Pre-Vascularized 3-Dimensional Skin Substitutes Promote Angiogenesis and Tissue Repair in a Murine Model of Refractory Skin Ulcers
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Construction of Vascularized 3D Skin Substitute
2.3. Animal Experiments
2.4. Evaluation of Wound State
2.5. Histological Observation and Immunohistochemistry
2.6. Statistical Analysis
3. Results
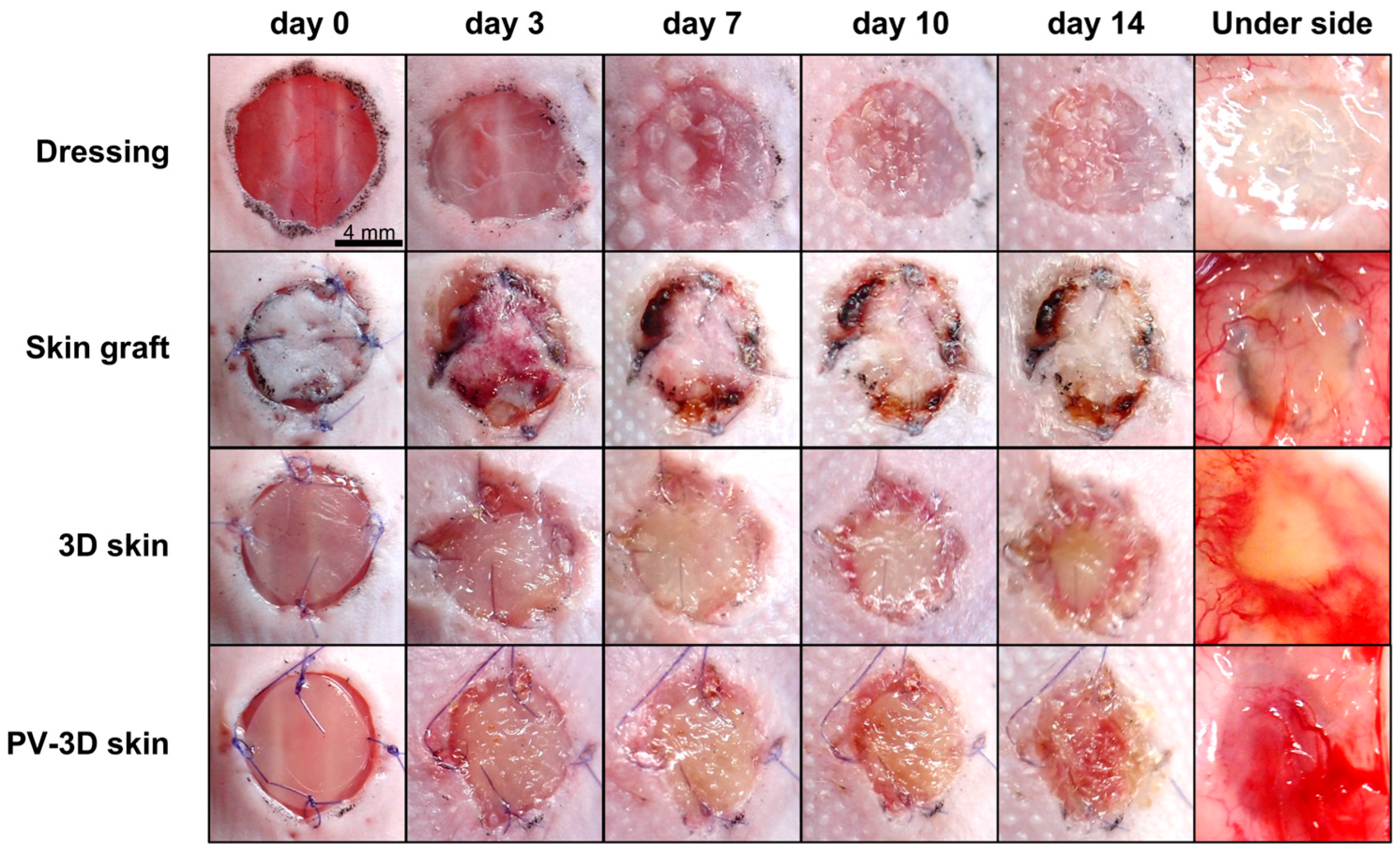
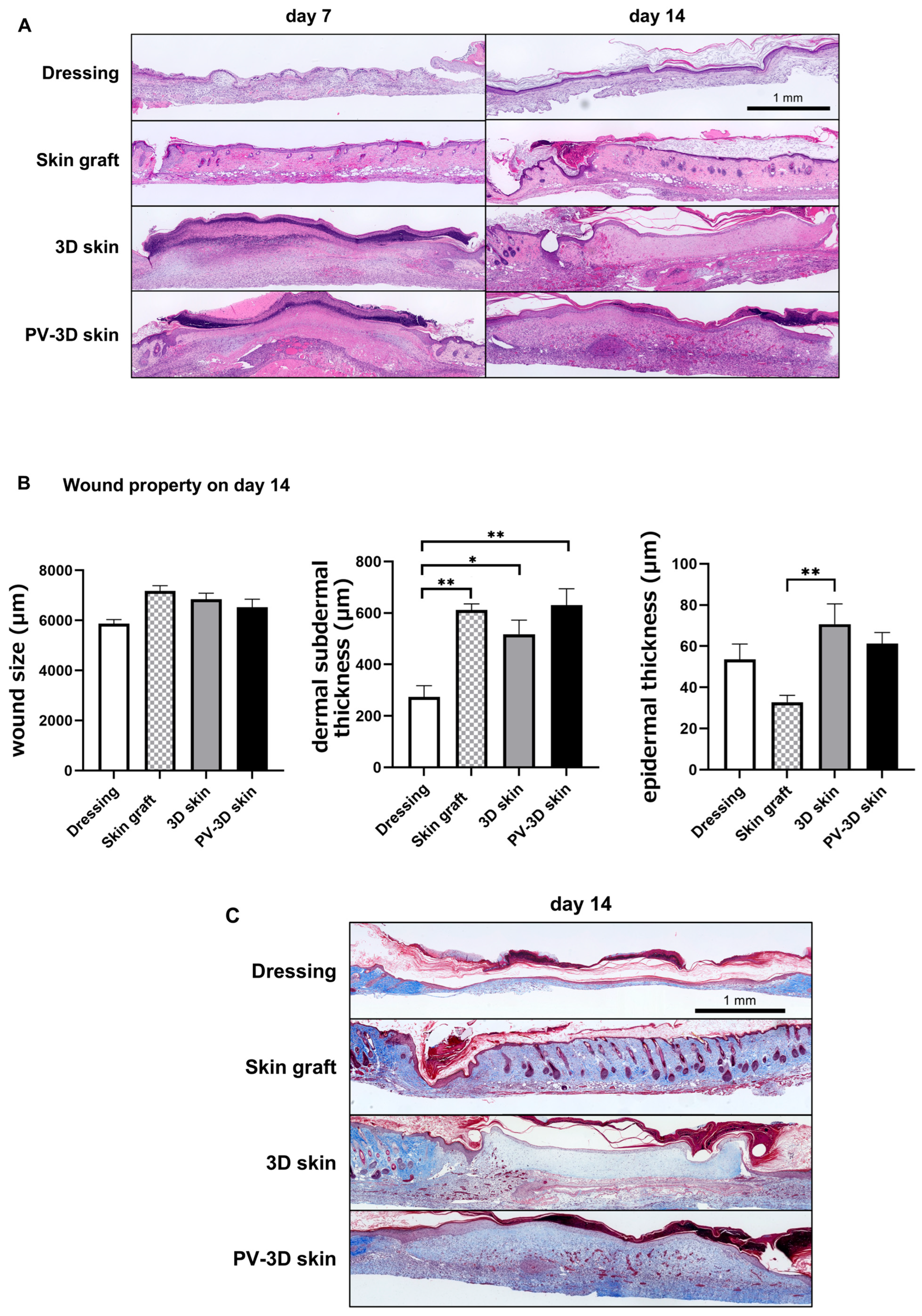
3.1. PV-3D Skin Accelerated Wound Healing in Refractory Ulcer
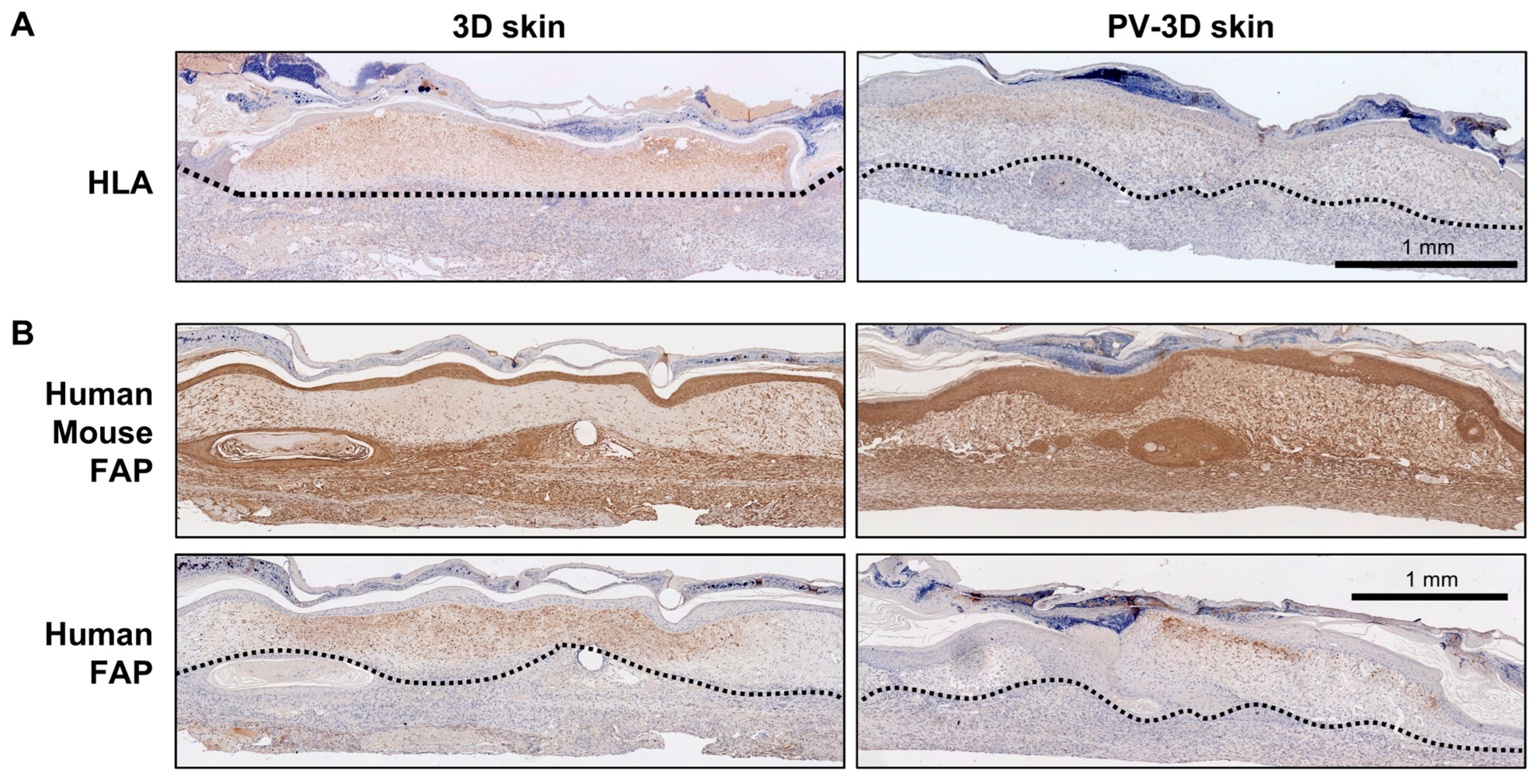
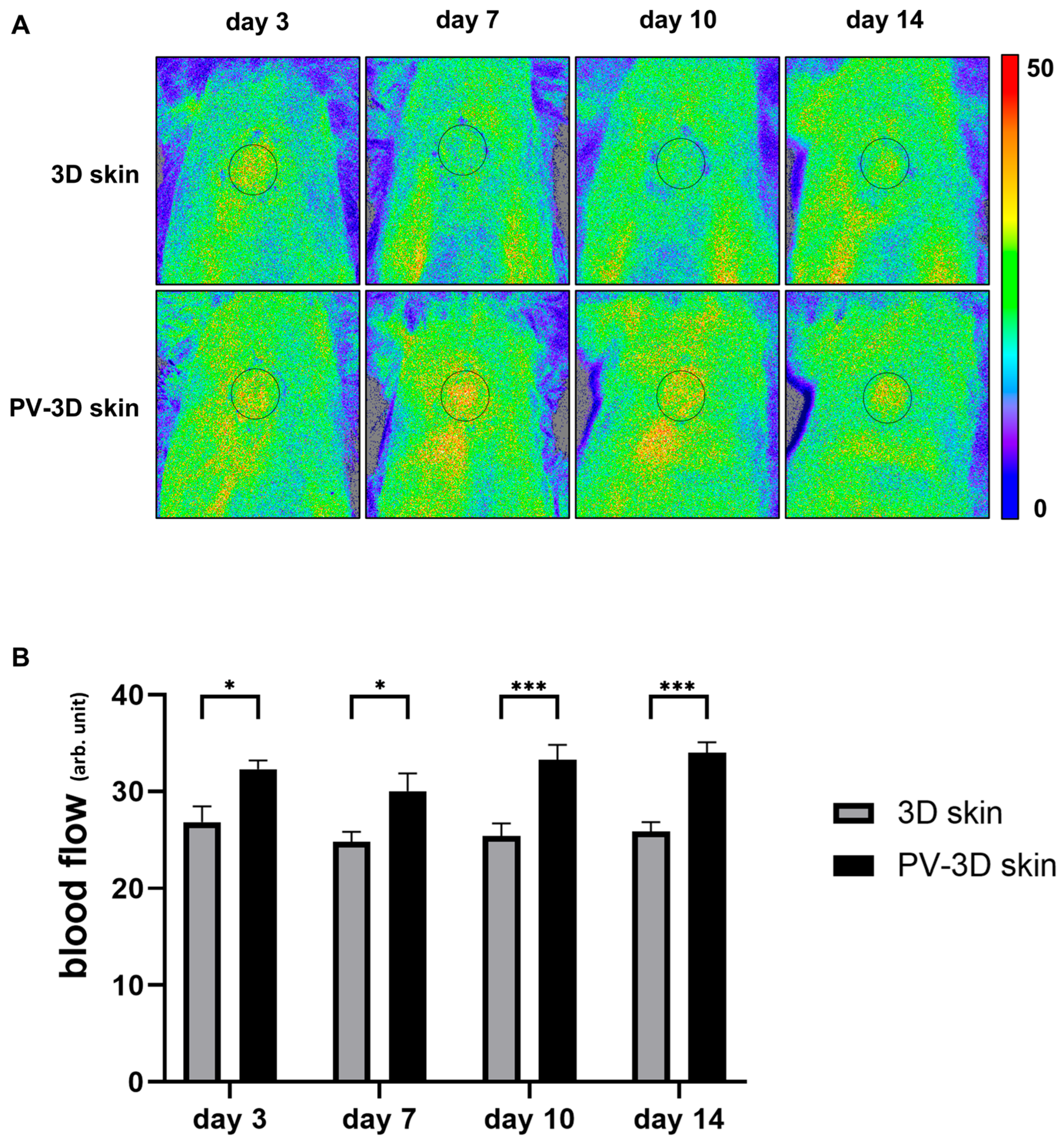
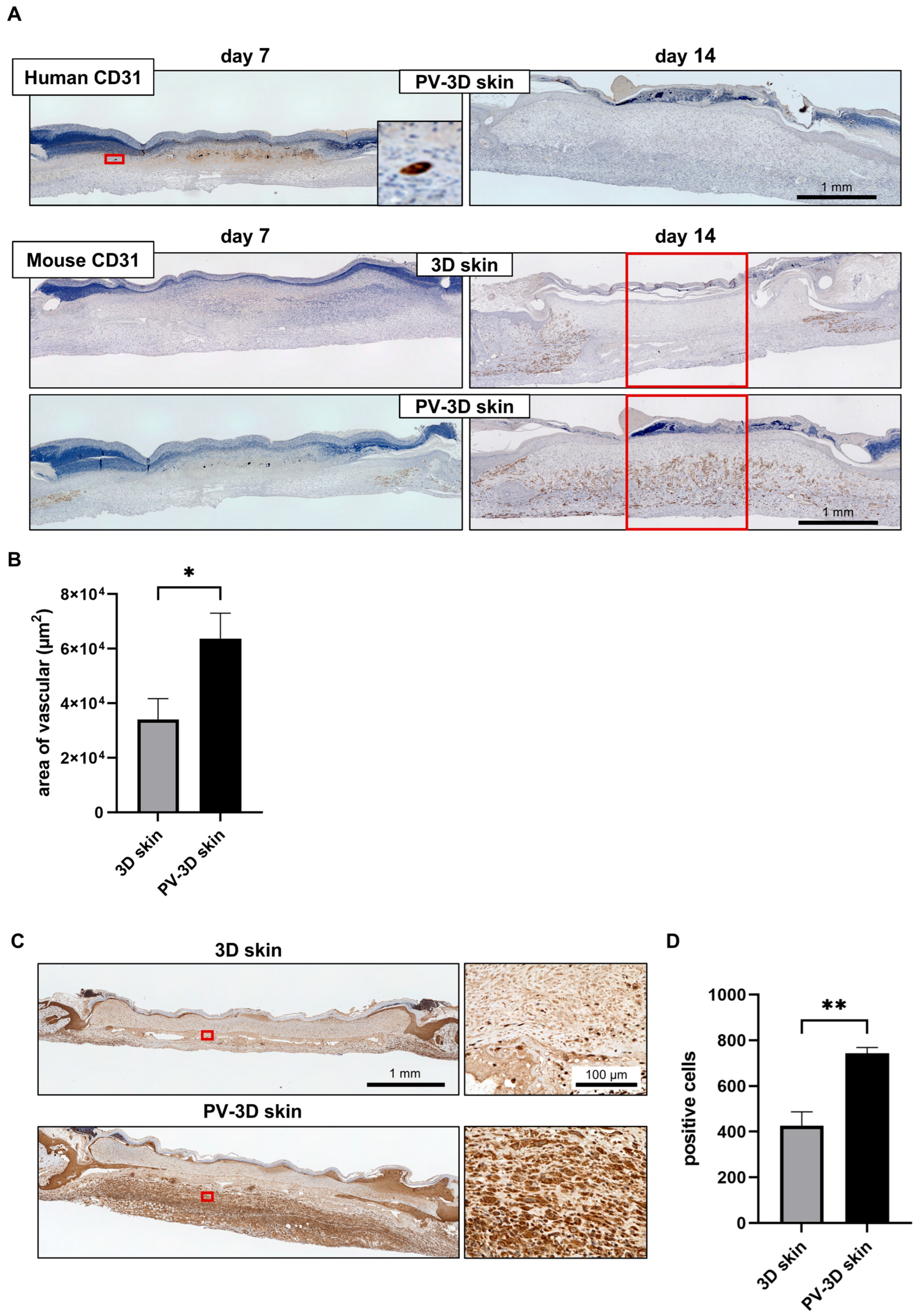
3.2. Influence of Vascular Structure on Wound Healing
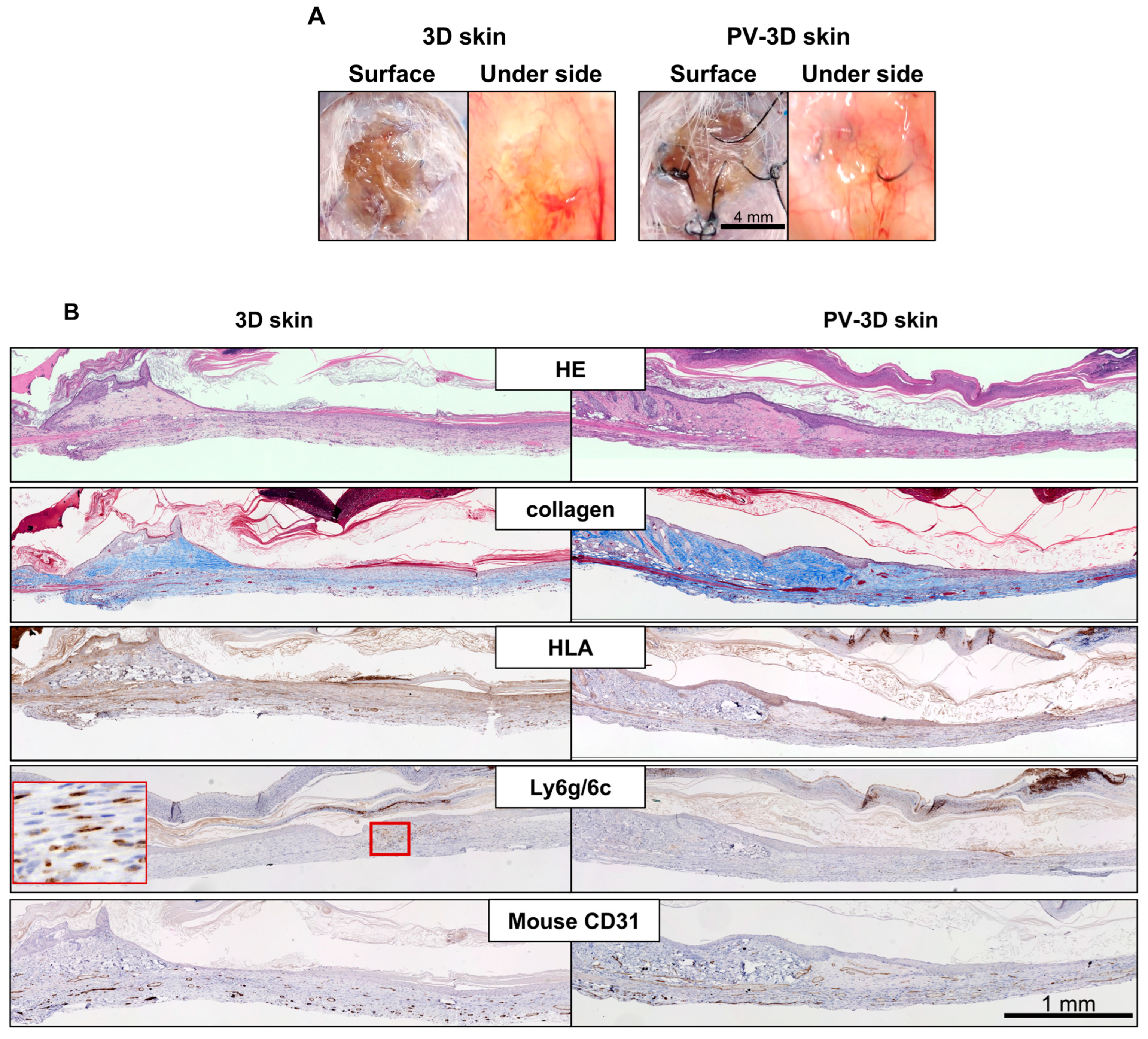
3.3. Long Term Observation of PV-3D Skin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| cAMP | cyclic adenosine monophosphate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FAP | Fibroblast activation protein |
| FGF | fibroblast growth factor |
| FN | fibronectin |
| G | gelatin |
| H&E | Hematoxylin and Eosin |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| HLA | Human leukocyte antigen |
| HMVECs | Human dermal microvascular endothelial cells |
| IgG | immunoglobulin G |
| MMC | mitomycin C |
| NHDFs | Neonatal normal human dermal fibroblasts |
| NHEKs | Neonatal human epidermal keratinocytes |
| PBS | phosphate-buffered saline |
| PGs | prostaglandins |
| PV-3D skin | pre-vascularized three-dimensional skin substitutes |
| SCID | severe combined immunodeficiency |
References
