Biomimetic Coatings in Implant Dentistry: A Quick Update
Abstract
:1. Introduction
2. Implant Surface Modifications
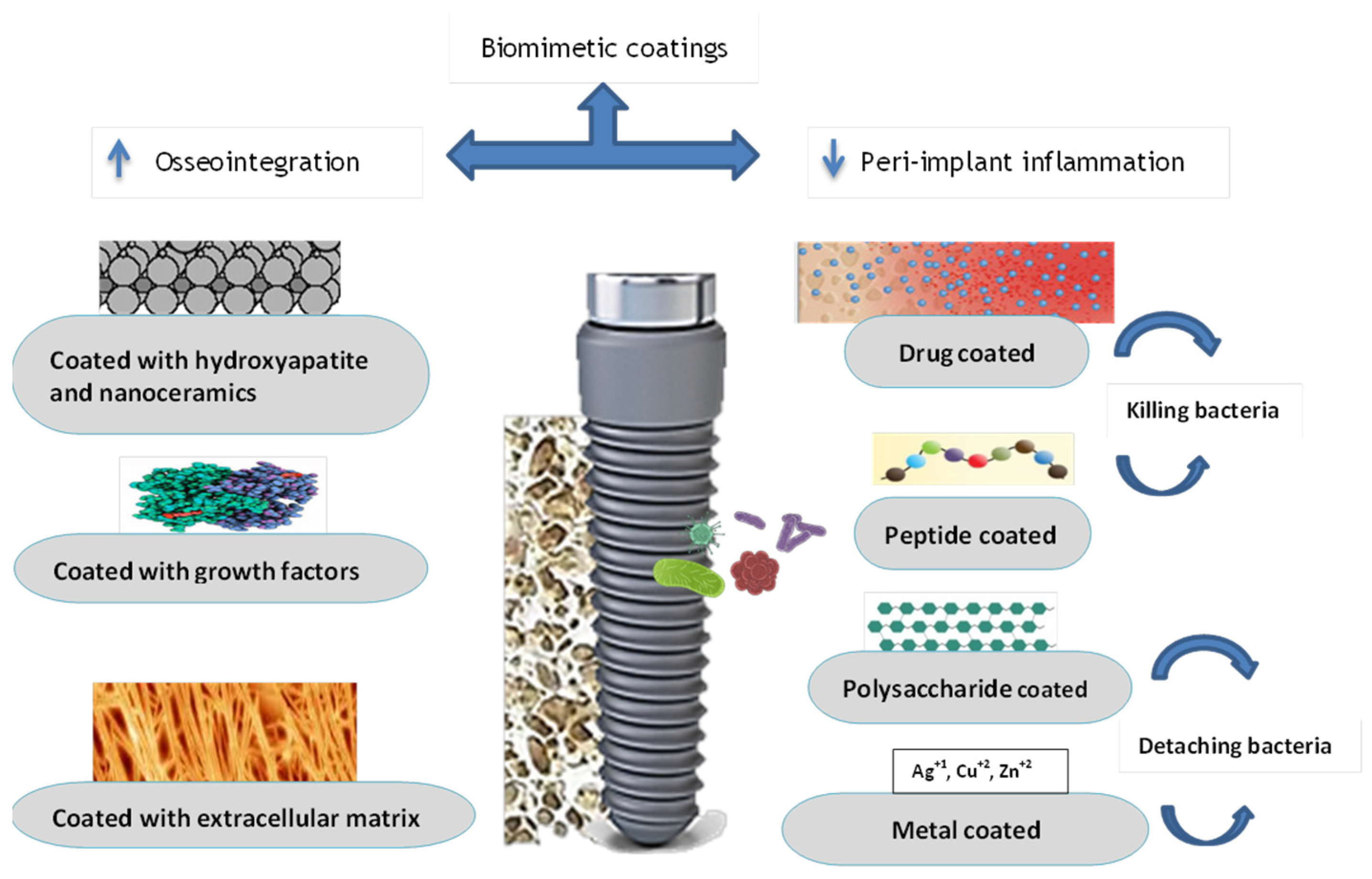
3. Biomimetic Coating
3.1. Coating to Improve Osseointegration
3.1.1. Hydroxyapatite Layer and Nanocomposites
3.1.2. Growth Factors
3.1.3. Extra Cellular Matrix
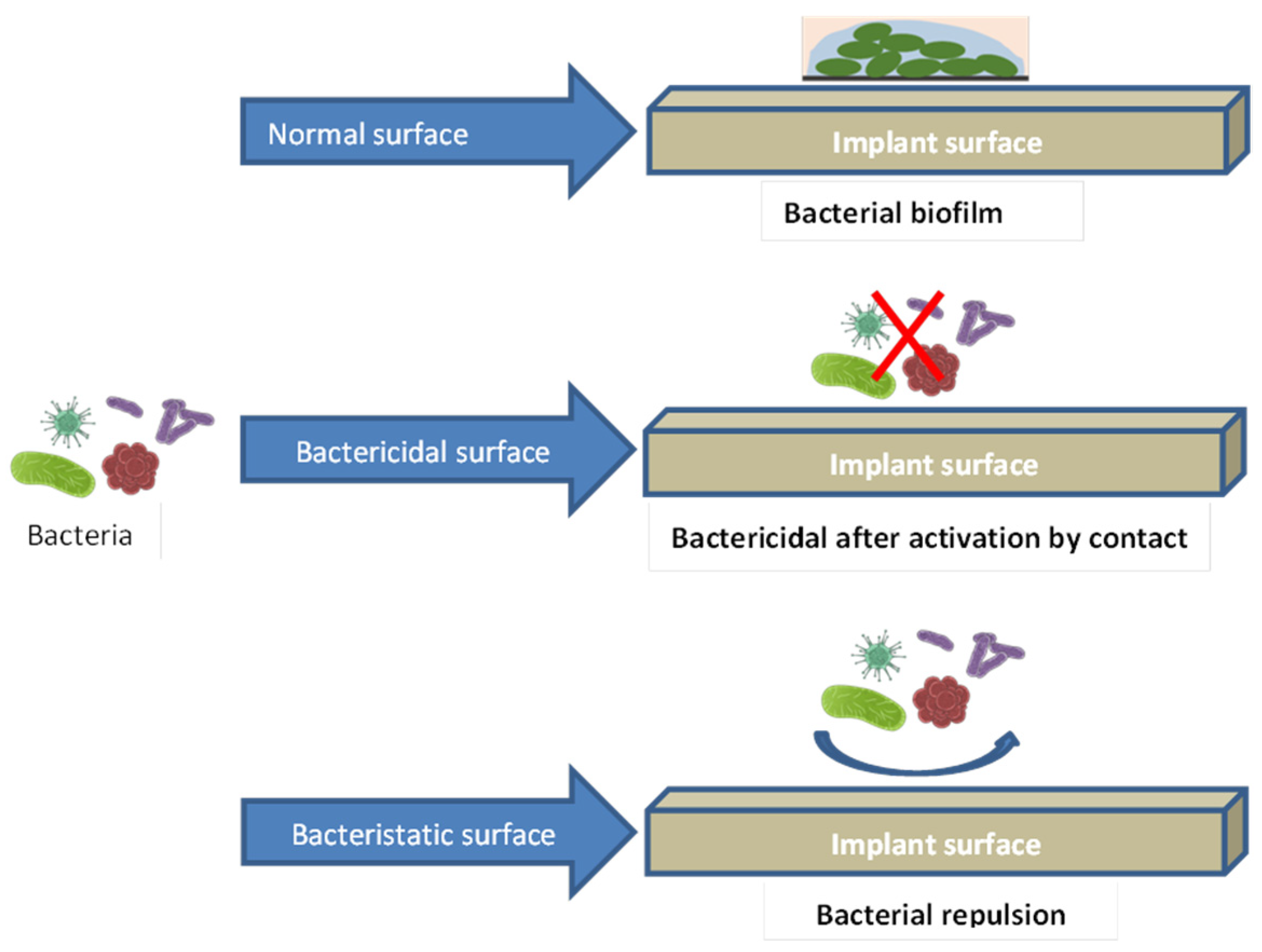
3.2. The Antibacterial Performance of Coating
3.2.1. Drug-Coated Dental Implant
3.2.2. Antimicrobial Peptide Coating
3.2.3. Polysaccharide Antibacterial Coating
3.2.4. Antibacterial Properties of Metal-Element Components
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Thanissorn, C.; Guo, J.; Jing Ying Chan, D.; Koyi, B.; Kujan, O.; Khzam, N.; Miranda, L.A. Success rates and complications associated with single immediate implants: A systematic review. Dent. J. 2022, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Leal, J.I.; Rodríguez-Valverde, M.A.; Mazzaglia, G.; Ramón-Torregrosa, P.J.; Díaz-Rodríguez, L.; García-Martínez, O.; Vallecillo-Capilla, M.; Ruiz, C.; Cabrerizo-Vílchez, M. Effect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesion. Colloids Surf. A Physicochem. Eng. Asp. 2010, 365, 222–229. [Google Scholar] [CrossRef]
- Hudieb, M.; AlKhader, M.; Mortaja, S.; Abusamak, M.; Wakabayashi, N.; Kasugai, S. Impact of bone augmentation of facial bone defect around osseointegrated implant: A three dimensional finite element analysis. Dent. J. 2021, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.Q.; Nath, S.D.; Akilan, A.A.; Khanjar, S.; Balla, V.K.; Grant, G.T.; Atre, S.V. Investigation of patient-specific maxillofacial implant prototype development by metal fused filament fabrication (MF3) of Ti-6Al-4V. Dent. J. 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, A.; Bissa, M.W.; Khurshid, Z.; Zohaib, S.; Asiri, F.Y.I.; Zafar, M.S. Surface modification techniques of dental implants. In Dental Implants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–68. [Google Scholar]
- Albrektsson, T.; Buser, D.; Sennerby, L. Crestal bone loss and oral implants. Clin. Implant Dent. Relat. Res. 2012, 14, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Shemtov-Yona, K.; Rittel, D. Fatigue of dental implants: Facts and fallacies. Dent. J. 2016, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Hieu, T.; Borghetti, A.; Aboudharam, G. Peri-implantitis: From diagnosis to therapeutics. J. Investig. Clin. Dent. 2012, 3, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Jaquiéry, C.; Ilgenstein, B.; Jungo, M.; Rüeger, K.; Chenaux, S.; Papadimitropoulos, A.; Jäger, K. Clinical and radiological outcome of titanium implants in clinical practice: A 5 year, prospective, multicenter case series. Dent. J. 2014, 2, 106–117. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Alghamdi, H.; Cuijpers, V.; Wolke, J.; Van den Beucken, J.; Jansen, J. Calcium-phosphate-coated oral implants promote osseointegration in osteoporosis. J. Dent. Res. 2013, 92, 982–988. [Google Scholar] [CrossRef]
- Stadlinger, B.; Korn, P.; Tödtmann, N.; Eckelt, U.; Range, U.; Bürki, A.; Ferguson, S.; Kramer, I.; Kautz, A.; Schnabelrauch, M. Osseointegration of biochemically modified implants in an osteoporosis rodent model. Eur. Cells Mater. 2013, 25, 326–340; discussion 339. [Google Scholar] [CrossRef]
