Modulated-Diameter Zirconia Nanotubes for Controlled Drug Release—Bye to the Burst
Abstract
1. Introduction
2. Materials and Methods
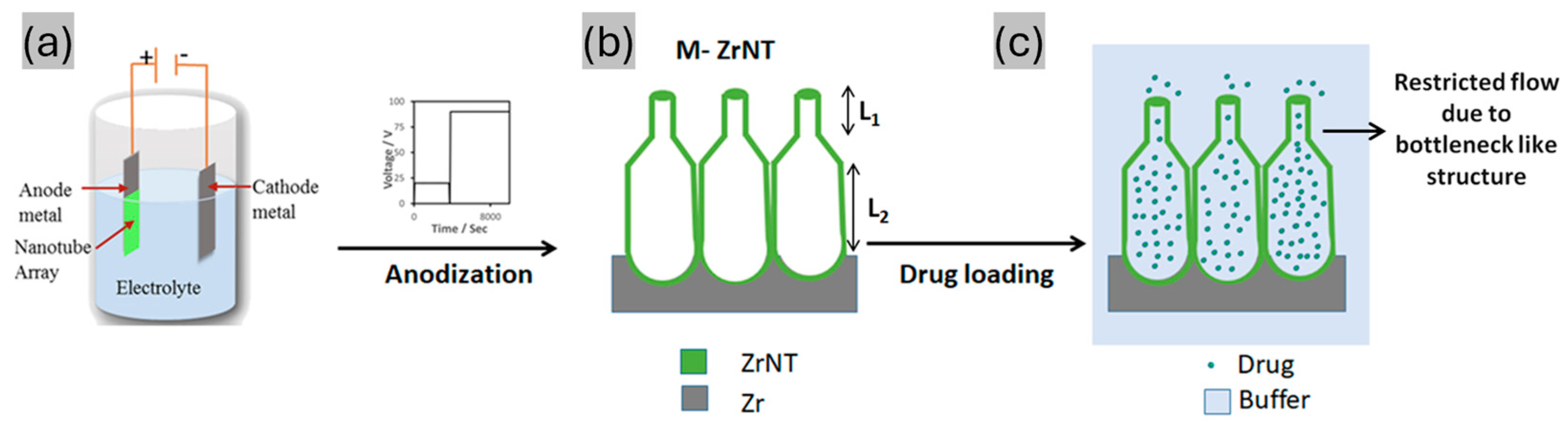
2.1. Fabrication of Zirconia Nanotubes (ZrNTs)
2.2. Drug Loading and Release
2.3. Chemical Characterization of Drug-Loaded ZrNTs
3. Results
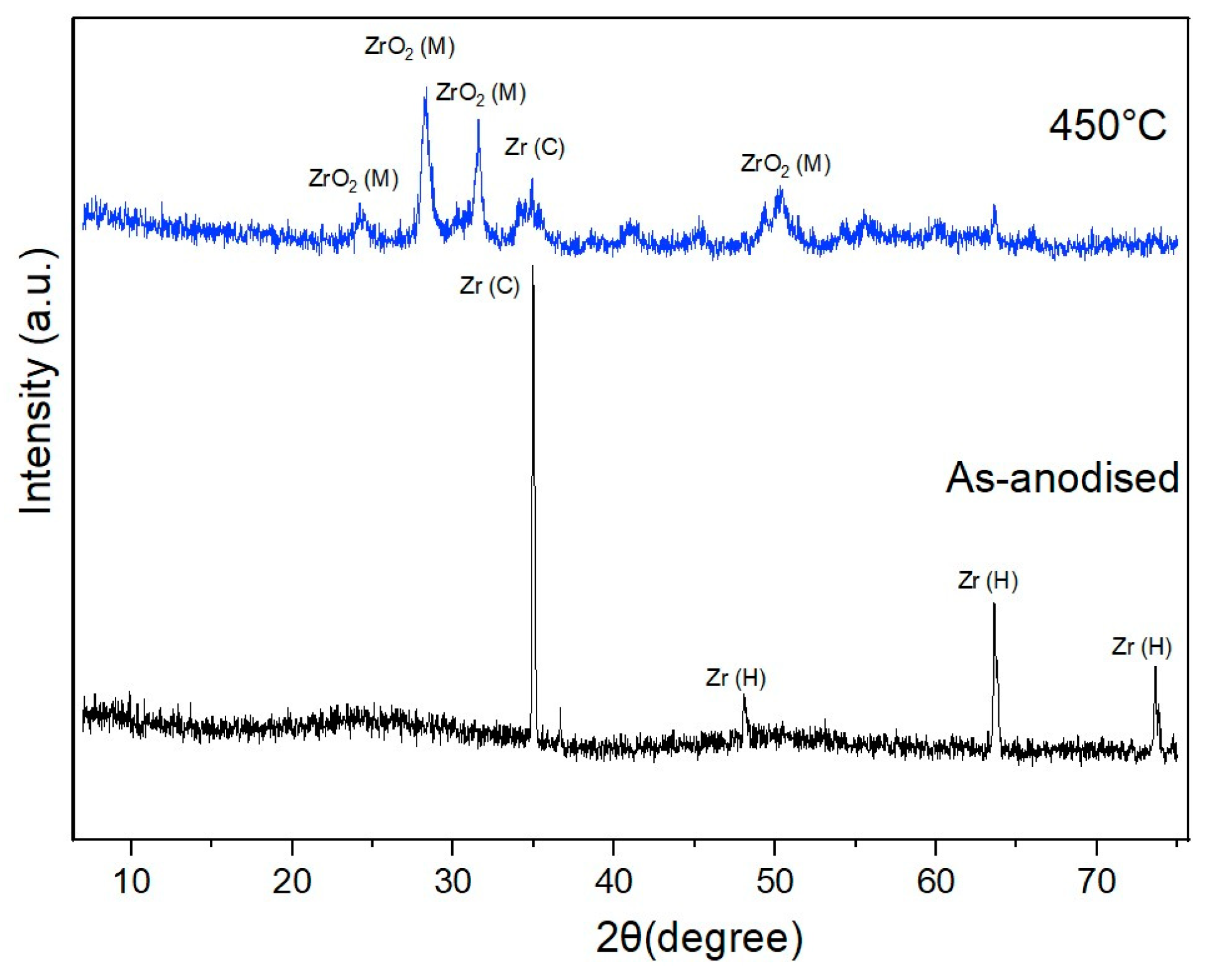
3.1. Fabrication and Characterization of ZrNT
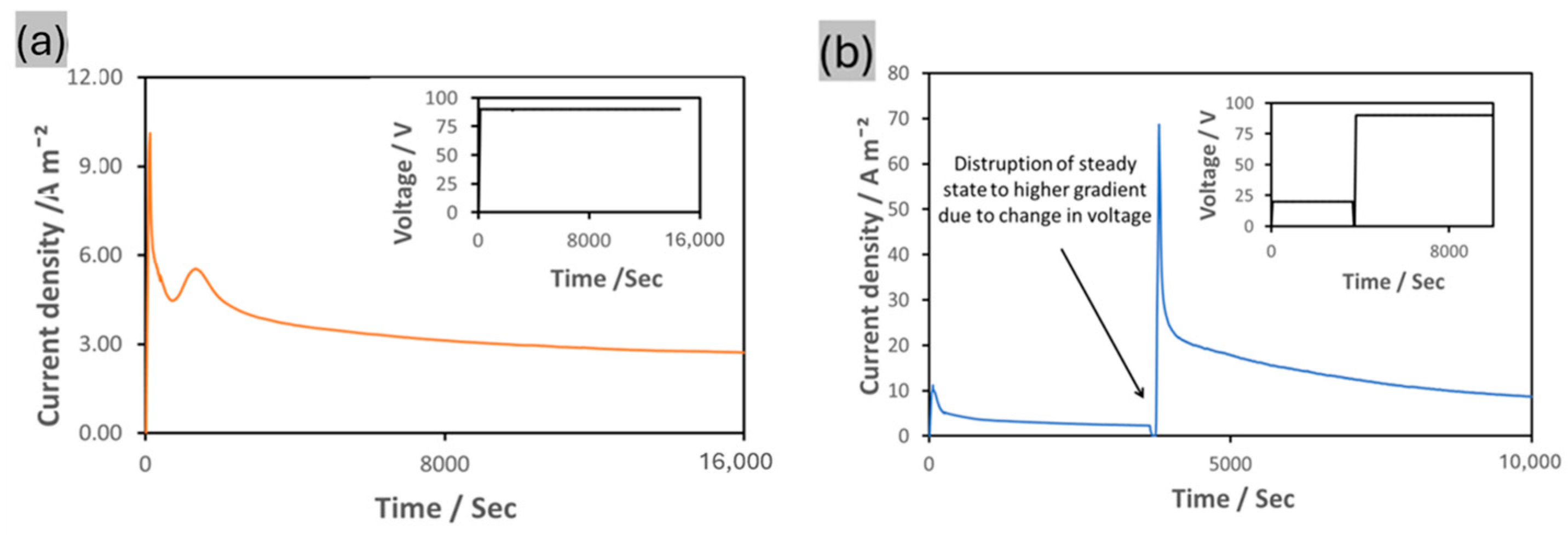
3.2. Drug Loading and Release Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. The Origins and Evolution of “Controlled” Drug Delivery Systems. J. Control. Release 2008, 132, 153–163. [Google Scholar] [CrossRef]
- Folkman, J.; Long, D.M. The Use of Silicone Rubber as a Carrier for Prolonged Drug Therapy. J. Surg. Res. 1964, 4, 139–142. [Google Scholar] [CrossRef]
- Sinn Aw, M.; Kurian, M.; Losic, D. Non-Eroding Drug-Releasing Implants with Ordered Nanoporous and Nanotubular Structures: Concepts for Controlling Drug Release. Biomater. Sci. 2014, 2, 10–34. [Google Scholar] [CrossRef]
- Pioletti, D.; Gauthier, O.; Stadelmann, V.; Bujoli, B.; Guicheux, J.; Zambelli, P.-Y.; Bouler, J.-M. Orthopedic Implant Used as Drug Delivery System: Clinical Situation and State of the Research. Curr. Drug Deliv. 2008, 5, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Zafaroni, A. Bandage for Administering Drugs. U.S. Patent 3,598,123, 10 August 1971. Available online: https://patents.google.com/patent/US3598123A/en (accessed on 10 January 2025).