- Mudge, E.; Price, P.; Walkley, N.; Harding, K.G. A randomized controlled trial of larval therapy for the debridement of leg ulcers: Results of a multicenter, randomized, controlled, open, observer blind, parallel group study. Wound Repair Regen. 2014, 22, 43–51. [Google Scholar] [CrossRef]
- Wilcox, J.R.; Carter, M.J.; Covington, S. Frequency of debridements and time to heal: A retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013, 149, 1050–1058. [Google Scholar] [CrossRef]
- Banakh, I.; Cheshire, P.; Rahman, M.; Carmichael, I.; Jagadeesan, P.; Cameron, N.R.; Cleland, H.; Akbarzadeh, S. A Comparative Study of Engineered Dermal Templates for Skin Wound Repair in a Mouse Model. Int. J. Mol. Sci. 2020, 21, 4508. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, D.; Zeng, J.; Fu, Q.; Chen, Z.; Sun, X.; Yang, Y.; Li, S.; Chen, M. Application of 3D-printed tissue-engineered skin substitute using innovative biomaterial loaded with human adipose-derived stem cells in wound healing. Int. J. Bioprint 2023, 9, 674. [Google Scholar] [CrossRef]
- Romanelli, M.; Dini, V.; Bertone, M.S. Randomized comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult-to-heal wounds of mixed arterial/venous etiology. Adv. Skin Wound Care 2010, 23, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.B.; Zheng, X. Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. S1), 179–185. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625, quiz 625-606. [Google Scholar] [CrossRef]
- Miyazaki, H.; Tsunoi, Y.; Akagi, T.; Sato, S.; Akashi, M.; Saitoh, D. A novel strategy to engineer pre-vascularized 3-dimensional skin substitutes to achieve efficient, functional engraftment. Sci. Rep. 2019, 9, 7797. [Google Scholar] [CrossRef]
