Advances in Bioactive Dental Adhesives for Caries Prevention: A State-of-the-Art Review
Abstract
1. Introduction
2. Materials and Methods
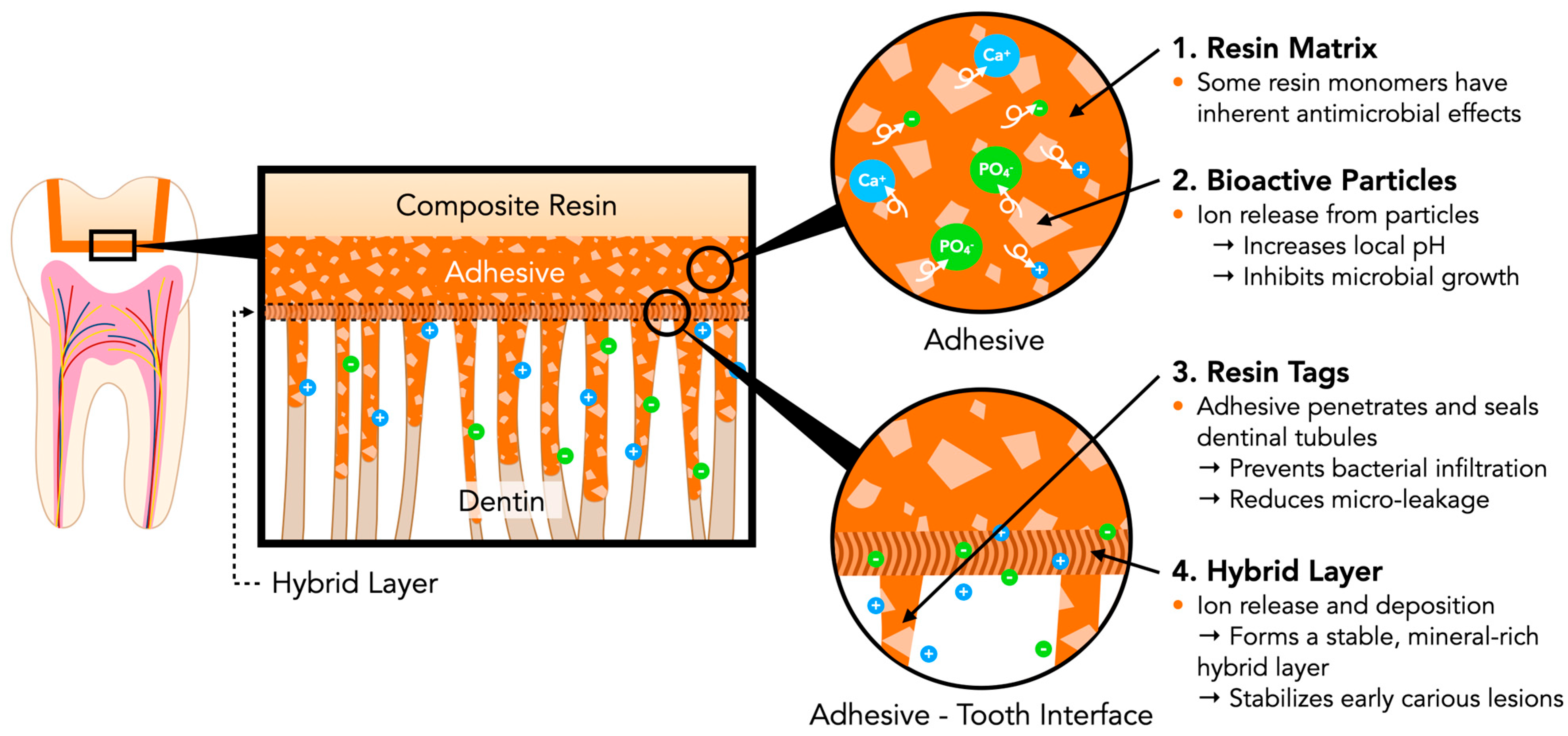
3. Principle of Bioactive Dental Adhesives
3.1. Function of Bioactive Materials
3.2. Mechanisms of Caries Prevention in Bioactive Dental Adhesives
3.2.1. Bioactive Ion Release and Mineral Deposition at the Adhesive–Tooth Interface
3.2.2. Dynamic pH-Responsive Buffering Function
3.2.3. Inhibition of Microbial Adhesion and Biofilm Formation
3.2.4. Stabilization of Hybrid Layer to Enhance Bonding Durability
4. Overview of Bioactive Dental Adhesives
4.1. Chemical Composition of Dental Adhesives
4.2. Current Challenges in Dental Adhesive Systems
4.3. Bioactive Agents in Dental Adhesive Systems
| Types | Materials | Bioactive Function * | Mechanism of Action | Limitations | Adhesive Integration |
|---|---|---|---|---|---|
| Section 4.3.1 Calcium Phosphate Particles | Amorphous Calcium Phosphate (ACP) [14] | Remineralization | Ion Exchange; Disruption of bacterial cell membrane | Limited long-term stability | Additives |
| Hydroxyapatite Nanoparticles (n-HAp) |
Antimicrobial
Remineralization | Ion Exchange; Disruption of bacterial cell membrane | Prone to degradation upon moisture exposure | Additives | |
| Section 4.3.2 Bioactive Glass (BAG) | Calcium Sodium Phosphosilicate [61] |
Remineralization
Antimicrobial Regenerative | Ion exchange elevates pH and activates reparative cellular responses. | Potential to interfere with resin components | Additives |
| Section 4.3.3 Antimicrobial Agents | Nanoparticles of silver (NAg) [62] | Antimicrobial | Disrupt bacterial membranes and generate ROS | May cause discoloration and cytotoxicity at high doses | Additives |
| Quaternary ammonium dimethacrylate (QADM) [58] | Antimicrobial | Contact kills by disrupting bacterial membranes | Limited sustained antimicrobial effect. | Resin Monomer | |
| Methacryloyloxydodecylpyridinium bromide (MDPB) [59] | Antimicrobial | Sustained contact-based membrane disruption | Reduce the degree of conversion | Resin Monomer | |
| Nisin peptide [63] | Antimicrobial | Form pores in bacterial membranes | Unstable; enzyme-prone degradation | Additives | |
| Glutaraldehyde [64] | Antimicrobial | Cross-links bacterial proteins, leading to cell death | Tissue toxicity; polymerization interference | Additives | |
| Chlorhexidine [65] | Antimicrobial | Disrupts membranes; precipitates cytoplasm | Short-term effectiveness; Leaching over time | Additives |
4.3.1. Calcium Phosphate Particles
4.3.2. Bioactive Glass
- 1.
- Ion Exchange: The glass releases sodium and calcium ions into the surrounding fluid, while hydrogen ions (H+ or H3O+) enter the glass. This raises the local pH and begins breaking the silicon-oxygen (Si–O–Si) bonds.
- 2.
- Silica Dissolution: The breaking of Si–O–Si bonds releases silicon into the fluid as silanol (Si(OH)4) molecules.
- 3.
- Silica Gel Layer Formation: If the pH stays below 9.5, silanol molecules condense to form a porous silica gel layer on the glass surface, allowing further ion exchange.
- 4.
- Calcium Phosphate Layer Formation: Calcium and phosphate ions from both the glass and fluid accumulate on the silica gel, creating a layer of amorphous calcium phosphate (ACP).
- 5.
- HCA Crystallization: Carbonate ions incorporate into the ACP layer, which gradually crystallizes into hydroxycarbonate apatite (HCA), closely resembling the mineral phase of natural teeth and bone.
