1. Introduction
Though largely preventable, oral diseases remain a significant global health burden, impacting individual and public health, quality of life, and economies [
1]. Additionally, poor oral health is one of the main causes of the loss of healthy longevity in older people [
2]. Considering that many developed countries are experiencing rapidly aging populations, actions aimed at achieving a high healthy age should become key initiatives [
3].
Dental composites have become integral to modern restorative dentistry due to their esthetic advantages and good mechanical properties [
4]. Nevertheless, the longevity and durability of these materials remain subjects of ongoing research and clinical concern [
5,
6]. Dental composites in the oral environment are exposed to both chemical and mechanical degradation processes, with hydrolysis playing a crucial role in this mechanism. A key phenomenon occurring in dental restorations is the absorption of water and other substances from the surrounding environment, resulting in the plasticization, swelling, and softening of the polymer matrix [
7]. The aqueous environment can cause oxidation and hydrolysis of the key components of dental composites. Siloxane bonds between the filler and the coupling agent, and ester bonds between the filler and the polymer matrix or those present within the polymer matrix are particularly susceptible to degradation [
8]. Hydrolysis can be catalyzed by enzymes produced by bacteria present in the oral cavity. Accelerated degradation of dental composites has also been observed in high alkaline (pH 13.0) or very low (pH < 2.0) acidic environments, due to the increased presence and interactions of hydroxide (OH
-) and hydrogen (H
+) ions [
9]. Emerging research directions focused on enhancing the longevity of dental restorations explore the application of novel, often complex technologies, such as modifications to the polymer matrix (e.g., click chemistry), advancements in fillers (e.g., bactericidal composite fillers), and the optimization of coupling agents [
10]. Furthermore, another line of research is focused on the development of self-healing materials [
11].
Dental composites are continuously exposed to the challenging conditions of the oral environment, including fluctuations in temperature, pH, and mechanical stresses [
12,
13,
14]. Therefore, it is imperative to develop standardized methods to predict their long-term performance in vitro accurately. Another indisputable value of such standardization is predicting how significantly the properties of the tested materials will deteriorate.
Artificial aging protocols are essential tools in many industries for simulating the long-term effects of usage environment on products [
15]. In dentistry, there is still a lack of consensus on the most representative in vitro aging protocols [
16]. This absence of standardization impedes the ability to accurately compare different materials’ performance and confidently predict their clinical longevity. Previous research [
17,
18], and this study, aim to address this critical gap. Considering that the degradation of dental composites occurs via hydrolysis processes, a suitable medium for in vitro aging should be a solution characterized by elevated hydroxyl ion from, i.e., sodium hydroxide (NaOH) [
9,
19]. Thermal fluctuations (approximately 20 to 50 times daily [
20]) in the oral environment should also be considered. The findings from a previous study [
17,
18] led to the selection of an aging protocol that involved thermocycles and exposure to a harsh environment inducing accelerated hydrolytic degradation (0.1 M NaOH solution). The repeated heating and cooling cycles during thermocycling caused increased flexibility and swelling of the polymer structure, making it more susceptible to penetration by a corrosive aging agent. Thermocycles were replaced with water aging at higher temperatures (55 °C) to streamline this method. These complex aging protocols aim to replicate extended periods of material exposure in the oral cavity, potentially spanning several years, offering valuable insights into the anticipated clinical performance in laboratory settings.
In the final stage of efforts to contribute to the foundation for standardized aging protocols, twelve commercially available resin-based composites were selected, representing various types of dental materials. The null hypothesis posits that the properties of the tested materials will remain stable following in vitro aging.
2. Materials and Methods
Three different artificial aging protocols were selected for this study based on previous research [
17,
18]. The primary aging factor is a 0.1 M NaOH solution, which, due to its high concentration of hydroxyl ions, serves as an effective agent for accelerating hydrolysis. Two aging protocols include more complex procedures, where thermocycling or aging at elevated temperatures precedes exposure to the degrading medium. These preliminary steps are designed to enhance the flexibility and swelling of the polymer structure, potentially amplifying the material’s response to hydrolytic degradation. A detailed description of chosen aging methods is shown in
Table 1.
Twelve various resin composites were used to evaluate the proposed aging protocols. The composition and additional information about the selected materials are presented in
Table 2.
The materials were irradiated during sample preparation according to the manufacturer’s recommendations (
Table 2) using an LED lamp (The Cure—TC—01, Spring Health Products, Norristown, PA, USA). The lamp’s power was verified with a curing light meter (Light Meter 200, Jovident System, Eindhoven, The Netherlands) and measured to be over 1250 mW/cm
2.
The effect of the selected aging protocols was evaluated by examining flexural strength (FS), diametral tensile strength (DTS), Vickers hardness (HV), fracture toughness (FT), and microstructure analysis.
2.1. Flexural Strength and Modulus
Flexural strength (FS) was assessed using the three-point bending test in accordance with the ISO 4049:2019 standard [
22]. The flexural modulus (FM) was determined by the software using the stress–strain curve within the strain range of 0.1% to 0.5%. Seven rectangular samples (2 mm × 2 mm × 25 mm) were prepared per group. A universal testing machine (Z020, Zwick–Roell, Ulm, Germany) was used for the tests.