- Felix Theeuwes, Los Altos, Calif, Takeru Higuchi, Lawrence, Kans. Osmatic dispensing device for release of beneficial agent. U.S. Patent 19,259,469, 5 November 1974.
- Vainionpää, S.; Rokkanen, P.; Törmälä, P. Surgical Applications of Biodegradable Polymers in Human Tissues. Prog. Polym. Sci. 1989, 14, 679–716. [Google Scholar] [CrossRef]
- Vakamulla Raghu, S.N.; Chuluunbandi, K.; Killian, M.S. Zirconia Nanotube Coatings-UV-Resistant Superhydrophobic Surfaces. Surf. Interfaces 2021, 26, 101357. [Google Scholar] [CrossRef]
- Vakamulla Raghu, S.N.; Killian, M.S. Wetting Behavior of Zirconia Nanotubes. RSC Adv. 2021, 11, 29585–29589. [Google Scholar] [CrossRef]
- Losic, D.; Aw, M.S.; Santos, A.; Gulati, K.; Bariana, M. Titania Nanotube Arrays for Local Drug Delivery: Recent Advances and Perspectives. Expert Opin. Drug Deliv. 2015, 12, 103–127. [Google Scholar] [CrossRef]
- Gulati, K.; Aw, M.S.; Findlay, D.; Losic, D. Local Drug Delivery to the Bone by Drug-Releasing Implants: Perspectives of Nano-Engineered Titania Nanotube Arrays. Ther. Deliv. 2012, 3, 857–873. [Google Scholar] [CrossRef]
- Ganguly, D.; Shahbazian-yassar, R.; Shokuhfar, T. Recent Advances in Nanotubes for Orthopedic Implants. Jnsm 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased Osteoblast Adhesion on Nanophase Metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef]
- Martin, C.R.; Kohli, P. The Emerging Field of Nanotube Biotechnology. Nat. Rev. Drug Discov. 2003, 2, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Raghu, S.N.V.; Onyenso, G.; Mohajernia, S.; Killian, M.S. Functionalization Strategies to Facilitate Multi-Depth, Multi-Molecule Modifications of Nanostructured Oxides for Triggered Release Applications. Surf. Sci. 2022, 719, 122024. [Google Scholar] [CrossRef]
- Raghu, S.N.V.; Hartwich, P.; Patalas, A.; Marczewski, M.; Talar, R.; Pritzel, C.; Killian, M.S. Nanodentistry Aspects Explored towards Nanostructured ZrO2: Immobilizing Zirconium-Oxide Nanotube Coatings onto Zirconia Ceramic Implant Surfaces. Open Ceram. 2023, 14, 100340. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Patel, R. Infection Associated with Prosthetic Joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Barth, E.; Myrvik, Q.M.; Wagner, W.; Gristina, A.G. In Vitro and in Vivo Comparative Colonization of Staphylococcus Aureus and Staphylococcus Epidermidis on Orthopaedic Implant Materials. Biomaterials 1989, 10, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.F.; Schlegel, U.; Perren, S.; Richards, R.G. Infection in Fracture Fixation: Can We Influence Infection Rates through Implant Design? J. Mater. Sci. Mater. Med. 2010, 21, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Simovic, S. Self-Ordered Nanopore and Nanotube Platforms for Drug Delivery Applications. Expert Opin. Drug Deliv. 2009, 6, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Christel, P.; Meunier, A.; Dorlot, J.-M.; Crolet, J.-M.; Witvoet, J.; Sedel, L.; Boutin, P. Biomechanical Compatibility and Design of Ceramic Implants for Orthopedic Surgery. Ann. N. Y. Acad. Sci. 1988, 523, 234–256. [Google Scholar] [CrossRef]
- Chevalier, J. What Future for Zirconia as a Biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Depprich, R.; Ommerborn, M.; Zipprich, H.; Naujoks, C.; Handschel, J.; Wiesmann, H.P.; Kübler, N.R.; Meyer, U. Behavior of Osteoblastic Cells Cultured on Titanium and Structured Zirconia Surfaces. Head Face Med. 2008, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G. Zirconia as a Ceramic Biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Apratim, A.; Eachempati, P.; Krishnappa Salian, K.; Singh, V.; Chhabra, S.; Shah, S. Zirconia in Dental Implantology: A Review. J. Int. Soc. Prev. Community Dent. 2015, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I. Yttrium-Partially Stabilized Zirconium Dioxide Posts: An Approach to Restoring Coronally Compromised Nonvital Teeth. Int. J. Periodontics Restor. Dent. 1998, 18, 454–465. [Google Scholar]