- Tsujino, T.; Takahashi, A.; Watanabe, T.; Isobe, K.; Kitamura, Y.; Okuda, K.; Nakata, K.; Kawase, T. Platelet adhesion on commercially pure titanium plates in vitro II. Immunofluorescence visualization of PDGF-B, TGFβ1, and PPARγ released from activated adherent platelets. Dent. J. 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, V.; Pezzetti, F.; Scarano, A.; Piattelli, A.; Bignozzi, C.A.; Massari, L.; Brunelli, G.; Carinci, F. Zirconium oxide coating improves implant osseointegration in vivo. Dent. Mater. 2008, 24, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Abdullatif, F.A.; Al-Askar, M. Does Ultraviolet Radiation Exhibit Antimicrobial Effect against Oral Pathogens Attached on Various Dental Implant Surfaces? A Systematic Review. Dent. J. 2022, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Pilloni, A. Current Molecular, Cellular and Genetic Aspects of Peri-Implantitis Disease: A Narrative Review. Dent. J. 2023, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Fakheran, O.; Fischer, K.R.; Schmidlin, P.R. Enamel Matrix Derivatives as an Adjunct to Alveolar Ridge Preservation—A Systematic Review. Dent. J. 2023, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with reinforced materials and modifications for dental implant applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, M.; Martinolli, M.; Kim, Y.-J.; Cocchi, F.; Meloni, S.M.; Alushi, A.; Xhanari, E. Accuracy of computer-assisted template-based implant placement using two different surgical templates designed with or without metallic sleeves: A randomized controlled trial. Dent. J. 2019, 7, 41. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface modified techniques and emerging functional coating of dental implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Stanford, C.M. Surface modification of biomedical and dental implants and the processes of inflammation, wound healing and bone formation. Int. J. Mol. Sci. 2010, 11, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Biofunctionalization of titanium for dental implant. Jpn. Dent. Sci. Rev. 2010, 46, 93–101. [Google Scholar] [CrossRef]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest trends in surface modification for dental implantology: Innovative developments and analytical applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef]
- Rampado, R.; Caliceti, P.; Agostini, M. Latest advances in biomimetic cell membrane-coated and membrane-derived nanovectors for biomedical applications. Nanomaterials 2022, 12, 1543. [Google Scholar] [CrossRef] [PubMed]
- Harkness, J.M. An idea man (the life of Otto Herbert Schmitt). IEEE Eng. Med. Biol. Mag. 2004, 23, 20–41. [Google Scholar] [CrossRef]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic aspects of restorative dentistry biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Kottoor, J. Biomimetic endodontics: Barriers and strategies. Health Sci. 2013, 2, 7–12. [Google Scholar]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Jakšić, Z.; Jakšić, O. Biomimetic nanomembranes: An overview. Biomimetics 2020, 5, 24. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Koziaeva, V.V.; Sitkov, N.O.; Gao, H.; Zimina, T.M.; Shevtsov, M. Biomimetic nanomaterials: Diversity, technology, and biomedical applications. Nanomaterials 2022, 12, 2485. [Google Scholar] [CrossRef]
- Mačković, M.; Hoppe, A.; Detsch, R.; Mohn, D.; Stark, W.J.; Spiecker, E.; Boccaccini, A. Bioactive glass (type 45S5) nanoparticles: In vitro reactivity on nanoscale and biocompatibility. J. Nanoparticle Res. 2012, 14, 1–22. [Google Scholar] [CrossRef]
