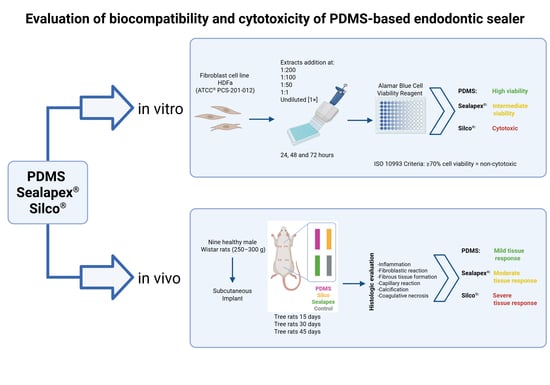
In Vitro and In Vivo Evaluation of a New Experimental Polydimethylsiloxane-Based Endodontic Sealer
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Study
2.1.1. Cell Line
2.1.2. Composition of Endodontic Sealers
2.1.3. Endodontic Sealer Extracts
2.1.4. In Vitro Cytotoxicity Assay
2.2. In Vivo Study
2.2.1. Murine Model
2.2.2. Sealer Preparation
2.2.3. Subcutaneous Implant
2.2.4. Histological Evaluation
2.2.5. Statistical Analysis
3. Results
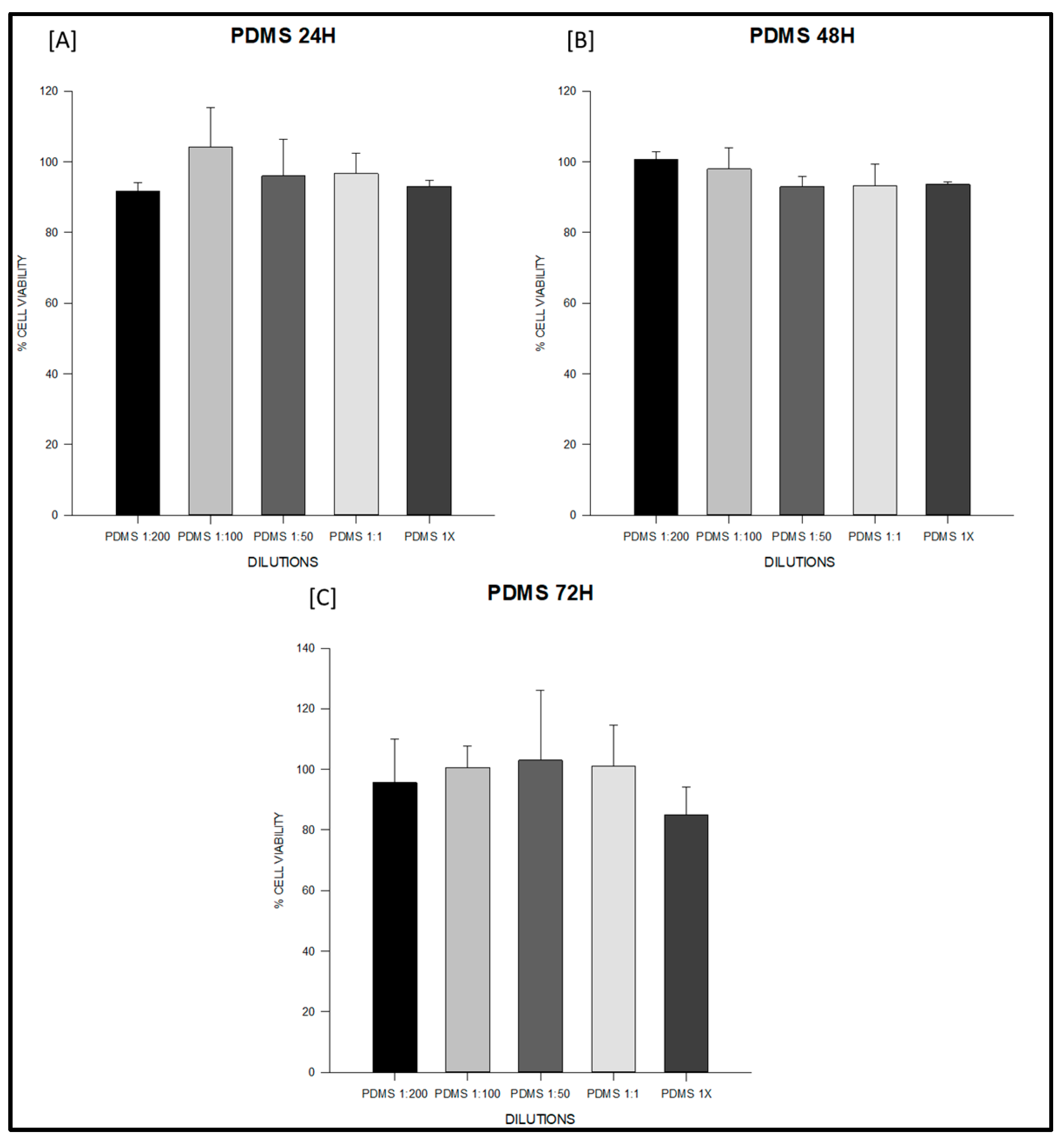
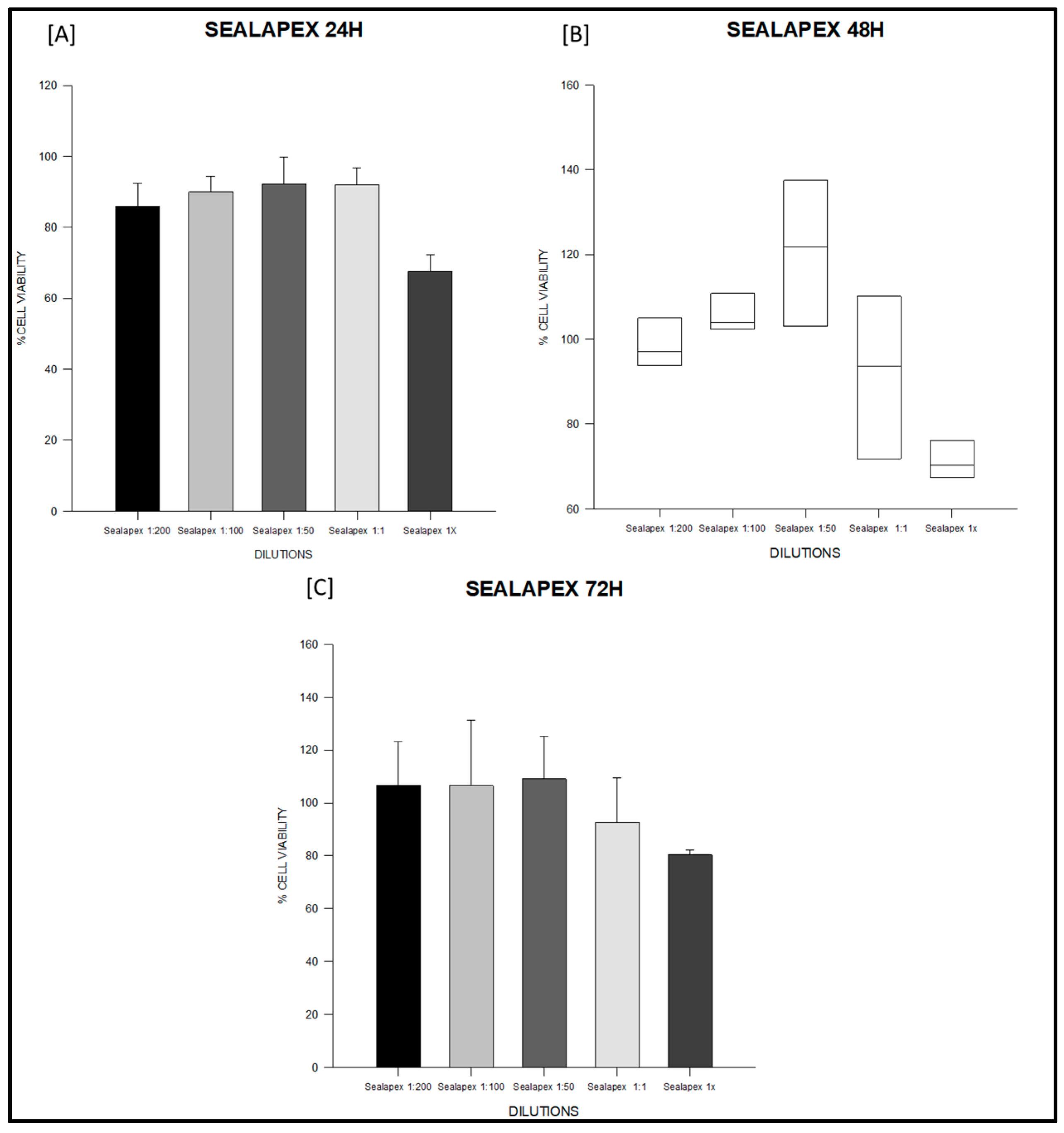
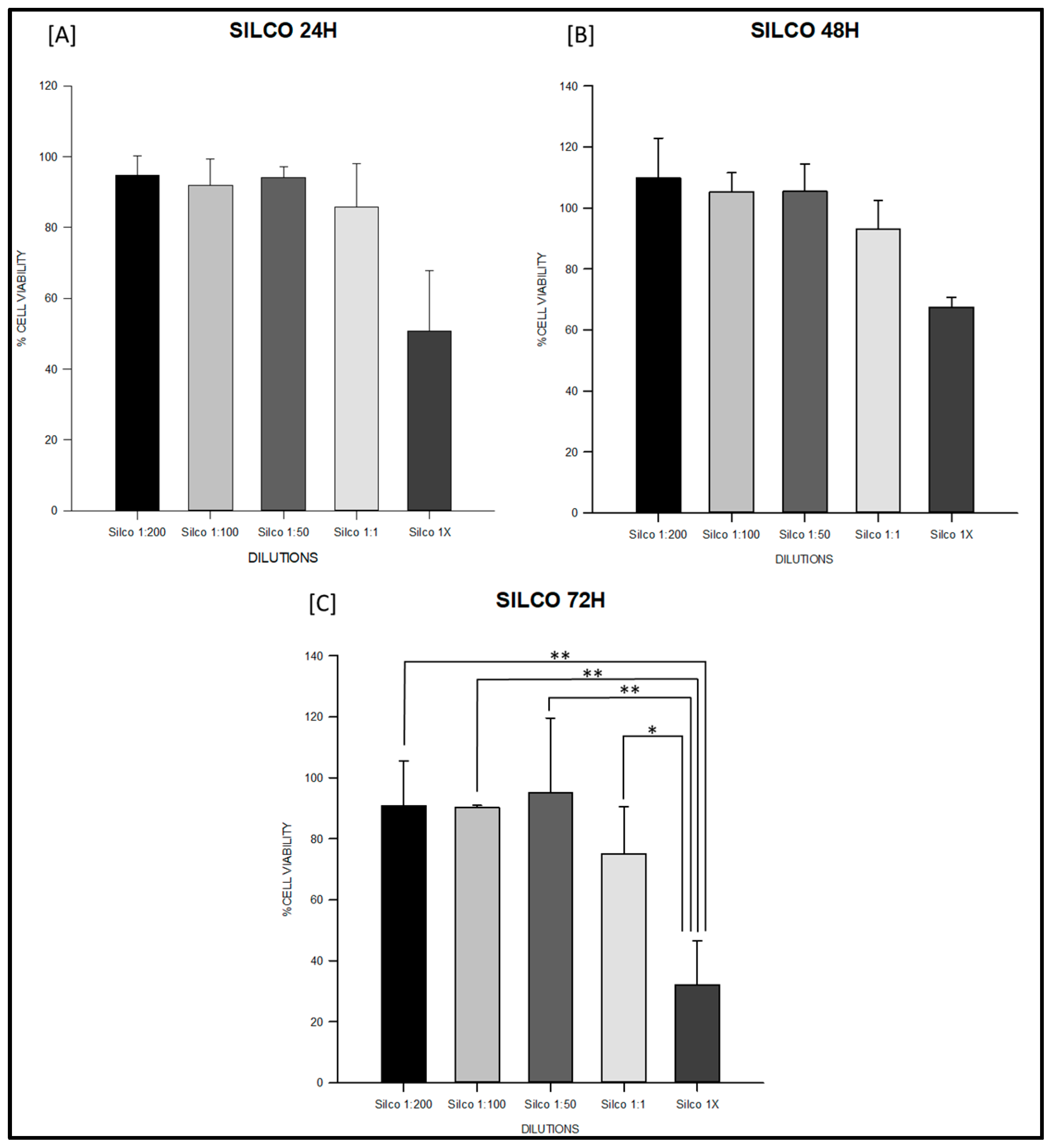
3.1. Cytotoxicity Test
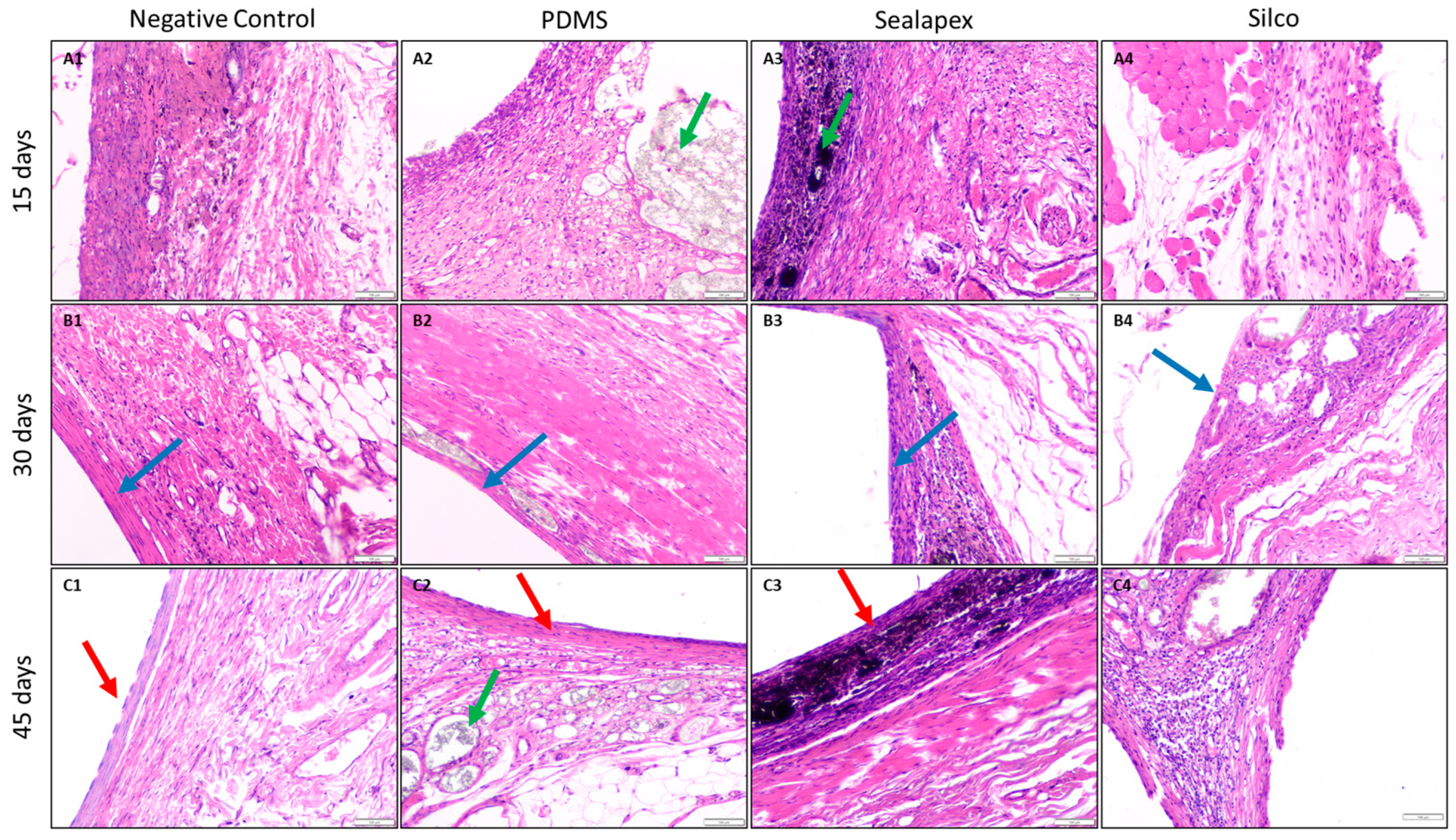
3.2. Biocompatibility Test
4. Discussion
5. Conclusions
6. Limitations and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kapralos, V.; Böcker, J.; Vach, K.; Altenburger, M.; Proksch, S.; Karygianni, L. On the biocompatibility of endodontic sealers. Swiss Dent. J. 2022, 132, 586–597. [Google Scholar] [CrossRef]
- Alrahabi, M.; Zafar, M.S.; Adanir, N. Aspects of Clinical Malpractice in Endodontics. Eur. J. Dent. 2019, 13, 450–458. [Google Scholar] [CrossRef]