- Mehra, M.; Vahidi, F. Complete Mouth Implant Rehabilitation with a Zirconia Ceramic System: A Clinical Report. J. Prosthet. Dent. 2014, 112, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, D.; Lei, J.; He, J.; Wu, S.; Pan, F. Fast Fabrication of Highly Regular and Ordered ZrO2 Nanotubes. Mater. Lett. 2011, 65, 1434–1437. [Google Scholar] [CrossRef]
- Stępień, M.; Handzlik, P.; Fitzner, K. Synthesis of ZrO2 nanotubes in Inorganic and Organic Electrolytes by Anodic Oxidation of Zirconium. J. Solid State Electrochem. 2014, 18, 3081–3090. [Google Scholar] [CrossRef]
- Fang, D.; Yu, J.; Luo, Z.; Liu, S.; Huang, K.; Xu, W. Fabrication Parameter-Dependent Morphologies of Self-Organized ZrO2 Nanotubes during Anodization. J. Solid State Electrochem. 2012, 16, 1219–1228. [Google Scholar] [CrossRef]
- Gulati, K.; Kogawa, M.; Maher, S.; Atkins, G.; Findlay, D.; Losic, D. Titania Nanotubes for Local Drug Delivery from Implant Surfaces. In Electrochemically Engineered Nanoporous Materials: Methods, Properties and Applications; Springer International Publishing: Cham, Switzerland, 2015; Volume 220. [Google Scholar] [CrossRef]
- Gultepe, E.; Nagesha, D.; Casse, B.D.F.; Banyal, R.; Fitchorov, T.; Karma, A.; Amiji, M.; Sridhar, S. Sustained Drug Release from Non-Eroding Nanoporous Templates. Small 2010, 6, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.; Laitinen, L.; Kaukonen, A.M.; Tuura, J.; Björkqvist, M.; Heikkilä, T.; Vähä-Heikkilä, K.; Hirvonen, J.; Lehto, V.P. Mesoporous Silicon Microparticles for Oral Drug Delivery: Loading and Release of Five Model Drugs. J. Control. Release 2005, 108, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Celi, N.; Zhang, D.; Cai, J. Magnetic Biohybrid Microrobot Multimers Based on Chlorella Cells for Enhanced Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2022, 14, 6320–6330. [Google Scholar] [CrossRef]
- Tu, L.; Liao, Z.; Luo, Z.; Wu, Y.L.; Herrmann, A.; Huo, S. Ultrasound-Controlled Drug Release and Drug Activation for Cancer Therapy. Exploration 2021, 1, 20210023. [Google Scholar] [CrossRef]
- Çalişkan, N.; Bayram, C.; Erdal, E.; Karahaliloǧlu, Z.; Denkbaş, E.B. Titania Nanotubes with Adjustable Dimensions for Drug Reservoir Sites and Enhanced Cell Adhesion. Mater. Sci. Eng. C 2014, 35, 100–105. [Google Scholar] [CrossRef]
- Hamlekhan, A.; Sinha-Ray, S.; Takoudis, C.; Mathew, M.T.; Sukotjo, C.; Yarin, A.L.; Shokuhfar, T. Fabrication of Drug Eluting Implants: Study of Drug Release Mechanism from Titanium Dioxide Nanotubes. J. Phys. D Appl. Phys. 2015, 48, 275401. [Google Scholar] [CrossRef]
- Peng, L.; Mendelsohn, A.D.; LaTempa, T.J.; Yoriya, S.; Grimes, C.A.; Desai, T.A. Long-Term Small Molecule and Protein Elution from Tio2 Nanotubes. Nano Lett. 2009, 9, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Gultepe, E.; Nagesha, D.; Sridhar, S.; Amiji, M. Nanoporous Inorganic Membranes or Coatings for Sustained Drug Delivery in Implantable Devices. Adv. Drug Deliv. Rev. 2010, 62, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Kant, K.; Findlay, D.; Losic, D. Periodically Tailored Titania Nanotubes for Enhanced Drug Loading and Releasing Performances. J. Mater. Chem. B 2015, 3, 2553–2559. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Addai-Mensah, J.; Losic, D. Polymeric Micelles in Porous and Nanotubular Implants as a New System for Extended Delivery of Poorly Soluble Drugs. J. Mater. Chem. 2011, 21, 7082–7089. [Google Scholar] [CrossRef]