- Li, S.; Yu, W.; Zhang, W.; Zhang, G.; Yu, L.; Lu, E. Evaluation of highly carbonated hydroxyapatite bioceramic implant coatings with hierarchical micro-/nanorod topography optimized for osseointegration. Int. J. Nanomed. 2018, 13, 3643. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wei, M. Cellular performance comparison of biomimetic calcium phosphate coating and alkaline-treated titanium surface. BioMed Res. Int. 2013, 2013, 832790. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, H.; Liu, L.; He, Q.; Zhao, L.; Huang, Z.; Yang, J.; Cao, C.; Chen, J.; Wang, A. Biomimetic titanium implant coated with extracellular matrix enhances and accelerates osteogenesis. Nanomedicine 2020, 15, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Sebdani, M.M.; Fathi, M. Novel hydroxyapatite–forsterite–bioglass nanocomposite coatings with improved mechanical properties. J. Alloys Compd. 2011, 509, 2273–2276. [Google Scholar] [CrossRef]
- Erol-Taygun, M.; Zheng, K.; Boccaccini, A.R. Nanoscale bioactive glasses in medical applications. Int. J. Appl. Glass Sci. 2013, 4, 136–148. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive titanium surfaces: Interactions of eukaryotic and prokaryotic cells of nano devices applied to dental practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.S. Methods to improve osseointegration of dental implants in low quality (type-IV) bone: An overview. J. Funct. Biomater. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Tang, Y.; Wang, K.; Zhou, X.; Xiang, L. Nanostructured titanium implant surface facilitating osseointegration from protein adsorption to osteogenesis: The example of TiO2 NTAs. Int. J. Nanomed. 2022, 17, 1865–1879. [Google Scholar] [CrossRef]
- Dorcioman, G.; Grumezescu, V.; Stan, G.E.; Chifiriuc, M.C.; Gradisteanu, G.P.; Miculescu, F.; Matei, E.; Popescu-Pelin, G.; Zgura, I.; Craciun, V. Hydroxyapatite Thin Films of Marine Origin as Sustainable Candidates for Dental Implants. Pharmaceutics 2023, 15, 1294. [Google Scholar] [CrossRef]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.M.; Macedo de Sousa, B.; Herrero-Hernández, S.; López-Valverde, A. Bioactive surfaces vs. conventional surfaces in titanium dental implants: A comparative systematic review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef]
- Taymour, N.; Fahmy, A.E.; Gepreel, M.A.H.; Kandil, S.; El-Fattah, A.A. Improved Mechanical Properties and Bioactivity of Silicate Based Bioceramics Reinforced Poly (ether-ether-ketone) Nanocomposites for Prosthetic Dental Implantology. Polymers 2022, 14, 1632. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Zhang, M.; Jin, L.; Zhao, J.; Gao, Q.; Ren, M.; Fan, Q. Assessment of osteoinduction using a porous hydroxyapatite coating prepared by micro-arc oxidation on a new titanium alloy. Int. J. Surg. 2015, 24, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Ben-Nissan, B.; Matinlinna, J.; Conway, R. Current perspectives: Calcium phosphate nanocoatings and nanocomposite coatings in dentistry. J. Dent. Res. 2013, 92, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Roden, L.C.; Renton, L.F. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 2012, 33, 3813–3823. [Google Scholar] [CrossRef] [PubMed]
- Carradò, A.; Perrin-Schmitt, F.; Le, Q.; Giraudel, M.; Fischer, C.; Koenig, G.; Jacomine, L.; Behr, L.; Chalom, A.; Fiette, L. Nanoporous hydroxyapatite/sodium titanate bilayer on titanium implants for improved osteointegration. Dent. Mater. 2017, 33, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hędzelek, W.; Dorocka-Bobkowska, B. Hydroxyapatite coating on titanium endosseous implants for improved osseointegration: Physical and chemical considerations. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2018, 27, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, X.; Xia, W.; Wang, Z.; Zhang, P.; Xia, L.; Lin, K.; Zhu, M. Nano-structure designing promotion osseointegration of hydroxyapatite coated Ti–6Al–4V alloy implants in diabetic model. J. Biomed. Nanotechnol. 2019, 15, 1701–1713. [Google Scholar] [CrossRef]
