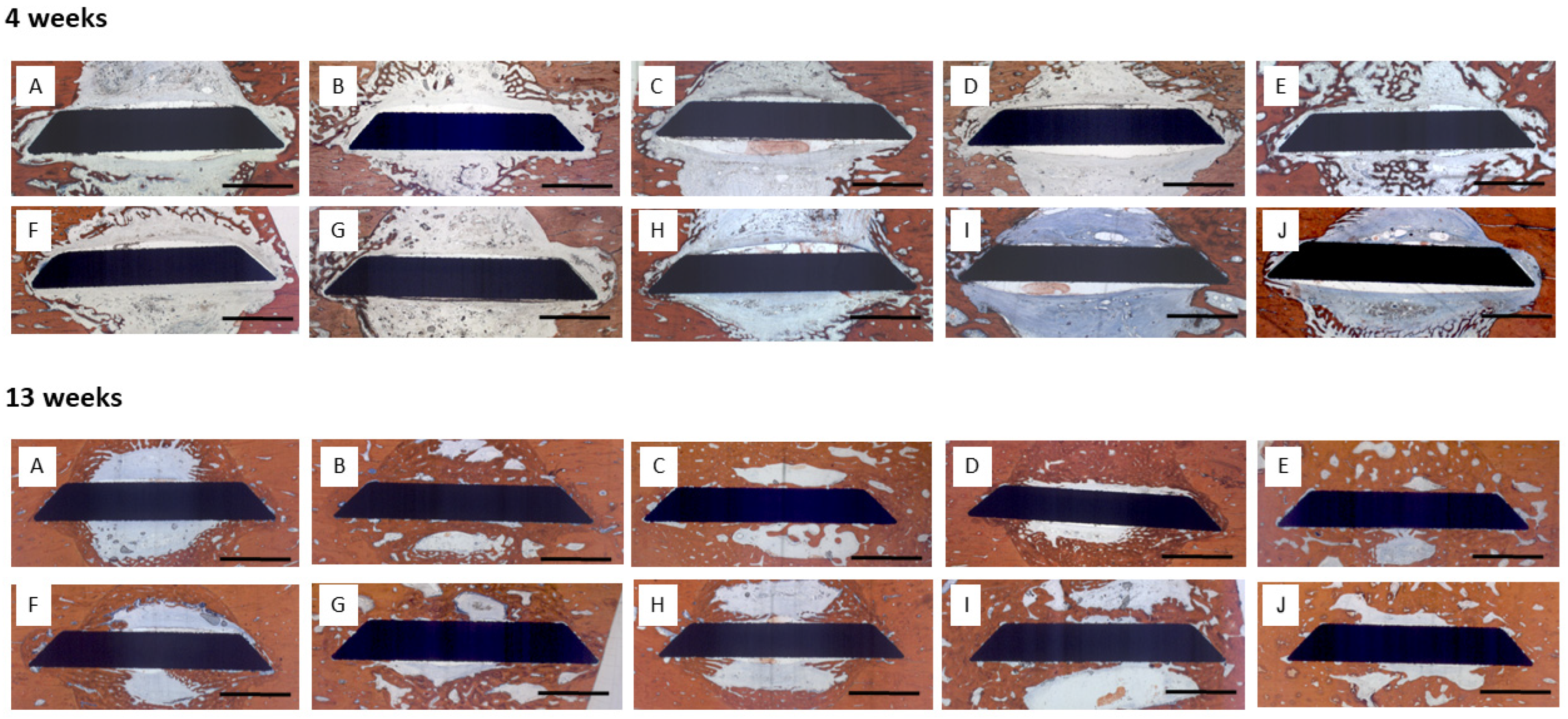
3.2.1. Histology
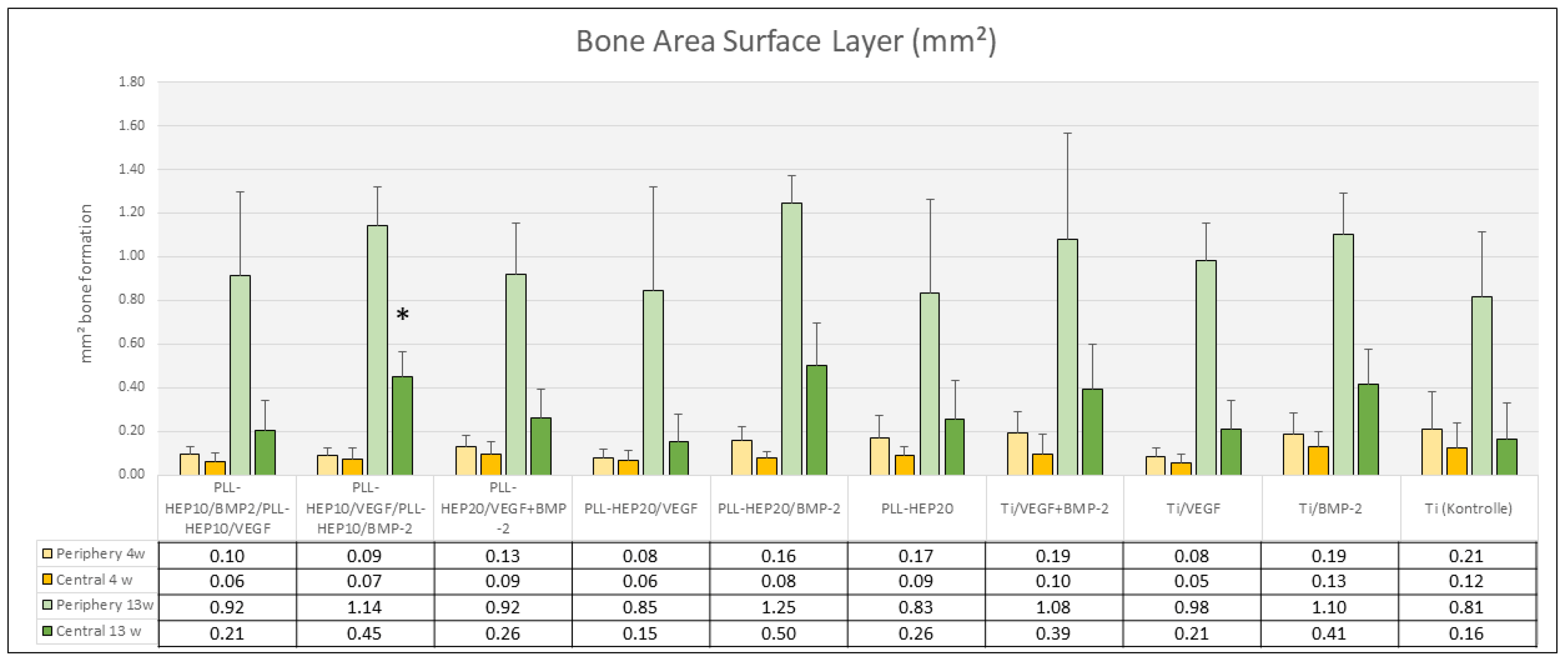
3.2.2. Histomorphometry
4 Weeks
13 Weeks
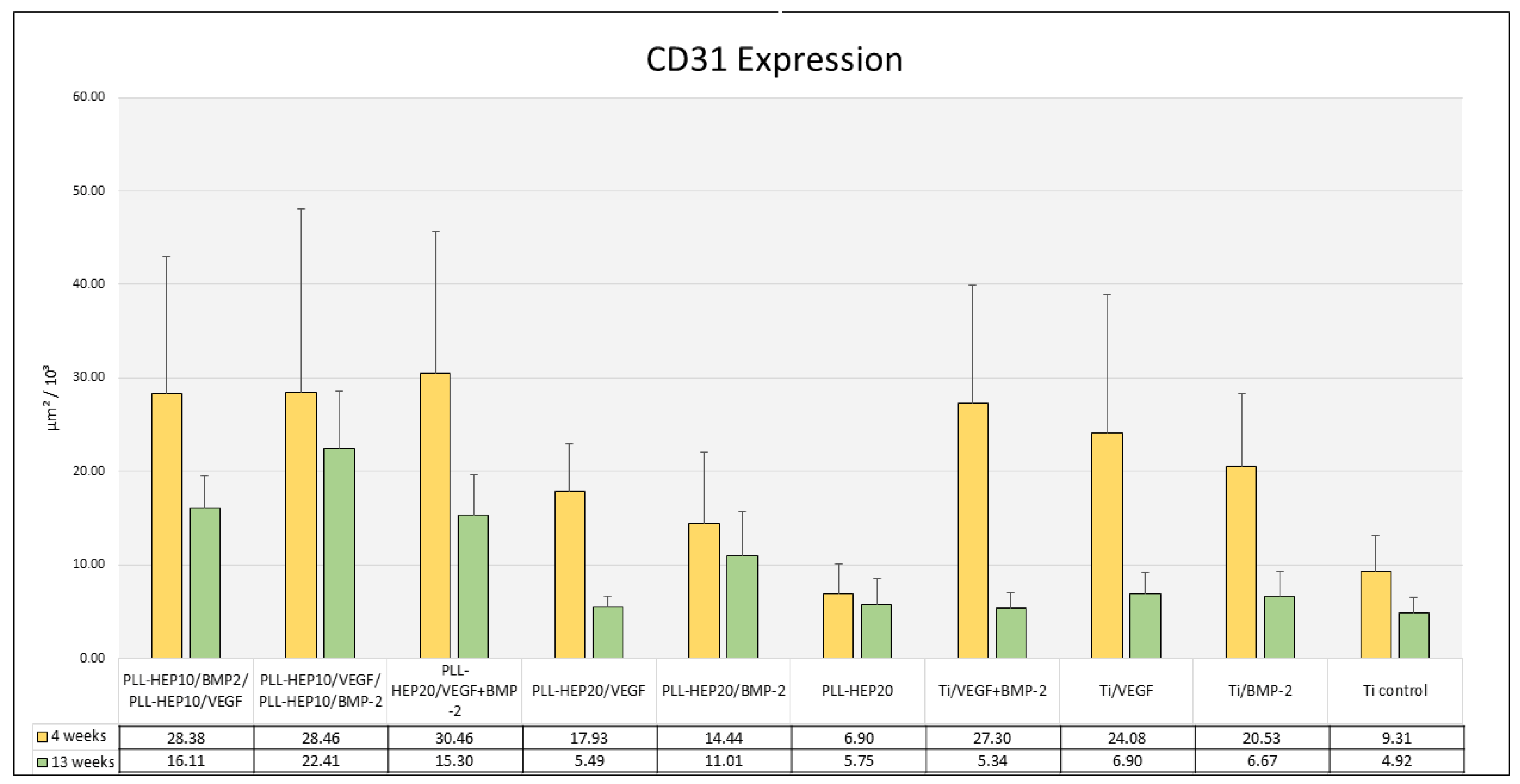
3.2.3. Immunofluorescence of CD31 Expression
3.2.4. Histomorphometry of Immunofluorescence of CD31 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| rhBMP2 | recombinant human bone morphogenic protein 2 |
| rhVEGF165 | recombinant human vascular endothelial growth factor 165 |
| PEM | poly-electrolyte multilayer |
| PLL | poly-L-lysine |
References
- Pan, J.; Shirota, T.; Ohno, K.; Michi, K. Effect of ovarectomy on bone remodelling adjacent to hydroxyapatite-coated implants in the tibia of mature rats. J. Oral. Maxillofacial Surg. 2000, 58, 877–8827. [Google Scholar] [CrossRef]
- McCracken, M.; Lemons, J.E.; Rahemtulla, F.; Prince, C.W.; Feldman, D. Bone response to titanium alloy implants placed in diabetic rats. Int. J. Oral Maxillofac. Implants 2000, 15, 345–354. [Google Scholar] [PubMed]
- Keller, J.C.; Stewart, M.; Roehm, M.; Schneider, G.B. Osteoporosis-like bone conditions affect osseointegration of implants. Int. J. Oral Maxillofac. Implants 2004, 19, 687–694. [Google Scholar]
- Li, J.; Zheng, Y.; Yu, Z.; Kankala, R.K.; Lin, Q.; Shi, J.; Chen, C.; Luo, K.; Chen, A.; Zhong, Q. Surface-modified titanium and titanium-based alloys for improved osteogenesis: A critical review. Heliyon 2023, 10, e23779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Che, Z.; Sun, Q.; Zhao, Z.; Wu, Y.; Xing, H.; Song, K.; Chen, A.; Wang, B.; Cai, M. Growth factor-functionalized titanium implants for enhanced bone regeneration: A review. Int. J. Biol. Macromol. 2024, 274 Pt 2, 133153. [Google Scholar] [CrossRef] [PubMed]
- Shayeb, M.A.; Elfadil, S.; Abutayyem, H.; Shqaidef, A.; Marrapodi, M.M.; Cicciù, M.; Minervini, G. Bioactive surface modifications on dental implants: A systematic review and meta-analysis of osseointegration and longevity. Clin. Oral Investig. 2024, 28, 592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, H.W.; Chien, E.Y.; Chien, H.H. Dental implant bioactive surface modifications and their effects on osseointegration: A review. Biomark. Res. 