- Komabayashi, T.; Colmenar, D.; Cvach, N.; Bhat, A.; Primus, C.; Imai, Y. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef]
- Özdemir, O.; Kopac, T. Cytotoxicity and biocompatibility of root canal sealers: A review on recent studies. J. Appl. Biomater. Funct. Mater. 2022, 20, 22808000221076325. [Google Scholar] [CrossRef]
- Gutmann, J.L.; Manjarrés, V.; De La Espriella, C.M. Revisiting the future of root canal obturation. Endodontology 2022, 34, 73–75. [Google Scholar] [CrossRef]
- Deniz Sungur, D.; Purali, N.; Cosgun, E.; Calt, S. Push-out bond strength and dentinal tubule penetration of different root canal sealers used with coated core materials. Restor. Dent. Endod. 2016, 41, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Suresh Chandra, B.; Gopikrishna, V. Grossman’s Endodontic Practice, 13th ed.; Wolters Kluwer: New Delhi, India, 2014. [Google Scholar]
- Eskandarinezhad, M.; Hooman Sadr Haghighi, A.; Khademnezhad, S.; Aghazadeh, Z.; Noruzani, F. Effect of incorporation of triphala into AH26 sealer on its cytotoxicity at different intervals. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Taddei, P.; Di Foggia, M.; Zamparini, F.; Prati, C.; Gandolfi, M.G. The Influence of the Matrix on the Apatite-Forming Ability of Calcium Containing Polydimethylsiloxane-Based Cements for Endodontics. Molecules 2022, 27, 5750. [Google Scholar] [CrossRef]
- Marconi, D.F.; da Silva, G.S.; Weissheimer, T.; Silva, I.A.; Só, G.B.; Jahnke, L.T.; Skupien, J.A.; Só, M.V.R.; da Rosa, R.A. Influence of the root canal filling technique on the success rate of primary endodontic treatments: A systematic review. Restor. Dent. Endod. 2022, 47, e40. [Google Scholar] [CrossRef]
- Endodontists, A.A. (Ed.) Glossary of Endodontic Terms, 10th ed.; American Association of Endodontists: Chicago, IL, USA, 2020. [Google Scholar]
- Bhandi, S.; Mashyakhy, M.; Abumelha, A.S.; Alkahtany, M.F.; Jamal, M.; Chohan, H.; Raj, A.T.; Testarelli, L.; Reda, R.; Patil, S. Complete Obturation-Cold Lateral Condensation vs. Thermoplastic Techniques: A Systematic Review of Micro-CT Studies. Materials 2021, 14, 4013. [Google Scholar] [CrossRef]