4.3.3. Antimicrobial Agents
4.3.4. Multifunctionality
5. Trends in Bioactive Dental Adhesives Development
5.1. Nanotech-Enhanced Dental Adhesive Systems
5.2. Smart Polymers and Hydrogels-Based Therapeutic Dental Adhesives
5.3. Enzymatic Inhibitors Incorporated Bioactive Dental Adhesives
| Types | Agents | Target Functions | Added Benefits |
|---|---|---|---|
| Nanomaterials | Nano-Hydroxyapatite (n-HAp) [76,94,110,111,112] | Biomimetic remineralization | Facilitates mineral deposition and reduces hypersensitivity |
| Nano-Silica (SiO2) [113,114,115,116] | Mechanical reinforcement | Boosts bond strength, wear resistance, and marginal seal | |
| Nano-Zirconia (ZrO2) [117,118,119] | Toughening agent | Enhances fracture toughness and mechanical stability | |
| Titanium Dioxide (TiO2) [117,120] | Mechanical reinforcement | Increases bond strength; reduces microleakage and microbial adhesion | |
| Silver Nanoparticles (NAg) [123,124] | Antimicrobial | Suppresses biofilm; prevents recurrent caries | |
| Zinc Oxide Nanoparticles [23,46] | Antimicrobial and anti-inflammatory | Inhibits microbial colonization; modulates inflammation | |
| Copper Oxide Nanoparticles [125,126,127] | Antimicrobial | Boosts antimicrobial activity and bond durability | |
| Chitosan Nanoparticles [128,129] | Antimicrobial; drug delivery carrier | Facilitates healing with controlled drug release | |
| Dendrimers [130,131] | Self-healing | Enables pH-driven ion release and self-repair of microcracks | |
| Nanogels [132,133,134] | Smart delivery system | Enables pH-responsive ion and drug release | |
| Nanotubes (e.g., Halloysite) [135,136,137] | Therapeutic agent reservoirs | Enables prolonged antimicrobial release | |
| Smart Polymers /Hydrogels | pH-Responsive Polymers [25,100] | Ion release in acidic pH | Promotes remineralization and acid protection |
| Diels–Alder/Self-Healing Polymers [142,143] | Autonomous microcrack repair | Improves longevity and strength at the adhesive interface | |
| Enzyme-Responsive Systems [146] | Targeted therapeutic release | Release agents in response to bacterial enzymatic action | |
| Gelatin Methacryloyl (GelMA) [150] | Light-curable hydrogel matrix | Enables moisture-resistant curing and tissue bonding | |
| Thermoresponsive Hydrogels (PNIPAAm) [151] | Thermoresponsive viscosity | Facilitates handling and better intraoral adaptation | |
| Chitosan-Based Hydrogels [43] | Antimicrobial, drug delivery carrier | Inhibits microbe; facilitates prolonged therapeutic delivery. | |
| ACP/nano-HAp Hydrogels [19,155] | Calcium/phosphate delivery | Enhances remineralization; decreases hypersensitivity | |
|
Enzymatic
Inhibitors | MMP Inhibitors (e.g., Chlorhexidine, Galardin) [49,158,159] | Inhibits matrix metalloproteinases | Preserves hybrid layer; reduces adhesive degradation |
| Cathepsin Inhibitors (e.g., EGCG, Tannic Acid) [160,161] | Blocks collagen-degrading cathepsins | Strengthens bond over time; protects interface integrity | |
| Peptide-Based Inhibitors (e.g., Synthetic MMP-inhibitory peptides) [152] | Selective MMP inhibition | Provides sustained enzyme inhibition with low toxicity | |
| Bioactive Fillers + Inhibitors (e.g., n-HAp + ACP) [162,163]. | Dual action: remineralization and enzymatic inhibition | Reinforces framework and inhibits enzymatic degradation |
5.4. Clinical Performance Studies
| Engineered Performance | Experimental Outcomes | Clinical Benefits |
|---|---|---|
| Remineralization [165] | BAG and nHAp induce apatite formation, restoring mineral content in dentin/enamel | Aids tooth preservation and repair |
| Postoperative Sensitivity [169] | Ion release mitigates dentinal hypersensitivity in deep cavities | Improves comfort and compliance. |
| Secondary Caries Prevention [169] | Fluoride/calcium ions prevent demineralization and bacterial ingress | Strengthens caries defense at margins |
| Biocompatibility [172] | Safe and well-tolerated; fluoride and BAG offer low-toxicity therapeutic effects | Enables safe application in pulp and deep cavities |
| Bond Strength [175] | Strong initial bond; ion release boosts durability in moist conditions | Ensures reliable adhesion in challenging clinical settings |
| Marginal Integrity [176] | Minimizes microleakage with enhanced sealing and adaptation | Prevents bacterial ingress and recurrent caries |
| Longevity [177] | Stable adhesion for 5+ years, dependent on hygiene and site | Minimizes long-term replacement needs |
6. Current Challenges and Limitations in Advanced Bioactive Dental Adhesive Systems
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, K.H.; Mai, Y.; Kim, H.; Tong, K.C.; Ng, D.; Hsiao, J.C. Resin composite filling. Materials 2010, 3, 1228–1243. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F. Adhesive/dentin interface: The weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef]
- Demarco, F.F.; Corrêa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J. Longevity of posterior composite restorations: Not only a matter of materials. Dent. Mat. 2012, 28, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Opdam, N.; Van De Sande, F.; Bronkhorst, E.; Cenci, M.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.; Van Dijken, J. Longevity of posterior composite restorations: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef]
- Shah, Y.R.; Shiraguppi, V.L.; Deosarkar, B.A.; Shelke, U.R. Long-term survival and reasons for failure in direct anterior composite restorations: A systematic review. J. Conserv. Dent. Endod. 2021, 24, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Wang, X.; Egusa, H.; Sun, J. High-performance dental adhesives containing an ether-based monomer. J. Dent. Res. 2020, 99, 189–195. [Google Scholar] [CrossRef]
- Price, R.; Roulet, J. The value of consensus conferences: Peer review by 50 key opinion leaders! Stoma 2020, 7, 1–80. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Sidhu, S.K.; Melo, M.A.S.; Yeo, I.-S.L.; Diogenes, A.; Darvell, B.W. Bioactive dental materials: Developing, promising, confusing. JADA Found. Sci. 2023, 2, 100022. [Google Scholar]
- Melo, M.A.S.; Garcia, I.M.; Mokeem, L.; Weir, M.D.; Xu, H.H.K.; Montoya, C.; Orrego, S. Developing Bioactive Dental Resins for Restorative Dentistry. J. Dent. Res. 2023, 102, 1180–1190. [Google Scholar] [CrossRef]
- Cocco, A.R.; da Rosa, W.L.d.O.; da Silva, A.F.; Lund, R.G.; Piva, E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dent. Mater. 2015, 31, 1345–1362. [Google Scholar] [CrossRef]
- Münchow, E.A.; da Silva, A.F.; Piva, E.; Cuevas-Suárez, C.E.; de Albuquerque, M.T.P.; Pinal, R.; Gregory, R.L.; Breschi, L.; Bottino, M.C. Development of an antibacterial and anti-metalloproteinase dental adhesive for long-lasting resin composite restorations. J. Mater. Chem. B 2020, 8, 10797–10811. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Dental adhesives of the future. J. Adhes. Dent. 2002, 4, 91–103. [Google Scholar]