2.2. Diametral Tensile Strength
For the diametral tensile strength (DTS) tests, nine cylindrical samples per group were used with a diameter (
d) of 6 mm and a height (
h) of 3 mm. The tests were conducted based on the American Dental Association Specification No. 27 [
23]. The test was conducted with 2 mm/min crosshead speed using a universal testing machine (Z020, Zwick–Roell, Ulm, Germany). The
DTS value based on the maximum force applied (
F) was calculated using Formula (1).
2.3. Vickers Hardness
To evaluate hardness, the Vickers method was employed using a Zwick hardness tester (ZHVµm, Zwick–Roell, Ulm, Germany). The measurement load was 1000 g for 10 s. For each experimental group, three randomly selected DTS samples were used, with three measurements taken on each sample, resulting in a total of nine measurements per group.
2.4. Fracture Toughness
Six notched rectangular specimens (2 mm × 4 mm × 20 mm) per group were prepared in a metal mold. At the midpoint of the mold, a precisely fabricated slot was utilized to insert a blade, thereby creating a sharp central notch in the sample. Samples were subjected to a three-point bending test in a universal testing machine (Zwick Roell Z005, Ulm, Germany) with 1 mm/min crosshead speed.
The fracture toughness (
FT) was calculated using Formula (2):
where
P is the peak load at fracture;
S is the span (14 mm);
B is the specimen thickness;
W is the specimen width;
a is notch length; and
is the function based on formula:
[
24].
2.5. Water Absorption
Water absorption was determined using five cylindrical samples (15 mm in diameter and 1 mm in height) per dental composite. The samples were cured in nine partially overlapping zones as described in ISO 4049 in sorption/solubility tests [
22]. The weight measurements were performed (AS 160/C/2, Radwag, Radom, Poland) immediately after preparation and after 28 consecutive days (4 weeks). According to Formula (3) the absorbency was calculated:
