Assessing Microstructural, Biomechanical, and Biocompatible Properties of TiNb Alloys for Potential Use as Load-Bearing Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Ti-Based Alloys
2.2. Microstructural and Biomechanical Characterization
2.3. In Vitro Testing of TiNb Alloys
3. Results
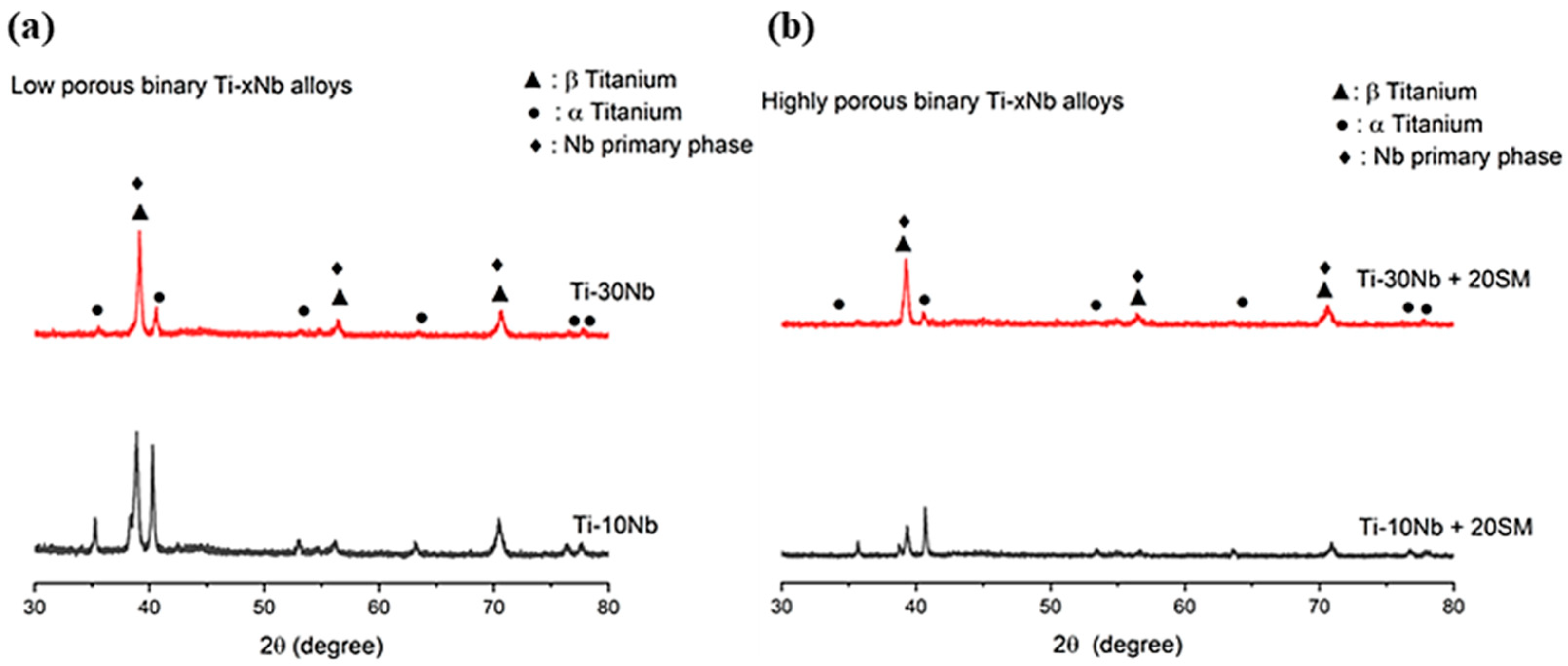
3.1. Material Characterization
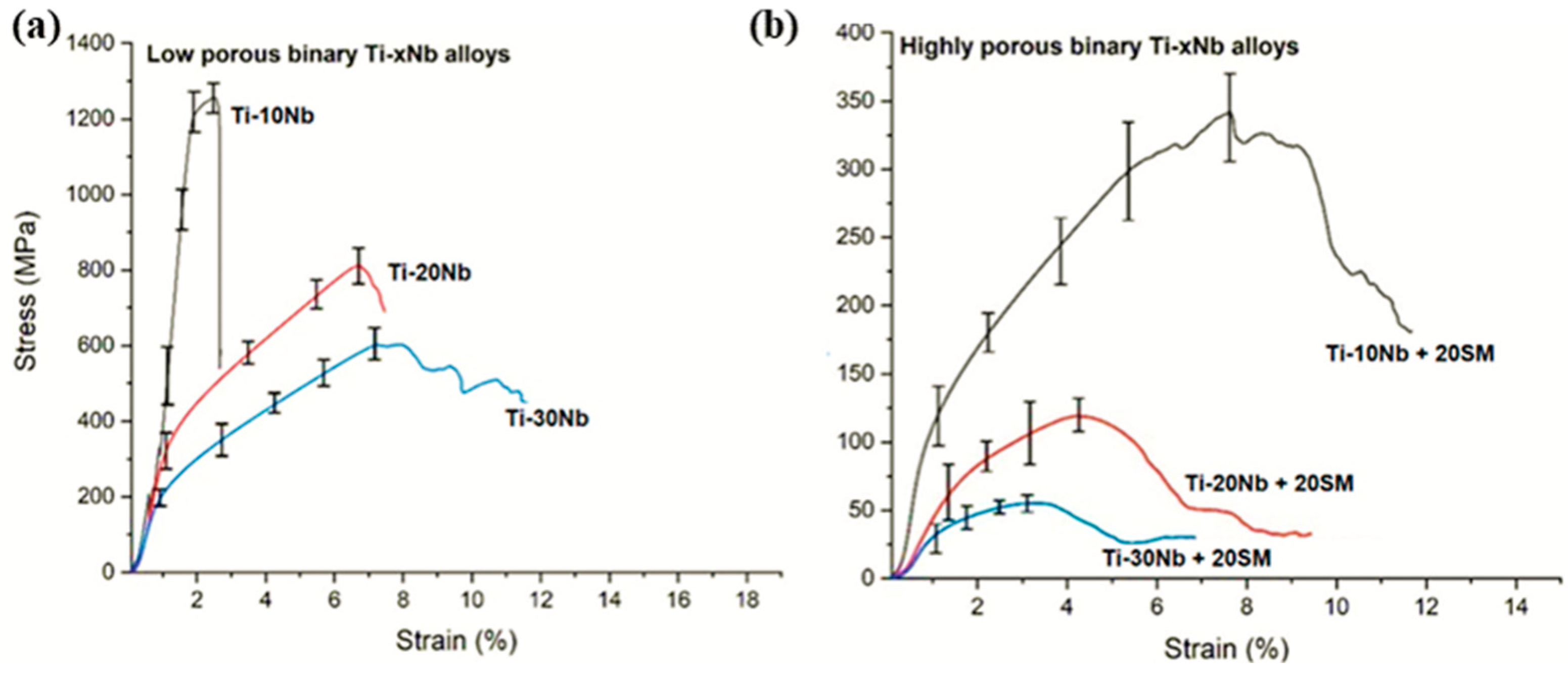
3.2. Compressive Performance
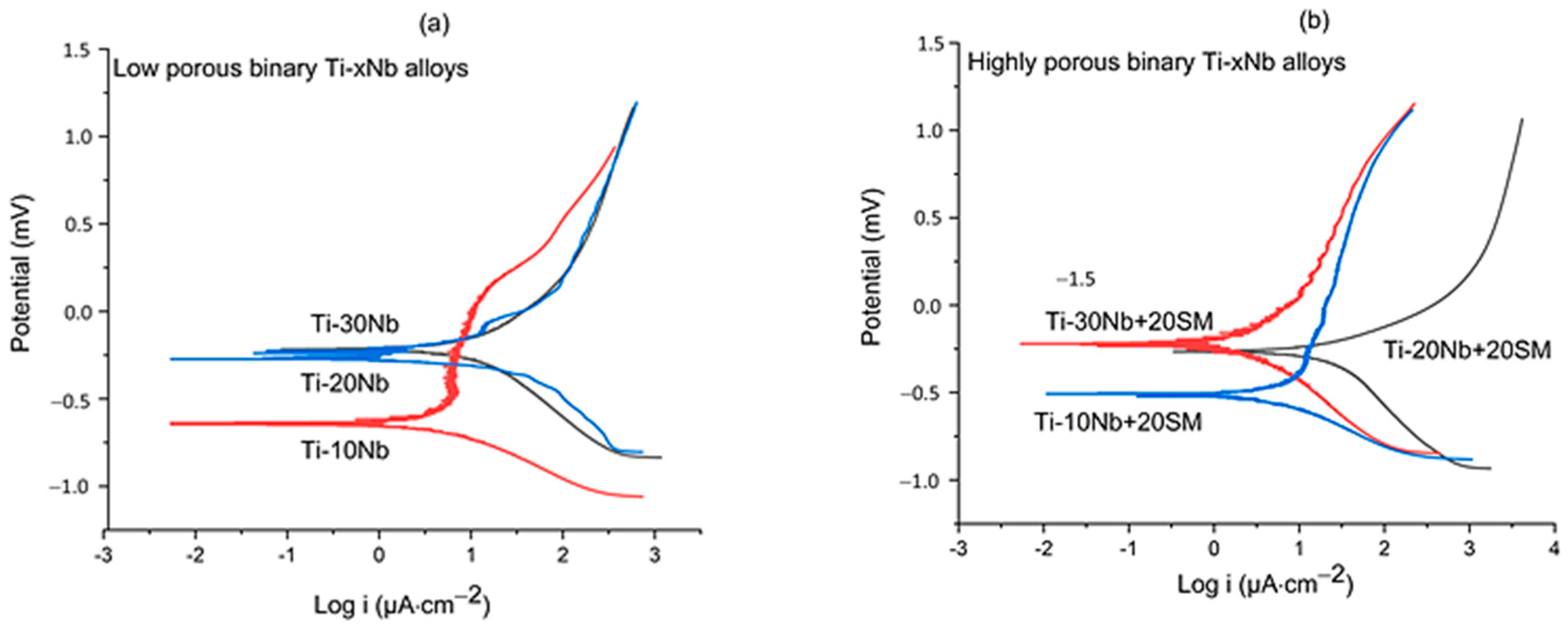
3.3. Corrosion Performance
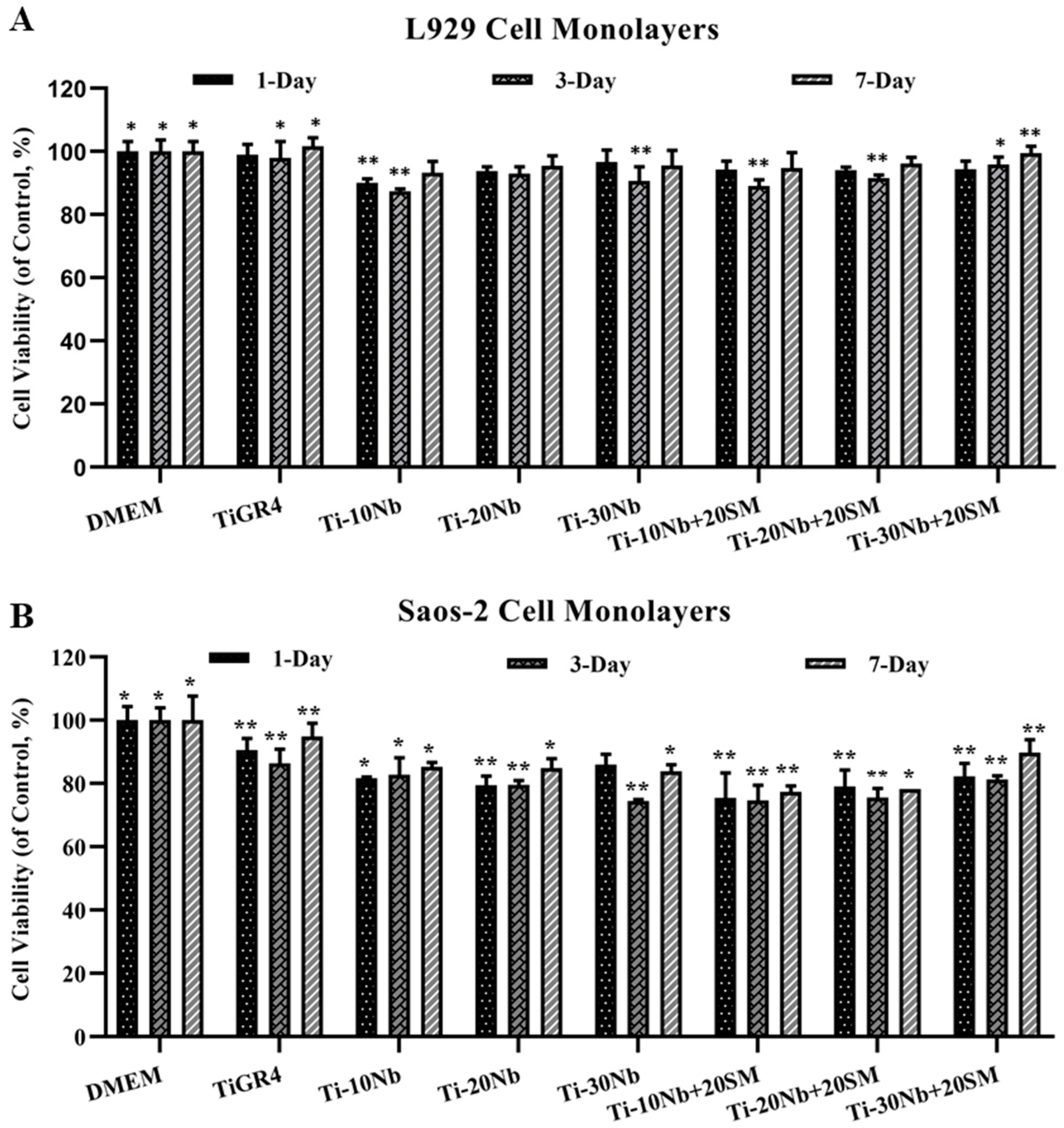
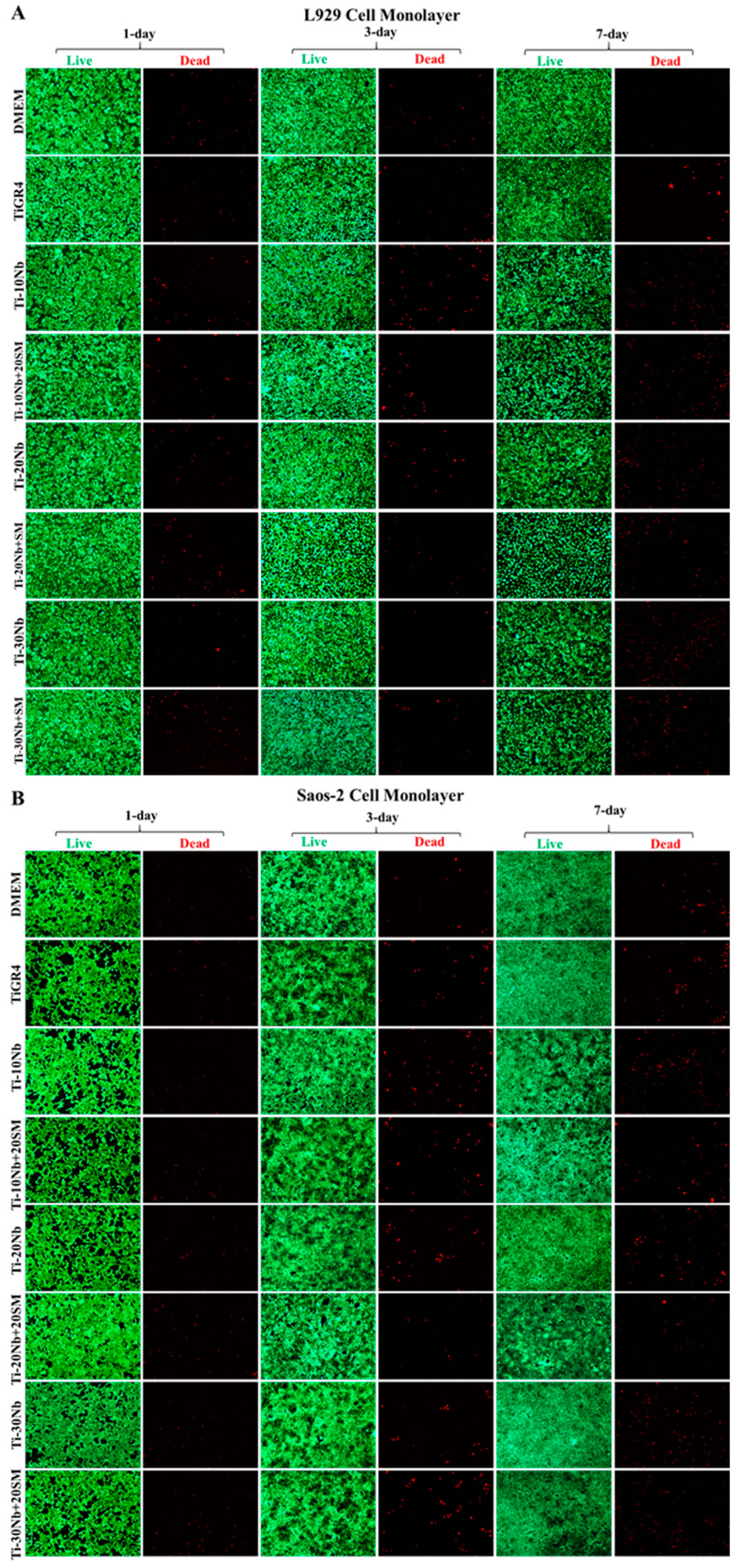
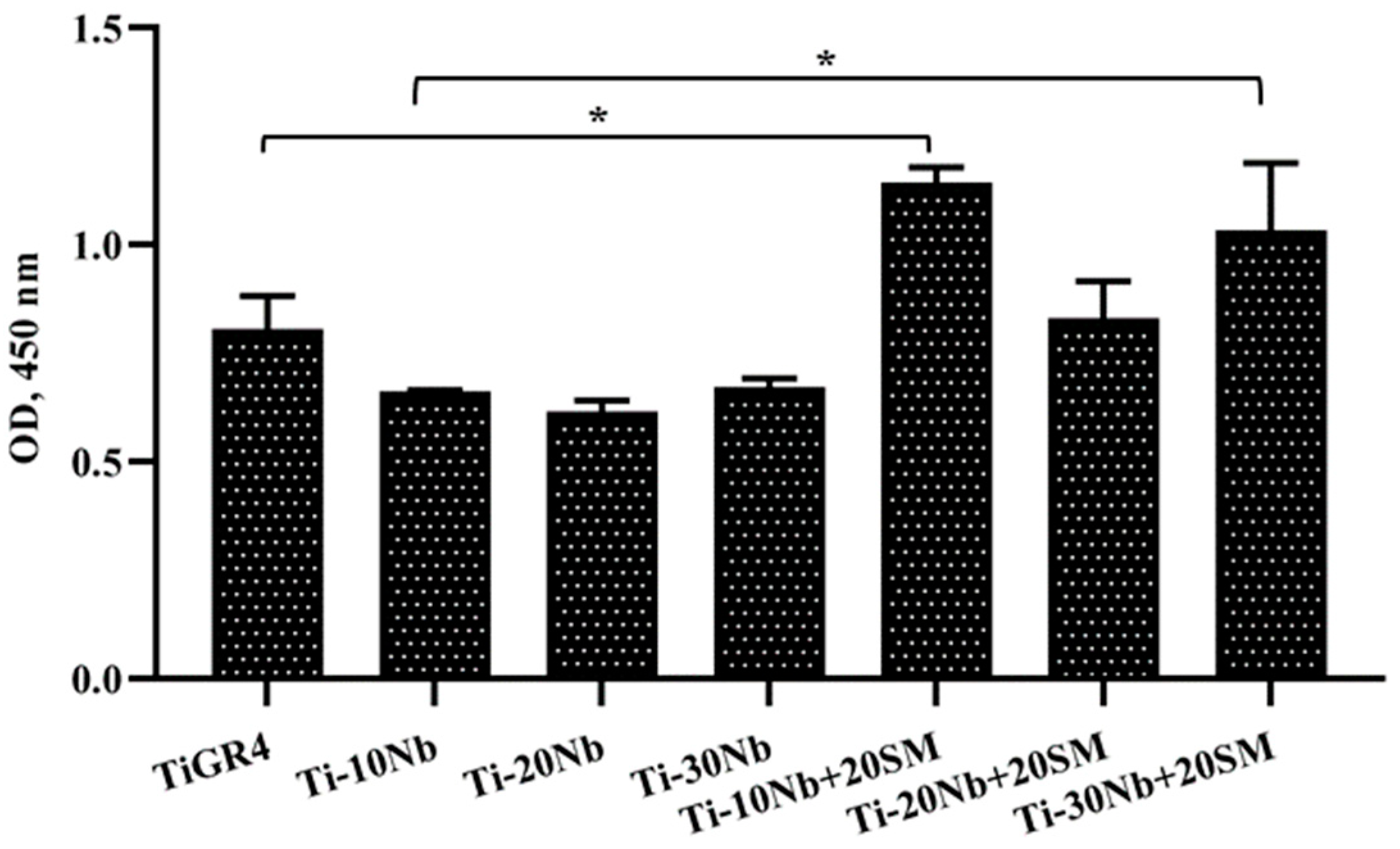
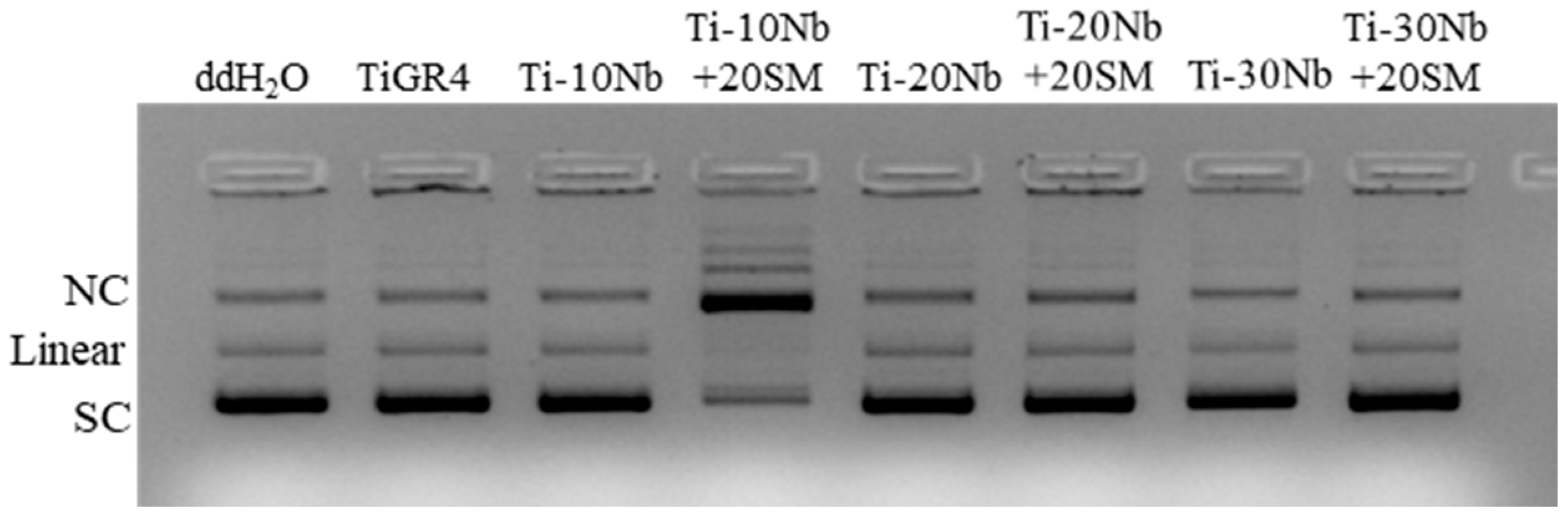
3.4. Biocompatibility of TiNb Alloys
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordeiro, J.M.; Barão, V.A. Is there scientific evidence favouring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants. Mater. Sci. Eng. C 2017, 71, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Yang, C. A review on high-strength titanium alloys: Microstructure, strengthening, and properties. Adv. Eng. Mater. 2019, 21, 1801359. [Google Scholar] [CrossRef]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Goodman, S.B. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J. Biomed. Mater. Res. 2017, 105, 2162–2173. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Woodrow, K.A. Medical applications of porous biomaterials: Features of porosity and tissue-specific ımplications for biocompatibility. Adv. Healthc. Mater. 2022, 11, 2102087. [Google Scholar] [CrossRef]
- Guerra, C.; Sancy, M.; Walczak, M.; Martínez, C.; Ringuedé, A.; Cassir, M.; Aguilar, C. Effect of added porosity on a novel porous Ti-Nb-Ta-Fe-Mn alloy exposed to simulated body fluid. Mater. Sci. Eng. C 2020, 111, 110758. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef]