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Thorey, F.; Menzel, H.; Lorenz, C.; Gross, G.; Hoffmann, A.; Windhagen, H. Osseointegration by bone morphogenetic protein-2 an transforming growth factor beta 2 coated titanium implants in femora of New Zealand white rabbits. Indian J. Orthop. 2011, 45, 57–62. [Google Scholar] [CrossRef]
- Ramanzanoglu, M.; Lutz, R.; Ergun, C.; Wilmowsky, C.; Nkenke, E.; Schlegel, K.A. The effect of combined delivery of recombinant human bone morphogenic protein-2 and recombinant human vascular endothelial growth factor 165 from biomimetic calcium-phosphate coated implants. Clin. Oral Implants Res. 2011, 22, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Leknes, K.N.; Yang, J.; Qahash, M.; Polimeni, G.; Susin, C.; Wikesjö, U.M. Alveolar ridge augmentation using implants coated with recombinant human growth/differentiation factor 5 (rhGDF-5). Radiographic observations. Clin. Oral Implants Res. 2013, 24, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.M.; Thorwarth, M.; Schmidt, M.; Schlegel, K.A.; Schultze-Mosgau, S. Comparative analysis of osseointegration of titanium implants with acid-etched surfaces and different biomolecular coatings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Beidas, O.E.; Deschamps-Braly, J.C.; Morgan, A.M.; Workman, M.C.; Knotts, C.D.; Denny, A.D.; El Amm, C.A. Safety and efficacy of recombinant human bone morphogenetic protein 2 on cranial defect closure in the pediatric population. J. Craniofac Surg. 2013, 24, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Shweikeh, F.; Hanna, G.; Bloom, L.; Sayegh, E.T.; Liu, J.; Acosta, F.L.; Drazin, D. Assessment of outcome following the use of recombinant human bone morphogeneticprotein-2 for spinal fusion in the elderly population. J. Neurosurg. Sci. 2014; ahead of print. [Google Scholar]
- Merrick, M.T.; Hamilton, K.D.; Russo, S.S. Acute epidural lipedema: A novel entity and potential complication of bonemorphogenetic protein use in lumbar spine fusion. Spine J. 2013, 13, e15–e19. [Google Scholar] [CrossRef]
