Bioengineering Approaches and Novel Biomaterials to Enhance Sternal Wound Healing after Cardiac Surgery: A Crosstalk between Innovation and Surgical Practice
Abstract
1. Introduction
2. Patients and Risk Factors for Postoperative Complications
2.1. Preoperative Risk Factors
2.2. Intraoperative Risk Factors
2.3. Postoperative Risk Factors
3. “Traditional” Alternatives to “Traditional” Wires: Current Evidence
Mechanical Properties
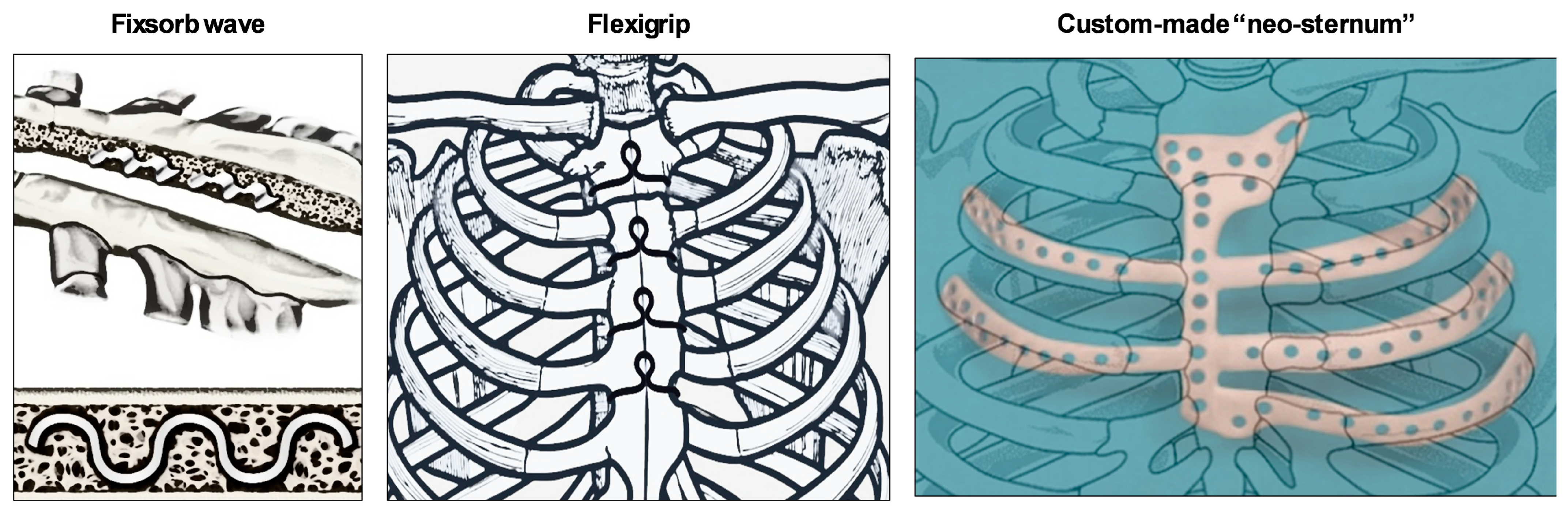
4. New Methods and Devices for Sternal Closure
4.1. Fixsorb Wave
4.2. Flexigrip
4.3. Custom-Made “Neo-sternum”
4.4. Alternatives to Bone Wax
5. Bone Adhesives
6. Fibrous Sheets and Electrospun Fibers
7. Growth Factor Therapies
8. Biophysical Stimulation Techniques
9. Cell-Based Therapies
10. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Abassi, A.; Papini, M.; Towler, M. Review of Biomechanical Studies and Finite Element Modeling of Sternal Closure Using Bio-Active Adhesives. Bioengineering 2022, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Khoynezhad, A.; Jahangiri, M. Surgical Site Infections in Cardiac Surgery. Crit. Care Clin. 2020, 36, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Zukowska, A.; Zukowski, M. Surgical Site Infection in Cardiac Surgery. J. Clin. Med. 2022, 11, 6991. [Google Scholar] [CrossRef]
- Balachandran, S.; Lee, A.; Denehy, L.; Lin, K.Y.; Royse, A.; Royse, C.; El-Ansary, D. Risk Factors for Sternal Complications After Cardiac Operations: A Systematic Review. Ann. Thorac. Surg. 2016, 102, 2109–2117. [Google Scholar] [CrossRef]
- Nenna, A.; Nappi, F.; Dougal, J.; Satriano, U.; Chello, C.; Mastroianni, C.; Lusini, M.; Chello, M.; Spadaccio, C. Sternal wound closure in the current era: The need of a tailored approach. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Copeland, M.; Senkowski, C.; Ulcickas, M.; Mendelson, M.; Griepp, R.B. Breast size as a risk factor for sternal wound complications following cardiac surgery. Arch. Surg. 1994, 129, 757–759. [Google Scholar] [CrossRef]
- Crabtree, T.D.; Codd, J.E.; Fraser, V.J.; Bailey, M.S.; Olsen, M.A.; Damiano, R.J., Jr. Multivariate analysis of risk factors for deep and superficial sternal infection after coronary artery bypass grafting at a tertiary care medical center. Semin. Thorac. Cardiovasc. Surg. 2004, 16, 53–61. [Google Scholar] [CrossRef]
- Colombier, S.; Kessler, U.; Ferrari, E.; von Segesser, L.K.; Berdajs, D.A. Influence of deep sternal wound infection on long-term survival after cardiac surgery. Med. Sci. Monit. 2013, 19, 668–673. [Google Scholar] [CrossRef]
- Trick, W.E.; Scheckler, W.E.; Tokars, J.I.; Jones, K.C.; Reppen, M.L.; Smith, E.M.; Jarvis, W.R. Modifiable risk factors associated with deep sternal site infection after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2000, 119, 108–114. [Google Scholar] [CrossRef]
- De Paulis, R.; de Notaris, S.; Scaffa, R.; Nardella, S.; Zeitani, J.; Del Giudice, C.; De Peppo, A.P.; Tomai, F.; Chiariello, L. The effect of bilateral internal thoracic artery harvesting on superficial and deep sternal infection: The role of skeletonization. J. Thorac. Cardiovasc. Surg. 2005, 129, 536–543. [Google Scholar] [CrossRef]