- Du, P.; Xiang, T.; Cai, Z.; Xie, G. The influence of porous structure on the corrosion behavior and biocompatibility of bulk Ti-based metallic glass. J. Alloys Compd. 2022, 906, 164326. [Google Scholar] [CrossRef]
- Barberi, J.A.C.O.P.O.; Mandrile, L.; Napione, L.; Giovannozzi, A.M.; Rossi, A.M.; Vitale, A.; Spriano, S. Albumin and fibronectin adsorption on treated titanium surfaces for osseointegration: An advanced investigation. Appl. Surf. Sci. 2022, 599, 154023. [Google Scholar] [CrossRef]
- Bai, Y.; Deng, Y.; Zheng, Y.; Li, Y.; Zhang, R.; Lv, Y.; Wei, S. Characterization, corrosion behavior, cellular response, and in vivo bone tissue compatibility of titanium–niobium alloy with low Young’s modulus. Mater. Sci. Eng. C 2016, 59, 565–576. [Google Scholar] [CrossRef]
- Yolun, A.; Simşek, M.; Kaya, M.; Annaç, E.E.; Kom, M.; Çakmak, Ö. Fabrication, characterization, and in vivo biocompatibility evaluation of titanium-niobium implants. Proc. Inst. Mech. Eng. H. 2021, 235, 99–108. [Google Scholar] [CrossRef]
- Cetin, Y.; Ibrahim, A.M.H.; Gungor, A.; Yildizhan, Y.; Balog, M.; Krizik, P. In-vitro evaluation of a partially biodegradable TiMg dental implant: The cytotoxicity, genotoxicity, and oxidative stress. Materialia 2020, 14, 100899. [Google Scholar] [CrossRef]
- Couto, B.A.D.A.; Fernandes, J.C.H.; Saavedra-Silva, M.; Roca, H.; Castilho, R.M.; Fernandes, G.V.D.O. Antisclerostin effect on osseointegration and bone remodeling. J. Clin. Med. 2023, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, H.E.; Rodriguez-Romero, M.; Razavi, M.; Tahriri, M.; Ganjawalla, K.; Rasoulianboroujeni, M.; Tayebi, L. The cross-disciplinary emergence of 3D printed bioceramic scaffolds in orthopedic bioengineering. Ceram. Int. 2018, 44, 1–9. [Google Scholar] [CrossRef]