- Fang, C.-H.; Lin, Y.-W.; Lin, F.-H.; Sun, J.-S.; Chao, Y.-H.; Lin, H.-Y.; Chang, Z.-C. Biomimetic synthesis of nanocrystalline hydroxyapatite composites: Therapeutic potential and effects on bone regeneration. Int. J. Mol. Sci. 2019, 20, 6002. [Google Scholar] [CrossRef]
- Eawsakul, K.; Tancharoen, S.; Nasongkla, N. Combination of dip coating of BMP-2 and spray coating of PLGA on dental implants for osseointegration. J. Drug Deliv. Sci. Technol. 2021, 61, 102296. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Li, J.; Li, D.; Wang, Q.; Teng, W. Dopamine-assisted co-deposition of hydroxyapatite-functionalised nanoparticles of polydopamine on implant surfaces to promote osteogenesis in environments with high ROS levels. Mater. Sci. Eng. C 2021, 131, 112473. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zheng, A.; Cao, L.; Peng, L.; Wang, X.; Wang, J.; Xin, X.; Jiang, X. Adhesion-enhancing coating embedded with osteogenesis-promoting PDA/HA nanoparticles for peri-implant soft tissue sealing and osseointegration. Bio-Des. Manuf. 2022, 5, 233–248. [Google Scholar] [CrossRef]
- Alcudia, A.; Begines, B.; Rodriguez-Lejarraga, P.; Greyer, V.; Godinho, V.C.F.; Pajuelo, E.; Torres, Y. Development of porous silver nanoparticle/polycaprolactone/polyvinyl alcohol coatings for prophylaxis in titanium interconnected samples for dental implants. Colloid Interface Sci. Commun. 2022, 48, 100621. [Google Scholar] [CrossRef]
- Mokobia, K.E.; Ifijen, I.H.; Ikhuoria, E.U. ZnO-NPs-coated implants with osteogenic properties for enhanced osseointegration. In Proceedings of the TMS 2023 152nd Annual Meeting & Exhibition, San Diego, CA, USA, 19–23 March 2023; pp. 288–300. [Google Scholar]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration–communication of cells. Clin. Oral Implant. Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Barbu, H.M.; Iancu, S.A.; Rapani, A.; Stacchi, C. Guided bone regeneration with concentrated growth factor enriched bone graft matrix (sticky bone) vs. bone-shell technique in horizontal ridge augmentation: A retrospective study. J. Clin. Med. 2021, 10, 3953. [Google Scholar] [CrossRef]
- Guang, M.; Huang, B.; Yao, Y.; Zhang, L.; Yang, B.; Gong, P. Effects of vascular endothelial growth factor on osteoblasts around dental implants in vitro and in vivo. J. Oral Sci. 2017, 59, 215–223. [Google Scholar] [CrossRef]
- Palermo, A.; Ferrante, F.; Stanca, E.; Damiano, F.; Gnoni, A.; Batani, T.; Carluccio, M.A.; Demitri, C.; Siculella, L. Release of VEGF from dental implant surface (IML® Implant) coated with Concentrated Growth Factors (CGF) and the Liquid Phase of CGF (LPCGF): In vitro results and future expectations. Appl. Sci. 2019, 9, 2114. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Santos-Ruiz, L.; Becerra, J.; Feito, M.; Fernández-Villa, D.; Serrano, M.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F. Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomater. 2019, 83, 456–466. [Google Scholar] [CrossRef]
