Anti-Inflammatory, Antipyretic, and Analgesic Potential of Chitin and Chitosan Derived from Cockroaches (Periplaneta americana) and Termites
Abstract
1. Introduction
2. Methods and Materials
2.1. Collection of Isoptera (Termite) and Periplaneta americana
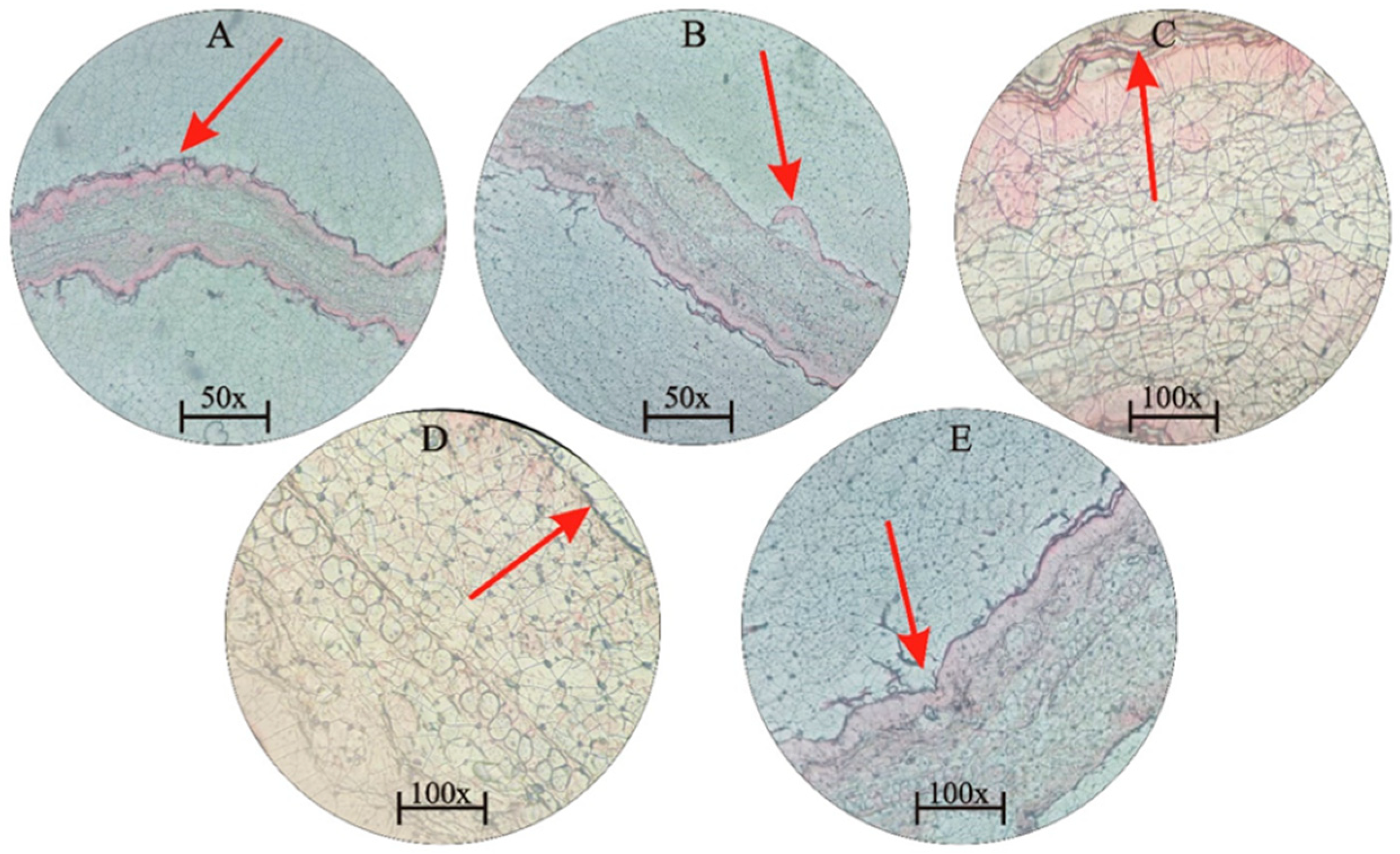
2.2. Preparation of Insect Extracts
2.3. Physical Characterization
2.3.1. FTI-R Analysis
2.3.2. X-ray Powder Diffraction (XRD) Analysis
2.4. Biological Activities
2.4.1. Assays for Analgesic Activity
2.4.2. In Vivo Assay for Anti-Inflammatory Effect
2.4.3. Antipyretic Activity
2.5. Statistical Analysis
3. Results and Discussion
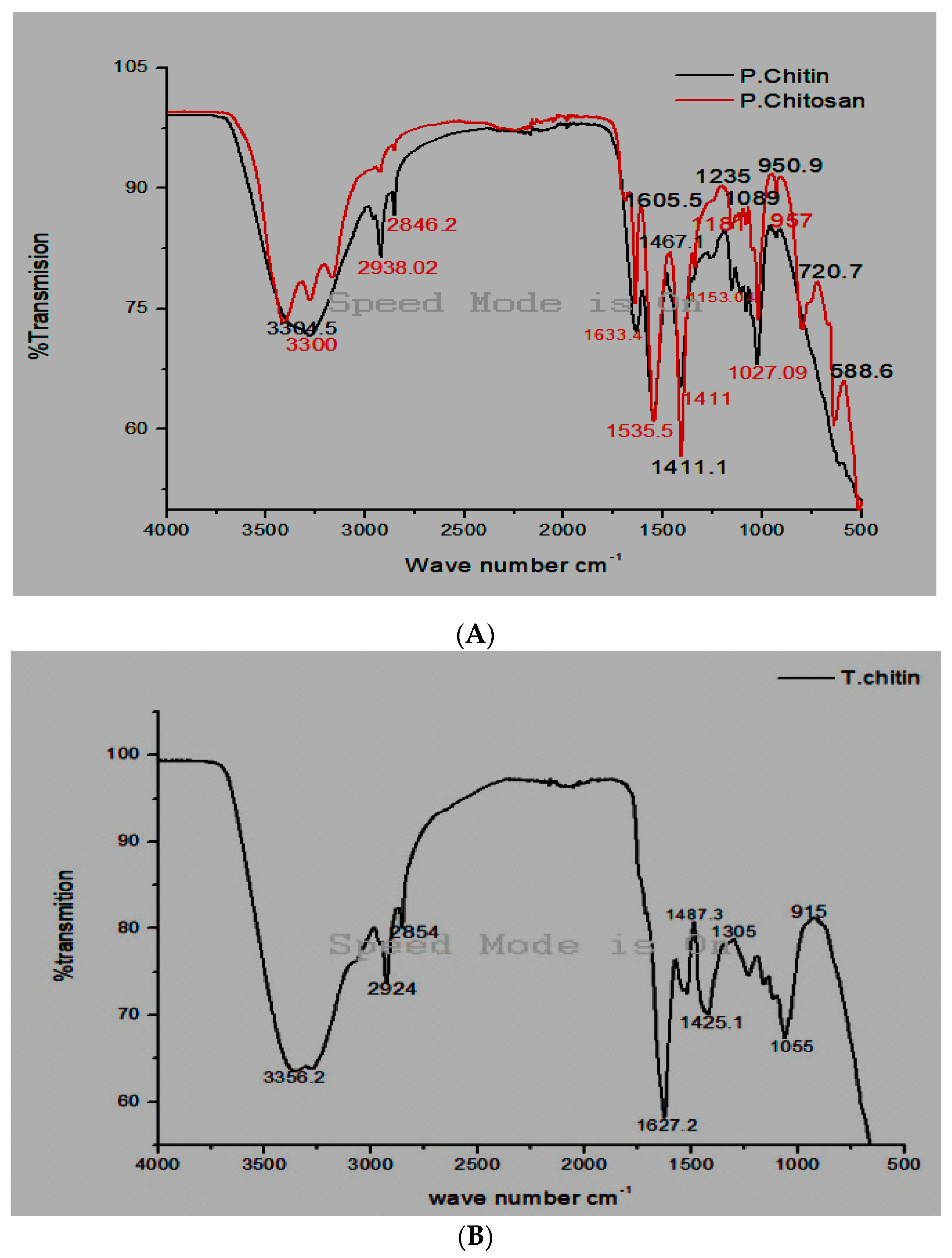
3.1. Fourier Transforms Infrared Spectroscopy Analysis
3.2. Termite Chitin FT-IR
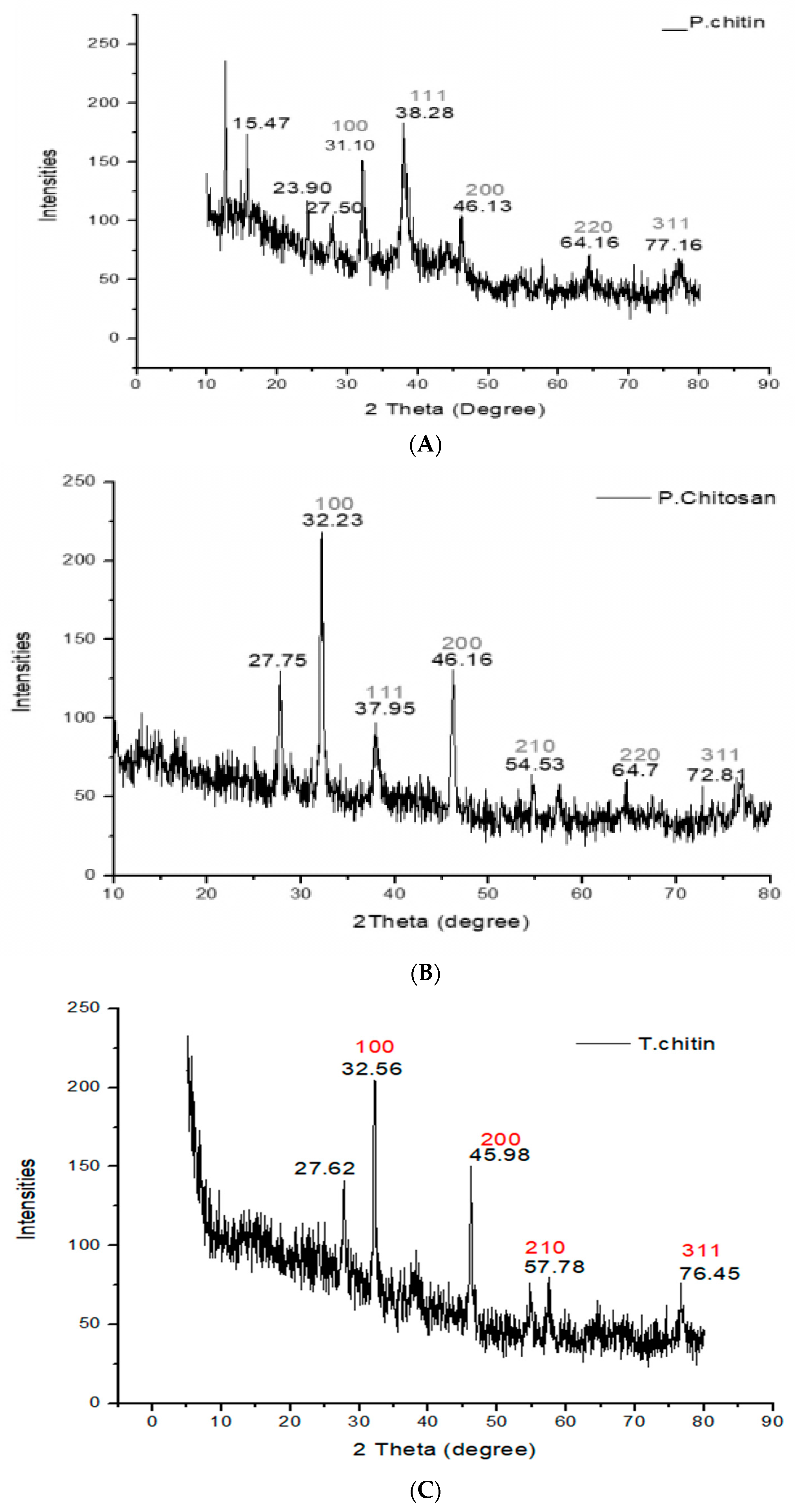
3.3. X-rays Diffraction (XRD) Analysis
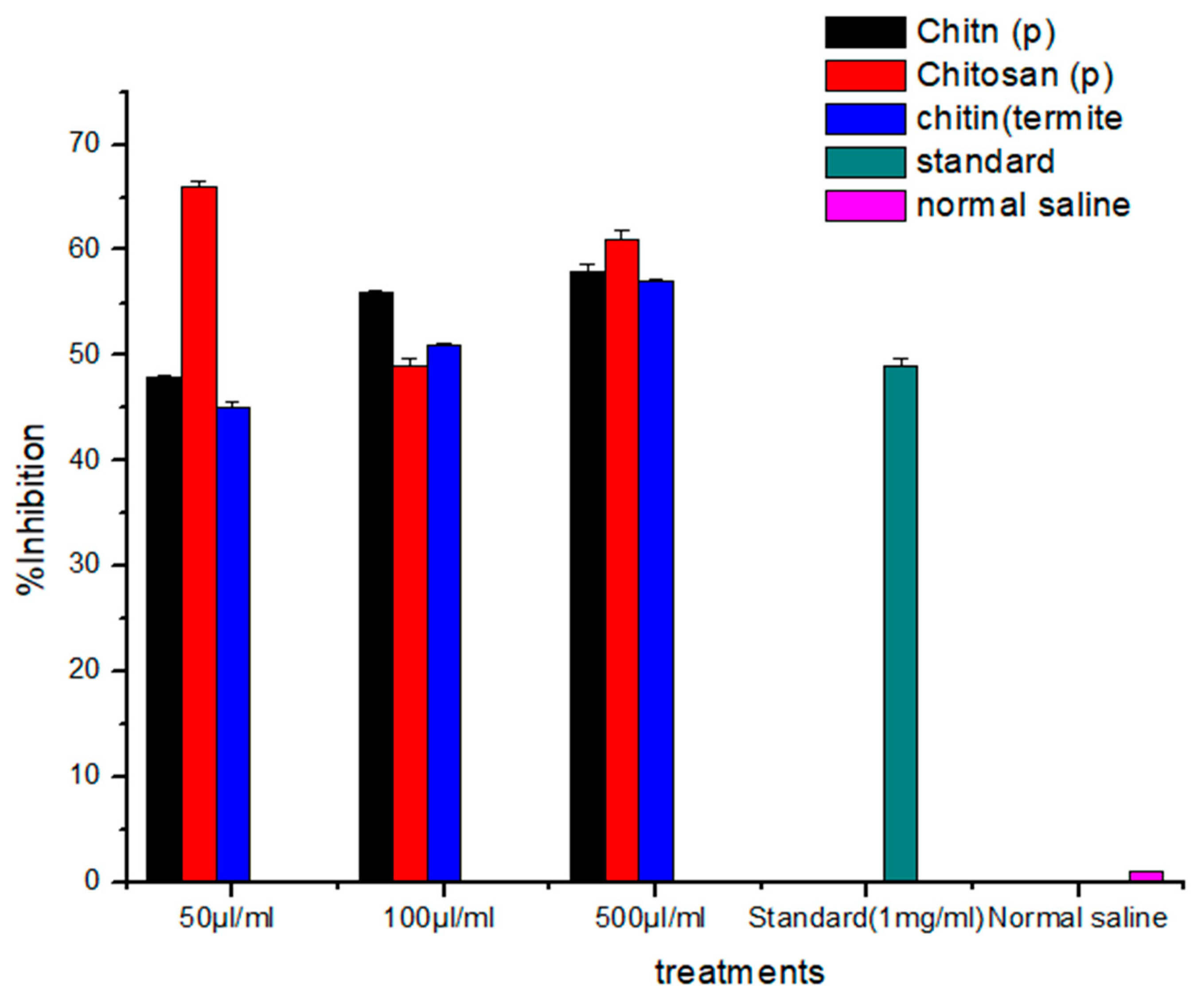
3.4. Analgesic Activity
3.5. Anti-Inflammatory In Vivo Assay
3.6. Antipyretic Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Kumar, A.S.H.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Berger, L.R.R.; Stamford, T.C.M.; de Oliveira, K.Á.R.; Pessoa, A.D.M.P.; de Lima, M.A.B.; Pintado, M.M.E.; Câmara, M.P.S.; de Oliveira Franco, L.; Magnani, M.; de Souza, E.L. Chitosan produced from Mucorales fungi using agroindustrial by-products and its efficacy to inhibit Colletotrichum species. Int. J. Biol. Macromol. 