- Kaya, M.; Yakuphanoğlu, F.; Elibol, E.; Köm, M. Microstructure characterization and biocompatibility behaviour of TiNbZr alloy fabricated by powder metallurgy. Mater. Res. Express 2019, 6, 126560. [Google Scholar] [CrossRef]
- Edosa, O.O.; Tekweme, F.K.; Gupta, K. A review on the influence of process parameters on powder metallurgy parts. Eng. Appl. Sci Res. 2022, 49, 433–443. [Google Scholar]
- Neto, J.V.C.; da Costa Valente, M.L.; Dos Reis, A.C. Effect of pores on cell adhesion to additively manufactured titanium implants: A systematic review. J. Prosthet. Dent. 2023. [Google Scholar] [CrossRef]
- Gou, J.; Wang, Z.; Hu, S.; Shen, J.; Tian, Y.; Zhao, G.; Chen, Y. Effects of trace Nb addition on microstructure and properties of Ti–6Al–4V thin-wall structure prepared via cold metal transfer additive manufacturing. J. Alloys Compd. 2020, 829, 154481. [Google Scholar] [CrossRef]
- Li, S.; Choi, M.S.; Nam, T.H. Phase stability of the amorphous phase and non-equilibrium phase in a β Ti-Zr-based shape memory alloy. Scr. Mater. 2021, 195, 113721. [Google Scholar] [CrossRef]
- Chang, J.H.; Liu, J.F.; Sun, Y.S.; Wu, C.P.; Huang, H.H.; Han, Y. Mesoporous surface topography promotes bone cell differentiation on low elastic modulus Ti–25Nb–25Zr alloys for bone implant applications. J. Alloys Compd. 2017, 707, 220–226. [Google Scholar] [CrossRef]
- Kariya, S.; Umeda, J.; Ma, Q.; Seiichi, K. Ductility improvement mechanism of pure titanium with excessive oxygen solid solution via rapid cooling process. J. Japan. Inst. Metals. 2018, 82, 390–395. [Google Scholar] [CrossRef]
- Lin, Y.C.; Huang, J.; He, D.G.; Zhang, X.Y.; Wu, Q.; Wang, L.H.; Zhou, K.C. Phase transformation and dynamic recrystallization behaviors in a Ti55511 titanium alloy during hot compression. J. Alloys Compd. 2019, 795, 471–482. [Google Scholar] [CrossRef]