- Fonseca, D.A.; Paula, A.B.; Marto, C.M.; Coelho, A.; Paulo, S.; Martinho, J.P.; Carrilho, E.; Ferreira, M.M. Biocompatibility of Root Canal Sealers: A Systematic Review of In Vitro and In Vivo Studies. Materials 2019, 12, 4113. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Narloch, R. Generation of compliant, pre-clinical, biocompatibility data during characterization of medical devices. 1Med SA: Agno, Switzerland, 2021; pp. 7–13. [Google Scholar]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Herrera, R.; Rueda, J.A.M.; Fonseca, C.G.; Cuisinier, F.; Pérez, E. Intraradicular dentine silanization by a new silicon-based endodontic sealer. Int. J. Adhes. Adhes. 2016, 69, 115–124. [Google Scholar] [CrossRef]
- Collart Dutilleul, P.Y.; Fonseca, C.G.; Zimányi, L.; Romieu, O.; Pozos-Guillén, A.J.; Semetey, V.; Cuisinier, F.; Pérez, E.; Levallois, B. Root canal hydrophobization by dentinal silanization: Improvement of silicon-based endodontic treatment tightness. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 721–728. [Google Scholar] [CrossRef]
- Gaitan-Fonseca, C.; Collart-Dutilleul, P.Y.; Semetey, V.; Romieu, O.; Cruz, R.; Flores, H.; Cuisinier, F.; Pérez, E.; Pozos-Guillen, A. Chemical treatment of the intra-canal dentin surface: A new approach to modify dentin hydrophobicity. J. Appl. Oral Sci. Rev. FOB 2013, 21, 63–67. [Google Scholar] [CrossRef]
- Dem, K.; Wu, Y.; Kaminga, A.C.; Dai, Z.; Cao, X.; Zhu, B. The push out bond strength of polydimethylsiloxane endodontic sealers to dentin. BMC Oral Health 2019, 19, 181. [Google Scholar] [CrossRef]
- Jain, P.; Pruthi, V.; Sikri, V. An ex vivo evaluation of the sealing ability of polydimethylsiloxane-based root canal sealers. Indian J. Dent. Res. 2014, 25, 336–339. [Google Scholar] [CrossRef]
- Silva, E.J.; Neves, A.A.; De-Deus, G.; Accorsi-Mendonça, T.; Moraes, A.P.; Valentim, R.M.; Moreira, E.J. Cytotoxicity and gelatinolytic activity of a new silicon-based endodontic sealer. J. Appl. Biomater. Funct. Mater. 2015, 13, e376–e380. [Google Scholar] [CrossRef]
- ISO 6876:2012; Dentistry—Root Canal Sealing Materials. ISO: Geneva, Switzerland, 2012.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009; Volume 3, pp. 1–34.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021; Volume 5, pp. 1–21.
