A Comparison Between Two Bearing Surfaces for Total Hip Arthroplasty—Ceramic-on-Ceramic and Metal–Polycarbonate–Urethane—A Pseudo-Randomized Study
Abstract
1. Introduction
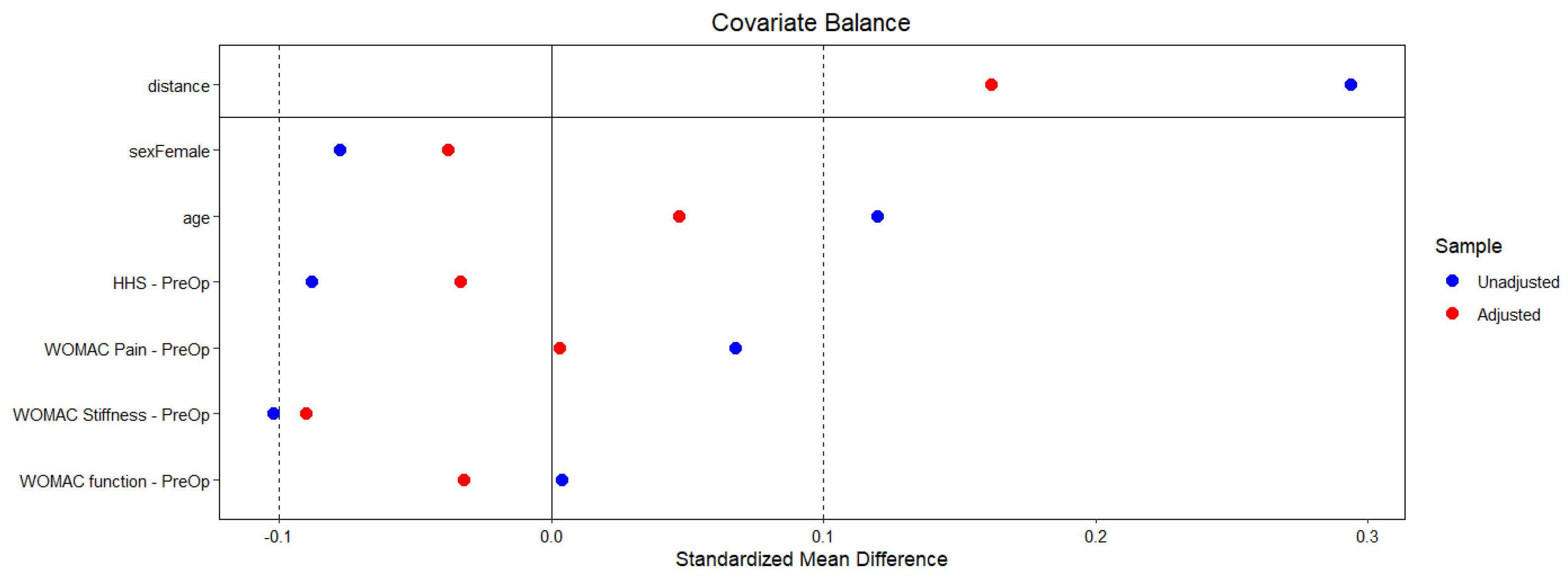
2. Materials and Methods
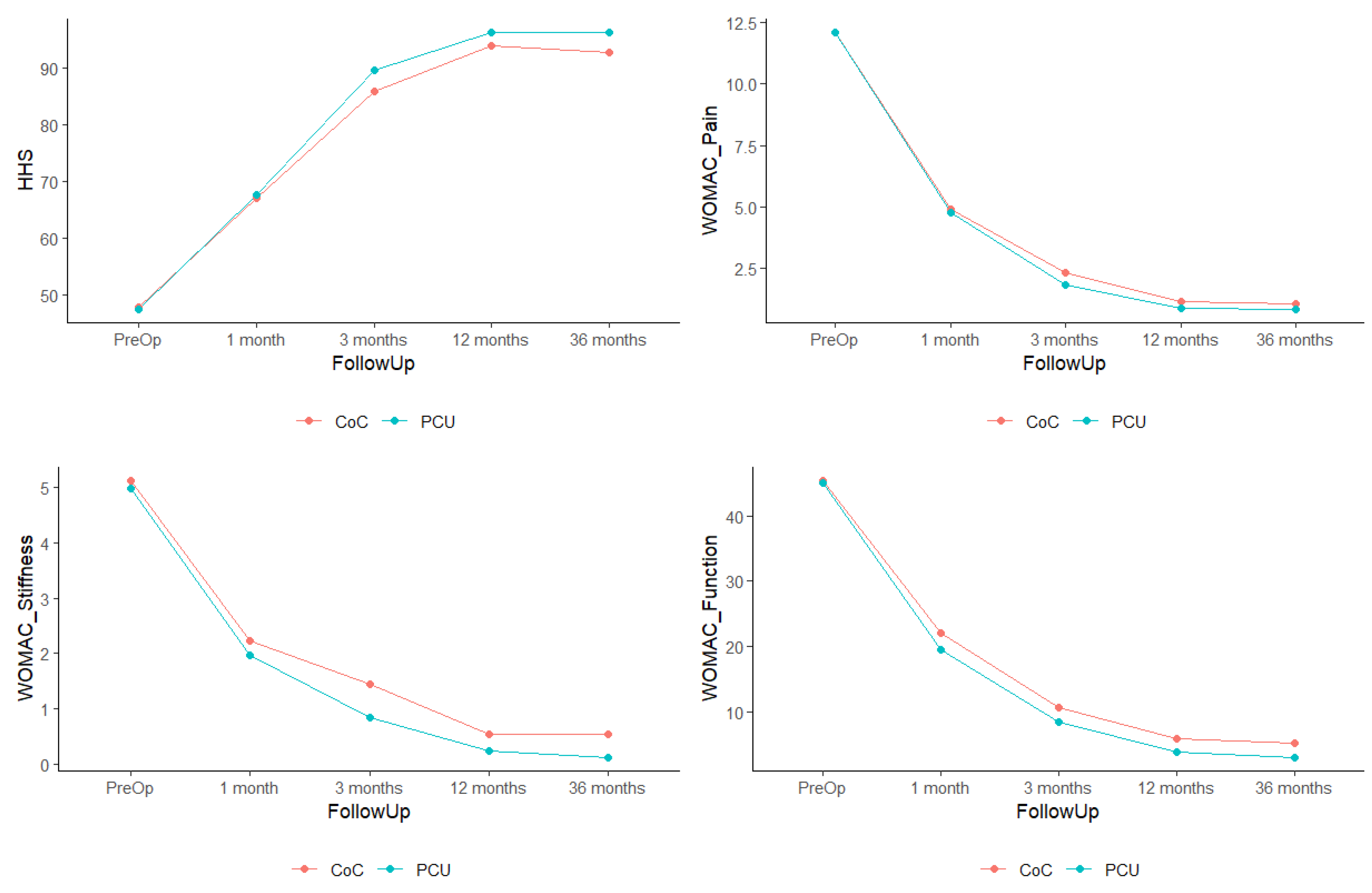
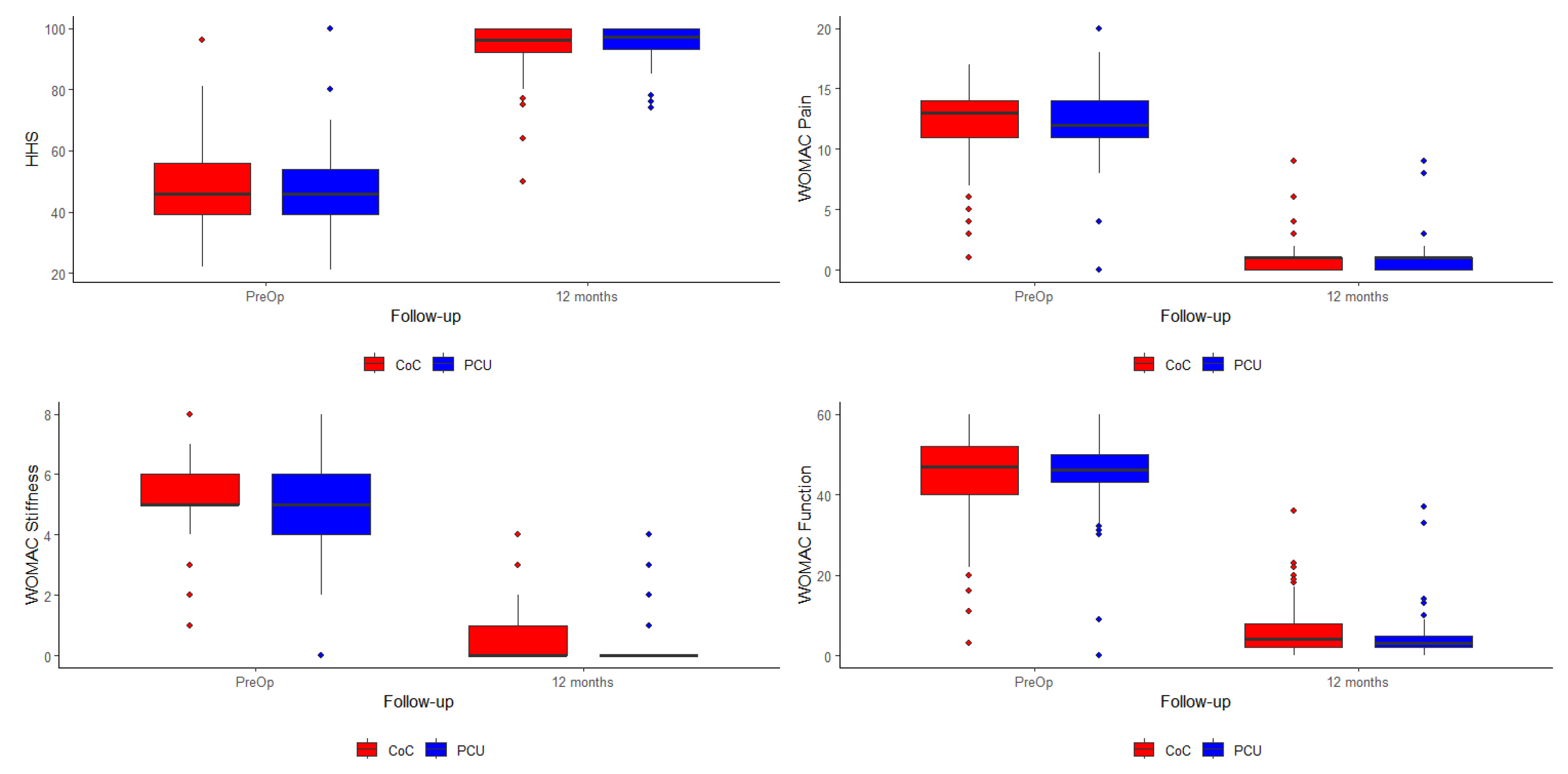
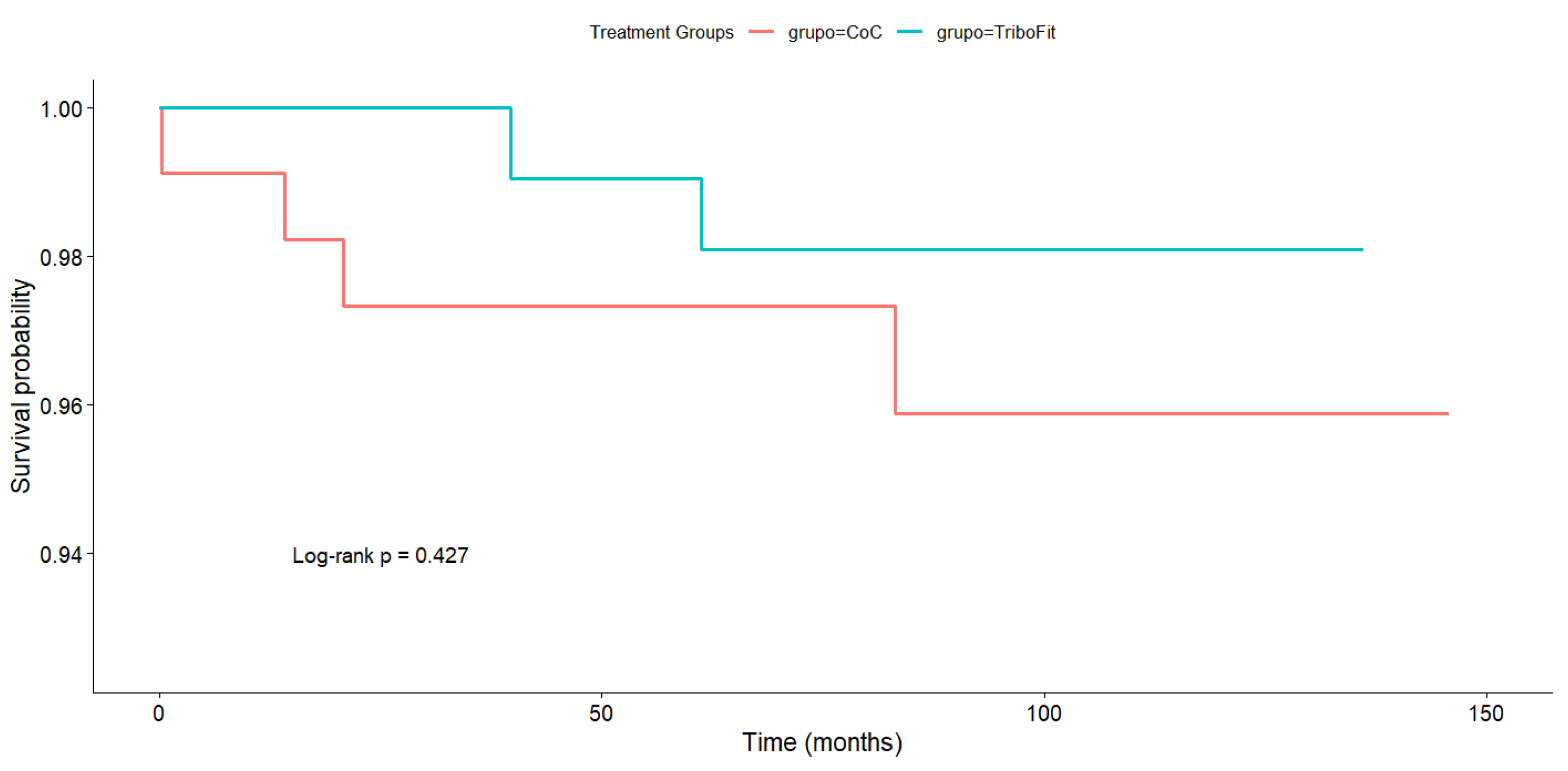
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| THA | Total Hip Arthroplasty |
| MoP | Metal-on-Polyethylene |
| PCU | Metal–Polycarbonate–Urethane |
| CoC | Ceramic-on-Ceramic |
| HHS | Harris Hip Score |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
| PSM | Propensity Score Matching |
References
- Lachiewicz, P.F.; Kleeman, L.T.; Seyler, T. Bearing Surfaces for Total Hip Arthroplasty. J. Am. Acad. Orthop. Surg. 2018, 26, 45–57. [Google Scholar] [CrossRef]
- Varnum, C. Outcomes of different bearings in total hip arthroplasty—Implant survival, revision causes, and patient-reported outcome. Dan. Med. J. 2017, 64, B5350. [Google Scholar]