- Hoppe, V.; SzymczykZiółkowska, P.; Rusi’nska, M.; Dybała, B.; Poradowski, D.; Janeczek, M. Assessment of mechanical, chemical, and biological properties of Ti-Nb-Zr alloy for medical applications. Materials 2021, 14, 126. [Google Scholar] [CrossRef]
- Ha, Y.; Ma, X.; Li, S.; Li, T.; Li, Z.; Qian, Y.; He, C. Bone microenvironment-mimetic scaffolds with hierarchical microstructure for enhanced vascularization and bone regeneration. Adv. Funct. Mater. 2022, 32, 2200011. [Google Scholar] [CrossRef]
- Bertassoni, L.E. In-vitro models of biocompatibility testing for restorative dental materials: From 2D cultures to organs on-a-chip. Acta Biomater. 2022, 150, 58–66. [Google Scholar]
- Kargozar, S.; Singh, R.K.; Kim, H.W.; Baino, F. “Hard” ceramics for “Soft” tissue engineering: Paradox or opportunity? Acta Biomater. 2020, 115, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Di, T.; Song, C.; Niu, F.; Lu, J.; Wu, D. Phase transformation mechanism and mechanical properties of Ti-45Al-8Nb alloy prepared by directed laser deposition. Mater. Charact. 2022, 193, 112256. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Koptyug, A.; Khrapov, D.; Ivanov, Y.F.; Mishurova, T.; Evsevleev, S.; Surmenev, R.A. In situ synthesis of a binary Ti–10at% Nb alloy by electron beam melting using a mixture of elemental niobium and titanium powders. J. Mater. Process. Technol. 2020, 282, 116646. [Google Scholar] [CrossRef]
- Zhao, D.; Chang, K.; Ebel, T.; Nie, H.; Willumeit, R.; Pyczak, F. Sintering behavior and mechanical properties of a metal injection molded Ti–Nb binary alloy as biomaterial. J. Alloy. Compd. 2015, 640, 393–400. [Google Scholar] [CrossRef]