- Matsusaki, M.; Kadowaki, K.; Nakahara, Y.; Akashi, M. Fabrication of cellular multilayers with nanometer-sized extracellular matrix films. Angew. Chem. Int. Ed. Engl. 2007, 46, 4689–4692. [Google Scholar] [CrossRef]
- Nagano, H.; Suematsu, Y.; Takuma, M.; Aoki, S.; Satoh, A.; Takayama, E.; Kinoshita, M.; Morimoto, Y.; Takeoka, S.; Fujie, T.; et al. Enhanced cellular engraftment of adipose-derived mesenchymal stem cell spheroids by using nanosheets as scaffolds. Sci. Rep. 2021, 11, 14500. [Google Scholar] [CrossRef]
- Seno, S.I.; Shimazu, H.; Kobayashi, H.; Kogure, E.; Watanabe, A.; Isoyama, T. Quantitative evaluation of skin barrier function using water evaporation time related to transepidermal water loss. Skin Res. Technol. 2023, 29, e13242. [Google Scholar] [CrossRef]
- Surinchak, J.S.; Malinowski, J.A.; Wilson, D.R.; Maibach, H.I. Skin wound healing determined by water loss. J. Surg. Res. 1985, 38, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Byeon, J.Y.; Lee, D.W.; Kim, J.H.; Lim, S.; Choi, H.J. Skin graft monitoring using forward-looking infrared thermal imaging. Int. Wound J. 2024, 21, e70107. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.L.; Gaillard-Bigot, F.; Cracowski, C.; Roustit, M.; Millet, C. Skin microdialysis coupled with laser speckle contrast imaging to assess microvascular reactivity. Microvasc. Res. 2011, 82, 333–338. [Google Scholar] [CrossRef]
- Linkous, C.; Pagan, A.D.; Shope, C.; Andrews, L.; Snyder, A.; Ye, T.; Valdebran, M. Applications of Laser Speckle Contrast Imaging Technology in Dermatology. JID Innov. 2023, 3, 100187. [Google Scholar] [CrossRef] [PubMed]