- Cadenaro, M.; Josic, U.; Maravić, T.; Mazzitelli, C.; Marchesi, G.; Mancuso, E.; Breschi, L.; Mazzoni, A. Progress in dental adhesive materials. J. Dent. Res. 2023, 102, 254–262. [Google Scholar] [CrossRef]
- Tung, M.S. Amorphous Calcium Phosphates for Tooth. Compendium 2004, 25, 9–13. [Google Scholar] [PubMed]
- National Cancer Institute. NCI Dictionary of Cancer Terms; National Cancer Institute: Bethesda, MD, USA, 2023. [Google Scholar]
- Arifa, M.K.; Ephraim, R.; Rajamani, T. Recent advances in dental hard tissue remineralization: A review of literature. Inter. J. Clin. Pediatr. Dent. 2019, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Quigley, R.M.; Kearney, M.; Kennedy, O.D.; Duncan, H.F. Tissue engineering approaches for dental pulp regeneration: The development of novel bioactive materials using pharmacological epigenetic inhibitors. Bioact. Mater. 2024, 40, 182. [Google Scholar] [CrossRef]
- Schmalz, G.; Hickel, R.; Price, R.B.; Platt, J.A. Bioactivity of dental restorative materials: FDI policy statement. Inter. Dent. J. 2023, 73, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; He, L.; Xu, H.H.K.; Weir, M.D.; Fan, M.; Yu, Z.; Zhang, M.; Zhou, X.; Liang, K.; Li, J. Dentin remineralization via adhesive containing amorphous calcium phosphate nanoparticles in a biofilm-challenged environment. J. Dent. 2019, 89, 103193. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, J.H.; Woo, S.U.; Choi, K.K.; Kim, S.Y.; Ferracane, J.L.; Lee, J.H.; Choi, D.; Choi, S.; Kim, S.; et al. Effect of Novel Bioactive Glass-Containing Dentin Adhesive on the Permeability of Demineralized Dentin. Materials 2021, 14, 5423. [Google Scholar] [CrossRef]
- Alhussein, A.; Alsahafi, R.; Balhaddad, A.A.; Mokeem, L.; Schneider, A.; Jabra-Rizk, M.-A.; Masri, R.; Hack, G.D.; Oates, T.W.; Sun, J. Novel bioactive nanocomposites containing calcium fluoride and calcium phosphate with antibacterial and low-shrinkage-stress capabilities to inhibit dental caries. Bioengineering 2023, 10, 991. [Google Scholar] [CrossRef]
- Zhang, K.; Melo, M.A.; Cheng, L.; Weir, M.D.; Bai, Y.; Xu, H.H. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent. Mater. 2012, 28, 842–852. [Google Scholar] [CrossRef]
- Garcia, I.M.; Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Antibacterial response of oral microcosm biofilm to nano-zinc oxide in adhesive resin. Dent. Mater. 2021, 37, e182–e193. [Google Scholar] [CrossRef]
- Boutsiouki, C.; Frankenberger, R.; Lücker, S.; Krämer, N. Effect of Chlorhexidine-containing Etch-and-Rinse Adhesives on Dentin Microtensile Bond Strength after Biological Loading. J. Adhes. Dent. 2023, 25, 13–22. [Google Scholar]
- Ibrahim, M.S.; Balhaddad, A.A.; Garcia, I.M.; Collares, F.M.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. pH-responsive calcium and phosphate-ion releasing antibacterial sealants on carious enamel lesions in vitro. J. Dent. 2020, 97, 103323. [Google Scholar] [CrossRef]
- He, Y.; Vasilev, K.; Zilm, P. pH-responsive biomaterials for the treatment of dental caries—A focussed and critical review. Pharmaceutics 2023, 15, 1837. [Google Scholar] [CrossRef] [PubMed]
- Kachoei, M.; Divband, B.; Rahbar, M.; Esmaeilzadeh, M.; Ghanizadeh, M.; Alam, M. A Novel Developed Bioactive Composite Resin Containing Silver/Zinc Oxide (Ag/ZnO) Nanoparticles as an Antimicrobial Material against Streptococcus mutans, Lactobacillus, and Candida albicans. Evid. Based. Complement. Alternat. Med. 2021, 2021, 4743411. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liang, J.; Zhou, X.; Ren, B.; Wang, H.; Han, Q.; Li, H.; Cheng, L. Effects of a Novel, Intelligent, pH-Responsive Resin Adhesive on Cariogenic Biofilms In Vitro. Pathogens 2022, 11, 1014. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Abbassy, M.A.; Bakry, A.S.; Alshehri, N.I.; Alghamdi, T.M.; Rafiq, S.A.; Aljeddawi, D.H.; Nujaim, D.S.; Hassan, A.H. 45S5 Bioglass paste is capable of protecting the enamel surrounding orthodontic brackets against erosive challenge. J. Orthod. Sci. 2019, 8, 5. [Google Scholar] [CrossRef]
- Zhang, R.; Qi, J.; Gong, M.; Liu, Q.; Zhou, H.; Wang, J.; Mei, Y. Effects of 45S5 bioactive glass on the remineralization of early carious lesions in deciduous teeth: An in vitro study. BMC Oral Health 2021, 21, 576. [Google Scholar] [CrossRef]
- Juntavee, A.; Juntavee, N.; Sinagpulo, A.N. Nano-Hydroxyapatite Gel and Its Effects on Remineralization of Artificial Carious Lesions. Int. J. Dent. 2021, 2021, 7256056. [Google Scholar] [CrossRef] [PubMed]
- Kunert, M.; Piwonski, I.; Hardan, L.; Bourgi, R.; Sauro, S.; Inchingolo, F.; Lukomska-Szymanska, M. Dentine remineralisation induced by “Bioactive” materials through mineral deposition: An in vitro study. Nanomaterials 2024, 14, 274. [Google Scholar] [CrossRef]
- Lertwisitphon, P.; Worapasphaiboon, Y.; Champakanan, N.; Toneluck, A.; Naruphontjirakul, P.; Young, A.M.; Chinli, R.; Chairatana, P.; Sucharit, S.; Panpisut, P. Enhancing elemental release and antibacterial properties of resin-based dental sealants with calcium phosphate, bioactive glass, and polylysine. BMC Oral Health 2025, 25, 96. [Google Scholar] [CrossRef]
- Jia, A.; Wang, P.; Tong, F.; Chen, Z.; Deng, Y.; Yao, H.; Wang, L.; Liu, Y.; Ge, H. Developing a Novel Enamel Adhesive with Amorphous Calcium Phosphate and Silver Nanoparticles to Prevent Demineralization during Orthodontic Treatment. J. Funct. Biomater. 2023, 14, 77. [Google Scholar] [CrossRef]
- Zhang, L.; Weir, M.D.; Hack, G.; Fouad, A.F.; Xu, H.H. Rechargeable dental adhesive with calcium phosphate nanoparticles for long-term ion release. J. Dent. 2015, 43, 1587–1595. [Google Scholar] [CrossRef]
- Li, S.; Qi, L.; Liu, Z. Antimicrobial properties and bonding durability of pH-responsive dentin adhesives with chlorhexidine-load mesoporous silica nanoparticles. Front. Mater. 2024, 11, 1379941. [Google Scholar] [CrossRef]
- Lobo, M.M.; Goncalves, R.B.; Pimenta, L.A.F.; Bedran-Russo, A.K.B.; Pereira, P.N. In vitro evaluation of caries inhibition promoted by self-etching adhesive systems containing antibacterial agents. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2005, 75, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.R.; Matuda, A.G.; Campos, R.P.; Mafetano, A.P.V.; Barnabe, A.H.M.; Chagas, G.S.; Barcellos, D.C.; Niu, L.-N.; Tay, F.R.; Pucci, C.R. Development of an antibacterial dentin adhesive. Polymers 2022, 14, 2502. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef]
- Thaweboon, S.; Saito, T.; Mateekusontan, S.; Thaweboon, B. Antimicrobial Effect of Dental Adhesive on Cariogenic Multi-Species Biofilm. Key Eng. Mater. 2024, 977, 129–134. [Google Scholar] [CrossRef]