- Chen, Y.; Han, P.; Dehghan-Manshadi, A.; Kent, D.; Ehtemam-Haghighi, S.; Jowers, C.; Dargusch, M.S. Sintering and biocompatibility of blended elemental Ti-xNb alloys. J. Mech. Behav. Biomed. Mater. 2020, 104, 103691. [Google Scholar] [CrossRef]
- Zhao, D.P.; Chen, Y.K.; Chang, K.K.; Thomas, E.B.E.L.; Luthrigner-Feyerabend, B.J.; Willumeit-Roemer, R.; Pyczak, F. Surface topography and cytocompatibility of metal injection molded Ti–22Nb alloy as biomaterial. TNMSC 2018, 28, 1342–1350. [Google Scholar] [CrossRef]
- Li, B.Y.; Rong, L.J.; Li, Y.Y.; Gjunter, V.E. Synthesis of porous Ni–Ti shape-memory alloys by self-propagating high-temperature synthesis: Reaction mechanism and anisotropy in pore structure. Acta Biomater. 2000, 48, 3895–3904. [Google Scholar] [CrossRef]
- Karakurt, E.M. Microstructural, Biomechanical and Biocompatibility Studies of Porous Ti-Nb-Zr Alloys Fabricated by Powder Metallurgy. Ph.D. Thesis, Brunel University, London, UK, June 2023. [Google Scholar]
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, E.; Gökçe, A.; Findik, F.; Gulsoy, H.Ö. Assessment of Ti–16Nb–xZr alloys produced via PIM for implant applications. J. Therm. Anal. Calorim. 2018, 134, 7–14. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, H.Y.; Miyazaki, S. Effect of Zr content on phase stability, deformation behavior, and Young’s modulus in Ti–Nb–Zr alloys. Materials 2020, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Singh, S.; Ramakrishna, S.; Królczyk, G.; Le, C.H. Microwave sintering of porous Ti–Nb-HA composite with high strength and enhanced bioactivity for implant applications. J. Alloys Compd. 2020, 824, 153774. [Google Scholar] [CrossRef]
- Khrunyk, Y.Y.; Ehnert, S.; Grib, S.V.; Illarionov, A.G.; Stepanov, S.I.; Popov, A.A.; Nüssler, A.K. Synthesis and characterization of a novel biocompatible alloy, ti-nb-zr-ta-sn. Int. J. Mol. Sci. 2021, 22, 10611. [Google Scholar] [CrossRef]