- Allen, K.B.; Thourani, V.H.; Naka, Y.; Grubb, K.J.; Grehan, J.; Patel, N.; Guy, T.S.; Landolfo, K.; Gerdisch, M.; Bonnell, M.; et al. Randomized, multicenter trial comparing sternotomy closure with rigid plate fixation to wire cerclage. J. Thorac. Cardiovasc. Surg. 2017, 153, 888–896.e1. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.B.; Thourani, V.H.; Naka, Y.; Grubb, K.J.; Grehan, J.; Patel, N.; Guy, T.S.; Landolfo, K.; Gerdisch, M.; Bonnell, M.; et al. Rigid Plate Fixation Versus Wire Cerclage: Patient-Reported and Economic Outcomes from a Randomized Trial. Ann. Thorac. Surg. 2018, 105, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Madjarov, J.M.; Katz, M.G.; Fazal, S.; Kumar, A.; Madzharov, S.; Handa, A.; Madjarova, S.J.; Robicsek, F. Use of longitudinal rigid sternal fixation in prevention and treatment of wound complications among high-risk patients after cardiac surgery. J. Card. Surg. 2021, 36, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, N.; Wolfe, B.; Mathes, D.W.; Malgor, R.D.; Kaoutzanis, C. Vacuum-assisted closure therapy for the management of deep sternal wound complications: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2023, 94, 251–260. [Google Scholar] [CrossRef]
- Sjogren, J.; Malmsjo, M.; Gustafsson, R.; Ingemansson, R. Poststernotomy mediastinitis: A review of conventional surgical treatments, vacuum-assisted closure therapy and presentation of the Lund University Hospital mediastinitis algorithm. Eur. J. Cardiothorac. Surg. 2006, 30, 898–905. [Google Scholar] [CrossRef]
- Yu, C.M.; Yu, C.M.; Yao, W.T.; Chen, Y.F.; Lee, A.L.; Liu, Y.C.; Tu, C.; Huang, W.; Tung, K.; Tsai, M. Efficacy and safety of pectoralis muscle flap combined rectus abdominis muscle sheath fasciocutaneous flap for reconstruction of sternal infection. Int. Wound J. 2022, 19, 1829–1837. [Google Scholar] [CrossRef]
- Trumble, D.R.; McGregor, W.E.; Magovern, J.A. Validation of a bone analog model for studies of sternal closure. Ann. Thorac. Surg. 2002, 74, 739–744. [Google Scholar] [CrossRef]
- Nagaoka, E.; Arai, H. Mechanical simulation study of reapproximated sternum rigidity comparing sternal fixation devices. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 98–103. [Google Scholar] [CrossRef]
- Park, Y.G.; Jo, J.H.; Lee, J.K.; Kim, J.M.; Lee, S.J.; Kim, H.Y. Biomechanical comparison of bone staple fixation methods with suture material for median sternotomy closure using 3D-printed bone models. N. Z. Vet. J. 2024, 72, 265–274. [Google Scholar] [CrossRef]
- Jutley, R.S.; Watson, M.A.; Shepherd, D.E.; Hukins, D.W. Finite element analysis of stress around a sternum screw used to prevent sternal dehiscence after heart surgery. Proc. Inst. Mech. Eng. H. 2002, 216, 315–321. [Google Scholar] [CrossRef]
- Oishi, K.; Arai, H.; Kuroki, H.; Fujioka, T.; Tomita, M.; Tasaki, D.; Oi, K.; Nagaoka, E.; Fujiwara, T.; Takeshita, M.; et al. A prospective randomized controlled study to assess the effectiveness of super FIXSORB WAVE((R)) for sternal stabilization after sternotomy. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 665–673. [Google Scholar] [CrossRef]