- Luiz, M.T.; di Filippo, L.D.; Dutra, J.A.P.; Viegas, J.S.R.; Silvestre, A.L.P.; Anselmi, C.; Duarte, J.L.; Calixto, G.M.F.; Chorilli, M. New technological approaches for dental caries treatment: From liquid crystalline systems to nanocarriers. Pharmaceutics 2023, 15, 762. [Google Scholar] [CrossRef]
- Elsaka, S.E. Antibacterial activity and adhesive properties of a chitosan-containing dental adhesive. Quintessence Int. 2012, 43, 603–613. [Google Scholar]
- Wang, S.; Zhang, K.; Zhou, X.; Xu, N.; Xu, H.H.; Weir, M.D.; Ge, Y.; Wang, S.; Li, M.; Li, Y.; et al. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of Streptococcus mutans biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef]
- Thomas, R.; Snigdha, S.; Bhavitha, K.; Babu, S.; Ajith, A.; Radhakrishnan, E. Biofabricated silver nanoparticles incorporated polymethyl methacrylate as a dental adhesive material with antibacterial and antibiofilm activity against Streptococcus mutans. 3 Biotech 2018, 8, 404. [Google Scholar] [CrossRef]
- Saffarpour, M.; Rahmani, M.; Tahriri, M.; Peymani, A. Antimicrobial and bond strength properties of a dental adhesive containing zinc oxide nanoparticles. Braz. J. Oral Sci. 2016, 15, 66–69. [Google Scholar] [CrossRef]
- Betancourt, D.; Baldion, P.; Castellanos, J. Resin-dentin bonding interface: Mechanisms of degradation and strategies for stabilization of the hybrid layer. Int. J. Biomater. 2019, 2019, 5268342. [Google Scholar] [CrossRef]
- Hebling, J.; Pashley, D.H.; Tjäderhane, L.; Tay, F.R. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J. Dent. Res. 2005, 84, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, C.; Patel, S.K. Matrix metalloproteinase inhibitory properties of benzalkonium chloride stabilizes adhesive interfaces. Eur. J. Oral Sci. 2013, 121, 610–616. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.; Pashley, D.; Tay, F.; Toledano, M. Zinc reduces collagen degradation in demineralized human dentin explants. J. Dent. 2011, 39, 148–153. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Prosthodontics, A.O. The Glossary of Prosthodontic Terms; Mosby: Maryland Heights, MO, USA, 1999. [Google Scholar]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The photoinitiators used in resin based dental composite—A review and future perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef]
- Yoshida, K.; Greener, E.H. Effect of photoinitiator on degree of conversion of unfilled light-cured resin. J. Dent. 1994, 22, 296–299. [Google Scholar] [CrossRef]
- Rueggeberg, F.A.; Craig, R.G. Correlation of parameters used to estimate monomer conversion in a light-cured composite. J. Dent. Res. 1988, 67, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP based dental adhesives: Adhesive interface characterization and adhesive stability—A systematic review. Materials 2019, 12, 790. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef]
- Imazato, S.; Torii, Y.; Takatsuka, T.; Inoue, K.; Ebi, N.; Ebisu, S. Bactericidal effect of dentin primer containing antibacterial monomer methacryloyloxydodecylpyridinium bromide (MDPB) against bacteria in human carious dentin. J. Oral. Rehabil. 2001, 28, 314–319. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; Dorigo, E.D.S. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef]
- Hill, R.G.; Stevens, M.M. Bioactive Glass. US20090208428A1, 20 August 2009. [Google Scholar]
- de Souza Balbinot, G.; Leitune, V.C.B.; Montenegro, S.D.; Collares, F.M. Silver core-shells particles as antibacterial filler for adhesive resins. Int. J. Adhes. Adhes. 2025, 138, 103916. [Google Scholar] [CrossRef]
- Su, M.; Yao, S.; Gu, L.; Huang, Z.; Mai, S. Antibacterial effect and bond strength of a modified dental adhesive containing the peptide nisin. Peptides 2018, 99, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.R.; Zehnder, M.; Göhring, T.N.; Waltimo, T.M. Glutaraldehyde in bonding systems disinfects dentin in vitro. J. Adhes. Dent. 2004, 6, 61–64. [Google Scholar]
- Jing, D.; Wang, L. Effect of Chlorhexidine on the Bonding Effect of an Etch-and-Rinse Adhesive to Pretreatment Dentin. Int. J. Periodontics Restor. Dent. 2023, 43, s146–s155. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous calcium (ortho) phosphates. Acta biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Degli Esposti, L.; Iafisco, M.; Brambilla, E. Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulations. Sci. Rep. 2022, 12, 5994. [Google Scholar] [CrossRef] [PubMed]
- Maillard, M.; Bandiaky, O.N.; Maunoury, S.; Alliot, C.; Alliot-Licht, B.; Serisier, S.; Renard, E. The effectiveness of calcium phosphates in the treatment of dentinal hypersensitivity: A systematic review. Bioengineering 2023, 10, 447. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Sun, W.-b.; Yang, X. First detection, characterization, and application of amorphous calcium phosphate in dentistry. J. Den. Sci. 2012, 7, 316–323. [Google Scholar] [CrossRef]
- Hegde, M.N.; Moany, A. Remineralization of enamel subsurface lesions with casein phosphopeptide-amorphous calcium phosphate: A quantitative energy dispersive X-ray analysis using scanning electron microscopy: An: In vitro: Study. J. Conserv. Dent. 2012, 15, 61–67. [Google Scholar] [CrossRef]
- Nozari, A.; Haji Abbas Oghli, F.; Parvizi, F.; Jowkar, Z.; Pakniyat Jahromi, M.; Hamidi, S.A. Impact of Casein Phosphopeptide Amorphous Calcium Phosphate and Proanthocyanidin on Bond Strength of Universal Adhesives to Caries-Affected Dentin in Primary Teeth: An In Vitro Study. Clin. Exp. Dent. Res. 2025, 11, e70131. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Degli Esposti, L.; Ramírez-Rodríguez, G.B.; Carella, F.; Gómez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-López, J.M. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater. 2011, 27, 762–769. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Anil, A.; Ibraheem, W.I.; Meshni, A.A.; Preethanath, R.S.; Anil, S. Nano-hydroxyapatite (nHAp) in the remineralization of early dental caries: A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 5629. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Atai, M.; Nodehi, A.; Khanlar, L.N. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent. Mater. 2010, 26, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.M.; Huynh, E.; Tang, S.; Uskoković, V. Calcium phosphate nanoparticles as intrinsic inorganic antimicrobials: Mechanism of action. Biomed. Mater. 2020, 16, 015018. [Google Scholar] [CrossRef]
- Skaria, S.; Berk, K.J. Experimental dental composites containing a novel methacrylate-functionalized calcium phosphate component: Evaluation of bioactivity and physical properties. Polymers 2021, 13, 2095. [Google Scholar] [CrossRef]