- Alabort, E.; Tang, Y.T.; Barba, D.; Reed, R.C. Alloys-by-design: A low-modulus titanium alloy for additively manufactured biomedical implants. Acta Mater. 2022, 229, 117749. [Google Scholar] [CrossRef]
- Cakmak, O.; Kaya, M. Effect of sintering procedure on microstructure and mechanical properties of biomedical TiNbSn alloy produced via powder metallurgy. Appl. Phys. A Mater. Sci. Process. 2021, 127, 561. [Google Scholar] [CrossRef]
- Zheng, J.; Xia, R.; Sun, C.; Yaqoob, N.; Qiu, Q.; Zhong, L.; Huijben, M. Fast and durable lithium storage enabled by tuning entropy in wadsley–roth phase titanium niobium oxides. Small 2023, 19, 2301967. [Google Scholar] [CrossRef]
- Rao, X.; Chu, C.L.; Zheng, Y.Y. Phase composition, microstructure, and mechanical properties of porous Ti–Nb–Zr alloys prepared by a two-step foaming powder metallurgy method. J. Mech. Behav. Biomed. Mater. 2014, 34, 27–36. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Kamarul, T. The effect of tortuosity on permeability of porous scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liao, Q.; Gong, M.; Fu, Q. Corrosion performances of selective laser melting ti6al4v alloy in different solutions. Metals 2023, 13, 192. [Google Scholar] [CrossRef]
- Majumdar, D.D.; Sahu, S.; Mondal, D.P.; Roychowdhury, A.; Jha, A.K.; Ghosh, M. Microstructural analysis and corrosion behavior of a titanium cenosphere composite foam fabricated by powder metallurgy route. ChemistrySelect 2023, 8, e202203581. [Google Scholar] [CrossRef]
- Pandey, A.K.; Gautam, R.K.; Behera, C.K. Microstructure, mechanical strength, chemical resistance, and antibacterial behavior of Ti–5Cu–x% Nb biomedical alloy. Biomed. Mater. 2022, 17, 045022. [Google Scholar] [CrossRef]