- Tsunekawa, T.; Usui, A.; Oshima, H.; Mizutani, S.; Araki, Y.; Okada, N.; Ueda, Y. A bioresorbable osteosynthesis device can induce an earlier sternal fusion after median sternotomy. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Takami, Y.; Maekawa, A.; Yamana, K.; Akita, K.; Amano, K.; Sakurai, Y.; Takagi, Y. Early Sternal Bone Healing after Thermoreactive Nitinol Flexigrip Sternal Closure. Ann. Thorac. Cardiovasc. Surg. 2022, 28, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Intihar, U.; Zeleznik, J.; Brajlih, T.; Drstvensek, I.; Hudak, R.; Antonic, M. Sternal Reconstruction Using 3D-Printed Titanium Custom-Made Prosthesis for Sternal Dehiscence After Cardiac Surgery. Heart Surg. Forum 2023, 26, E160–E163. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.K.; Cheng, A.; Vaughan, B.; Stiles, B.; Altorki, N.; Spector, J.A.; Port, J.L. Sternal Reconstruction Using Customized 3D-Printed Titanium Implants. Ann. Thorac. Surg. 2020, 109, e411–e414. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Chen, C.; Liu, M.; Xiao, Y.; Zhang, J.; Yu, G.; Jiang, G. Sternal Resection and Reconstruction With a Novel Modularized Prosthesis. Ann. Thorac. Surg. 2020, 110, 1412–1416. [Google Scholar] [CrossRef]
- Allie, D.E.; Hebert, C.J.; Lirtzman, M.D.; Wyatt, C.H.; Keller, V.A.; Souther, S.M.; A Allie, A.; Mitran, E.V.; Walker, C.M. Novel treatment strategy for leg and sternal wound complications after coronary artery bypass graft surgery: Bioengineered Apligraf. Ann. Thorac. Surg. 2004, 78, 673–678. [Google Scholar] [CrossRef]
- Pradeep, A.; Rangasamy, J.; Varma, P.K. Recent developments in controlling sternal wound infection after cardiac surgery and measures to enhance sternal healing. Med. Res. Rev. 2021, 41, 709–724. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Zhu, T.; Le, H.; Wang, X.; Guo, J.; Liu, G.; Ding, J. Functional macromolecular adhesives for bone fracture healing. ACS Appl. Mater. Interfaces 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Sidhu, V.P.S.; Towler, M.R.; Papini, M. Measurement of Adhesion of Sternal Wires to a Novel Bioactive Glass-Based Adhesive. J. Funct. Biomater. 2019, 10, 37. [Google Scholar] [CrossRef]
- Sa, M.P.; Van den Eynde, J.; Cavalcanti, L.R.; Kadyraliev, B.; Enginoev, S.; Zhigalov, K.; Ruhparwar, A.; Weymann, A.; Dreyfus, G. Mitral valve repair with minimally invasive approaches vs sternotomy: A meta-analysis of early and late results in randomized and matched observational studies. J. Card. Surg. 2020, 35, 2307–2323. [Google Scholar] [CrossRef] [PubMed]
- Mehrvar, C.; Deignan, E.; Hurtig, M.; Cohen, G.; Zalzal, P.; Safir, O.; Alhalawani, A.; Papini, M.; Towler, M.R. In vivo analysis of a proprietary glass-based adhesive for sternal fixation and stabilization using rabbit and sheep models. J. Mater. Sci. Mater. Med. 2021, 32, 53. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, M.R.; Tambare, O.H.; More, A.P. Performance of various fillers in adhesives applications: A review. Polym. Bull. 2022, 79, 1–63. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric tissue adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef]
- Shokri, M.; Dalili, F.; Kharaziha, M.; Eslaminejad, M.B.; Tafti, H.A. Strong and bioactive bioinspired biomaterials, next generation of bone adhesives. Adv. Colloid. Interface Sci. 2022, 305, 102706. [Google Scholar] [CrossRef]
- Zaokari, Y.; Persaud, A.; Ibrahim, A. Biomaterials for adhesion in orthopedic applications: A review. Eng. Regen. 2020, 1, 51–63. [Google Scholar] [CrossRef]