- Al-Qarni, F.; Weir, M.; Melo, M.A.; Al-Dulaijan, Y.; Almulhim, K.S.; Xu, H.H. Novel calcium phosphate ion-rechargeable and antibacterial adhesive to inhibit dental caries. Clin. Oral Investig. 2022, 26, 313–323. [Google Scholar] [CrossRef]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J. Non-Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Hench, L.L. Introduction to Bioceramics, An; World Scientific Publishing Company: Singapore, 2013. [Google Scholar]
- Xu, Y.-T.; Wu, Q.; Chen, Y.-M.; Smales, R.J.; Shi, S.-Y.; Wang, M.-T. Antimicrobial effects of a bioactive glass combined with fluoride or triclosan on Streptococcus mutans biofilm. Arch. Oral Biol. 2015, 60, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Kido, H.W.; Oliveira, P.; Parizotto, N.A.; Crovace, M.C.; Zanotto, E.D.; Peitl-Filho, O.; Fernandes, K.P.; Mesquita-Ferrari, R.A.; Ribeiro, D.A.; M. Renno, A.C. Histopathological, cytotoxicity and genotoxicity evaluation of Biosilicate® glass–ceramic scaffolds. J. Biomed. Mater. Res. Part A 2013, 101, 667–673. [Google Scholar] [CrossRef]
- Suzuki, T.Y.U.; Gallego, J.; Assunção, W.G.; Briso, A.L.F.; Dos Santos, P.H. Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLoS ONE 2019, 14, e0217750. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.E.; Zarow, M.; Kharouf, N.; Mancino, D.; Villares, C.F.; Skaba, D.; Lukomska-Szymanska, M. The bond strength and antibacterial activity of the universal dentin bonding system: A systematic review and meta-analysis. Microorganisms 2021, 9, 1230. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef]
- Gonzalez-Bonet, A.; Kaufman, G.; Yang, Y.; Wong, C.; Jackson, A.; Huyang, G.; Bowen, R.; Sun, J. Preparation of Dental Resins Resistant to Enzymatic and Hydrolytic Degradation in Oral Environments. Biomacromolecules 2015, 16, 3381–3388. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakajima, M.; Hosaka, K.; Ikeda, M.; Foxton, R.M.; Tagami, J. Long-term evaluation of water sorption and ultimate tensile strength of HEMA-containing/-free one-step self-etch adhesives. J. Dent. 2011, 39, 506–512. [Google Scholar] [CrossRef]
- Park, J.G.; Ye, Q.; Topp, E.M.; Misra, A.; Spencer, P. Water sorption and dynamic mechanical properties of dentin adhesives with a urethane-based multifunctional methacrylate monomer. Dent. Mater. 2009, 25, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Guedes, S.F.; Xu, H.H.; Rodrigues, L.K. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral Health 2023, 23, 105. [Google Scholar] [CrossRef]
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Durán, G.; Paula, A.J.; Durán, N. Advances in Dental Materials through Nanotechnology: Facts, Perspectives and Toxicological Aspects. Trends Biotechnol. 2015, 33, 621–636. [Google Scholar] [CrossRef]
- da Costa, K.N.B.; Veras, N.P.; Carvalho, C.N.; de Oliveira Bauer, J.R.; Loguercio, A.D.; Cardenas, A.F.M.; de Siqueira, F.S.F.; Ferreira, M.C. Adhesive system containing Bioglass 45S5 particles in teeth affected by molar-incisor hypomineralization and quality of life impact: Study protocol for a randomized clinical trial. Trials 2025, 26, 151. [Google Scholar] [CrossRef] [PubMed]
- AlQhtani, F.; Abdulla, A.M.; Kamran, M.A.; Luddin, N.; Abdelrahim, R.K.; Samran, A.; AlJefri, G.H.; Niazi, F.H. Effect of adding sodium fluoride and nano-hydroxyapatite nanoparticles to the universal adhesive on bond strength and microleakage on caries-affected primary molars. J. Clin. Pediatr. Dent. 2024, 48, 79–85. [Google Scholar]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano-Particles-An SEM, EDX, Micro-Raman, and Microtensile Bond Strength Study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef]
- Aljamhan, A.S.; Alrefeai, M.H.; Alhabdan, A.; Alhusseini, S.A.; Farooq, I.; Vohra, F.; Naseem, M.; Alkhudhairy, F. Influence of ER-CR-YSGG Laser and Photodynamic Therapy on the Dentin Bond Integrity of Nano-Hydroxyapatite Containing Resin Dentin Adhesive: SEM-EDX, Micro-Raman, Micro-Tensile, and FTIR Evaluation. Polymers 2021, 13, 1903. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, W.; Liang, J.; Ran, S. Influence of silver nanoparticles on the resin-dentin bond strength and antibacterial activity of a self-etch adhesive system. J. Prosthet. Dent. 2022, 128, 1363.e1–1363.e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y.; Deng, J.; Nie, R.; Meng, X. Antibacterial one-step self-etching dental adhesive with silver nanoparticles synthesized in situ. J. Dent. 2023, 129, 104411. [Google Scholar] [CrossRef]
- Aguiar, J.D.; Pedrosa, M.D.S.; Toma, S.H.; Araki, K.; Marques, M.M.; Medeiros, I.S. Antibacterial effect, cytotoxicity, and bond strength of a modified dental adhesive containing silver nanoparticles. Odontology 2023, 111, 420–427. [Google Scholar] [CrossRef]
- Liang, J.; Liu, F.; Zou, J.; Xu, H.H.K.; Han, Q.; Wang, Z.; Li, B.; Yang, B.; Ren, B.; Li, M.; et al. pH-Responsive Antibacterial Resin Adhesives for Secondary Caries Inhibition. J. Dent. Res. 2020, 99, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Dionysopoulos, D. Effect of digluconate chlorhexidine on bond strength between dental adhesive systems and dentin: A systematic review. J. Conserv. Dent. Endod. 2016, 19, 11–16. [Google Scholar] [CrossRef]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Esmerino, L.A.; Maluf, D.F.; Zawadzki, S.F.; Michél, M.D.; Santos, F.A.d.; Gomes, O.M.M.; Gomes, J.C. An innovative quaternary ammonium methacrylate polymer can provide improved antimicrobial properties for a dental adhesive system. J. Biomater. Sci. Polym. Ed 2013, 24, 1443–1458. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Silva, E.M.; Carvalho, C.M.; Miranda, M.; Portela, M.B.; Amaral, C.M. Characterization and Anti-Caries Effect of an Experimental Adhesive Containing Natural Antimicrobial Agents. J. Adhes. Dent. 2021, 23, 527–537. [Google Scholar]
- Sarikaya, R.; Song, L.; Yuca, E.; Xie, S.X.; Boone, K.; Misra, A.; Spencer, P.; Tamerler, C. Bioinspired multifunctional adhesive system for next generation bio-additively designed dental restorations. J. Mech. Behav. Biomed. Mater. 2021, 113, 104135. [Google Scholar] [CrossRef]
- Schröter, F.-J.; Moldovan, M.; Sarosi, C.; Ilie, N. Enhancing dentin bonding through new adhesives formulations with natural polyphenols, tricalcium phosphate and chitosan. Dent. Mater. 2024, 40, 276–284. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Weir, M.D.; Melo, M.A.S.; Zhou, X.; Xu, H.H. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine 2015, 10, 627–641. [Google Scholar] [CrossRef]
- Nassif, M.; El Askary, F. Nanotechnology and nanoparticles in contemporary dental adhesives. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–198. [Google Scholar]