- Tanji, A.; Fan, X.; Sakidja, R.; Liaw, P.K.; Hermawan, H. Niobium addition improves the corrosion resistance of TiHfZrNbx high-entropy alloys in Hanks’ solution. Electrochim. Acta 2022, 424, 140651. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, Z.; Zhang, B.; Kundu, T.; Sevostianov, I. The effect of porosity on the elastic properties of cortical bone and ultrasound propagation. Int. J. Eng. Sci. 2023, 182, 103772. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder metallurgy with space holder for porous titanium implants: A review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- Chézeau, L.; Tchinda, A.; Pierson, G.; Bravetti, P.; Ferrari, L.; Joubert, O.; Zaiou, M.; Rihn, B.H. In vitro molecular study of titanium-niobium alloy biocompatibility. Biomedicines 2022, 10, 1898. [Google Scholar] [CrossRef]
- Karrea, R.; Kodlia, B.K.; Rajendranb, A.; Nivedhithab, J.; Pattanayak, D.K.; Ameyamac, K.; Dey, S.R. Comparative study on Ti-Nb binary alloys fabricated through spark plasma sintering and conventional P/M routes for biomedical application. Mater. Sci. Eng. C 2019, 94, 619–627. [Google Scholar] [CrossRef]
- Annur, D.; Kartika, I.; Supriadi, S.; Soharno, B. Titanium and titanium based alloy prepared by spark plasma sintering method for biomedical implant applications—A review. Mater. Res. Express 2021, 8, 012001. [Google Scholar] [CrossRef]





| Alloy Group | Chemical Formula (at. %) |
|---|---|
| Low porous alloys | Ti-10Nb |
| Ti-20Nb | |
| Ti-30Nb | |
| Highly porous alloys | Ti-10Nb + 20 (wt.%) SM |
| Ti-20Nb + 20 (wt.%) SM | |
| Ti-30Nb + 20 (wt.%) SM |
| Alloys | Area 1 (at. %) | Area 2 (at. %) | Area 3 (at. %) | |||
|---|---|---|---|---|---|---|
| Ti | Nb | Ti | Nb | Ti | Nb | |