- Sheikh, Z.; Javaid, M.A.; Hamdan, N.; Hashmi, R. Bone regeneration using bone morphogenetic proteins and various biomaterial carriers. Materials 2015, 8, 1778–1816. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.; Lojudice, F.H.; Halcsik, E.; Navarro, R.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Ramazanoglu, M.; Lutz, R.; Ergun, C.; von Wilmowsky, C.; Nkenke, E.; Schlegel, K.A. The effect of combined delivery of recombinant human bone morphogenetic protein-2 and recombinant human vascular endothelial growth factor 165 from biomimetic calcium-phosphate-coated implants on osseointegration. Clin. Oral Implant. Res. 2011, 22, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Kang, S.-S.; Choi, K.-H.; Shim, J.-S.; Jeong, C.-M.; Shin, S.-W.; Huh, J.-B. The effect of anodized implants coated with combined rhBMP-2 and recombinant human vascular endothelial growth factors on vertical bone regeneration in the marginal portion of the peri-implant. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e24–e31. [Google Scholar] [CrossRef] [PubMed]
- Schierano, G.; Canuto, R.A.; Mauthe von Degerfeld, M.; Navone, R.; Peirone, B.; Preti, G.; Muzio, G. Role of rhBMP-7, fibronectin, and type I collagen in dental implant osseointegration process: An initial pilot study on minipig animals. Materials 2021, 14, 2185. [Google Scholar] [CrossRef] [PubMed]
- Al-Jarsha, M.; Moulisová, V.; Leal-Egaña, A.; Connell, A.; Naudi, K.B.; Ayoub, A.F.; Dalby, M.J.; Salmerón-Sánchez, M. Engineered coatings for titanium implants to present ultralow doses of BMP-7. ACS Biomater. Sci. Eng. 2018, 4, 1812–1819. [Google Scholar] [PubMed]
- Lee, S.Y.; Koak, J.Y.; Heo, S.J.; Kim, S.K.; Lee, S.J.; Nam, S.Y. Osseointegration of anodized titanium implants coated with poly (lactide-co-glycolide)/basic fibroblast growth factor by electrospray. Int. J. Oral Maxillofac. Implant. 2010, 25, 315–320. [Google Scholar]
- Schliephake, H.; Rublack, J.; Aeckerle, N.; Förster, A.; Schwenzer, B.; Reichert, J.; Scharnweber, D. In vivo effect of immobilisation of bone morphogenic protein 2 on titanium implants through nano-anchored oligonucleotides. Eur. Cells Mater. 2015, 30, 28–40. [Google Scholar] [CrossRef]
- Yang, D.H.; Moon, S.W.; Lee, D.-W. Surface modification of titanium with BMP-2/GDF-5 by a heparin linker and its efficacy as a dental implant. Int. J. Mol. Sci. 2017, 18, 229. [Google Scholar] [CrossRef]
- Keceli, H.G.; Akman, A.C.; Bayram, C.; Nohutcu, R.M. Tissue engineering applications and nanobiomaterials in periodontology and implant dentistry. In Nanobiomaterials in Dentistry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 337–387. [Google Scholar]
- Maekawa, S.; Cho, Y.D.; Kauffmann, F.; Yao, Y.; Sugai, J.V.; Zhong, X.; Schmiedeler, C.; Kinra, N.; Moy, A.; Larsson, L. BMP Gene-Immobilization to Dental Implants Enhances Bone Regeneration. Adv. Mater. Interfaces 2022, 9, 2200531. [Google Scholar] [CrossRef]
- Adanir, N.; Khurshid, Z.; Ratnayake, J. The Regenerative Potential of Decellularized Dental Pulp Extracellular Matrix: A Systematic Review. Materials 2022, 15, 6386. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Ito, A.; Kimura, T.; Kishida, A. Extracellular matrix induces periodontal ligament reconstruction in vivo. Int. J. Mol. Sci. 2019, 20, 3277. [Google Scholar] [CrossRef] [PubMed]
- Scotchford, C.A.; Ball, M.; Winkelmann, M.; Vörös, J.; Csucs, C.; Brunette, D.; Danuser, G.; Textor, M. Chemically patterned, metal-oxide-based surfaces produced by photolithographic techniques for studying protein-and cell-interactions. II: Protein adsorption and early cell interactions. Biomaterials 2003, 24, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Della Rocca, Y.; Oliva, S.; Rajan, T.S.; Trubiani, O.; Murmura, G.; Diomede, F.; Pizzicannella, J. Enhanced Extracellular Matrix Deposition on Titanium Implant Surfaces: Cellular and Molecular Evidences. Biomedicines 2021, 9, 1710. [Google Scholar] [CrossRef] [PubMed]