A—water absorption,
m0—the initial mass of the sample,
mi—the mass of the sample after storage in water for a specified (i) period of time.
2.6. Microstructure Evaluation
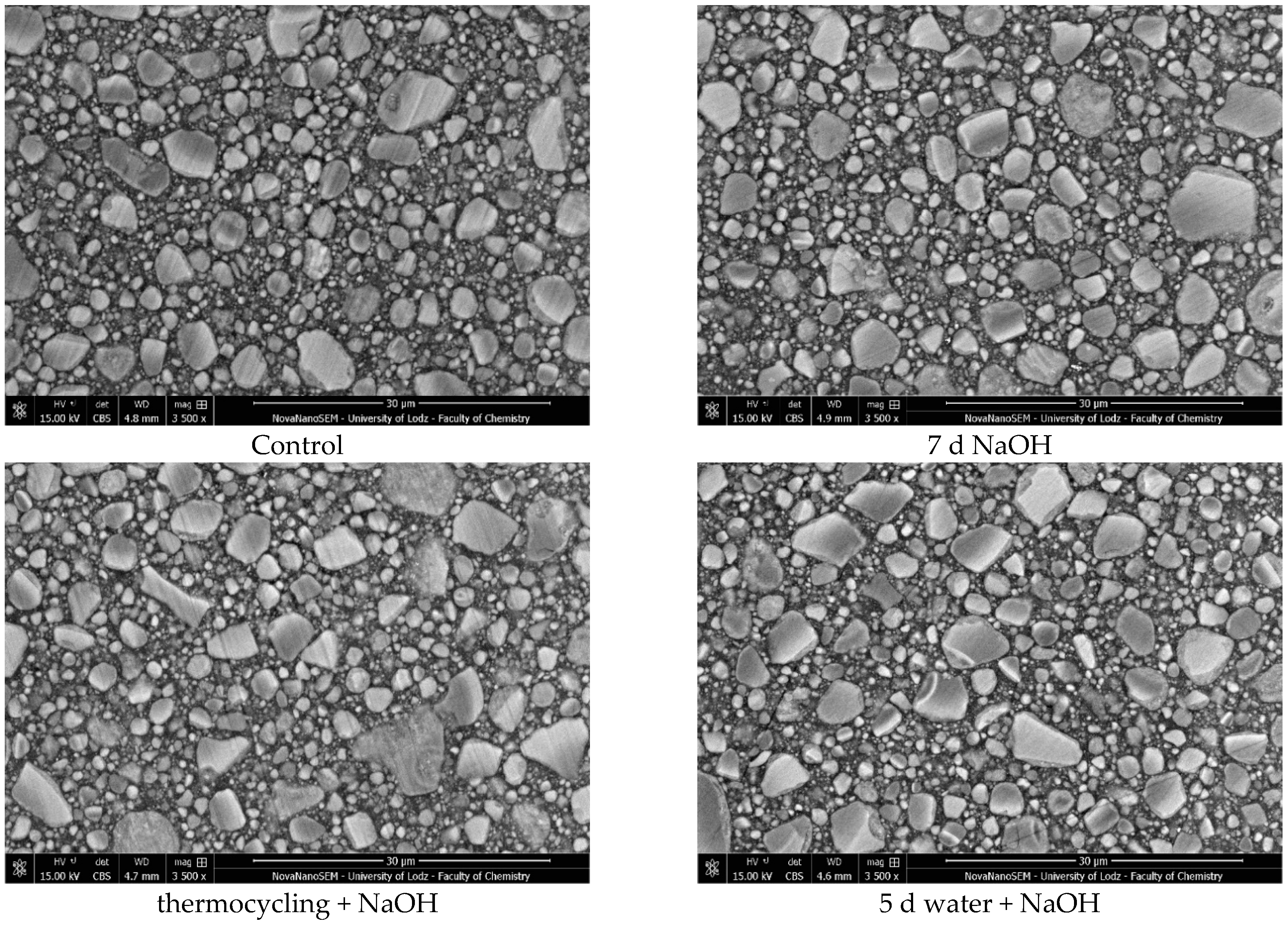
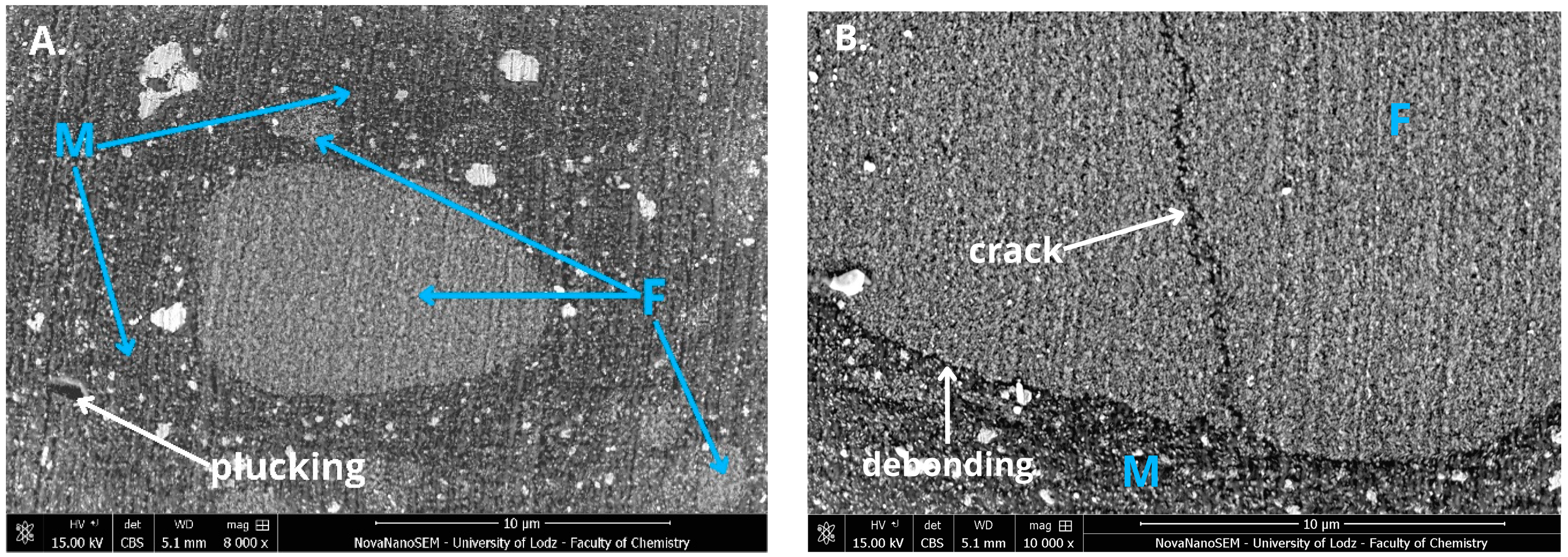
A high-resolution scanning electron microscope (HR-SEM) (FEI Nova NanoSEM 450, FEI, Hillsboro, OR, USA) equipped with a high-sensitivity circular backscatter (CBS) detector was used for a microstructure evaluation of selected samples. Samples were polished and coated with a 10 nm layer of gold (Q15OT ES, Quorum Technologies, Laughton, UK; sputter conditions: material—gold; coating thickness –10 nm; sputter current—40 mA; tooling factor—2.70) before analysis.
2.7. Statistical Analysis
Data were analyzed using Statistica version 13 software (StatSoft, Kraków, Poland). The Shapiro–Wilk test was employed to assess the normal distribution of the variables. The data were appropriately analyzed with a significance level set at p = 0.05, using the appropriate parametric (ANOVA with a post hoc test (Fisher’s least significant difference)) or non-parametric (Kruskal–Wallis test with multiple comparisons of mean ranks) tests. Additionally, Spearman’s rank order correlations between analyzed research methods were determined.
4. Discussion
Studies conducted in vitro should provide essential information to accurately assess the material’s suitability under its intended conditions of use. The lack of a standardized aging protocol complicates the evaluation of long-term properties of dental composites under laboratory conditions [
16]. This study aimed to assess the impact of various aging protocols on selected dental composites’ mechanical properties, which may contribute to developing a unified method for determining their durability in vitro.