- Campbell, P.; Shen, F.-W.; McKellop, H. Biologic and Tribologic Considerations of Alternative Bearing Surfaces. Clin. Orthop. Relat. Res. 2004, 418, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Di Puccio, F. Biotribology of artificial hip joints. World J. Orthop. 2015; 6, 77. [Google Scholar] [CrossRef]
- Heckmann, N.D.; Sivasundaram, L.; Stefl, M.D.; Kang, H.P.; Basler, E.T.; Lieberman, J.R. Total Hip Arthroplasty Bearing Surface Trends in the United States From 2007 to 2014: The Rise of Ceramic on Polyethylene. J. Arthroplast. 2018, 33, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Grieco, P.W.; Pascal, S.; Newman, J.M.; Shah, N.V.; Stroud, S.G.; Sheth, N.P.; Maheshwari, A.V. New alternate bearing surfaces in total hip arthroplasty: A review of the current literature. J. Clin. Orthop. Trauma. 2018, 9, 7–16. [Google Scholar] [CrossRef] [PubMed]
- St. John, K.; Gupta, M. Evaluation of the wear performance of a polycarbonate-urethane acetabular component in a hip joint simulator and comparison with UHMWPE and cross-linked UHMWPE. J. Biomater. Appl. 2012, 27, 55–65. [Google Scholar] [CrossRef]
- St. John, K.R. The use of compliant layer prosthetic components in orthopedic joint repair and replacement: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1332–1341. [Google Scholar] [CrossRef]
- Elsner, J.J.; Mezape, Y.; Hakshur, K.; Shemesh, M.; Linder-Ganz, E.; Shterling, A.; Eliaz, N. Wear rate evaluation of a novel polycarbonate-urethane cushion form bearing for artificial hip joints. Acta. Biomater. 2010, 6, 4698–4707. [Google Scholar] [CrossRef]
- Moroni, A.; Nocco, E.; Hoque, M.; Diremigio, E.; Buffoli, D.; Cantù, F.; Catalani, S.; Apostoli, P. Cushion bearings versus large diameter head metal-on-metal bearings in total hip arthroplasty: A short-term metal ion study. Arch. Orthop. Trauma. Surg. 2012, 132, 123–129. [Google Scholar] [CrossRef]