- Petrie, T.A.; Raynor, J.E.; Dumbauld, D.W.; Lee, T.T.; Jagtap, S.; Templeman, K.L.; Collard, D.M.; García, A.J. Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration. Sci. Transl. Med. 2010, 2, 45ra60. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Cassinelli, C.; Cascardo, G.; Bollati, D.; Rodriguez y Baena, R. Multifunctional implant surfaces: Surface characterization and bone response to acid-etched Ti implants surface-modified by fibrillar collagen I. J. Biomed. Mater. Res. Part A 2010, 94, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.S.; van Oirschot, B.A.; Bosco, R.; van den Beucken, J.J.; Aldosari, A.A.F.; Anil, S.; Jansen, J.A. Biological response to titanium implants coated with nanocrystals calcium phosphate or type 1-collagen in a dog implantation model. Surf. Modif. Endosseous Implant Mater. 2015, 2015, 129. [Google Scholar]
- Lee, S.-W.; Hahn, B.-D.; Kang, T.Y.; Lee, M.-J.; Choi, J.-Y.; Kim, M.-K.; Kim, S.-G. Hydroxyapatite and collagen combination-coated dental implants display better bone formation in the peri-implant area than the same combination plus bone morphogenetic protein-2–coated implants, hydroxyapatite only coated implants, and uncoated implants. J. Oral Maxillofac. Surg. 2014, 72, 53–60. [Google Scholar] [CrossRef]
- Korn, P.; Schulz, M.; Hintze, V.; Range, U.; Mai, R.; Eckelt, U.; Schnabelrauch, M.; Möller, S.; Becher, J.; Scharnweber, D. Chondroitin sulfate and sulfated hyaluronan-containing collagen coatings of titanium implants influence peri-implant bone formation in a minipig model. J. Biomed. Mater. Res. Part A 2014, 102, 2334–2344. [Google Scholar] [CrossRef]
- de Barros, R.R.; Novaes Jr, A.B.; Korn, P.; Queiroz, A.; de Almeida, A.L.; Hintze, V.; Scharnweber, D.; Bierbaum, S.; Stadlinger, B. Bone formation in a local defect around dental implants coated with extracellular matrix components. Clin. Implant Dent. Relat. Res. 2015, 17, 742–757. [Google Scholar] [CrossRef]
- Raphel, J.; Karlsson, J.; Galli, S.; Wennerberg, A.; Lindsay, C.; Haugh, M.G.; Pajarinen, J.; Goodman, S.B.; Jimbo, R.; Andersson, M. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials 2016, 83, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Komasa, S.; Yoshimine, S.; Sekino, T.; Okazaki, J. Effect of mussel adhesive protein coating on osteogenesis in vitro and osteointegration in vivo to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2019, 14, 3831–3843. [Google Scholar] [CrossRef] [PubMed]
- Syam, S.; Wu, C.-J.; Lan, W.-C.; Ou, K.-L.; Huang, B.-H.; Lin, Y.-Y.; Saito, T.; Tsai, H.-Y.; Chuo, Y.-C.; Yen, M.-L. The potential of a surface-modified titanium implant with tetrapeptide for osseointegration enhancement. Appl. Sci. 2021, 11, 2616. [Google Scholar] [CrossRef]
- Rappe, K.S.; Ortiz-Hernandez, M.; Punset, M.; Molmeneu, M.; Barba, A.; Mas-Moruno, C.; Guillem-Marti, J.; Caparrós, C.; Rupérez, E.; Calero, J. On-growth and in-growth osseointegration enhancement in PM porous Ti-scaffolds by two different bioactivation strategies: Alkali thermochemical treatment and RGD peptide coating. Int. J. Mol. Sci. 2022, 23, 1750. [Google Scholar] [CrossRef] [PubMed]
- Esteves, G.M.; Esteves, J.; Resende, M.; Mendes, L.; Azevedo, A.S. Antimicrobial and antibiofilm coating of dental implants—Past and new perspectives. Antibiotics 2022, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Wychowański, P.; Starzyńska, A.; Adamska, P.; Słupecka-Ziemilska, M.; Sobocki, B.K.; Chmielewska, A.; Wysocki, B.; Alterio, D.; Marvaso, G.; Jereczek-Fossa, B.A. Methods of topical administration of drugs and biological active substances for dental implants—A narrative review. Antibiotics 2021, 10, 919. [Google Scholar] [CrossRef]