Performing in vitro aging significantly reduced the mechanical properties of most of the materials (
Table 2,
Table 3 and
Table 4,
Figure 2 and
Figure 3), allowing for the rejection of the stated null hypothesis. Our studies are consistent with other research that showed mechanical properties decrease after artificial aging [
19,
25,
26,
27]. Unfortunately, due to the lack of standardized aging methods, accurate comparison of individual studies is challenging. However, the observed changes arise from processes triggered by the exposure of materials to a harsh environment.
Dental composites used for lost tooth tissue restorations are exposed in the oral environment to agents that contribute to their chemical degradation [
28]. A primary mechanism is diffusion, wherein water molecules are absorbed by the polymer material, occupying microvoids and free spaces within the polymer matrix. This process causes the plasticization and swelling of the material, with residual monomer particles potentially leaching out and creating additional voids that can further absorb the surrounding medium [
7,
28,
29]. The consequences of diffusion are not only physical—such as plasticization, softening, and swelling of the polymer matrix—but also chemical, as exposure to the aqueous environment leads to the hydrolytic degradation of key bonds in the resin composite, including ester, urethane, amide, and siloxane bonds [
30,
31]. Although degradation in pure water occurs relatively slowly (depending on time and temperature) [
32], it is important to recognize that factors in the oral environment, such as enzymes, temperature fluctuations, chemical substances, and variable pH levels, can accelerate these processes [
33].
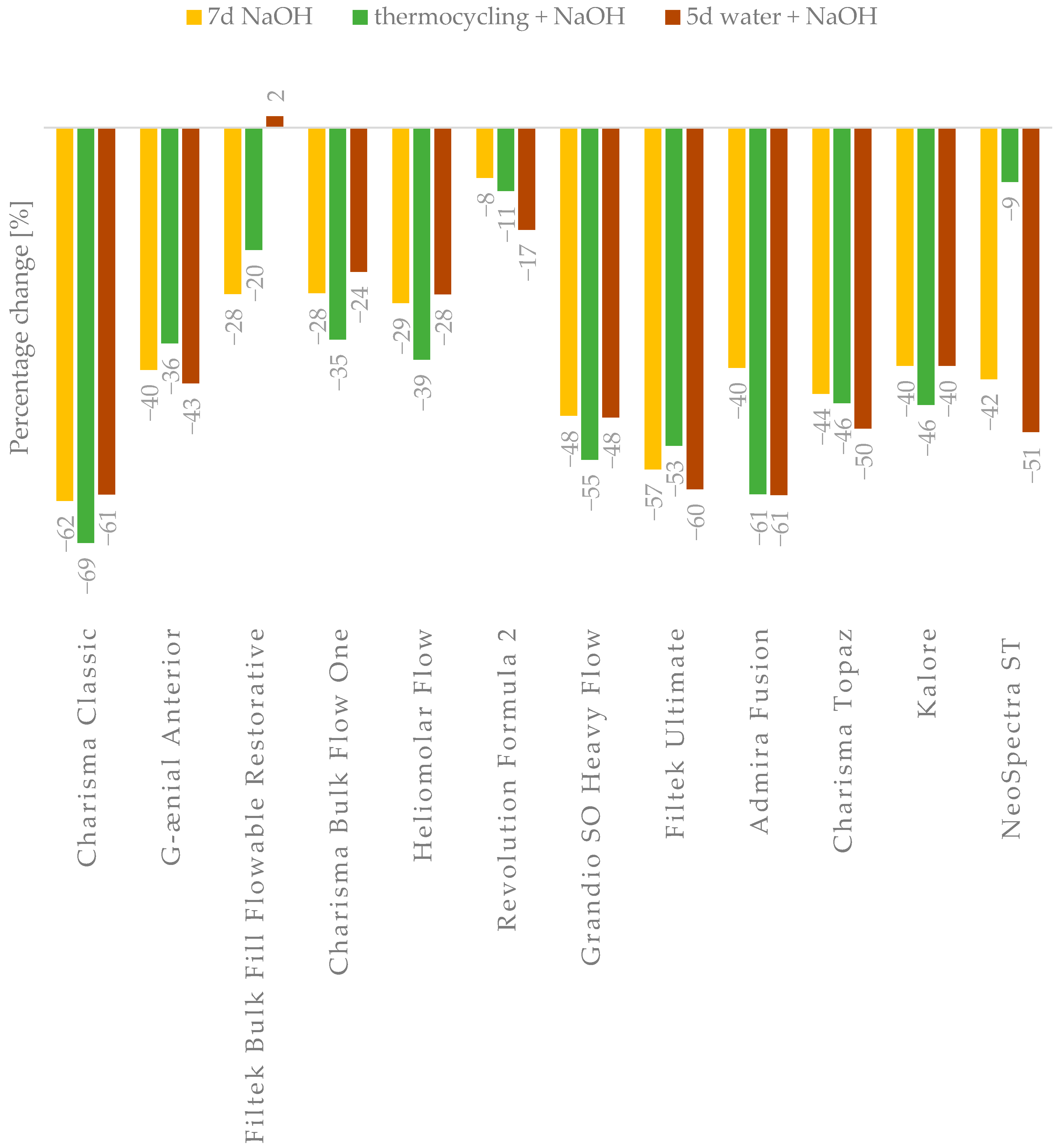
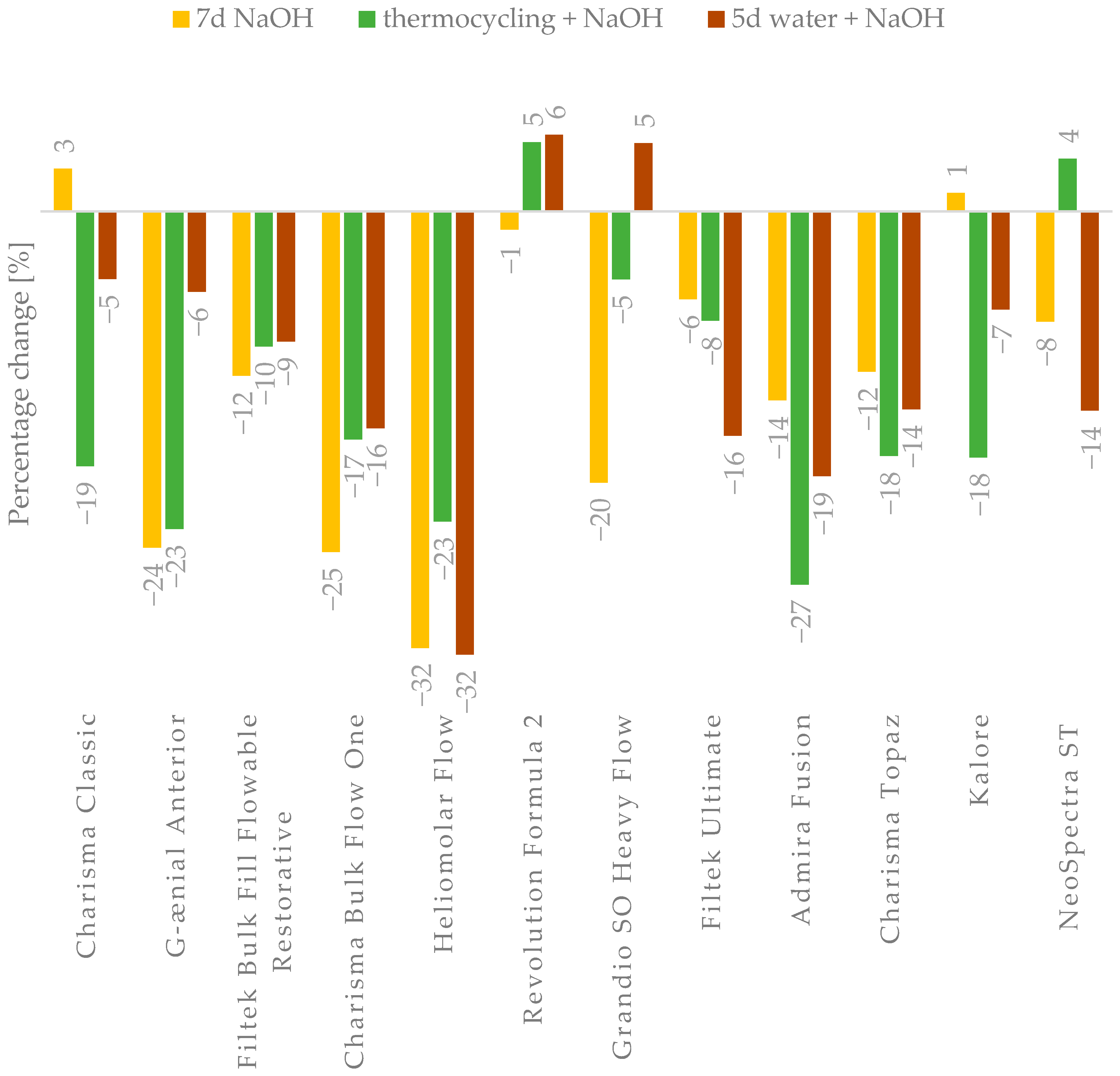
In general, aging processes affecting composite materials ultimately reduce their strength properties. However, the obtained results indicate that not all materials experience the same degree of property degradation (
Figure 4,
Figure A3,
Figure A4,
Figure A5,
Figure A6 and
Figure A7).