- Nagano, F.; Selimovic, D.; Noda, M.; Ikeda, T.; Tanaka, T.; Miyamoto, Y.; Koshiro, K.-i.; Sano, H. Improved bond performance of a dental adhesive system using nano-technology. BioMed. Mater. Eng. 2009, 19, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Stape, T.H.S.; Mutluay, M.M.; Tjäderhane, L.; Uurasjärvi, E.; Koistinen, A.; Tezvergil-Mutluay, A. The pursuit of resin-dentin bond durability: Simultaneous enhancement of collagen structure and polymer network formation in hybrid layers. Dent. Mater. 2021, 37, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Lezaja, M.; Jokic, B.M.; Veljovic, D.N.; Miletic, V. Shear bond strength to dentine of dental adhesives containing hydroxyapatite nano-fillers. J. Adhes. Sci. Technol. 2016, 30, 2678–2689. [Google Scholar] [CrossRef]
- Mutar, M.A.; Mahdi, M.S. Synthesis and characterization of novel nanocomposites with nanofillers particles and their applications as dental materials. Period. Eng. Nat. Sci. (PEN) 2019, 7, 1512–1538. [Google Scholar] [CrossRef]
- Shekofteh, K.; Boruziniat, A.; Moghaddas, M.-J.; Namdar, F.; Zahabi, E.; Bagheri, H. Formulation and mechanical characterization of a semi-crystalline nano-fluorine hydroxyapatite-filled dental adhesive. J. Aust. Ceram. Soc. 2018, 54, 731–738. [Google Scholar]
- Mazloom-Jalali, A.; Taromi, F.A.; Atai, M.; Solhi, L. Dual modified nanosilica particles as reinforcing fillers for dental adhesives: Synthesis, characterization, and properties. J. Mech. Behav. Biomed. Mater. 2020, 110, 103904. [Google Scholar] [CrossRef]
- Solhi, L.; Atai, M.; Nodehi, A.; Imani, M. On the properties of nanosilicate-based filled dental adhesives: Synthesis, characterization, and optimized formulation. J. Mech. Behav. Biomed. Mater. 2021, 119, 104498. [Google Scholar] [CrossRef]
- Jahromia, S.F.; Naimi-Jamala, M.; Ataib, M. Preparation of dental polymeric nano-adhesives with silica nano-porous (MCM-41) as novel fillers for improving the adhesive mechanical properties: Synthesis and application. In Proceedings of the The 18th International Electronic Conference on Synthetic Organic Chemistry, Bergen, Norway, 1–6 August 2010; MDPI: Basel, Switzerland, 2014. [Google Scholar]
- Sabir, M.; Muhammad, N.; Siddiqui, U.; Khan, A.S.; Syed, M.R.; Rahim, A.; Liaqat, S.; Shah, A.T.; Sharif, F.; Khan, M.A. Effect of nanocrystalline cellulose/silica-based fillers on mechanical properties of experimental dental adhesive. Polym. Bull. 2023, 80, 9131–9148. [Google Scholar]
- Felemban, N.H.; Ebrahim, M.I. The influence of adding modified zirconium oxide-titanium dioxide nano-particles on mechanical properties of orthodontic adhesive: An in vitro study. BMC Oral Health 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Lohbauer, U.; Wagner, A.; Belli, R.; Stoetzel, C.; Hilpert, A.; Kurland, H.-D.; Grabow, J.; Müller, F.A. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010, 6, 4539–4546. [Google Scholar] [CrossRef]
- Alkhudhairy, F.; Vohra, F. Adhesive bond strength and compressive strength of a novel bulk fill composite with zirconia nano-hybrid filler. J. Adhes. Sci. Technol. 2017, 31, 450–463. [Google Scholar] [CrossRef]
- Florez, F.L.E.; Hiers, R.D.; Larson, P.; Johnson, M.; O’Rear, E.; Rondinone, A.J.; Khajotia, S.S. Antibacterial dental adhesive resins containing nitrogen-doped titanium dioxide nanoparticles. Mater. Sci. Eng. C 2018, 93, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez y Baena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel Before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. BioMed Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [PubMed]
- Kavrik, F.; Kucukyilmaz, E. Effect of Nano-sized Hydroxyapatite Filler on Remineralization Efficacy of an Adhesive Resin: An in vitro study. J. Adhes. Sci. Technol. 2024, 38, 1334–1352. [Google Scholar] [CrossRef]
- Peralta, L.C.F.; Almeida, N.L.M.; Pontes, F.M.L.; Rinaldo, D.; Carneiro, C.A.; Neppelenbroek, K.H.; Lara, V.S.; Porto, V.C. Silver nanoparticles in denture adhesive: An antimicrobial approach against Candida albicans. J. Dent. 2023, 131, 104445. [Google Scholar] [CrossRef]
- Dutra-Correa, M.; Leite, A.A.; de Cara, S.P.; Diniz, I.M.; Marques, M.M.; Suffredini, I.B.; Fernandes, M.S.; Toma, S.H.; Araki, K.; Medeiros, I.S. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J. Dent. 2018, 77, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.F.; Malaquias, P.; Hass, V.; Matos, T.P.; Lourenço, L.; Reis, A.; Loguercio, A.D.; Farago, P.V. The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resin-dentine interfaces. J. Dent. 2017, 61, 12–20. [Google Scholar] [CrossRef]
- Argueta-Figueroa, L.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Olea-Mejía, O.F. An evaluation of the antibacterial properties and shear bond strength of copper nanoparticles as a nanofiller in orthodontic adhesive. Aust. Orthod. J. 2015, 31, 42–48. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Alegría-Acevedo, L.F.; Méndez-Bauer, L.; Bermudez, J.; Dávila-Sánchez, A.; Buvinic, S.; Hernández-Moya, N.; Reis, A.; Loguercio, A.D.; Farago, P.V. Biological, mechanical and adhesive properties of universal adhesives containing zinc and copper nanoparticles. J. Dent. 2019, 82, 45–55. [Google Scholar] [CrossRef]
- Neves, J.; Marcato, P.; e Silva, F.d.P.; Mantovani, C.; Prado, H.; Aires, C.; Massaro, T.; Borsato, M. Synthesis and characterization of an experimental primer containing chitosan nanoparticles–Effect on the inactivation of metalloproteinases, antimicrobial activity and adhesive strength. Arch. Oral Biol. 2021, 127, 105148. [Google Scholar] [CrossRef]
- Sodagar, A.; Bahador, A.; Jalali, Y.F.; Gorjizadeh, F.; Baghaeian, P. Effect of chitosan nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in orthodontics. Iran J. Orth.o 2016, 21, 81–88. [Google Scholar]
- Cai, X.; Wang, X. Chlorhexidine-loaded poly (amido amine) dendrimer and a dental adhesive containing amorphous calcium phosphate nanofillers for enhancing bonding durability. Dent. Mater. 2022, 38, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yang, X.; Liao, L.; Yang, J.; Liang, K.; Zeng, S.; Zhou, J.; Zhang, M.; Li, J. A novel anticaries agent, honokiol-loaded poly (amido amine) dendrimer, for simultaneous long-term antibacterial treatment and remineralization of demineralized enamel. Dent. Mater. 2021, 37, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Gotti, V.B.; Correr, A.B.; Lewis, S.H.; Feitosa, V.P.; Correr-Sobrinho, L.; Stansbury, J.W. Influence of nanogel additive hydrophilicity on dental adhesive mechanical performance and dentin bonding. Dent. Mater. 2016, 32, 1406–1413. [Google Scholar] [CrossRef]
- Morães, R.; Garcia, J.; Wilson, N.; Lewis, S.; Barros, M.; Yang, B.; Pfeifer, C.; Stansbury, J. Improved dental adhesive formulations based on reactive nanogel additives. J. Dent. Res. 2012, 91, 179–184. [Google Scholar] [CrossRef]