- Muchow, R.D.; Hsu, W.K.; Anderson, P.A. Histopathologic inflammatory response induced by recombinant bone morphogeneticprotein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010, 10, e1–e6. [Google Scholar] [CrossRef]
- Seol, Y.J.; Park, Y.J.; Lee, S.C.; Kim, K.H.; Lee, J.Y.; Kim, T.I.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Han, S.B.; et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J. Biomed. Mater. Res. A 2006, 77, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Adden, N.; Gamble, L.J.; Castner, D.G.; Hoffmann, A.; Gross, G.; Menzel, H. Phosphonic acid monolayers for binding of bioactive molecules to titanium surfaces. Langmuir 2006, 22, 8197–8204. [Google Scholar] [CrossRef]
- Schliephake, H.; Bötel, C.; Förster, A.; Schwenzer, B.; Reichert, J.; Scharnweber, D. Effect of oligonucleotide mediated immobilization of bone morphogenic proteins on titanium surfaces—An in vitro study. Biomaterials 2012, 33, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, M.L.; Rodriguez, N.M.; Shah, N.J.; Hammond, P.T. Characterization of tunable FGF-2 releasing polyelectrolyte multilayers. Biomacromolecules 2010, 11, 2053–2059. [Google Scholar] [CrossRef]
- Guduru, D.; Niepel, M.S.; Gonzalez-Garcia, C.; Salmeron-Sanchez, M.; Groth, T. Comparative Study of Osteogenic Activity of Multilayers Made of Synthetic and Biogenic Polyelectrolytes. Macromol. Biosci. 2017, 17, 1700078. [Google Scholar] [CrossRef]
- Guillot, R.; Gilde, F.; Becquart, P.; Sailhan, F.; Lapeyrere, A.; Logeart-Avramoglou, D.; Picart, C. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials 2013, 34, 5737–5746. [Google Scholar] [CrossRef]
- Crouzier, T.; Szarpak, A.; Boudou, T.; Auzély-Velty, R.; Picart, C. Polysaccharide-blend multilayers containing hyaluronan and heparin as a delivery system for rhBMP2. Small 2010, 6, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, M.; Guillot, R.; Lavaud, J.; Plettinx, C.; Olivier, C.; Curry, V.; Boutonnat, J.; Coll, J.L.; Peyrin, F.; Josserand, V.; et al. Surface delivery of tunable doses of