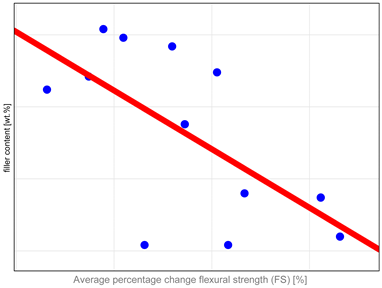
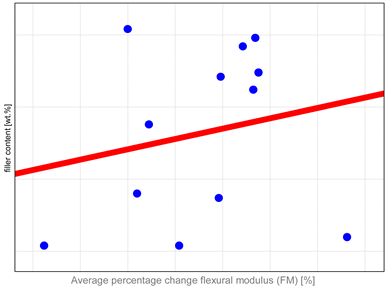
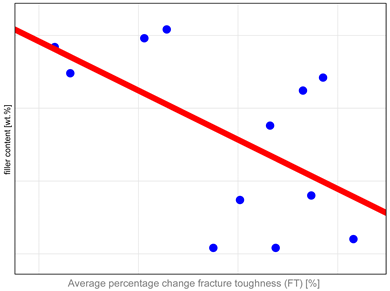
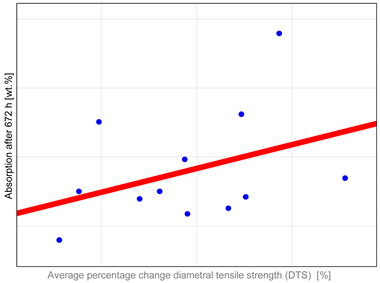
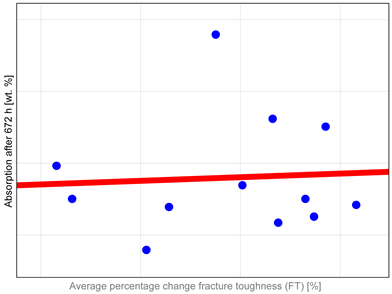
The study investigated whether these changes correlate with key factors influencing material properties: composition and water sorption. Unfortunately, manufacturers do not provide detailed compositional information, with filler content as the only quantitative data available. It has been shown that mechanical properties such as hardness, flexural strength, and fracture toughness depend both on the amount and type of filler. High filler content, homogeneous particle dispersion, nanofillers’ inclusion, and the filler–matrix interface’s good quality contribute to improved mechanical properties [
10,
34,
35,
36,
37]. Correlation analysis revealed (
Table A1) that mechanical properties, particularly DTS and FS, tend to undergo more significant changes in materials with higher filler content. The authors identified only one study in the literature that compared changes after aging to the amount of organic content in resin composites. In that study [
38], no similar correlations were observed. However, the aging protocol involved the use of ethanol as the medium, with observed changes compared to samples stored in water for an equivalent period, rather than to control samples as in the present research. The results revealed that materials with higher filler content demonstrate increased resistance to degradation. However, the study also highlighted the significant impact of coupling agent content on material stability, with higher amounts adversely affecting long-term performance [
38]. It is essential to emphasize that increasing the filler content beyond 60% by volume has no measurable impact on enhancing the materials’ mechanical properties [
39]. This observation aligns with our findings showing that materials with exceptionally high filler content, such as Grandio, Filtek Ultimate, Admira Fusion, and Kalore, tend to exhibit reduced stability after aging. A dual mechanism can explain the observed behavior. First, the high filler content increases the probability of defects forming within the composite material, compromising its structural integrity [
39]. Second, the large interface surface area in such materials, especially those used in nanotechnology, is considered a critical weak point, as reported in the literature. It was shown that exposure to hydroxyl ion-rich solutions, such as 0.1N NaOH, accelerates the hydrolysis of siloxane bonds and filler dissolution. Degradation of this interfacial layer results in the deboning of fillers and rinsing them out, compromising material integrity [
9,
40,
41]. In addition, this process creates new pathways for medium infiltration, further accelerating the deterioration of the material’s performance [
30,