- Oladele, I.O.; Omotosho, T.F.; Adediran, A.A. Polymer-based composites: An Measurement of Adhesion of Sternal Wires to a Novel Bioactive Glass-Based Adhesiveindispensable material for present and future applications. Int. J. Polym. Sci. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Radulescu, D.E.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. Novel trends into the development of natural hydroxyapatite-based polymeric composites for bone tissue engineering. Polymers 2022, 14, 899. [Google Scholar] [CrossRef]
- Guo, Z.; Poot, A.A.; Grijpma, D.W. Advanced polymer-based composites and structures for biomedical applications. Eur. Polym. J. 2021, 149, 110388. [Google Scholar] [CrossRef]
- Shibata, M.; Takagi, G.; Kudo, M.; Kurita, J.; Kawamoto, Y.; Miyagi, Y.; Kanazashi, M.; Sakatani, T.; Naito, Z.; Tabata, Y.; et al. Enhanced Sternal Healing Through Platelet-Rich Plasma and Biodegradable Gelatin Hydrogel. Tissue Eng. Part A 2018, 24, 1406–1412. [Google Scholar] [CrossRef]
- Rainer, A.; Spadaccio, C.; Sedati, P.; De Marco, F.; Carotti, S.; Lusini, M.; Vadalà, G.; Di Martino, A.; Morini, S.; Chello, M.; et al. Electrospun hydroxyapatite-functionalized PLLA scaffold: Potential applications in sternal bone healing. Ann. Biomed. Eng. 2011, 39, 1882–1890. [Google Scholar] [CrossRef]
- Iwakura, A.; Tabata, Y.; Koyama, T.; Doi, K.; Nishimura, K.; Kataoka, K.; Fujita, M.; Komeda, M. Gelatin sheet incorporating basic fibroblast growth factor enhances sternal healing after harvesting bilateral internal thoracic arteries. J. Thorac. Cardiovasc. Surg. 2003, 126, 1113–1120. [Google Scholar] [CrossRef][Green Version]
- Zhu, S.; Gao, J.; Yu, W.; Xiong, J. Platelet-rich plasma influence on the sternal wounds healing: A meta-analysis. Int. Wound J. 2023, 20, 3794–3801. [Google Scholar] [CrossRef]
- Song, Y.; Chu, W.; Zhang, M.; Liu, Z.; Li, D.; Zhao, F.; Zhang, B.; Gao, M.; Yuan, H.; Shen, C. A study on the preoperative risk factors for primary healing failure in the reconstruction of deep sternal wound infection with platelet-rich plasma and negative pressure trauma therapy. Int. Wound J. 2023, 20, 3457–3466. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z. Effect of topical application of autologous platelet gel on sternal wound infection after cardiac surgery: A meta-analysis. Int. Wound J. 2024, 21, e14761. [Google Scholar] [CrossRef] [PubMed]
- Kirmani, B.H.; Jones, S.G.; Datta, S.; McLaughlin, E.K.; Hoschtitzky, A.J. A meta-analysis of platelet gel for prevention of sternal wound infections following cardiac surgery. Blood Transfus. 2017, 15, 57–65. [Google Scholar] [CrossRef]
- Gallo, I.; Saenz, A.; Artinano, E.; Esquide, J. Autologous platelet-rich plasma: Effect on sternal healing in the sheep model. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, A.; Tabata, Y.; Tamura, N.; Doi, K.; Nishimura, K.; Nakamura, T.; Shimizu, Y.; Fujita, M.; Komeda, M. Gelatin sheet incorporating basic fibroblast growth factor enhances healing of devascularized sternum in diabetic rats. Circulation 2001, 104, I325–I329. [Google Scholar] [CrossRef]