BMP2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials 2016, 104, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Nakamura, S.; Sato, Y.; Takayama, T.; Fukuda, K.; Fujita, M.; Murakami, K.; Yokoe, H. Heparinoid Complex-Based Heparin-Binding Cytokines and Cell Delivery Carriers. Molecules 2019, 24, 4630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilde, F.; Fourel, L.; Guillot, R.; Pignot-Paintrand, I.; Okada, T.; Fitzpatrick, V.; Boudou, T.; Albiges-Rizo, C.; Picart, C. Stiffness-dependent cellular internalization of matrix-bound BMP-2 and its relation to Smad and non-Smad signalling. Acta Biomater. 2016, 46, 55–67. [Google Scholar] [CrossRef]
- Kienle, D.F.; Chaparro Sosa, A.F.; Kaar, J.L.; Schwartz, D.K. Polyelectrolyte Multilayers Enhance the Dry Storage and pH Stability of Physically Entrapped Enzymes. ACS Appl. Mater. Interfaces 2020, 12, 22640–22649. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Macdonald, M.L.; Beben, Y.M.; Padera, R.F.; Samuel, R.E.; Hammond, P.T. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials 2011, 32, 6183–6193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Behrens, C.; Kauffmann, P.; von Hahn, N.; Giesecke, A.; Schirmer, U.; Liefeith, K.; Schliephake, H. Development of a system of heparin multilayers on titanium surfaces for dual growth factor release. J. Biomed. Mater. Res. A 2022, 110, 1599–1615. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, U.; Ludolph, J.; Rothe, H.; Hauptmann, N.; Behrens, C.; Bittrich, E.; Schliephake, H.; Liefeith, K. Tailored Polyelectrolyte Multilayer Systems by Variation of Polyelectrolyte Composition and EDC/NHS Cross-Linking: Physicochemical Characterization and In Vitro Evaluation. Nanomaterials 2022, 12, 2054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kauffmann, P.; Wolfer, S.; Behrens, C.; Schlosser, P.; Dullin, C.; SChirmer, U.; Liefeieith, K.; Schliephake, H. Dual growth factor release from collagen based polyelectrolyte multilayer films on Ti-implant enhances periimplant bone formation and angiogenic activity. Biomaterials, 2025; submitted. [Google