2018, 108, 635–641. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Saad, A.Z.M. Chitosan: A promising marine polysaccharide for biomedical research. Pharmacogn. Rev. 2016, 10, 39. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.D.; Assis, O.B. Assis, A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Q.; Huo, J.; Yang, B. Green preparation of a cellulose nanocrystals/polyvinyl alcohol composite superhydrophobic coating. RSC Adv. 2017, 7, 20152–20159. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Vinsova, J.; Vavrikova, E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—A review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Philippova, O.E.; Korchagina, E.V.; Volkov, E.V.; Smirnov, V.A.; Khokhlov, A.R.; Rinaudo, M. Aggregation of some water-soluble derivatives of chitin in aqueous solutions: Role of the degree of acetylation and effect of hydrogen bond breaker. Carbohydr. Polym. 2012, 87, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of chitin: Solvents, solution behaviors, and their related mechanisms. Solubility Polysacch. 2017, 3, 20–60. [Google Scholar]
- Mullins, D.E. Physiology of environmental adaptations and resource acquisition in cockroaches. Annu. Rev. Entomol. 2015, 60, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Wanule, D.; Balkhande, J.V.; Ratnakar, P.U.; Kulkarni, A.N.; Bhowate, C.S. Extraction, and FTIR analysis of chitosan from American cockroach, Periplaneta americana. Extraction 2014, 3, 299–304. [Google Scholar]