- Mahé, G.; Humeau-Heurtier, A.; Durand, S.; Leftheriotis, G.; Abraham, P. Assessment of skin microvascular function and dysfunction with laser speckle contrast imaging. Circ. Cardiovasc. Imaging 2012, 5, 155–163. [Google Scholar] [CrossRef]
- Mahé, G.; Rousseau, P.; Durand, S.; Bricq, S.; Leftheriotis, G.; Abraham, P. Laser speckle contrast imaging accurately measures blood flow over moving skin surfaces. Microvasc. Res. 2011, 81, 183–188. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef]
- Nambu, M.; Ishihara, M.; Nakamura, S.; Mizuno, H.; Yanagibayashi, S.; Kanatani, Y.; Hattori, H.; Takase, B.; Ishizuka, T.; Kishimoto, S.; et al. Enhanced healing of mitomycin C-treated wounds in rats using inbred adipose tissue-derived stromal cells within an atelocollagen matrix. Wound Repair Regen. 2007, 15, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.T.; Gadre, S.A.; Calhoun, K.H. The effects of intradermal and topical mitomycin C on wound healing. Otolaryngol. Head Neck Surg. 2006, 135, 56–60. [Google Scholar] [CrossRef]
- Ribeiro, F.e.A.; Guaraldo, L.; Borges, J.e.P.; Vianna, M.R.; Eckley, C.A. Study of wound healing in rats treated with topical and injected mitomycin C. Ann. Otol. Rhinol. Laryngol. 2008, 117, 786–790. [Google Scholar] [CrossRef]
- Takikawa, M.; Ishihara, M.; Takabayashi, Y.; Sumi, Y.; Yoshida, R.; Nakamura, S.; Hattori, H.; Yanagibayashi, S.; Yamamoto, N.; Kiyosawa, T. Enhanced healing of mitomycin C-treated healing-impaired wounds in rats with PRP-containing fragmin/protamine microparticles (PRP&F/P MPs). J. Plast. Surg. Hand Surg. 2015, 49, 268–274. [Google Scholar] [CrossRef]
- Dachlan, I.; Kurniawan, H.S.; Wicaksana, A.; Fauzi, A.R.; Makrufardi, F.; Seswandhana, R. The effect of platelet-rich fibrin on normal dermal fibroblast proliferation after mitomycin-c treatment: An in vitro study. Ann. Med. Surg. 2021, 62, 473–476. [Google Scholar] [CrossRef]