Scholar]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Gronowicz, G.; Jacobs, E.; Peng, T.; Zhu, L.; Hurley, M.; Kuhn, L.T. Calvarial Bone Regeneration Is Enhanced by Sequential Delivery of FGF-2 and BMP-2 from Layer-by-Layer Coatings with a Biomimetic Calcium Phosphate Barrier Layer. Tissue Eng. Part A 2017, 23, 1490–1501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacobs, E.E.; Gronowicz, G.; Hurley, M.M.; Kuhn, L.T. Biomimetic calcium phosphate/polyelectrolyte multilayer coatings for sequential delivery of multiple biological factors. J. Biomed. Mater. Res. A 2017, 105, 1500–1509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Min, J.; Braatz, R.D.; Hammond, P.T. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials 2014, 35, 2507–2517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almquist, B.D.; Castleberry, S.A.; Sun, J.B.; Lu, A.Y.; Hammond, P.T. Combination Growth Factor Therapy via Electrostatically Assembled Wound Dressings Improves Diabetic Ulcer Healing In Vivo. Adv. Healthc. Mater. 2015, 4, 2090–2099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Liu, W.C.; Wang, H.Y.; Li, V.L.; Chen, Y.C.; Wang, A.N.; Wu, C.J.; Li, Y.; Zhao, G.; Lin, C.; et al. Polyelectrolyte multilayer composite coating on 316 L stainless steel for controlled release of dual growth factors accelerating restoration of bone defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112187. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; McShane, M.J. Dual-function nanofilm coatings with diffusion control and protein resistance. ACS Appl. Mater. Interfaces 2010, 2, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.D.; Bennett, E.E.; Ellis, E.A.; Gumienny, T.L. Regulation of extracellular matrix organization by BMP signaling in Caenorhabditis elegans. PLoS ONE 2014, 11, e1011929. [Google Scholar] [CrossRef] [PubMed]