- Sharma, V.; Dash, S.K.; Govarthanan, K.; Gahtori, R.; Negi, N.; Barani, M.; Tomar, R.; Chakraborty, S.; Mathapati, S.; Bishi, D.K.; et al. Recent advances in cardiac tissue engineering for the management of myocardium infarction. Cells 2021, 10, 2538. [Google Scholar] [CrossRef]
- Augustine, R.; Dan, P.; Hasan, A.; Khalaf, I.M.; Prasad, P.; Ghosal, K.; Gentile, C.; McClements, L.; Maureira, P. Stem cell-based approaches in cardiac tissue engineering: Controlling the microenvironment for autologous cells. Biomed. Pharmacother. 2021, 138, 111425. [Google Scholar] [CrossRef] [PubMed]
- Garoffolo, G.; Ferrari, S.; Rizzi, S.; Barbuto, M.; Bernava, G.; Pesce, M. Harnessing mechanosensation in next generation cardiovascular tissue engineering. Biomolecules 2020, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical stimulation and cellular behaviors in electric field in biomedical research. Materials 2021, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Afjeh-Dana, E.; Naserzadeh, P.; Moradi, E.; Hosseini, N.; Seifalian, A.M.; Ashtari, B. Stem cell differentiation into cardiomyocytes: Current methods and emerging approaches. Stem Cell Rev. Rep. 2022, 18, 2566–2592. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Gronthos, S. Clinical application of bone marrow mesenchymal stem/stromal cells to repair skeletal tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Challapalli, R.S.; Dwyer, R.M.; McInerney, N.; Kerin, M.J.; Lowery, A.J. Effect of breast cancer and adjuvant therapy on adipose-derived stromal cells: Implications for the role of ADSCs in regenerative strategies for breast reconstruction. Stem Cell Rev. Rep. 2021, 17, 523–538. [Google Scholar] [CrossRef]
- Iwakura, A.; Tabata, Y.; Miyao, M.; Ozeki, M.; Tamura, N.; Ikai, A.; Nishimura, K.; Nakamura, T.; Shimizu, Y.; Fujita, M.; et al. Novel method to enhance sternal healing after harvesting bilateral internal thoracic arteries with use of basic fibroblast growth factor. Circulation 2000, 102, III307–III311. [Google Scholar] [CrossRef]


| Wire Closure Style | Modeled Illustration | Advantages | Disadvantages |
|---|---|---|---|
| Alternating peri-sternal and trans-sternal | 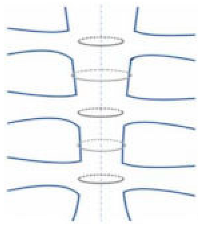 | Superior strength and stability | Can easily injure osteoporotic bones |
| Single trans-sternal | 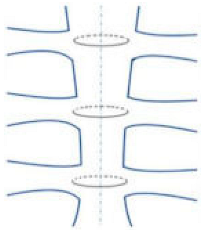 | Easy to use Allows for a good body union in most patients | The twisted free ends of the wire may penetrate the sternum (due to osteoporosis or other factors) Requires seven or more wires |
| Single peri-sternal |  | Reduces risk of deep sternal wound infection by reinforcing the sternum Safe for solid internal fixation Sternal stability was higher in single wire vs. figure-of-eight wire in high-risk obese patients | Requires seven or more wires |
| Figure-of-eight |  | Potential benefit in osteoporotic bone Biomechanical benefit (larger surface area) | Conflicting results in the literature |
| Modified figure-of-eight | 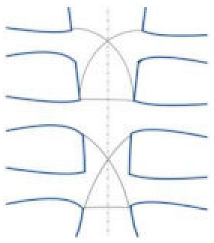 | Effective and safe method for limiting sternal dehiscence by limiting the penetration in the intercostal spaces | Conflicting results in the literature |
| Longitudinal parasternal (Robicsek cage) | 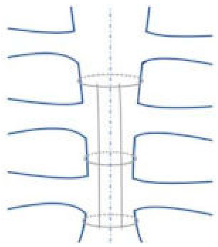 | Used for high-risk patients (chronic pulmonary disease, obesity, bilateral mammary artery harvesting, diabetes, off-midline sternotomy, and patients undergoing reoperations) | Increased risk of sternal de-vascularization due to antero-posterior compression |