- Mireles-Carlos, Á.; Aguilera-Galaviz, L.A.; Robles-Martínez, M.; Villanueva-Sánchez, G.; Cepeda Argüelles, O.; Araujo-Espino, R.; Bermúdez-Jiménez, C.; Gaitán-Fonseca, C. Cytotoxicity and biocompatibility of a new endodontic sealant with polymethylsiloxane and Tyzor AA. Int. J. Polym. Mater. Polym. Biomater. 2021, 72, 40–48. [Google Scholar] [CrossRef]
- Santos, J.M.; Pereira, S.; Sequeira, D.B.; Messias, A.L.; Martins, J.B.; Cunha, H.; Palma, P.J.; Santos, A.C. Biocompatibility of a bioceramic silicone-based sealer in subcutaneous tissue. J. Oral Sci. 2019, 61, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Baldasso, F.E.; Kopper, P.M.; Morgental, R.D.; Steier, L.; Figueiredo, J.A.; Scarparo, R.K. Biological Tissue Response to a New Formulation of a Silicone Based Endodontic Sealer. Braz. Dent. J. 2016, 27, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Rangel Cobos, O.M.; Lara, C.A.L.; Téllez Garza, A.; Ley Fong, M.T. Obturación del sistema de conductos radiculares: Revisión de literatura. Rev. Asoc. Dent. Mex. 2018, 75, 269–272. [Google Scholar]
- Kevin Genez, V.P.; Posada Pérez, V.M.; Patricia Fernández-Morales, G.; Ramírez Patiño, J.F. Cytotoxic evaluation and biocompatibility of AZ31B alloy for applications in bone tissue engineering. Prospect 2016, 14, 7–12. [Google Scholar] [CrossRef]
- Collado-González, M.; Tomás-Catalá, C.J.; Oñate-Sánchez, R.E.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Cytotoxicity of GuttaFlow Bioseal, GuttaFlow2, MTA Fillapex, and AH Plus on Human Periodontal Ligament Stem Cells. J. Endod. 2017, 43, 816–822. [Google Scholar] [CrossRef]
- Szczurko, G.; Pawińska, M.; Łuczaj-Cepowicz, E.; Kierklo, A.; Marczuk-Kolada, G.; Hołownia, A. Effect of root canal sealers on human periodontal ligament fibroblast viability: Ex vivo study. Odontology 2018, 106, 245–256. [Google Scholar] [CrossRef]
- Accardo, C.; Himel, V.T.; Lallier, T.E. A novel GuttaFlow sealer supports cell survival and attachment. J. Endod. 2014, 40, 231–234. [Google Scholar] [CrossRef]
- Sequeira, D.B.; Seabra, C.M.; Palma, P.J.; Cardoso, A.L.; Peça, J.; Santos, J.M. Effects of a New Bioceramic Material on Human Apical Papilla Cells. J. Funct. Biomater. 2018, 9, 74. [Google Scholar] [CrossRef]
- Chavarria-Bolanos, D.; Komabayashi, T.; Shen, I.; Vega-Baudrit, J.; Gandolfi, M.G.; Prati, C.; Montero-Aguilar, M. Effects of heat on seven endodontic sealers. J. Oral Sci. 2022, 64, 33–39. [Google Scholar] [CrossRef]
| Feature | Category | Score | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Inflammation | I. Type | Absence of acute and chronic | Chronic | Acute | Presence of both | |
| II. Severity | No detected inflammatory cells | Cell count < 25 (sparse) | Cell count 25 –50 (mild) | Cell count > 50–75 (moderate) | Cell count > 75 (severe) | |
| III. Extent | No detected inflammatory cells | Inflammatory cells just at the superficial layer of the capsule | Cells limited to the fibrous capsule | Cells beyond the fibrous capsule | No capsulation/cells are limited to interface | |