- Bradner, W.T. Mitomycin C: A clinical update. Cancer Treat. Rev. 2001, 27, 35–50. [Google Scholar] [CrossRef]
- McKenna, E.; Traganos, F.; Zhao, H.; Darzynkiewicz, Z. Persistent DNA damage caused by low levels of mitomycin C induces irreversible cell senescence. Cell Cycle 2012, 11, 3132–3140. [Google Scholar] [CrossRef]
- Wang, Y.W.; Ren, J.H.; Xia, K.; Wang, S.H.; Yin, T.F.; Xie, D.H.; Li, L.H. Effect of mitomycin on normal dermal fibroblast and HaCat cell: An in vitro study. J. Zhejiang Univ. Sci. B 2012, 13, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Frueh, F.S.; Spater, T.; Korbel, C.; Scheuer, C.; Simson, A.C.; Lindenblatt, N.; Giovanoli, P.; Menger, M.D.; Laschke, M.W. Prevascularization of dermal substitutes with adipose tissue-derived microvascular fragments enhances early skin grafting. Sci. Rep. 2018, 8, 10977. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.L.; Song, S.H.; Choi, K.S.; Suh, W. Cooperation of endothelial and smooth muscle cells derived from human induced pluripotent stem cells enhances neovascularization in dermal wounds. Tissue Eng. Part A 2013, 19, 2478–2485. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Huang, N.F.; Jamé, S.; Lee, J.C.; Nguyen, H.N.; Byers, B.; De, A.; Okogbaa, J.; Rollins, M.; Reijo-Pera, R.; et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e72–e79. [Google Scholar] [CrossRef]
- Chiu, A.; Sharma, D.; Zhao, F. Tissue Engineering-Based Strategies for Diabetic Foot Ulcer Management. Adv. Wound Care 2023, 12, 145–167. [Google Scholar] [CrossRef]