- Kopf, J.; Paarmann, P.; Hiepen, J.; Horbelt, D.; Knaus, P. BMP growth factor signaling in a biomechanical context. Biofactors 2014, 40, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Moser, N.; Lohse, N.; Golstein, J.; Kauffmann, P.; Sven, B.; Epple, M.; Schliephake, H. Do we need retarded delivery of bone growth factors in facial bone repair? An experimental study in rats. Eur. Cell Mater. 2017, 34, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, M.; Garot, C.; Machillot, P.; Vollaire, J.; Fitzpatrick, V.; Morand, S.; Boutonnat, J.; Josserand, V.; Bettega, G.; Picart, C. 3D-printed scaffold combined to 2D osteoinductive coatings to repair a critical-size mandibular bone defect. Mater. Today Bio. 2021, 11, 100113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| rhBMP2 (μg/cm2) | rhVEGF165 (μg/cm2) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| PLL-HEP10/BMP-2&PLL-HEP10/VEGF | 3.8 | 1.3 | 5.5 | 0.4 |
| PLL-HEP10/VEGF&PLL-HEP10/BMP-2 | 5.9 | 0.6 | 5.0 | 0.7 |
| PLL-HEP20/VEGF + BMP-2 | 3.5 | 1.3 | 4.5 | 2.1 |
| PLL-HEP20/VEGF | 4.4 | 2.0 | ||
| PLL-HEP20/BMP-2 | 5.7 | 0.4 | ||
| PLL-HEP20 | ||||
| Ti/VEGF + BMP-2 | 2.9 | 1.8 | 4.6 | 0.4 |
| Ti/VEGF | 4.9 | 1.9 | ||
| Ti/BMP-2 | 3.1 | 2.2 | ||
| Ti Control | ||||
| r hBMP2 (μg/cm2) | rhVEGF165 (μg/cm2) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| PLL-HEP10/BMP-2&PLL-HEP10/VEGF | 0.06 | 0.01 | 2.63 | 0.27 |
| PLL-HEP10/VEGF&PLL-HEP10/BMP-2 | 3.03 | 0.47 | 0.01 | 0.00 |
| PLL-HEP20/VEGF + BMP-2 | 1.96 | 0.28 | 1.49 | 0.29 |
| PLL-HEP20/VEGF | 1.70 | 0.28 | ||
| PLL-HEP20/BMP-2 | 2.59 | 0.43 | ||
| PLL-HEP20 | ||||
| Ti/VEGF + BMP-2 | 0.89 | 0.66 | 0.16 | 0.05 |
| Ti/VEGF | 0.27 | 0.02 | ||
| Ti/BMP-2 | 0.36 | 0.03 | ||
| Bone Area Trephine Defect vs. Bone–Implant Contact 4 Weeks | Bone Area Surface Layer vs. Bone–Implant Contact 4 Weeks | Bone Area Trephine Defect vs. Bone–Implant Contact 13 Weeks | Bone Area Surface Layer vs. Bone–Implant Contact 13 Weeks | Bone Area Surface Layer vs. CD31 Expression 4 Weeks | Bone Area Surface Layer vs. CD31 Expression 13 Weeks | |
|---|---|---|---|---|---|---|
| PLL-HEP10/BMP-2 and PLL-HEP10/VEGF | 0.266 | 0.329 | 0.397 | 0.266 | 0.544 | 0.397 |
| PLL-HEP10/VEGF andPLL-HEP10/BMP-2 | 0.787 | 0.787 | 0.005 | 0.019 | 0.042 | 0.019 |
| PLL-HEP20/VEGF +BMP-2 | 0.466 | 0.148 | 0.019 | 0.072 | 0.461 | 0.111 |