- Wang, T.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Controlled Release and Biocompatibility of Polymer/Titania Nanotube Array System on Titanium Implants. Bioact. Mater. 2017, 2, 44–50. [Google Scholar] [CrossRef]
- Gulati, K.; Ramakrishnan, S.; Aw, M.S.; Atkins, G.J.; Findlay, D.M.; Losic, D. Biocompatible Polymer Coating of Titania Nanotube Arrays for Improved Drug Elution and Osteoblast Adhesion. Acta Biomater. 2012, 8, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Van der Giessen, W.J.; Lincoff, A.M.; Schwartz, R.S.; Van Beusekom, H.M.M.; Serruys, P.W.; Holmes, D.R.; Ellis, S.G.; Topol, E.J. Marked Inflammatory Sequelae to Implantation of Biodegradable and Nonbiodegradable Polymers in Porcine Coronary Arteries. Circulation 1996, 94, 1690–1697. [Google Scholar] [CrossRef]
- Santos, A.; Sinn Aw, M.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-Releasing Implants: Current Progress, Challenges and Perspectives. J. Mater. Chem. B 2014, 2, 6157–6182. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Pittrof, A.; Killian, M.S.; Schmuki, P.; Von Der Mark, K. Synergistic Control of Mesenchymal Stem Cell Differentiation by Nanoscale Surface Geometry and Immobilized Growth Factors on TiO2 Nanotubes. Small 2012, 8, 98–107. [Google Scholar] [CrossRef]
- Wieneke, H.; Dirsch, O.; Sawitowski, T.; Gu, Y.L.; Brauer, H.; Dahmen, U.; Fischer, A.; Wnendt, S.; Erbel, R. Synergistic Effects of a Novel Nanoporous Stent Coating and Tacrolimus on Intima Proliferation in Rabbits. Catheter. Cardiovasc. Interv. 2003, 60, 399–407. [Google Scholar] [CrossRef]
- Albu, S.P.; Kim, D.; Schmuki, P. Growth of Aligned TiO2 Bamboo-Type Nanotubes and Highly Ordered Nanolace. Angew. Chem. 2008, 120, 1942–1945. [Google Scholar] [CrossRef]
- Lin, J.; Liu, K.; Chen, X. Synthesis of Periodically Structured Titania Nanotube Films and Their Potential for Photonic Applications. Small 2011, 7, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Ahmad, Z.A.; Berenov, A.; Lockman, Z. Effect of Applied Voltage and Fluoride Ion Content on the Formation of Zirconia Nanotube Arrays by Anodic Oxidation of Zirconium. Corros. Sci. 2011, 53, 1156–1164. [Google Scholar] [CrossRef]
- Riboni, F.; Nguyen, N.T.; So, S.; Schmuki, P. Aligned Metal Oxide Nanotube Arrays: Key-Aspects of Anodic TiO2 Nanotube Formation and Properties. Nanoscale Horiz. 2016, 1, 445–466. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Lillo, M. Porous Alumina with Shaped Pore Geometries and Complex Pore Architectures Fabricated by Cyclic Anodization. Small 2009, 5, 1392–1397. [Google Scholar] [CrossRef]
- Sultan, U.; Ahmadloo, F.; Cha, G.; Gökcan, B.; Hejazi, S.; Yoo, J.E.; Nguyen, N.T.; Altomare, M.; Schmuki, P.; Killian, M.S. A High-Field Anodic NiO Nanosponge with Tunable Thickness for Application in p-Type Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2020, 3, 7865–7872. [Google Scholar] [CrossRef]
- Skinner, G.B.; Johnston, H.L. Thermal Expansion of Zirconium between 298°K and 1600°K. J. Chem. Phys. 1953, 21, 1383–1384. [Google Scholar] [CrossRef]
- Swanson, H.E.; Tatge, E. Standard X-Ray Diffraction Powder Patterns (Data for 54 Inorganic Substances). NBS J. Res. 1953, 3. Available online: https://books.google.de/books?hl=en&lr=&id=CtVf1TRPoWcC&oi=fnd&pg=PA1&dq=Swanson,+H.+E.%3B+Tatge,+E.+Standard+X-Ray+Diffraction+Powder+Patterns+(Data+for+54+Inorganic+Substances).+NBS+J.+Res.+1953,+3.+&ots=8jCivz-HOP&sig=CmknIKCsC4BrRua5mY6AcDCrJpU&redir_esc=y#v=onepage&q&f=false (accessed on 10 January 2025).