| Preoperative Factors | Intraoperative Factors | Postoperative Factors |
|---|---|---|
| Macromastia | Bilateral internal mammary artery harvesting | Reoperation |
| Large chest circumference | Paramedian sternotomy | Blood transfusion |
| Obesity | Sterility breaks | Longer hospital stay |
| Active smoking | Prolonged operation time | |
| Diabetes mellitus | Poor closure technique | |
| Osteoporosis | ||
| Chronic pulmonary disease | ||
| Corticosteroid use |
| Concept | Materials | Positive Factors | Negative Factors | References |
|---|---|---|---|---|
| Bone Adhesives | Medical acrylate | Easy to apply Ready to use Biocompatible and does not release any toxic components | Solid adhesives often do not penetrate into the porous structure of bone Transition from high flexibility (modulus of ≤25 MPa) to low flexibility (modulus of ≥500 MPa) | [35,36,37,38,39] |
| Fibrous Sheets and Electrospun Fibers | Cotton-like hydrophilic hydroxyapatite (HA) | Ready to use Does not increase surgical time | / | [40] |
| Electrospun scaffold poly (lactic acid) (PLLA) | Reduction in postoperative complications and a greater rate of sternal healing Properties similar to native collagen Mitigate the inflammatory reaction | / | [41] | |
| Growth Factor Therapies | Gelatin hydrogel with drug-delivery system (DDS) | Release of growth factors Gelatin is converted into amino acids after its application, without adverse reactions | / | [42] |
| PRP (platelet-rich plasma) | Excellent alternative for bone wax Promoting bone healing Combined with fibrin or thrombin, it provided a lower rate of infections without side effects Angiogenic properties | When injected in free from, it did not guarantee a proper regeneration process because of its short-acting activity | [43,44,45] | |
| Cell-Based Therapies | Mesenchymal stem cells (MSCs) | Contribute to the overall success of the regenerative process | / | [54,55] |
| Biomaterial | Use | Action |
|---|---|---|
| Calcium sulfate with hydroxypropyl methylcellulose (HPMC) or sodium alginate | preclinical | The released calcium ions from the material can activate the coagulation cascade when it comes in contact with the blood thereby preventing bleeding. |
| Chitin–fibrin gel incorporated with tigecycline nanoparticles | preclinical | An adhesive gel with hemostatic properties and controlled drug release for 21 days. |
| Chitosan, oxidized starch, and hydroxyapatite | preclinical | Wax-like material with viscoelastic properties and biocompatibility. |
| Electrospun material Poly (l-lactide)/hydroxyapatite | preclinical | The scaffold was found to enhance sternal healing in the rabbit. |
| Gelatin hydrogel sheet with PRP/beta –FGF | preclinical | PRP/beta –FGF release was found to enhance sternal healing |
| Hydroxyapatite sheet with beta-tricalcium phosphate | preclinical | The sheet was sandwiched between the sternal halves, and their effects on sternal healing were studied in the canine model. |
| PEG- PPG- PEG with pregelatinized starch | preclinical | Has tamponade effects to prevent bleeding and shows good biocompatibility with osteoblast cells. |