- Lopez-Valverde, N.; Macedo-de-Sousa, B.; Lopez-Valverde, A.; Ramirez, J.M. Effectiveness of antibacterial surfaces in osseointegration of titanium dental implants: A systematic review. Antibiotics 2021, 10, 360. [Google Scholar] [CrossRef]
- Hickok, N.; Shapiro, I.; Chen, A. The impact of incorporating antimicrobials into implant surfaces. J. Dent. Res. 2018, 97, 14–22. [Google Scholar] [CrossRef]
- Patil, C.; Agrawal, A.; Abullais, S.S.; Arora, S.; Khateeb, S.U.; Elagib, M.F.A. Effectiveness of Different Chemotherapeutic Agents for Decontamination of Infected Dental Implant Surface: A Systematic Review. Antibiotics 2022, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Bormann, N.; Schwabe, P.; Smith, M.; Wildemann, B. Analysis of parameters influencing the release of antibiotics mixed with bone grafting material using a reliable mixing procedure. Bone 2014, 59, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Antoci Jr, V.; Adams, C.S.; Hickok, N.J.; Shapiro, I.M.; Parvizi, J. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin. Orthop. Relat. Res. 2007, 462, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Davidson, H.; Poon, M.; Saunders, R.; Shapiro, I.M.; Hickok, N.J.; Adams, C.S. Tetracycline tethered to titanium inhibits colonization by G ram-negative bacteria. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Barr, S.; Engiles, J.; Hickok, N.J.; Shapiro, I.M.; Richardson, D.W.; Parvizi, J.; Schaer, T.P. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: A proof-of-concept study. J. Bone Jt. Surg. 2012, 94, 1406. [Google Scholar] [CrossRef] [PubMed]
- Suchý, T.; Vištejnová, L.; Šupová, M.; Klein, P.; Bartoš, M.; Kolinko, Y.; Blassová, T.; Tonar, Z.; Pokorný, M.; Sucharda, Z. Vancomycin-loaded collagen/hydroxyapatite layers electrospun on 3D printed titanium implants prevent bone destruction associated with S. epidermidis infection and enhance osseointegration. Biomedicines 2021, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Focus: Infectious diseases: Vancomycin resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269. [Google Scholar]
- Rebelo, C.G.; Fernandes, J.C.H.; Bernardo, N.; Couto, P.; Fernandes, G.V.O. Bisphosphonates and Their Influence on the Implant Failure: A Systematic Review. Appl. Sci. 2023, 13, 3496. [Google Scholar] [CrossRef]
- Sreelakshmi, C.; Arunima, P.; Ambili, R.; Reeja Mol, M.; Arun, B. Biomimetic coatings for Dental Implant: An update. J. Int. Soc. Prev. Community Dent. 2021, 13, 37–46. [Google Scholar]
- Parfenova, L.V.; Galimshina, Z.R.; Gil’fanova, G.U.; Alibaeva, E.I.; Danilko, K.V.; Pashkova, T.M.; Kartashova, O.L.; Farrakhov, R.G.; Mukaeva, V.R.; Parfenov, E.V. Hyaluronic acid bisphosphonates as antifouling antimicrobial coatings for PEO-modified titanium implants. Surf. Interfaces 2022, 28, 101678. [Google Scholar] [CrossRef]
- Sendyk, D.I.; Deboni, M.C.Z.; Pannuti, C.M.; Naclério-Homem, M.d.G.; Wennerberg, A. The influence of statins on osseointegration: A systematic review of animal model studies. J. Oral Rehabil. 2016, 43, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, S.-f.; Zhang, F.; He, F.-m.; Yang, G.-l. Simvastatin-loaded porous implant surfaces stimulate preosteoblasts differentiation: An in vitro study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 111, 551–556. [Google Scholar] [CrossRef]