| PLL-HEP20/VEGF | 0.285 | 0.037 | 0.468 | 0.001 | 0.391 | 0.257 |
| PLL-HEP20/BMP-2 | 0.623 | 0.042 | 0.704 | 0.042 | 0.266 | 0.266 |
| PLL-HEP20 | 0.042 | 0.738 | 0.072 | 0.072 | 0.076 | 0.072 |
| Ti/VEGF + BMP-2 | 0.872 | 0.329 | 0.156 | 0.042 | 0.266 | 0.787 |
| Ti/VEGF | 0.329 | 0.019 | 0.544 | 0.019 | 0.957 | 0.957 |
| Ti/BMP-2 | 0.623 | 0.005 | 0.787 | 0.397 | 0.707 | 0.468 |
| Ti Control | 0.329 | 0.329 | 0.872 | 0.072 | 0.468 | 0.266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauffmann, P.; Wolfer, S.; Behrens, C.; Schlosser, P.; Dullin, C.; Schirmer, U.; Liefeith, K.; Schliephake, H. Effect of Sequential vs. Simultaneous Dual Growth Factor Release from Structured Heparin-Poly-Electrolyte Multilayer Coatings on Peri-Implant Bone Formation and Angiogenesis in Pig Mandibles. J. Funct. Biomater. 2025, 16, 67. https://doi.org/10.3390/jfb16020067
Kauffmann P, Wolfer S, Behrens C, Schlosser P, Dullin C, Schirmer U, Liefeith K, Schliephake H. Effect of Sequential vs. Simultaneous Dual Growth Factor Release from Structured Heparin-Poly-Electrolyte Multilayer Coatings on Peri-Implant Bone Formation and Angiogenesis in Pig Mandibles. Journal of Functional Biomaterials. 2025; 16(2):67. https://doi.org/10.3390/jfb16020067
Chicago/Turabian StyleKauffmann, Philipp, Susanne Wolfer, Christina Behrens, Pauline Schlosser, Christian Dullin, Uwe Schirmer, Klaus Liefeith, and Henning Schliephake. 2025. "Effect of Sequential vs. Simultaneous Dual Growth Factor Release from Structured Heparin-Poly-Electrolyte Multilayer Coatings on Peri-Implant Bone Formation and Angiogenesis in Pig Mandibles" Journal of Functional Biomaterials 16, no. 2: 67. https://doi.org/10.3390/jfb16020067
APA StyleKauffmann, P., Wolfer, S., Behrens, C., Schlosser, P., Dullin, C., Schirmer, U., Liefeith, K., & Schliephake, H. (2025). Effect of Sequential vs. Simultaneous Dual Growth Factor Release from Structured Heparin-Poly-Electrolyte Multilayer Coatings on Peri-Implant Bone Formation and Angiogenesis in Pig Mandibles. Journal of Functional Biomaterials, 16(2), 67. https://doi.org/10.3390/jfb16020067