- Hann, R.E.; Suitch, P.R.; Pentecost, J.L. Monoclinic Crystal Structures of ZrO2 and HfO2 Refined from X-ray Powder Diffraction Data. J. Am. Ceram. Soc. 1985, 68, C-285–C-286. [Google Scholar] [CrossRef]
- Hartwich, P.; Vakamulla Raghu, S.N.; Mogwitz, B.; Onyenso, G.; Rohnke, M.; Killian, M.S. FIB’n’SIMS–Advanced Depth Analysis on Hybrid Organic-Inorganic Nanomaterials. ChemRxiv Prepr. 2024. [Google Scholar] [CrossRef]
- Adeyeye, C.M.; Li, P.K. Diclofenac Sodium. Anal. Profiles Drug Subst. Excip. 1990, 19, 123–144. [Google Scholar] [CrossRef]
- Lee, J.L.S.; Gilmore, I.S.; Seah, M.P.; Fletcher, I.W. Topography and Field Effects in Secondary Ion Mass Spectrometry-Part I: Conducting Samples. J. Am. Soc. Mass Spectrom. 2011, 22, 1718–1728. [Google Scholar] [CrossRef]
- Rangarajan, S.; Tyler, B.J. Topography in Secondary Ion Mass Spectroscopy Images. J. Vac. Sci. Technol. A Vac. Surf. Film. 2006, 24, 1730–1736. [Google Scholar] [CrossRef]
- Muratore, F.; Baron-Wiecheć, A.; Hashimoto, T.; Skeldon, P.; Thompson, G.E. Anodic Zirconia Nanotubes: Composition and Growth Mechanism. Electrochem. Commun. 2010, 12, 1727–1730. [Google Scholar] [CrossRef]