| Fibroblastic reaction | Connective tissue capsule | Absent- without capsule | Immature form Dispersed fiber | Thin capsule (<150 µm) | Thick capsule (≥150 µm) | |
| Fibrous tissue formation | Thickness of connective tissue capsule | Normal collagen fiber morphology | Mild collagen fiber irregularity | Moderate collage fiber irregularity | Severe collagen fiber irregularity | |
| Capillary reaction | Congested blood vessels | Absent | Mild | Moderate | Severe | |
| Perivascular material | Absent | Present | ||||
| Vascular proliferation | No significant vascular proliferation | Number of vascular structures in field > 25 | Number of vascular structures in field 25–50 | Number of vascular structures in field > 50 | ||
| Calcification | Absent | Present | ||||
| Coagulative necrosis | Absent | Present | ||||
| Sealer | Time | n | 1:200 (Mean ± SD) | 1:100 (Mean ± SD) | 1:50 (Mean ± SD) | 1:1 (Mean ± SD) | 1× (Mean ± SD) |
|---|---|---|---|---|---|---|---|
| PDMS | 24 h | 3 | 91.64 ± 2.43 | 104.05 ± 11.28 | 95.94 ± 10.30 | 96.64 ± 5.62 | 92.97 ± 1.82 |
| 48 h | 3 | 100.52 ± 2.15 | 97.91 ± 6.02 | 92.93 ± 2.82 | 93.17 ± 6.18 | 93.57 ± 0.77 | |
| 72 h | 3 | 95.65 ± 14.29 | 100.63 ± 7.12 | 103.00 ± 23.10 | 100.94 ± 13.55 | 84.91 ± 9.15 | |
| Sealapex | 24 h | 3 | 85.90 ± 6.54 | 90.01 ± 4.35 | 92.14 ± 7.58 | 92.07 ± 4.61 | 67.54 ± 4.79 |
| 48 h | 3 | 99.17 ± 7.68 | 106.33 ± 5.98 | 120.43 ± 22.99 | 91.25 ± 25.65 | 71.56 ± 5.97 | |
| 72 h | 3 | 106.55 ± 16.66 | 106.47 ± 24.72 | 109.08 ± 16.11 | 92.65 ± 16.84 | 80.33 ± 1.70 | |
| Silco | 24 h | 3 | 94.81 ± 5.27 | 91.93 ± 7.32 | 94.05 ± 2.99 | 85.66 ± 12.24 | 50.67 ± 17.16 |
| 48 h | 3 | 109.88 ± 13.02 | 105.23 ± 6.38 | 105.43 ± 9.05 | 93.12 ± 9.29 | 67.45 ± 3.34 | |
| 72 h | 3 | 90.82 ± 14.61 | 90.30 ± 0.67 | 95.15 ± 24.48 | 75.12 ± 15.43 | 32.04 ± 14.43 |
| Sealer | PDMS, n (%) | SILCO®, n (%) | SEALAPEX®, n (%) | CONTROL, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | 15 Days | 30 Days | 45 Days | 15 Days | 30 Days | 45 Days | 15 Days | 30 Days | 45 Days | 15 Days | 30 Days | 45 Days |
| Type of inflammation | ||||||||||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 | 0 (0) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 2 (66.6) | 1 (33.3) | 0 (0) | 3 (100) |
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 3 (100) | 3 (100) | 2 (66.6) | 2 (66.6) | 3 (100) | 0 (0) | 3 (100) | 3 (100) | 1 (33.3) | 2 (66.6) | 3 (100) | 0 (0) |
| Severity of inflammation | ||||||||||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (100) | 0 (0) | 0 (0) |
| 1 | 2 (66.6) | 3 (100) | 3 (100) | 2 (66.6) | 3 (100) | 3 (100) | 2 (66.6) | 3 (100) | 3 (100) | 0 (0) | 3 (100) | 3 (100) |
| 2 | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Extent of inflammation | ||||||||||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 | 2 (66.6) | 3 (100) | 2 (66.6) | 2 (66.6) | 3 (100) | 2 (66.6) | 2 (66.6) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| 2 | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Connective tissue capsule | ||||||||||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (66.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 | 3 (100) | 3 (100) | 2 (66.6) | 2 (66.6) | 1 (33.3) | 2 (66.6) | 2 (66.6) | 3 (100) | 2 (66.6) | 3 (100) | 3 (100) | 3 (100) |