- Bignell, D.E. The role of symbionts in the evolution of termites and their rise to ecological dominance in the tropics. In The Mechanistic Benefits of Microbial Symbionts; Springer: Cham, Switzerland, 2016; pp. 121–172. [Google Scholar]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Acay, H.; Baran, M.F. Investigating antimicrobial activity of silver nanoparticles produced through the green synthesis using leaf extract of common grape (Vitis vinifera). Appl. Ecol. Environ. Res. 2019, 17, 4539–4546. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Mentes, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Khan, M.S.; Ullah, S. Analgesic, anti-inflammatory, antioxidant activity and phytochemical screening of Dryopteris blanfordii plant. J. Pharmacogn. Phytochem. 2018, 115, 523–531. [Google Scholar]
- Oh, Y.C.; Jeong, Y.H.; Cho, W.K.; Ha, J.H.; Gu, M.J.; Ma, J.Y. Anti-inflammatory and analgesic effects of pyeongwisan on LPS-stimulated murine macrophages and mouse models of acetic acid-induced writhing response and xylene-induced ear edema. Int. J. Mol. Sci. 2015, 16, 1232–1251. [Google Scholar] [CrossRef]
- Sulaiman, S.; Ahmad, S.; Naz, S.S.; Qaisar, S.; Muhammad, S.; Ullah, R.; Al-Sadoon, M.K.; Gulnaz, A. Synthesis of zinc oxide-based etoricoxib and montelukast nanoformulations and their evaluation through analgesic, anti-inflammatory, antipyretic, and acute toxicity activities. J. King Saud. Univ. Sci. 2022, 34, 101938. [Google Scholar] [CrossRef]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef] [PubMed]
- Şenel, S.; McClure, S.J. Potential applications of chitosan in veterinary medicine. Adv. Drug Deliv. Rev. 2004, 56, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Tavaria, F.; Jorge, M.P.; Ruiz, L.T.; Pintado, M.E.; Carvalho, J.E. Anti-proliferative, anti-inflammatory, anti-ulcerogenic and wound healing properties of chitosan. Curr. Bioact. Compd. 2016, 12, 114–122. [Google Scholar] [CrossRef]
- Jin, S.E.; Jung, J.; Jun, J.; Jeon, D.W.; Kim, H.M.; Jeong, H.J. Anti-allergic activity of crystallinity controlled N-acetyl glucosamine. Immunopharmacol. Immunotoxicol. 2012, 34, 991–1000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Groups | Treatment | Dose mL or mg/kg | Initial Value | Final Value | % Analgesia | |
|---|---|---|---|---|---|---|
| Analgesic Chitin (chi) | Gr.1 | chi1 | 50 μL/mL | 68.25 ± 0.95 | 42.75 ± 1.70 | 48.02% |
| Gr.2 | chi2 | 100 μL/mL | 75.00 ± 2.94 | 50.00 ± 2.58 | 56.17% | |
| Gr.3 | chi3 | 500 μL/mL | 76.00 ± 5.35 | 52.25 ± 2.75 | 58.70% | |
| Analgesic chitosan (chs) | Gr.1 | chs1 | 50 μL/mL | 84.25 ± 3.94 | 59.25 ± 1.70 | 66.56% |