- Trivedi, R.; Gautam, D.; Kehe, G.M.; Escobedo, H.D.; Patel, K.; Stansbury, J.W.; Schurr, M.J.; Nair, D.P. Synthesis, characterization and evaluation of azobenzene nanogels for their antibacterial properties in adhesive dentistry. Eur. J. Oral Sci. 2022, 130, e12832. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Garcia, I.M.; Mokeem, L.; Alsahafi, R.; Collares, F.M.; Sampaio de Melo, M.A. Metal oxide nanoparticles and nanotubes: Ultrasmall nanostructures to engineer antibacterial and improved dental adhesives and composites. Bioengineering 2021, 8, 146. [Google Scholar] [CrossRef]
- Garcia, I.M.; Araújo-Neto, V.G.; Balbinot, G.; Souza, V.S.; Mokeem, L.; Scholten, J.D.; Melo, M.A.S.; Giannini, M.; Collares, F.M. Dental adhesives incorporated with alkyl trimethyl ammonium bromide-loaded titanium oxide nanotubes for sustained bioactive and anti-biofilm protection. Dent. Mater. 2025, 41, 721–729. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Genari, B.; Leitune, V.C.B.; Arthur, R.A.; Luxan, S.A.; Samuel, S.M.W.; Collares, F.M.; Sauro, S. Polymerisation, antibacterial and bioactivity properties of experimental orthodontic adhesives containing triclosan-loaded halloysite nanotubes. J. Dent. 2018, 69, 77–82. [Google Scholar] [CrossRef]
- Amirthalingam, S.; Rajendran, A.K.; Moon, Y.G.; Hwang, N.S. Stimuli-responsive dynamic hydrogels: Design, properties and tissue engineering applications. Mater. Horiz. 2023, 10, 3325–3350. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, L.; Xie, X.; Yang, X.; Liao, J. Recent advances in stimuli responsive hydrogels for oral disease treatment. Mater. Des. 2024, 112817. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Huang, W.; Lei, Y. Injectable smart stimuli-responsive hydrogels: Pioneering advancements in biomedical applications. Biomater. Sci. 2024, 12, 8–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Weir, M.D.; Melo, M.A.S.; Strassler, H.E.; Xu, H.H.K. Effects of water-aging on self-healing dental composite containing microcapsules. J. Dent. 2016, 47, 86–93. [Google Scholar] [CrossRef]
- Carbonell-Blasco, M.P.; Moyano, M.A.; Hernández-Fernández, C.; Sierra-Molero, F.J.; Pastor, I.M.; Alonso, D.A.; Arán-Aís, F.; Orgilés-Calpena, E. Polyurethane Adhesives with Chemically Debondable Properties via Diels–Alder Bonds. Polymers 2023, 16, 21. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining Diels–Alder click reaction and acylhydrazone bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, A.; Kharaziha, M.; Khoroushi, M. Dentin extracellular matrix loaded bioactive glass/GelMA support rapid bone mineralization for potential pulp regeneration. Int. J. Biol. Macromol 2023, 234, 123771. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; Xiao, W.; Wang, P.; Wang, J.; Zou, D.; Luo, X.; Shi, L.; Wu, J.; Guo, L.; et al. Mussel-inspired adhesive drug-loaded hydrogels for oral ulcers treatment. Acta Biomater. 2024, 187, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Holcroft, J.; Ikeda, E.; Ganss, B. Amelotin Promotes Mineralization and Adhesion in Collagen-Based Systems. Cell Mol. Bioeng. 2022, 15, 245–254. [Google Scholar] [CrossRef]
- An, Y.H.; Yu, S.J.; Kim, I.S.; Kim, S.H.; Moon, J.M.; Kim, S.L.; Choi, Y.H.; Choi, J.S.; Im, S.G.; Lee, K.E. Hydrogel functionalized Janus membrane for skin regeneration. Adv. Healthc. Mater. 2017, 6, 1600795. [Google Scholar] [CrossRef]
- Basak, S.; Bandyopadhyay, A. Solvent responsive shape memory polymers-evolution, current status, and future outlook. Macromol. Chem. Phys. 2021, 222, 2100195. [Google Scholar] [CrossRef]
- Zhao, H.; Akiba, N.; Tanimoto, H.; Yoshizaki, T.; Yalikun, K.; Minakuchi, S. Effects of temperature-responsive hydrogel on viscosity of denture adhesives. Dent. Mater. J. 2016, 35, 210–215. [Google Scholar] [CrossRef][Green Version]
- Alia, A.; Gao, F.; Mitchell, J.C.; Gasiorowski, J.; Ciancio, M.; Kuppast, B.; Pfeifer, C.; Carrilho, M.R. Dentin primer based on a highly functionalized gelatin-methacryloyl hydrogel. Dental Materials 2023, 39, 192–203. [Google Scholar] [CrossRef]
- Mohamed, M.; Wahied, D.M.; Mohsen, R.; El Wakeel, A.M. The Effect of Poly (N-Isopropylacrylamide) Based Microgel Application on the Microtensile Bond Strength to Dentin: An In Vitro Study. Egypt. Dent. J. 2024, 70, 2753–2765. [Google Scholar] [CrossRef]
- Xie, S.X.; Boone, K.; VanOosten, S.K.; Yuca, E.; Song, L.; Ge, X.; Ye, Q.; Spencer, P.; Tamerler, C. Peptide Mediated Antimicrobial Dental Adhesive System. Appl. Sci. 2019, 9, 557. [Google Scholar] [CrossRef]
- Purcell, B.P.; Lobb, D.; Charati, M.B.; Dorsey, S.M.; Wade, R.J.; Zellars, K.N.; Doviak, H.; Pettaway, S.; Logdon, C.B.; Shuman, J.A.; et al. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat. Mater. 2014, 13, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Muntean, A.; Sarosi, C.; Petean, I.; Cuc, S.; Carpa, R.; Chis, I.A.; Ilea, A.; Delean, A.G.; Moldovan, M. Developing Bioactive Hydrogels with Peptides for Dental Application. Biomedicines 2024, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Su, Z.; Xiang, Z.; Xu, H.H.; Weir, M.D.; Fan, M.; Yu, Z.; Zhou, X.; Liang, K.; Li, J. Nano-calcium phosphate and dimethylaminohexadecyl methacrylate adhesive for dentin remineralization in a biofilm-challenged environment. Dent. Mater. 2020, 36, e316–e328. [Google Scholar] [CrossRef] [PubMed]
- Almahdy, A.; Koller, G.; Sauro, S.; Bartsch, J.; Sherriff, M.; Watson, T.; Banerjee, A. Effects of MMP inhibitors incorporated within dental adhesives. J. Dent. Res. 2012, 91, 605–611. [Google Scholar] [CrossRef]
- Zheng, P.; Chen, H. Evaluate the effect of different mmps inhibitors on adhesive physical properties of dental adhesives, bond strength and mmp substarte activity. Sci. Rep. 2017, 7, 4975. [Google Scholar] [CrossRef]
- Breschi, L.; Martin, P.; Mazzoni, A.; Nato, F.; Carrilho, M.; Tjäderhane, L.; Visintini, E.; Cadenaro, M.; Tay, F.R.; Dorigo, E.D.S. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent. Mater. 2010, 26, 571–578. [Google Scholar] [CrossRef]
- Oguz Ahmet, S.; Mutluay, M.M.; Seyfioglu Polat, Z.; Seseogullari Dirihan, R.; Bek, B.; Tezvergil-Mutluay, A. Addition of benzalkonium chloride to self-adhesive resin-cements: Some clinically relevant properties. Acta Odontol. Scand 2014, 72, 831–838. [Google Scholar] [CrossRef]
- Khamverdi, Z.; Rezaei-Soufi, L.; Rostamzadeh, T. The effect of epigallocatechin gallate on the dentin bond durability of two self-etch adhesives. J. Dent. 2015, 16, 68. [Google Scholar]
- Bedran-Russo, A.; Yoo, K.; Ema, K.; Pashley, D. Mechanical properties of tannic-acid-treated dentin matrix. J. Dent. Res. 2009, 88, 807–811. [Google Scholar] [CrossRef]
- Zhang, J.; He, X.; Yu, S.; Zhu, J.; Wang, H.; Tian, Z.; Zhu, S.; Cui, Z. A novel dental adhesive containing Ag/polydopamine-modified HA fillers with both antibacterial and mineralization properties. J. Dent. 2021, 111, 103710. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Cheng, L.; Weir, M.D.; Hsia, R.C.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2013, 101, 620–629. [Google Scholar] [CrossRef]