42]. The selection of an appropriate combination of filler systems and coupling agents presents a significant challenge to the development of new dental materials. Some materials exhibit suboptimal hydrolytic resistance despite technological advancements, as observed in products like Admira Fusion and Kalore. This highlights the need for innovative approaches to improve the stability and durability of composite formulations under an aggressive oral environment.
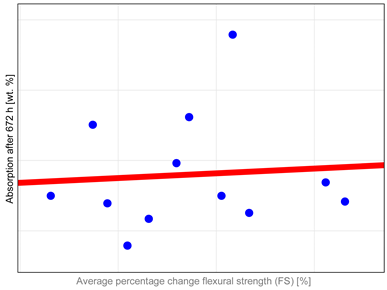
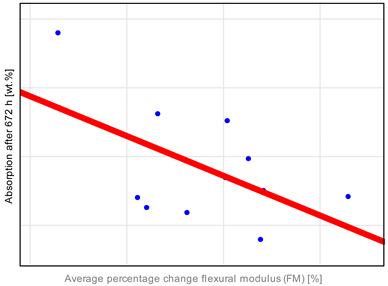
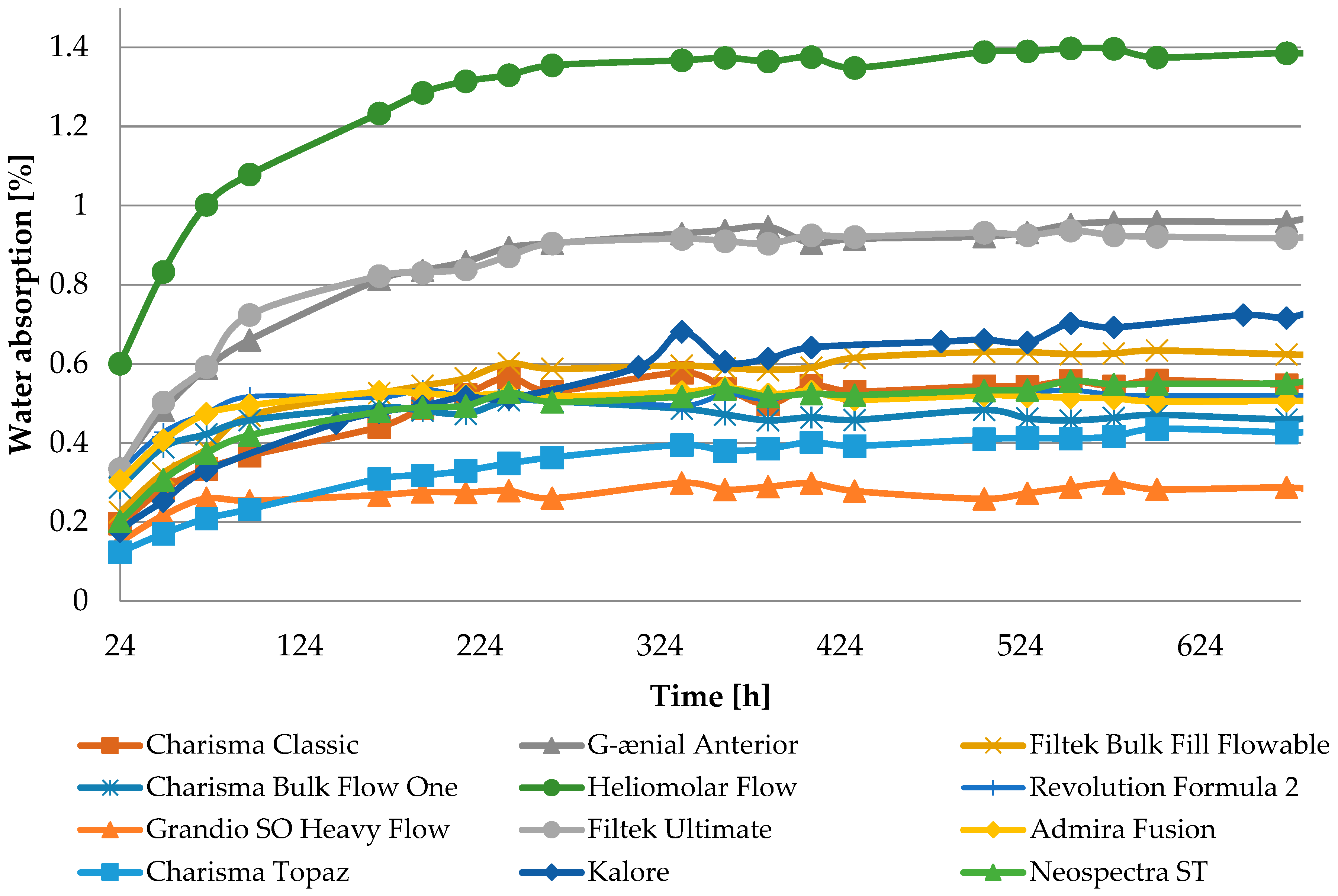
The observed properties changes do not correlate with water absorption (
Table A1). Heliomolar Flow, for example, exhibited the highest water sorption but did not demonstrate the most severe reduction in tested properties. This may be explained by increased material flexibility, which could enhance strength by allowing some plastic deformation during testing. In particular, DTS values may be inflated, as this test is intended for materials that remain brittle and do not undergo plastic deformation under load [
43]. A reduction in the modulus of elasticity was observed for most materials (
Table 4,
Figure A4). The most significant changes were noted for Heliomolar Flow, which may explain the smaller reductions in DTS and FS values after aging, likely due to some degree of material plasticization. Nevertheless, the significant drop in hardness following aging strongly suggests that the material undergoes degradation. Reduced surface hardness by material degradation can lead to increased abrasive wear, which may have adverse clinical implications by compromising the longevity and functionality of the restoration [
44,
45].
The Grandio SO HF material exhibits the lowest sorption values, likely due to its high filler content exceeding 80 wt.%, compared to 60 wt.% in Revolution Formula 2, despite their similar compositions [
46,
47]. However, considering overall stability, from our study objects, Kerr’s materials demonstrate superior performance, whereas Voco’s materials are less stable. It is most likely that in this material, the choice of filler and the quality of the filler–matrix interface were the issues. While Grandio SO HF shows lower sorption values, its significant hydrolytic instability is likely due to the high content of glass fillers. Unfortunately, no information is available on the type of coupling agent used, which also plays a key role. As discussed earlier, high filler content may result in worse resistance to hydrolysis due to the presence of internal flaws and a large interface that is susceptible to hydrolysis. Research also indicates that dental fillers can be leached out from the material, especially some glass fillers [
26]. Degradation of the filler–matrix interface compromises material integrity, and further debonding processes may result in filler particle leaching. This process creates voids in the composite material, allowing bacterial infiltration, altering the local pH, and accelerating composite hydrolysis during clinical use [
9,
40,
48].
