Enhancing the Biological Properties of Organic–Inorganic Hybrid Calcium Silicate Cements: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Experimental Specimens and Eluates
2.3. Cell Lines and Culture
2.4. Cell Viability
2.5. Cell Migration Assay
2.5.1. Wound Healing Assay
2.5.2. Transwell Assay
2.6. Osteogenic Capability
2.6.1. Alizarin Red S Staining Assay
2.6.2. Osteogenic Regulator Gene Expression Assay
2.7. Statistical Analysis
3. Results
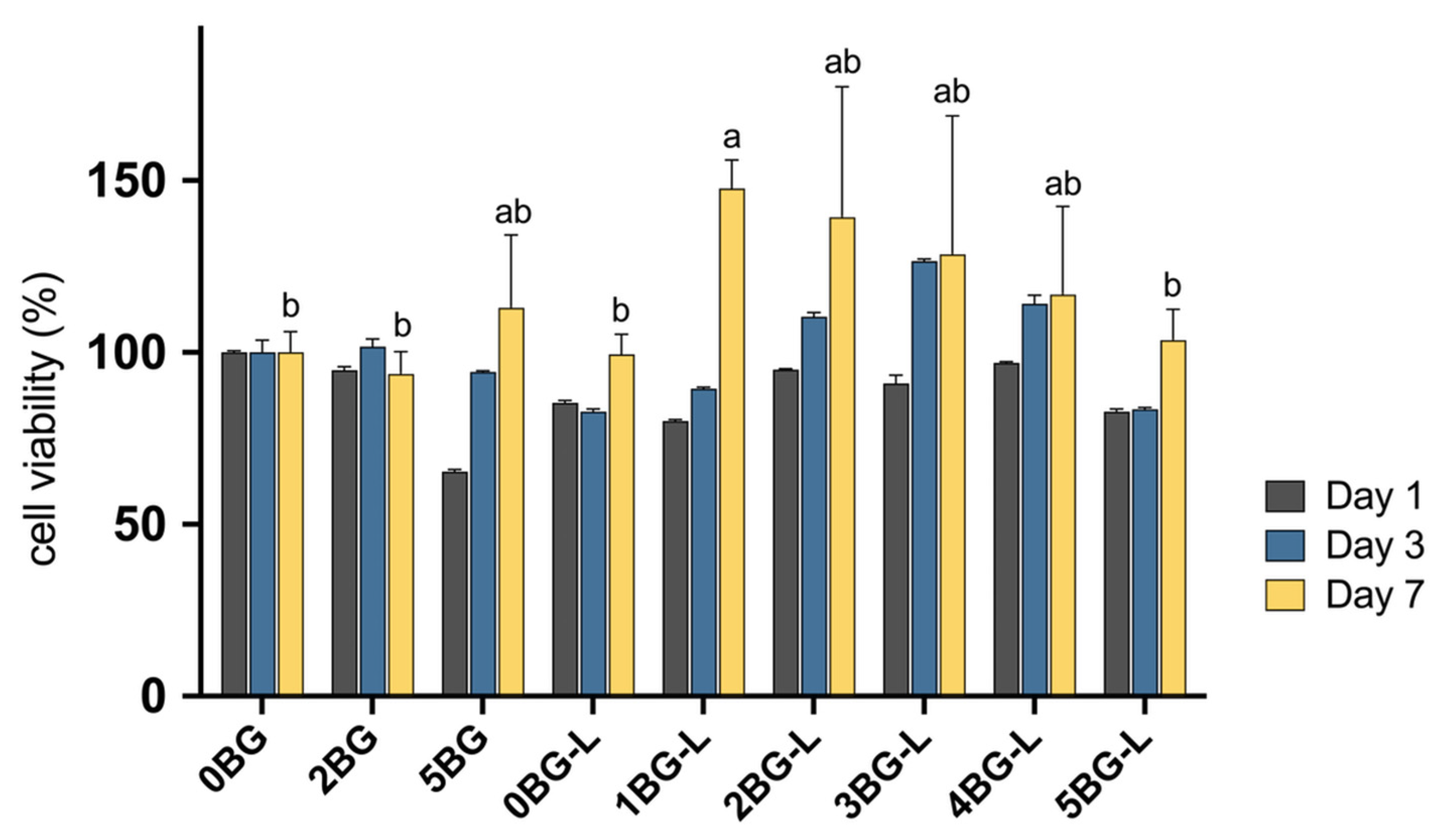
3.1. Cell Viability Test
3.2. Cell Migration Assay
3.2.1. Wound Healing Assay
3.2.2. Transwell Assay
3.3. Osteogenic Capability
3.3.1. Alizarin Red S Staining Assay
3.3.2. Osteogenic Regulator Gene Expression Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.-J.; Monsef, M.; Torabinejad, M. Sealing Ability of a Mineral Trioxide Aggregate for Repair of Lateral Root Perforations. J. Endod. 1993, 19, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Watson, T.F.; Pitt Ford, T.R. Sealing Ability of a Mineral Trioxide Aggregate When Used as a Root End Filling Material. J. Endod. 1993, 19, 591–595. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Yoon, H.; Jung, H.I.; Shin, D.-H.; Song, M. Comparison of Obturation Quality after MTA Orthograde Filling with Various Obturation Techniques. J. Clin. Med. 2021, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Jung, C.; Shin, D.-H.; Cho, Y.; Song, M. Calcium Silicate-Based Root Canal Sealers: A Literature Review. Restor. Dent. Endod. 2020, 45, e35. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; González, B. Medical Applications of Organic–Inorganic Hybrid Materials within the Field of Silica-Based Bioceramics. Chem. Soc. Rev. 2011, 40, 596–607. [Google Scholar] [CrossRef]
- Sarangthem, V.; Singh, T.D.; Dinda, A.K. Emerging Role of Elastin-Like Polypeptides in Regenerative Medicine. Adv. Wound Care 2021, 10, 257–269. [Google Scholar] [CrossRef]
- Despanie, J.; Dhandhukia, J.P.; Hamm-Alvarez, S.F.; MacKay, J.A. Elastin-like Polypeptides: Therapeutic Applications for an Emerging Class of Nanomedicines. J. Control. Release 2016, 240, 93–108. [Google Scholar] [CrossRef]
- Wang, E.; Lee, S.-H.; Lee, S.-W. Elastin-Like Polypeptide Based Hydroxyapatite Bionanocomposites. Biomacromolecules 2011, 12, 672–680. [Google Scholar] [CrossRef][Green Version]
- Jang, J.-H.; Lee, C.-O.; Kim, H.-J.; Kim, S.G.; Lee, S.-W.; Kim, S.-Y. Enhancing Effect of Elastinlike Polypeptide-Based Matrix on the Physical Properties of Mineral Trioxide Aggregate. J. Endod. 2018, 44, 1702–1708. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Bae, H.E.; Lee, J.-E.; Park, I.-S.; Kim, H.-G.; Kwon, J.; Kim, D.-S. Effects of Bioactive Glass Incorporation into Glass Ionomer Cement on Demineralized Dentin. Sci. Rep. 2021, 11, 7016. [Google Scholar] [CrossRef] [PubMed]