- Treadwell, T.; Sabolinski, M.L.; Skornicki, M.; Parsons, N.B. Comparative Effectiveness of a Bioengineered Living Cellular Construct and Cryopreserved Cadaveric Skin Allograft for the Treatment of Venous Leg Ulcers in a Real-World Setting. Adv. Wound Care 2018, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.C.; Stojadinovic, O.; Rosa, A.M.; Ramirez, H.A.; Badiavas, E.; Blumenberg, M.; Tomic-Canic, M. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci. Transl. Med. 2017, 9, eaaf8611. [Google Scholar] [CrossRef]
- Stone, R.C.; Stojadinovic, O.; Sawaya, A.P.; Glinos, G.D.; Lindley, L.E.; Pastar, I.; Badiavas, E.; Tomic-Canic, M. A bioengineered living cell construct activates metallothionein/zinc/MMP8 and inhibits TGFβ to stimulate remodeling of fibrotic venous leg ulcers. Wound Repair Regen. 2020, 28, 164–176. [Google Scholar] [CrossRef]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef]
- Mace, K.A.; Yu, D.H.; Paydar, K.Z.; Boudreau, N.; Young, D.M. Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 2007, 15, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Botusan, I.R.; Sunkari, V.G.; Savu, O.; Catrina, A.I.; Grünler, J.; Lindberg, S.; Pereira, T.; Ylä-Herttuala, S.; Poellinger, L.; Brismar, K.; et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. USA 2008, 105, 19426–19431. [Google Scholar] [CrossRef]
- Albina, J.E.; Mastrofrancesco, B.; Vessella, J.A.; Louis, C.A.; Henry, W.L.; Reichner, J.S. HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-alpha. Am. J. Physiol. Cell Physiol. 2001, 281, C1971–C1977. [Google Scholar] [CrossRef]
- Charles, J.; Harrison, C.; Britt, H. Chronic skin ulcers. Aust. Fam. Physician 2014, 43, 587. [Google Scholar] [PubMed]