Regarding flexural strength, most materials initially meet the requirements of ISO 4049:2019 (FS > 80 MPa) for composites intended for occlusal use [
22], with exceptions observed for Admira Fusion and G-ænial Anterior. In a previous paper [
18], there was a proposition that materials should achieve post-accelerated aging flexural strength of 32–48 MPa. The suggested range of limit values was based on the average masticatory forces (8–12 MPa) and the ISO 4049 standard, which require the flexural strength (FS) to exceed 80 MPa—approximately ten times the average chewing forces. In addition, an estimated fatigue strength threshold of approximately 40% of the FS after aging was considered. The material Admira Fusion falls below these proposed thresholds, and traditional, highly filled composites (e.g., Charisma Classic and Geanial Anterior) are near the lower limit. The significant percentage drop in FS before and after aging for Charisma Classic and Admira Fusion suggests that the applied aging methods particularly affected these two materials. Notably, materials of the Bulk Fill and Flow types displayed the least degradation and relatively high FS values post-aging. Admira Fusion is an innovative material utilizing ORMOCER
® resin (Pure Silicate Technology) as its matrix. Despite initially promising results in preliminary studies [
49], it has demonstrated reduced aging resistance [
50,
51]. This may be attributed to the low degree of conversion caused by the steric hindrance of the highly functionalized ORMOCER
® molecule [
52].
Diametral tensile strength (DTS), while commonly employed in studies of dental materials, raises concerns regarding the accuracy of its results. The stress–strain curves for most materials reveal certain plastic deformations, especially after aging, suggesting that modern composite materials do not exhibit the typical brittleness associated with traditional materials. Consequently, DTS values may be overestimated, leading to potential misinterpretations of the data. Selected sample strain–stress curves observed during DTS tests are presented in
Figure A8. This issue is particularly relevant when evaluating innovative materials and studies incorporating aging processes, which tend to plasticize the polymer matrix. Therefore, we recommend closely examining stress–strain curves and sample fractures, not just the DTS values themselves to assess the influence of plastic deformations on the results accurately.
Higher fracture toughness (FT) values indicate better clinical performance. It measures a material’s ability to resist crack propagation under stress, which may occur in materials due to exposure to cyclic loading, temperature fluctuations, and chemical challenges [
53]. Considering typical values in dentistry, enamel ranges from 0.7 to 1.3 MPa√m and dentin 1.7 to 3 MPa√m [
54], while composite materials generally show FT values of 1 to approximately 2 MPa√m [
55]. Therefore, if composites remain stable and their FT values exceed 1 MPa√m after aging, they are likely capable of withstanding loads encountered in the oral cavity. Conversely, lower FT values suggest a higher likelihood of material failure under stress. The obtained results confirm this hypothesis. The most significant differences in FT were observed for materials such as Grandio, Admira, Kalore, and Neospectra. These differences may be attributed to inadequate integration between the high filler content and the resin matrix. Fracture toughness is a particularly sensitive indicator of defects, making it an effective method for evaluating material performance and stability. Moreover, this parameter provides insights into the quality of the filler–matrix interface and the material’s resistance to defects, making it a vital criterion for assessing the long-term performance of composite materials in restorative dentistry [
55,
56,
57].
Given the complexity of the oral environment, accurately replicating all its variables is impossible. Therefore, a frequently used approach for in vitro aging involves the application of media such as ethanol or NaOH, which accelerate hydrolytic degradation processes affecting the polymer matrix, the coupling agent, and fillers, especially the glass-based ones [
9,
40,
58,
59]. Our findings demonstrate that using such media significantly impacts the mechanical properties of composites and can serve as a valuable method for in vitro studies to assess the hydrolytic stability of resin-based composite materials [
60,
61]. Additionally, thermocycling incorporation is an important aspect of aging studies. Temperature fluctuations during thermocycling can have a significant impact on the behavior of composite restorations. Thermal loads in the oral cavity arise from exposure to varying temperatures, such as hot or cold foods and beverages, creating thermal fatigue in restorative materials under moist conditions. Heat transfer occurs through convection, resulting in thermal boundary layers and uneven temperature distributions, causing thermal stresses due to differing thermal expansion coefficients or non-uniform material properties. In multiphase materials like composites, filler particles expand differently, further inducing internal stresses, especially at the matrix–filler interface. Those processes lead to the deterioration of the internal materials’ integrity. Cyclic temperature changes primarily affect the surface layer, where stress gradients are highest, increasing the risk of degradation. A reliable indicator of ongoing changes could be surface microhardness [
62,
63].
Drawing on the findings of this study and previous research [
17,
18], the application of aggressive media, such as NaOH, has proven effective in creating a consistent framework for evaluating the long-term stability of dental composites under in vitro conditions. This approach enables the observation of changes in mechanical properties resulting from the degradation of individual material components. When developing new composites, it is recommended that researchers conduct accelerated aging tests to identify the most durable ingredients and optimal formulations. Additionally, employing thermocycling during aging protocols can reveal interfacial issues between the matrix and filler that might remain undetected using simpler protocols. To provide a comprehensive evaluation of the material’s mechanical performance fracture, toughness testing should also be incorporated. It is worth emphasizing that results from diametral tensile strength (DTS) tests should be interpreted critically, as some plastic deformations can be induced during the test, potentially skewing the outcomes and limiting their reliability.
The findings highlight challenges associated with highly filled materials, which are currently widely promoted in the market. A particularly sensitive aspect is the matrix–filler interface, which significantly influences the material’s long-term performance. Notably, there is a scarcity of foundational studies addressing the behavior of new, proprietary products introduced by dental companies, underscoring the need for further research to evaluate their properties comprehensively.