- Inanc, B.; Elcin, A.E. Osteogenic Induction of Human Periodontal Ligament Fibroblasts Under Two- and Three-Dimensional Culture Conditions. Tissue Eng. 2006, 12, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, D.; Nebel, D.; Bratthall, G.; Nilsson, B.-O. The Human Periodontal Ligament Cell: A Fibroblast-like Cell Acting as an Immune Cell: Human Periodontal Ligament Cell Characteristics. J. Periodontal Res. 2011, 46, 153–157. [Google Scholar] [CrossRef]
- Ivanovski, S.; Li, H.; Haase, H.R.; Bartold, P.M. Expression of Bone Associated Macromolecules by Gingival and Periodontal Ligament Fibroblasts. J. Periodontal Res. 2001, 36, 131–141. [Google Scholar] [CrossRef]
- Balto, H.; Al-Nazhan, S. Attachment of Human Periodontal Ligament Fibroblasts to 3 Different Root-End Filling Materials: Scanning Electron Microscope Observation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2003, 95, 222–227. [Google Scholar] [CrossRef]
- Szczurko, G.; Pawińska, M.; Łuczaj-Cepowicz, E.; Kierklo, A.; Marczuk-Kolada, G.; Hołownia, A. Effect of Root Canal Sealers on Human Periodontal Ligament Fibroblast Viability: Ex Vivo Study. Odontology 2018, 106, 245–256. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Tucci, M.A.; Fan, L.; Benghuzzi, H.A.; Pal, P.; Bidwell, G.L.; Salazar Marocho, S.M.; Cason, Z.; Gordy, D.; Janorkar, A.V. Collagen-Elastin-Like Polypeptide-Bioglass Scaffolds for Guided Bone Regeneration. Adv. Healthc. Mater. 2020, 9, 1901385. [Google Scholar] [CrossRef]
- Huang, G.; Xu, L.; Wu, J.; Wang, S.; Dong, Y. Gelatin/Bioactive Glass Composite Scaffold for Promoting the Migration and Odontogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Polym. Test. 2021, 93, 106915. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, D.; Cho, S.; Jang, J.-H.; Kim, S.G.; Kim, S.-Y. Improvement of the Bonding Properties of Mineral Trioxide Aggregate by Elastin-Like Polypeptide Supplementation. Scanning 2019, 2019, 3484396. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce in Vivo Surface-structure Changes in Bioactive Glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- D’Antò, V.; Di Caprio, M.P.; Ametrano, G.; Simeone, M.; Rengo, S.; Spagnuolo, G. Effect of Mineral Trioxide Aggregate on Mesenchymal Stem Cells. J. Endod. 2010, 36, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In Vitro Cell Migration and Invasion Assays. J. Vis. Exp. 2014, 752, 51046. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Jeong, H.-J.; Lee, B.-N.; Lee, H.-S.; Kim, H.-J.; Kim, S.-Y.; Kim, D.-S.; Jang, J.-H. Effects of Various Mineral Trioxide Aggregates on Viability and Mineralization Potential of 3-Dimensional Cultured Dental Pulp Stem Cells. Appl. Sci. 2021, 11, 11381. [Google Scholar] [CrossRef]