- Lockman, Z.; Sreekantan, S.; Ismail, S.; Schmidt-Mende, L.; MacManus-Driscoll, J.L. Influence of Anodisation Voltage on the Dimension of Titania Nanotubes. J. Alloys Compd. 2010, 503, 359–364. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio-Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Butail, G.; Ganesan, P.G.; Teki, R.; Mahima, R.; Ravishankar, N.; Duquette, D.J.; Ramanath, G. Branched Titania Nanotubes through Anodization Voltage Control. Thin Solid Films 2011, 520, 235–238. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
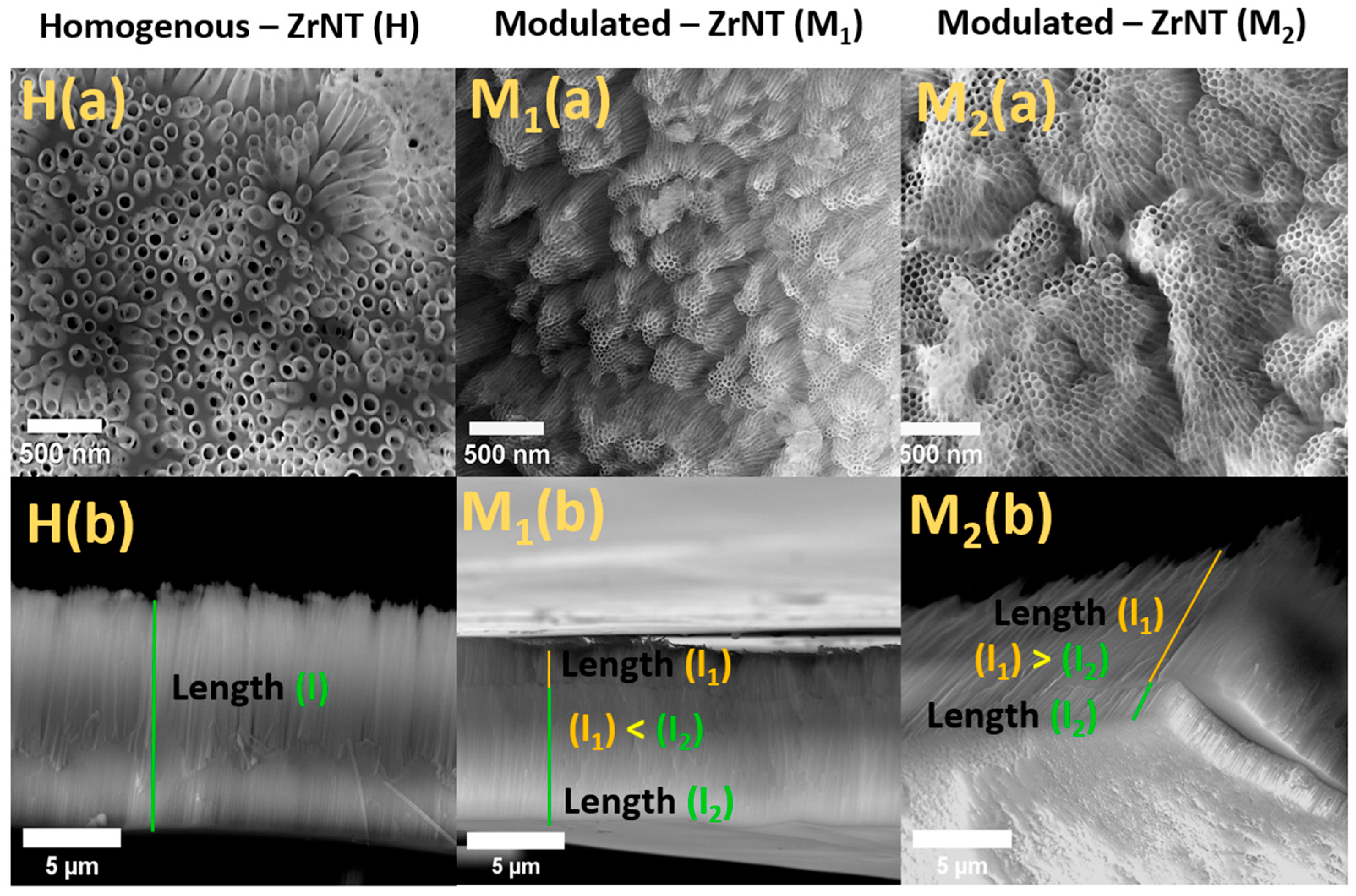
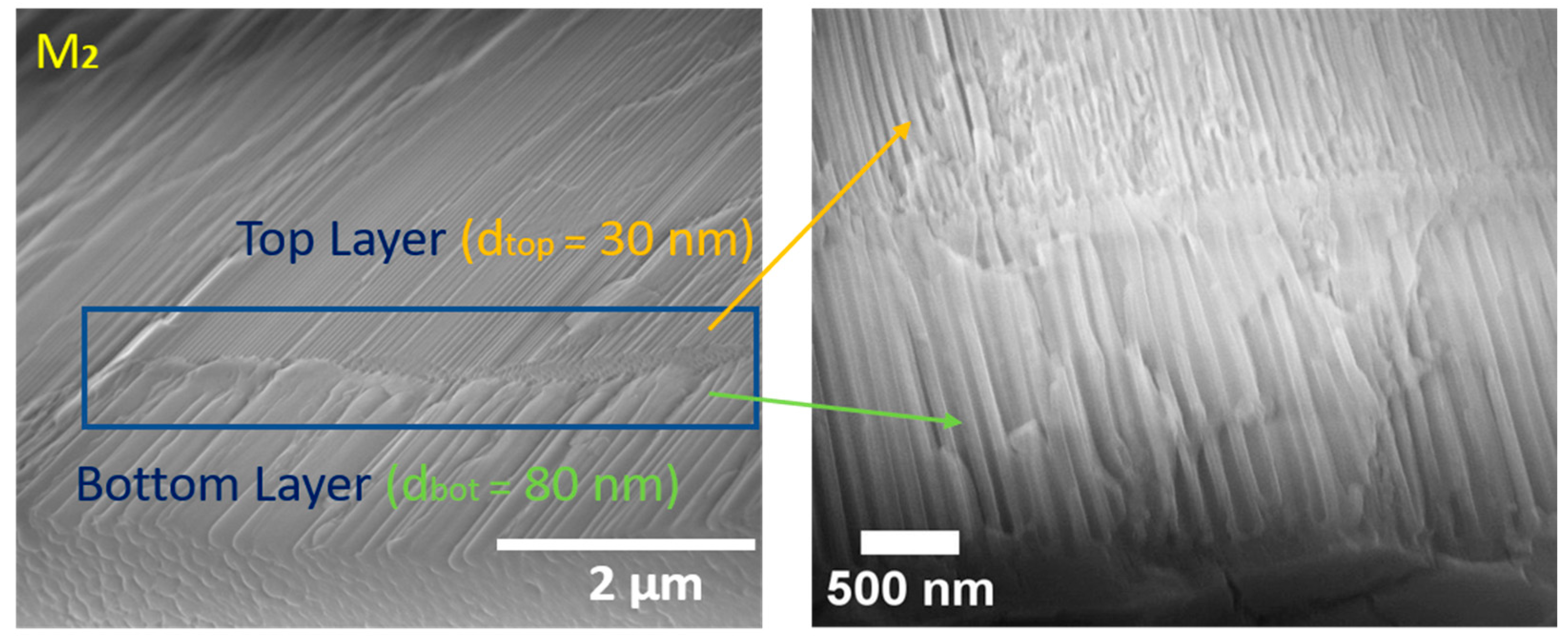
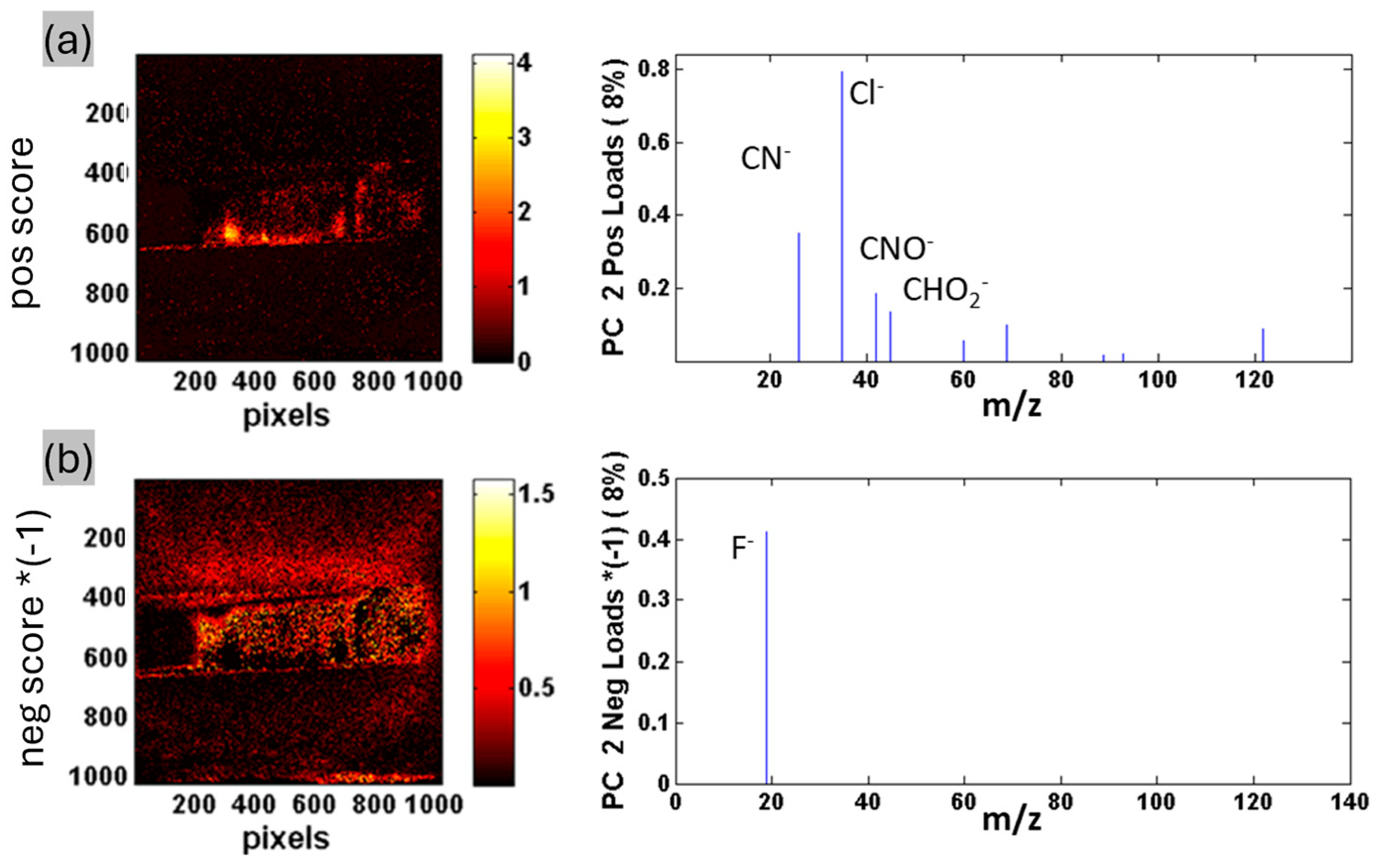

| Diameter (nm) (Top-Bottom) | Length (µm) (Top-Bottom) | |
|---|---|---|
| H—ZrNT | 80 | 9 |
| M1—ZrNT | 30 ± 5–80 ± 20 | 2–7 |
| M2—ZrNT | 30 ± 5–80 ± 20 | 7–2 |
| H—ZrNT | M1—ZrNT | M2—ZrNT | |
|---|---|---|---|
| Drug loading capacity (µg/cm2) | 4.96 | 8.76 | 10.22 |
| Loading efficency (%) | 34.63 | 61.17 | 71.37 |
| Initial burst release (wt%) | 61 | 43 | 16 |