- Mai, S.; Mai, B.; Siebert, W.E. The Use of Polycarbonate-Urethane as an Acetabular Shell Bearing Surface: A 5-Year Prospective Study. Hip Int. 2017, 27, 472–476. [Google Scholar] [CrossRef]
- Lazic, S.; Kellett, C.; Afzal, I.; Mohan, R.; Killampalli, V.; Field, R.E. Three-year results of a polycarbonate urethane acetabular bearing in total hip arthroplasty. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther 2020, 30, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: Treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J. Bone Jt. Surg. Am 1969, 51, 737–755. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.; Goldmith, C.; Campbell, J.; Stitt, L. Validation study of WOMAC: A health status instrument for measuring clinically-important patient-relevant outcomes following total hip or knee arthroplasty in osteoarthritis. J. Orthop. Rheumatol. 1988, 1, 95–108. [Google Scholar]
- Basgul, C.; Spece, H.; Kurtz, M.A.; Kurtz, S.M. Total Hip Arthroplasty Patient-Reported Outcome Measures Plateau by One Year: A Scoping Review and Meta-Analysis. J. Arthroplast. 2025, 1–10. [Google Scholar] [CrossRef]
- Ekhtiari, S.; Worthy, T.; Winemaker, M.J.; de V Beer, J.; Petruccelli, D.T.; Khanduja, V.; Citak, M.; Puri, L.; Wood, T.J. When does patient function “Plateau” after total joint arthroplasty? A cohort study. Int. Orthop. 2024, 48, 2283–2291. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Luo, J.C.; Su, Y.; Zhang, Y.J.; Tu, G.W.; Luo, Z. Propensity score matching with R: Conventional methods and new features. Ann. Transl. Med. 2021, 9, 812. [Google Scholar] [CrossRef]
- Chen, J.W.; Maldonado, D.R.; Kowalski, B.L.; Miecznikowski, K.B.; Kyin, C.; Gornbein, J.A.; Domb, B.G. Best Practice Guidelines for Propensity Score Methods in Medical Research: Consideration on Theory, Implementation, and Reporting. A Review. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 632–642. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 1 April 2019).