- Frank, O.; Heim, M.; Jakob, M.; Barbero, A.; Schäfer, D.; Bendik, I.; Dick, W.; Heberer, M.; Martin, I. Real-time Quantitative RT-PCR Analysis of Human Bone Marrow Stromal Cells during Osteogenic Differentiation in Vitro. J. Cell. Biochem. 2002, 85, 737–746. [Google Scholar] [CrossRef]
- Kamoun, A.; Landeau, J.-M.; Godeau, G.; Wallach, J.; Duchesnay, A.; Pellat, B.; Hornebeck, W. Growth Stimulation of Human Skin Fibroblasts by Elastin-Derived Peptides. Cell Adhes. Commun. 1995, 3, 273–281. [Google Scholar] [CrossRef]
- Kinikoglu, B.; Rodríguez-Cabello, J.C.; Damour, O.; Hasirci, V. The Influence of Elastin-like Recombinant Polymer on the Self-Renewing Potential of a 3D Tissue Equivalent Derived from Human Lamina Propria Fibroblasts and Oral Epithelial Cells. Biomaterials 2011, 32, 5756–5764. [Google Scholar] [CrossRef]
- Senior, R.M.; Griffin, G.L.; Mecham, R.P. Chemotactic Responses of Fibroblasts to Tropoelastin and Elastin-Derived Peptides. J. Clin. Investig. 1982, 70, 614–618. [Google Scholar] [CrossRef]
- Sarangthem, V.; Sharma, H.; Goel, R.; Ghose, S.; Park, R.-W.; Mohanty, S.; Chaudhuri, T.K.; Dinda, A.K.; Singh, T.D. Application of Elastin-like Polypeptide (ELP) Containing Extra-Cellular Matrix (ECM) Binding Ligands in Regenerative Medicine. Int. J. Biol. Macromol. 2022, 207, 443–453. [Google Scholar] [CrossRef]
- Sarangthem, V.; Sharma, H.; Mendiratta, M.; Sahoo, R.K.; Park, R.-W.; Kumar, L.; Singh, T.D.; Mohanty, S. Application of Bio-Active Elastin-like Polypeptide on Regulation of Human Mesenchymal Stem Cell Behavior. Biomedicines 2022, 10, 1151. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Bierdeman, P.C.; Janorkar, A.V. Composition of Elastin like Polypeptide–Collagen Composite Scaffold Influences in Vitro Osteogenic Activity of Human Adipose Derived Stem Cells. Dent. Mater. 2016, 32, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.; Westerlund, L.E.; Lovric, V.; Walsh, W. Controlling the Bone Regeneration Properties of Bioactive Glass: Effect of Particle Shape and Size. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Damen, J.J.M.; Ten Cate, J.M. Silica-Induced Precipitation of Calcium Phosphate in the Presence of Inhibitors of Hydroxyapatite Formation. J. Dent. Res. 1992, 71, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.C.; Radin, S.; Chen, A.T.; Ducheyne, P. Differential Alkaline Phosphatase Responses of Rat and Human Bone Marrow Derived Mesenchymal Stem Cells to 45S5 Bioactive Glass. Biomaterials 2007, 28, 4091–4097. [Google Scholar] [CrossRef]