| Gr.2 | chs2 | 100 μL/mL | 66.50 ± 3.31 | 44.25 ± 2.75 | 49.71% | |
| Gr.3 | chs3 | 500 μL/mL | 74.25 ± 2.75 | 55.00 ± 3.65 | 61.79% | |
| Analgesic termite’s chitin (ter chi) | Gr.1 | ter chi1 | 50 μL/mL | 62.50 ± 3.00 | 40.50 ± 1.29 | 45.50% |
| Gr.2 | ter chi2 | 100 μL/mL | 63.00 ± 3.16 | 45.50 ± 3.41 | 51.11% | |
| Gr.3 | ter chi3 | 500 μL/mL | 72.25 ± 6.50 | 51.00 ± 8.83 | 57.30% | |
| Gr.4 | Standard | 1 mL | 54.50 ± 4.14 | 44.25 ± 4.34 | 49.71% | |
| Gr.5 | Control | 1% v/v | 88.75 ± 0.50 | 87.25 ± 0.95 | 73.68% |
| Drug | Dose (μL/mL or mg/mL) | Temperature (°F) before Applying Yeast | Temperature (°F) after Using the Sample | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | |||
| Negative Control (Normal Saline) | 10% v/v | 98.7 | 101 | 101 | 101 | 101 | 101 |
| Positive Control (Paracetamol) | 1 mg/mL | 98.7 | 100 | 98.8 | 98.7 | 98.7 | 98.8 |
| Chitin | 50 µL/mL, 100 µL/mL and 500 µL/mL | 98.7 | 100, 99.5, 98.6 | 99.7, 99.5, 98.2 | 100, 99, 98.5 | 100, 98, 99.3 | 100.2, 99.1, 98.6 |
| Chitosan | 50 µL/mL, 100 µL/mL and 500 µL/mL | 98.7 | 100.5, 99, 100 | 100.1, 99.5, 99 | 100.1, 99.4, 98 | 100.6, 98, 99.1 | 100.3, 99, 98 |
| termite’s chitin | 50 µL/mL, 100 µL/mL and 500 µL/mL | 98.7 | 100.5, 99.9, 99.5 | 100, 99.6, 99.1 | 100, 99.1, 99 | 100.5, 99.9, 98 | 100, 99.2, 99.