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- Peters, R.M.; Van Steenbergen, L.N.; Stevens, M.; Rijk, P.C.; Bulstra, S.K.; Zijlstra, W.P. The effect of bearing type on the outcome of total hip arthroplasty. Acta. Orthop. 2018, 89, 163–169. [Google Scholar] [CrossRef]
- Khalifa, A.A.; Bakr, H.M. Updates in biomaterials of bearing surfaces in total hip arthroplasty. Arthroplasty 2021, 3, 32. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Kocagöz, S.; Arnholt, C.; Huet, R.; Ueno, M.; Walter, W.L. Advances in zirconia toughened alumina biomaterials for total joint replacement. J. Mech. Behav. Biomed. Mater. 2014, 31, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Gabarre, S.; Herrera, A.; Mateo, J.; Ibarz, E.; Lobo-Escolar, A.; Gracia, L. Study of the Polycarbonate-Urethane/Metal Contact in Different Positions during Gait Cycle. Biomed. Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Ash, H.E.; Unsworth, A. A tribological study of UHMWPE acetabular cups and polyurethane compliant layer acetabular cups. J. Biomed. Mater. Res. 2000, 53, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Hallab, N.J. In vitro macrophage response to polyethylene and polycarbonate-urethane particles. J. Biomed. Mater. Res. Part. A 2010, 93, 347–355. [Google Scholar] [CrossRef]
- Si, H.; Zeng, Y.; Cao, F.; Pei, F.; Shen, B. Is a Ceramic-on-Ceramic Bearing Really Superior to Ceramic-on-Polyethylene for Primary Total Hip Arthroplasty? A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther 2015, 25, 191–198. [Google Scholar] [CrossRef]
- Robertson, A.; Lavalette, D.; Morgan, S.; Angus, P.D. The hydroxyapatite-coated JRI-furlong hip. Outcome in patients under the age of 55 years. J. Bone Jt. Surg. Br. 2005, 87, 12–15. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Blom, A.; Boulton, C.; Brittain, R.; Clark, E.; Dawson-Bowling, S.; Deere, K.; Esler, C.; Espinoza, O.; Goldberg, A.; et al. The National Joint Registry 18th Annual Report 2021 [Internet]; National Joint Registry: London, UK, 2021. [Google Scholar] [PubMed]
- Tsikandylakis, G.; Mohaddes, M.; Cnudde, P.; Eskelinen, A.; Kärrholm, J.; Rolfson, O. Head size in primary total hip arthroplasty. EFORT Open Rev. 2018, 3, 225–231. [Google Scholar] [CrossRef]
- Sariali, E.; Lazennec, J.Y.; Khiami, F.; Catonné, Y. Mathematical evaluation of jumping distance in total hip arthroplasty. Acta. Orthop. 2009, 80, 277–282. [Google Scholar] [CrossRef]
- Burroughs, B.R.; Hallstrom, B.; Golladay, G.J.; Hoeffel, D.; Harris, W.H. Range of Motion and Stability in Total Hip Arthroplasty With 28-, 32-, 38-, and 44-mm Femoral Head Sizes. J. Arthroplast. 2005, 20, 11–19. [Google Scholar] [CrossRef]
- Kessler, O.; Patil, S.; Stefan, W.; Mayr, E.; Colwell, C.W.; D’Lima, D.D. Bony impingement affects range of motion after total hip arthroplasty: A subject-specific approach. J. Orthop. Res. 2008, 26, 443–452. [Google Scholar] [CrossRef]
- Bunn, A.; Colwell, C.W.; D’Lima, D.D. Effect of head diameter on passive and active dynamic hip dislocation. J. Orthop. Res. 2014, 32, 1525–1531. [Google Scholar] [CrossRef]
- Crowninshield, R.D.; Maloney, W.J.; Wentz, D.H.; Humphrey, S.M.; Blanchard, C.R. Biomechanics of Large Femoral Heads. Clin. Orthop. Relat. Res. 2004, 429, 102–107. [Google Scholar] [CrossRef]
- Rowan, F.E.; Benjamin, B.; Pietrak, J.R.; Haddad, F.S. Prevention of Dislocation After Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 1316–1324. [Google Scholar] [CrossRef]