| 2 | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Thickness of the connective tissue capsule | ||||||||||||
| 0 | 1 (33.3) | 3 (100) | 1 (33.3) | 2 (66.6) | 1 (33.3) | 2 (66.6) | 0 (0) | 3 (100) | 1 (33.3) | 2 (66.6) | 1 (33.3) | 3 (100) |
| 1 | 2 (66.6) | 0 (0) | 1 (33.3) | 0 (0) | 2 (66.6) | 1 (33.3) | 3 (100) | 0 (0) | 2 (66.6) | 1 (33.3) | 2 (66.6) | 0 (0) |
| 2 | 0 (0) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Congested blood vessels | ||||||||||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 3 (100) | 1 (33.3) |
| 1 | 3 (100) | 3 (100) | 1 (33.3) | 1 (33.3) | 3 (100) | 1 (33.3) | 2 (66.6) | 2 (66.6) | 1 (33.3) | 3 (100) | 0 (0) | 2 (66.6) |
| 2 | 0 (0) | 0 (0) | 2 (66.6) | 1 (33.3) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Perivascular material | ||||||||||||
| 0 | 2 (66.6) | 3 (100) | 1 (33.3) | 1 (33.3) | 3 (100) | 1 (33.3) | 3 (100) | 2 (66.6) | 2 (66.6) | 3 (100) | 3 (100) | 3 (100) |
| 1 | 1 (33.3) | 0 (0) | 2 (66.6) | 2 (66.6) | 0 (0) | 2 (66.6) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Vascular proliferation | ||||||||||||
| 0 | 3 (100) | 3 (100) | 0 (0) | 1 (33.3) | 3 (100) | 1 (33.3) | 3 (100) | 3 (100) | 1 (33.3) | 3 (100) | 3 (100) | 1 (33.3) |
| 1 | 0 (0) | 0 (0) | 3 (100) | 2 (66.6) | 0 (0) | 2 (66.6) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 2 (66.6) |
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Calcification | ||||||||||||
| 0 | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Coagulative necrosis | ||||||||||||
| 0 | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, F.C.; Fonseca, C.G.; Jimenez, C.B.; Galaviz, L.A.A.; Martinez-Fierro, M.L.; Vazquez, L.T.; Ortiz, M.E.R. In Vitro and In Vivo Evaluation of a New Experimental Polydimethylsiloxane-Based Endodontic Sealer. J. Funct. Biomater. 2025, 16, 402. https://doi.org/10.3390/jfb16110402
Maldonado FC, Fonseca CG, Jimenez CB, Galaviz LAA, Martinez-Fierro ML, Vazquez LT, Ortiz MER. In Vitro and In Vivo Evaluation of a New Experimental Polydimethylsiloxane-Based Endodontic Sealer. Journal of Functional Biomaterials. 2025; 16(11):402. https://doi.org/10.3390/jfb16110402
Chicago/Turabian StyleMaldonado, Fabiola Cardoso, Cesar Gaitan Fonseca, Carlos Bermudez Jimenez, Luis Alejandro Aguilera Galaviz, Margarita L. Martinez-Fierro, Lorena Troncoso Vazquez, and Martha Eugenia Reyes Ortiz. 2025. "In Vitro and In Vivo Evaluation of a New Experimental Polydimethylsiloxane-Based Endodontic Sealer" Journal of Functional Biomaterials 16, no. 11: 402. https://doi.org/10.3390/jfb16110402
APA StyleMaldonado, F. C., Fonseca, C. G., Jimenez, C. B., Galaviz, L. A. A., Martinez-Fierro, M. L., Vazquez, L. T., & Ortiz, M. E. R. (2025). In Vitro and In Vivo Evaluation of a New Experimental Polydimethylsiloxane-Based Endodontic Sealer. Journal of Functional Biomaterials, 16(11), 402. https://doi.org/10.3390/jfb16110402