- Wheeler, T.S.; Sbravati, N.D.; Janorkar, A.V. Mechanical & Cell Culture Properties of Elastin-Like Polypeptide, Collagen, Bioglass, and Carbon Nanosphere Composites. Ann. Biomed. Eng. 2013, 41, 2042–2055. [Google Scholar] [CrossRef]




| Groups | Liquid | Powder | |
|---|---|---|---|
| Positive control | 0BG | DW | 100% MTA |
| Inorganic hybrid HCSC | 2BG | DW | 98% MTA + 2% BAG |
| 5BG | DW | 95% MTA + 5% BAG | |
| Organic–inorganic hybrid HCSC | 0BG-L | ELP | 100% MTA |
| 1BG-L | ELP | 99% MTA + 1% BAG | |
| 2BG-L | ELP | 98% MTA + 2% BAG | |
| 3BG-L | ELP | 97% MTA + 3% BAG | |
| 4BG-L | ELP | 96% MTA + 4% BAG | |
| 5BG-L | ELP | 95% MTA + 5% BAG |
| Gene | Gene Name | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| ALP | Alkaline phosphatase | 5′-GACAAGAAGCCCTTCACTGC-3′ | 5′-AGACTGCGCCTGGTAGTTGT-3′ |
| RUNX-2 | Runt-related transcription factor 2 | 5′-GGTTAATCTCCGCAGGTCACT-3′ | 5′-CACTGTGCTGAAGAGGCTGTT-3′ |
| OCN | Osteocalcin | 5′-GGCGCTACCTGTATCAATGG-3′ | 5′-TCAGCCAACTCGTCACAGTC-3′ |
| Col1A2 | Collagen type I alpha 2 | 5′-CCTGGTGCTAAAGGAGAAAGAGG-3′ | 5′-ATCACCACGACTTCCAGCAGGA-3′ |
| hGAPDH | Glyceraldehyde 3-phosphate dehydrogenase | 5′-AACAGCGACACCCACTCCTC-3′ | 5′-CATACCAGGAAATGAGCTTGACAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.; Kwon, J.; Jang, J.-H.; Kim, D.-S.; Kim, H.-J. Enhancing the Biological Properties of Organic–Inorganic Hybrid Calcium Silicate Cements: An In Vitro Study. J. Funct. Biomater. 2024, 15, 337. https://doi.org/10.3390/jfb15110337
Choi M, Kwon J, Jang J-H, Kim D-S, Kim H-J. Enhancing the Biological Properties of Organic–Inorganic Hybrid Calcium Silicate Cements: An In Vitro Study. Journal of Functional Biomaterials. 2024; 15(11):337. https://doi.org/10.3390/jfb15110337
Chicago/Turabian StyleChoi, Minji, Jiyoung Kwon, Ji-Hyun Jang, Duck-Su Kim, and Hyun-Jung Kim. 2024. "Enhancing the Biological Properties of Organic–Inorganic Hybrid Calcium Silicate Cements: An In Vitro Study" Journal of Functional Biomaterials 15, no. 11: 337. https://doi.org/10.3390/jfb15110337
APA StyleChoi, M., Kwon, J., Jang, J.-H., Kim, D.-S., & Kim, H.-J. (2024). Enhancing the Biological Properties of Organic–Inorganic Hybrid Calcium Silicate Cements: An In Vitro Study. Journal of Functional Biomaterials, 15(11), 337. https://doi.org/10.3390/jfb15110337