| Poly-(ethylene glycol)–calcium phosphate cement with pregelatinized starch | preclinical | Like bone wax, it acts as a physical barrier to prevent bleeding. Tetracalcium phosphate provides osteogenic effects. |
| Polydopamine-co-acrylate and hydroxyapatite nanoparticles | preclinical | Material with controlled setting time, which can be used to enhance sternal healing. |
| Tricalcium silicate, 58S bioglass, chitosan, and carboxymethyl cellulose | preclinical | Injectable wax-like material with osteogenic and hemostatic effects. |
| AVITENE (microfibrillar collagen and antibiotic-containing fibrin glue) | clinical | It was applied at the sternum and was able to prevent bleeding and control infection. |
| BONESEAL (polylactic acid and hydroxyapatite) | clinical | It can act as a physical barrier against bleeding and enhance bone healing. |
| CALLOS (calcium phosphate cement) | clinical | It prevents bleeding and enhances better sternal and soft tissue healing with complete absorption of the material. |
| COLLOTAMP (gentamicin-impregnated collagen sponge) | clinical | The gentamicin-impregnated sponge placed in between the sternal halves helps in preventing infection. |
| HEMOBLAST (porcine collagen, bovine chondroitin sulfate, and human pooled plasma thrombin) | clinical | Explored for their effects in controlling sternal bleeding. |
| KRYPTONITE (castor oil-based adhesive) | clinical | It can enhance sternal union and stability. |
| OSTENE (alkylene oxide copolymer) | clinical | Water-soluble bone wax acts as a physical barrier against bleeding and is completely resorbable. |
| SPONGOSTAN (gelatin powder with rifamycin powder) | clinical | It is applied on the bone and helps in controlling bleeding. |
| VIVOSTAT (fibrin sealant with batroxobin) | clinical | The hemostasis effect of the material was studied, and clotting was observed within 43 s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrisi, C.; Loreni, F.; Nenna, A.; Giacinto, O.; Lusini, M.; Chello, M. Bioengineering Approaches and Novel Biomaterials to Enhance Sternal Wound Healing after Cardiac Surgery: A Crosstalk between Innovation and Surgical Practice. J. Funct. Biomater. 2024, 15, 254. https://doi.org/10.3390/jfb15090254
Ferrisi C, Loreni F, Nenna A, Giacinto O, Lusini M, Chello M. Bioengineering Approaches and Novel Biomaterials to Enhance Sternal Wound Healing after Cardiac Surgery: A Crosstalk between Innovation and Surgical Practice. Journal of Functional Biomaterials. 2024; 15(9):254. https://doi.org/10.3390/jfb15090254
Chicago/Turabian StyleFerrisi, Chiara, Francesco Loreni, Antonio Nenna, Omar Giacinto, Mario Lusini, and Massimo Chello. 2024. "Bioengineering Approaches and Novel Biomaterials to Enhance Sternal Wound Healing after Cardiac Surgery: A Crosstalk between Innovation and Surgical Practice" Journal of Functional Biomaterials 15, no. 9: 254. https://doi.org/10.3390/jfb15090254
APA StyleFerrisi, C., Loreni, F., Nenna, A., Giacinto, O., Lusini, M., & Chello, M. (2024). Bioengineering Approaches and Novel Biomaterials to Enhance Sternal Wound Healing after Cardiac Surgery: A Crosstalk between Innovation and Surgical Practice. Journal of Functional Biomaterials, 15(9), 254. https://doi.org/10.3390/jfb15090254