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asad, K.; Shams, S.; Ibáñez-Arancibia, E.; De los Ríos-Escalante, P.R.; Badshah, F.; Ahmad, F.; Khan, M.S.; Khan, A. Anti-Inflammatory, Antipyretic, and Analgesic Potential of Chitin and Chitosan Derived from Cockroaches (Periplaneta americana) and Termites. J. Funct. Biomater. 2024, 15, 80. https://doi.org/10.3390/jfb15030080
Asad K, Shams S, Ibáñez-Arancibia E, De los Ríos-Escalante PR, Badshah F, Ahmad F, Khan MS, Khan A. Anti-Inflammatory, Antipyretic, and Analgesic Potential of Chitin and Chitosan Derived from Cockroaches (Periplaneta americana) and Termites. Journal of Functional Biomaterials. 2024; 15(3):80. https://doi.org/10.3390/jfb15030080
Chicago/Turabian StyleAsad, Khushbakht, Sumaira Shams, Eliana Ibáñez-Arancibia, Patricio R. De los Ríos-Escalante, Farhad Badshah, Farooq Ahmad, Muhammad Salman Khan, and Asar Khan. 2024. "Anti-Inflammatory, Antipyretic, and Analgesic Potential of Chitin and Chitosan Derived from Cockroaches (Periplaneta americana) and Termites" Journal of Functional Biomaterials 15, no. 3: 80. https://doi.org/10.3390/jfb15030080
APA StyleAsad, K., Shams, S., Ibáñez-Arancibia, E., De los Ríos-Escalante, P. R., Badshah, F., Ahmad, F., Khan, M. S., & Khan, A. (2024). Anti-Inflammatory, Antipyretic, and Analgesic Potential of Chitin and Chitosan Derived from Cockroaches (Periplaneta americana) and Termites. Journal of Functional Biomaterials, 15(3), 80. https://doi.org/10.3390/jfb15030080