- Gou, Y.-P.; Meghil, M.M.; Pucci, C.R.; Breschi, L.; Pashley, D.H.; Cutler, C.W.; Niu, L.-n.; Li, J.-y.; Tay, F.R. Optimizing resin-dentin bond stability using a bioactive adhesive with concomitant antibacterial properties and anti-proteolytic activities. Acta Biomater. 2018, 75, 171–182. [Google Scholar] [CrossRef]
- Seifi, M.; Eskandarloo, F.; Amdjadi, P.; Farmany, A. Investigation of mechanical properties, remineralization, antibacterial effect, and cellular toxicity of composite orthodontic adhesive combined with silver-containing nanostructured bioactive glass. BMC Oral Health 2024, 24, 650. [Google Scholar] [CrossRef] [PubMed]
- Elasser, D.M.; Niazy, M.A.; Elsharkawy, D.A.; Mansour, M.S. The remineralizing potential of nano bioactive glass versus nanohydroxyapatite on dentine as affected by pH cycling. Al-Azhar Dent. J. Girls 2018, 5, 327–334. [Google Scholar] [CrossRef][Green Version]
- Khvostenko, D.; Hilton, T.; Ferracane, J.; Mitchell, J.; Kruzic, J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Farooq, I.; Moheet, I.A.; AlShwaimi, E. In vitro dentin tubule occlusion and remineralization competence of various toothpastes. Arch. Oral Biol. 2015, 60, 1246–1253. [Google Scholar] [CrossRef]
- Aboelenein, A.Z.; Riad, M.I.; Haridy, M.F. Effect of a self-etch adhesive containing nanobioglass on postoperative sensitivity of posterior composite restorations-a randomized trial. Maced. J. Med. Sci. 2019, 7, 2313. [Google Scholar] [CrossRef] [PubMed]
- Samir, N.K.M.; El-Hoshy, A.; Helal, H.; Sayed, Y. One-Year Clinical Evaluation of a Fluoride, Calcium and Phosphate-Releasing New Bioactive Material versus a Fluoride-Releasing Hybrid Restorative Material in Cervical Lesions: A Randomized Clinical Trial. Adv. Dent. J. 2025, 7, 457–472. [Google Scholar] [CrossRef]
- Machado, A.C.; Maximiano, V.; Yoshida, M.L.; Freitas, J.G.; Mendes, F.M.; Aranha, A.C.C.; Scaramucci, T. Efficacy of a calcium-phosphate/fluoride varnish and ionomeric sealant on cervical dentin hypersensitivity: A randomized, double-blind, placebo-controlled clinical study. J. Oral Rehabil. 2022, 49, 62–70. [Google Scholar] [CrossRef] [PubMed]
- De Caluwé, T.; Vercruysse, C.; Declercq, H.; Schaubroeck, D.; Verbeeck, R.; Martens, L. Bioactivity and biocompatibility of two fluoride containing bioactive glasses for dental applications. Dent. Mater. 2016, 32, 1414–1428. [Google Scholar] [CrossRef]
- Bahador, A.; Ayatollahi, B.; Akhavan, A.; Pourhajibagher, M.; Kharazifard, M.J.; Sodagar, A. Antimicrobial efficacy of silver nanoparticles incorporated in an orthodontic adhesive: An animal study. Front. Dent. 2020, 17, 14. [Google Scholar] [CrossRef]
- Ekrikaya, S.; Yilmaz, E.; Arslan, S.; Karaaslan, R.; Ildiz, N.; Celik, C.; Ocsoy, I. Dentin bond strength and antimicrobial activities of universal adhesives containing silver nanoparticles synthesized with Rosa canina extract. Clin. Oral Investig. 2023, 27, 6891–6902. [Google Scholar] [CrossRef]
- Cascales, Á.F.; Moscardó, A.P.; Toledano, M.; Banerjee, A.; Sauro, S. An in-vitro investigation of the bond strength of experimental ion-releasing dental adhesives to caries-affected dentine after 1 year of water storage. J. Dent. 2022, 119, 104075. [Google Scholar] [CrossRef]
- Tohidkhah, S.; Kermanshah, H.; Ahmadi, E.; Jalalian, B.; Ranjbar Omrani, L. Marginal microleakage and modified microtensile bond strength of Activa Bioactive, in comparison with conventional restorative materials. Clin. Exp. Dent. Rese. 2022, 8, 329–335. [Google Scholar] [CrossRef]
- Oltramare, R.; Par, M.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Short-and long-term dentin bond strength of bioactive glass-modified dental adhesives. Nanomaterials 2021, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Farooq, I.; Ali, S.; Al-Saleh, S.; AlHamdan, E.M.; AlRefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic effect of bioactive inorganic fillers in enhancing properties of dentin adhesives—A review. Polymers 2021, 13, 2169. [Google Scholar] [CrossRef]
- Almulhim, K.S.; Syed, M.R.; Alqahtani, N.; Alamoudi, M.; Khan, M.; Ahmed, S.Z.; Khan, A.S. Bioactive inorganic materials for dental applications: A narrative review. Materials 2022, 15, 6864. [Google Scholar] [CrossRef]
- Kreutz, M.; Kreutz, C.; Kanzow, P.; Tauböck, T.T.; Burrer, P.; Noll, C.; Bader, O.; Rohland, B.; Wiegand, A.; Rizk, M. Effect of Bioactive and Antimicrobial Nanoparticles on Properties and Applicability of Dental Adhesives. Nanomaterials 2022, 12, 3862. [Google Scholar] [CrossRef]
- Abedin, F.; Ye, Q.; Good, H.J.; Parthasarathy, R.; Spencer, P. Polymerization- and solvent-induced phase separation in hydrophilic-rich dentin adhesive mimic. Acta Biomater. 2014, 10, 3038–3047. [Google Scholar] [CrossRef]
- Belli, R.; Kreppel, S.; Petschelt, A.; Hornberger, H.; Boccaccini, A.R.; Lohbauer, U. Strengthening of dental adhesives via particle reinforcement. J. Mech. Behav. Biomed. Mater. 2014, 37, 100–108. [Google Scholar] [CrossRef]
- Aydınoğlu, A.; Yoruç, A.B.H. Effects of silane-modified fillers on properties of dental composite resin. Mater. Sci. Eng. C 2017, 79, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, K.; Wang, Z.; Li, B. Optimal resin monomer ratios for light-cured dental resins. Heliyon 2022, 8, e10554. [Google Scholar] [CrossRef] [PubMed]
- Mokeem, L.S.; Garcia, I.M.; Melo, M.A. Degradation and Failure Phenomena at the Dentin Bonding Interface. Biomedicines 2023, 11, 1256. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, R.; Yao, W.; Ma, L.; Li, B. Synergistic effect of ion-releasing fillers on the remineralization and mechanical properties of resin-dentin bonding interfaces. Biomed. Phys. Eng. Express 2023, 9, 062001. [Google Scholar] [CrossRef]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, e41–e53. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nizami, M.Z.I.; Jindarojanakul, A.; Ma, Q.; Lee, S.J.; Sun, J. Advances in Bioactive Dental Adhesives for Caries Prevention: A State-of-the-Art Review. J. Funct. Biomater. 2025, 16, 418. https://doi.org/10.3390/jfb16110418
Nizami MZI, Jindarojanakul A, Ma Q, Lee SJ, Sun J. Advances in Bioactive Dental Adhesives for Caries Prevention: A State-of-the-Art Review. Journal of Functional Biomaterials. 2025; 16(11):418. https://doi.org/10.3390/jfb16110418
Chicago/Turabian StyleNizami, Mohammed Zahedul Islam, Apissada Jindarojanakul, Qiang Ma, Sang J. Lee, and Jirun Sun. 2025. "Advances in Bioactive Dental Adhesives for Caries Prevention: A State-of-the-Art Review" Journal of Functional Biomaterials 16, no. 11: 418. https://doi.org/10.3390/jfb16110418
APA StyleNizami, M. Z. I., Jindarojanakul, A., Ma, Q., Lee, S. J., & Sun, J. (2025). Advances in Bioactive Dental Adhesives for Caries Prevention: A State-of-the-Art Review. Journal of Functional Biomaterials, 16(11), 418. https://doi.org/10.3390/jfb16110418