| Total release time (days) | 14 | 15 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyenso, G.; Vakamulla Raghu, S.N.; Hartwich, P.; Killian, M.S. Modulated-Diameter Zirconia Nanotubes for Controlled Drug Release—Bye to the Burst. J. Funct. Biomater. 2025, 16, 37. https://doi.org/10.3390/jfb16020037
Onyenso G, Vakamulla Raghu SN, Hartwich P, Killian MS. Modulated-Diameter Zirconia Nanotubes for Controlled Drug Release—Bye to the Burst. Journal of Functional Biomaterials. 2025; 16(2):37. https://doi.org/10.3390/jfb16020037
Chicago/Turabian StyleOnyenso, Gabriel, Swathi Naidu Vakamulla Raghu, Patrick Hartwich, and Manuela Sonja Killian. 2025. "Modulated-Diameter Zirconia Nanotubes for Controlled Drug Release—Bye to the Burst" Journal of Functional Biomaterials 16, no. 2: 37. https://doi.org/10.3390/jfb16020037
APA StyleOnyenso, G., Vakamulla Raghu, S. N., Hartwich, P., & Killian, M. S. (2025). Modulated-Diameter Zirconia Nanotubes for Controlled Drug Release—Bye to the Burst. Journal of Functional Biomaterials, 16(2), 37. https://doi.org/10.3390/jfb16020037