- Kaufmann, R.; Mielke, V.; Reimann, J.; Klein, C.E.; Sterry, W. Cellular and molecular composition of human skin in long-term xenografts on SCID mice. Exp. Dermatol. 1993, 2, 209–216. [Google Scholar] [CrossRef]
- Soria, A.; Boccara, D.; Chonco, L.; Yahia, N.; Dufossée, M.; Cardinaud, S.; Moris, A.; Liard, C.; Joulin-Giet, A.; Julithe, M.; et al. Long-term maintenance of skin immune system in a NOD-Scid IL2rγ(null) mouse model transplanted with human skin. Exp. Dermatol. 2014, 23, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, G.J.; Kelly, J.P. Macrophage activation and innate resistance to infection in SCID mice. Immunobiology 1994, 191, 424–431. [Google Scholar] [CrossRef]
- Jimi, S.; Kimura, M.; De Francesco, F.; Riccio, M.; Hara, S.; Ohjimi, H. Acceleration Mechanisms of Skin Wound Healing by Autologous Micrograft in Mice. Int. J. Mol. Sci. 2017, 18, 1675. [Google Scholar] [CrossRef]
- Kim, S.W.; Zhang, H.Z.; Guo, L.; Kim, J.M.; Kim, M.H. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS ONE 2012, 7, e41105. [Google Scholar] [CrossRef]
- Karim, A.S.; Liu, A.; Lin, C.; Uselmann, A.J.; Eliceiri, K.W.; Brown, M.E.; Gibson, A.L.F. Evolution of ischemia and neovascularization in a murine model of full thickness human wound healing. Wound Repair Regen. 2020, 28, 812–822. [Google Scholar] [CrossRef]
- Li, G.; Ko, C.N.; Li, D.; Yang, C.; Wang, W.; Yang, G.J.; Di Primo, C.; Wong, V.K.W.; Xiang, Y.; Lin, L.; et al. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat. Commun. 2021, 12, 3363. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hilmi, A.B.; Hassan, A.; Halim, A.S. A Bilayer Engineered Skin Substitute for Wound Repair in an Irradiation-Impeded Healing Model on Rat. Adv. Wound Care 2015, 4, 312–320. [Google Scholar] [CrossRef]