- Kummer, F.J.; Shah, S.; Iyer, S.; DiCesare, P.E. The effect of acetabular cup orientations on limiting hip rotation. J. Arthroplast. 1999, 14, 509–513. [Google Scholar] [CrossRef]

| CoC | PCU | p-Value | |
|---|---|---|---|
| Demographic Data | |||
| N | 105 (50%) | 105 (50%) | - |
| Height (cm) | 171.0 ± 7.8 | 172.5 ± 9.4 | 0.230 |
| BMI (m/kg2) | 27.4 ± 4 | 28.5 ± 4 | 0.053 |
| Sex (M/F) | 72/33 | 76/29 | 0.545 |
| Age (years) | 49.5± 8.7 | 49.9 ± 8.4 | 0.739 |
| Diagnosis | |||
| Primary hip arthritis | 41 (39.0%) | 44 (41.9%) | 0.360 |
| Secondary hip arthritis | 33 (31.4%) | 27 (25.7%) | |
| Avascular necrosis | 24 (22.9%) | 27 (27.5%) | |
| Fractures or sequelae | 3 (2.9%) | 1 (1.0%) | |
| Arthritis | 2 (1.9%) | 0 (0.0%) | |
| Unknown | 2 (1.9%) | 6 (5.7%) | |
| Scores | |||
| HHS—Pre-Op | 48.0 | 47.6 | 0.802 |
| WOMAC Pain—Pre-Op | 12.1 | 12.1 | 0.983 |
| WOMAC Stiffness—Pre-Op | 5.12 | 5.00 | 0.466 |
| WOMAC Function—Pre-Op | 45.4 | 45.0 | 0.815 |
| CoC | PCU | p-Value | |
|---|---|---|---|
| Satisfaction (1 year) | 8.75 ± 1.0 | 8.93 ± 0.8 | 0.138 |
| Scores | |||
| HHS—Pre-Op | 48.0 | 47.6 | 0.172 |
| HHS—12 Months | 94.0 | 96.2 | |
| WOMAC Pain—Pre-Op | 12.1 | 12.1 | 0.523 |
| WOMAC Pain—12 Months | 1.1 | 0.8 | |
| WOMAC Stiffness—Pre-Op | 5.12 | 5.00 | 0.448 |
| WOMAC Stiffness—12 Months | 0.5 | 0.2 | |
| WOMAC Function—Pre-Op | 45.4 | 45.0 | 0.255 |
| WOMAC Function—12 Months | 5.9 | 3.8 |
| Head Diameter | CoC | PCU | ||
|---|---|---|---|---|
| N | % | N | % | |
| Small heads (up to 36 mm) | 78 | 74.3% | 29 | 29.9% |
| Large heads (over 36 mm) | 27 | 25.7% | 68 | 70.1% |
| Complication | CoC | PCU | p-Value |
|---|---|---|---|
| Cup loosening | 1 | 0 | 1 |
| Deep infection | 2 | 0 | 0.497 |
| Dislocation | 1 | 0 | 1 |
| Periprosthetic fracture | 3 | 0 | 0.246 |
| Heterotopic ossification | 1 | 0 | 1 |
| Seroma | 2 | 0 | 0.497 |
| Squeaking | 1 | 9 | 0.010 * |
| Stem loosening | 1 | 1 | 1 |
| Superficial infection | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donaire Hoyas, D.; Jiménez Mejías, E.; Moreta, J.; Sumillera García, M.; Albert Ullibarri, A.; Albareda Albareda, J. A Comparison Between Two Bearing Surfaces for Total Hip Arthroplasty—Ceramic-on-Ceramic and Metal–Polycarbonate–Urethane—A Pseudo-Randomized Study. J. Funct. Biomater. 2025, 16, 371. https://doi.org/10.3390/jfb16100371
Donaire Hoyas D, Jiménez Mejías E, Moreta J, Sumillera García M, Albert Ullibarri A, Albareda Albareda J. A Comparison Between Two Bearing Surfaces for Total Hip Arthroplasty—Ceramic-on-Ceramic and Metal–Polycarbonate–Urethane—A Pseudo-Randomized Study. Journal of Functional Biomaterials. 2025; 16(10):371. https://doi.org/10.3390/jfb16100371
Chicago/Turabian StyleDonaire Hoyas, Daniel, Eladio Jiménez Mejías, Jesús Moreta, Manuel Sumillera García, Alberto Albert Ullibarri, and Jorge Albareda Albareda. 2025. "A Comparison Between Two Bearing Surfaces for Total Hip Arthroplasty—Ceramic-on-Ceramic and Metal–Polycarbonate–Urethane—A Pseudo-Randomized Study" Journal of Functional Biomaterials 16, no. 10: 371. https://doi.org/10.3390/jfb16100371
APA StyleDonaire Hoyas, D., Jiménez Mejías, E., Moreta, J., Sumillera García, M., Albert Ullibarri, A., & Albareda Albareda, J. (2025). A Comparison Between Two Bearing Surfaces for Total Hip Arthroplasty—Ceramic-on-Ceramic and Metal–Polycarbonate–Urethane—A Pseudo-Randomized Study. Journal of Functional Biomaterials, 16(10), 371. https://doi.org/10.3390/jfb16100371