| Ti-30Nb | 0.2 | 99.80 | 80.24 | 19.76 | 96.48 | 3.52 |
| Ti-10Nb + 20SM | 13.72 | 86.28 | 94.14 | 5.86 | 98.26 | 1.74 |
| Mechanical Properties | Ti-10Nb | Ti-20Nb | Ti-30Nb | |||
|---|---|---|---|---|---|---|
| Ultimate compressive strength (MPa) | 1295 | 802 | 618 | 331 | 127 | 48 |
| Yield strength (MPa) | 687 | 136 | 112 | 64 | 46 | 22 |
| Elastic modulus (GPa) | 41 | 32 | 28 | 12 | 6.7 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakurt, E.M.; Huang, Y.; Cetin, Y.; Incesu, A.; Demirtas, H.; Kaya, M.; Yildizhan, Y.; Tosun, M.; Akbas, G. Assessing Microstructural, Biomechanical, and Biocompatible Properties of TiNb Alloys for Potential Use as Load-Bearing Implants. J. Funct. Biomater. 2024, 15, 253. https://doi.org/10.3390/jfb15090253
Karakurt EM, Huang Y, Cetin Y, Incesu A, Demirtas H, Kaya M, Yildizhan Y, Tosun M, Akbas G. Assessing Microstructural, Biomechanical, and Biocompatible Properties of TiNb Alloys for Potential Use as Load-Bearing Implants. Journal of Functional Biomaterials. 2024; 15(9):253. https://doi.org/10.3390/jfb15090253
Chicago/Turabian StyleKarakurt, Eyyup Murat, Yan Huang, Yuksel Cetin, Alper Incesu, Huseyin Demirtas, Mehmet Kaya, Yasemin Yildizhan, Merve Tosun, and Gulsah Akbas. 2024. "Assessing Microstructural, Biomechanical, and Biocompatible Properties of TiNb Alloys for Potential Use as Load-Bearing Implants" Journal of Functional Biomaterials 15, no. 9: 253. https://doi.org/10.3390/jfb15090253
APA StyleKarakurt, E. M., Huang, Y., Cetin, Y., Incesu, A., Demirtas, H., Kaya, M., Yildizhan, Y., Tosun, M., & Akbas, G. (2024). Assessing Microstructural, Biomechanical, and Biocompatible Properties of TiNb Alloys for Potential Use as Load-Bearing Implants. Journal of Functional Biomaterials, 15(9), 253. https://doi.org/10.3390/jfb15090253