- Zhu, N.N.; Lu, M.J.; Chen, Y.Q.; Jin, X.J.; Zhou, X.; Wei, H.W.; Liu, X.Q.; Duan, L.S.; Yin, L.; Guo, J.R. Autologous blood transfusion stimulates wound healing in diabetic mice through activation of the HIF-1α pathway by improving the blood preservation solution. FASEB J. 2020, 34, 6038–6054. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tojo, S.; Miyazaki, H.; Saiki, T.; Tsunoi, Y.; Nakamura, S.; Azuma, R. Pre-Vascularized 3-Dimensional Skin Substitutes Promote Angiogenesis and Tissue Repair in a Murine Model of Refractory Skin Ulcers. J. Funct. Biomater. 2025, 16, 409. https://doi.org/10.3390/jfb16110409
Tojo S, Miyazaki H, Saiki T, Tsunoi Y, Nakamura S, Azuma R. Pre-Vascularized 3-Dimensional Skin Substitutes Promote Angiogenesis and Tissue Repair in a Murine Model of Refractory Skin Ulcers. Journal of Functional Biomaterials. 2025; 16(11):409. https://doi.org/10.3390/jfb16110409
Chicago/Turabian StyleTojo, Shota, Hiromi Miyazaki, Takami Saiki, Yasuyuki Tsunoi, Shingo Nakamura, and Ryuichi Azuma. 2025. "Pre-Vascularized 3-Dimensional Skin Substitutes Promote Angiogenesis and Tissue Repair in a Murine Model of Refractory Skin Ulcers" Journal of Functional Biomaterials 16, no. 11: 409. https://doi.org/10.3390/jfb16110409
APA StyleTojo, S., Miyazaki, H., Saiki, T., Tsunoi, Y., Nakamura, S., & Azuma, R. (2025). Pre-Vascularized 3-Dimensional Skin Substitutes Promote Angiogenesis and Tissue Repair in a Murine Model of Refractory Skin Ulcers. Journal of Functional Biomaterials, 16(11), 409. https://doi.org/10.3390/jfb16110409