- Sun, T.; Huang, J.; Zhang, W.; Zheng, X.; Wang, H.; Liu, J.; Leng, H.; Yuan, W.; Song, C. Simvastatin-hydroxyapatite coatings prevent biofilm formation and improve bone formation in implant-associated infections. Bioact. Mater. 2023, 21, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of antimicrobial peptides against bacterial biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Feng, T.; Huang, D.; Liu, P.; Lin, P.; Wu, Y.; Ye, Z.; Ji, J.; Li, P.; Huang, W. Antibacterial and hydroxyapatite-forming coating for biomedical implants based on polypeptide-functionalized titania nanospikes. Biomater. Sci. 2020, 8, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent advances in the development of antimicrobial and antifouling biocompatible materials for dental applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef]
- Balhara, V.; Schmidt, R.; Gorr, S.-U.; DeWolf, C. Membrane selectivity and biophysical studies of the antimicrobial peptide GL13K. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2193–2203. [Google Scholar] [CrossRef]
- Holmberg, K.V.; Abdolhosseini, M.; Li, Y.; Chen, X.; Gorr, S.-U.; Aparicio, C. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013, 9, 8224–8231. [Google Scholar] [CrossRef]
- Garaicoa, J.L.; Bates, A.M.; Avila-Ortiz, G.; Brogden, K.A. Antimicrobial prosthetic surfaces in the oral cavity—A perspective on creative approaches. Microorganisms 2020, 8, 1247. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.H.; Voss, E.; Russo, P.A.; Stephens, S.; Kleine, M.; Terheyden, H.; Liu, Q. Antimicrobial peptide coating of dental implants: Biocompatibility assessment of recombinant human beta defensin-2 for human cells. Int. J. Oral Maxillofac. Implant. 2013, 28, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Taher, B.B.; Rasheed, T.A. The Impact of Adding Chitosan Nanoparticles on Biofilm Formation, Cytotoxicity, and Certain Physical and Mechanical Aspects of Directly Printed Orthodontic Clear Aligners. Nanomaterials 2023, 13, 2649. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Zhou, L.; Deng, Y.; Chen, X.; Xiong, X.; Deng, F.; Wei, S. A carboxymethyl chitosan and peptide-decorated polyetheretherketone ternary biocomposite with enhanced antibacterial activity and osseointegration as orthopedic/dental implants. J. Mater. Chem. B 2016, 4, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Buxadera-Palomero, J.; Godoy-Gallardo, M.; Molmeneu, M.; Punset, M.; Gil, F.J. Antibacterial properties of triethoxysilylpropyl succinic anhydride silane (TESPSA) on titanium dental implants. Polymers 2020, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.M.; Toury, B.; D’almeida, M.; Attik, G.N.; Ferrand, A.; Renoud, P.; Grosgogeat, B. Acidic pH resistance of grafted chitosan on dental implant. Odontology 2015, 103, 210–217. [Google Scholar] [CrossRef]
- Palla-Rubio, B.; Araújo-Gomes, N.; Fernández-Gutiérrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goñi, I. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef]
- Valverde, A.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Olivenza, M.A.P.; Blanco, M.B.G.; Díaz-Fuentes, M.; Vilas-Vilela, J.L. Antibacterial hyaluronic acid/chitosan multilayers onto smooth and micropatterned titanium surfaces. Carbohydr. Polym. 2019, 207, 824–833. [Google Scholar] [CrossRef]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Alkheraif, A.A.; Haleem, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Zhang, H.; Xu, Z.; Zhao, L.; Wang, J.; Qiu, Y.; Liu, B. Improvements on biological and antimicrobial properties of titanium modified by AgNPs-loaded chitosan-heparin polyelectrolyte multilayers. J. Mater. Sci. Mater. Med. 2019, 30, 52. [Google Scholar] [CrossRef] [PubMed]
- Norowski, P.A.; Courtney, H.S.; Babu, J.; Haggard, W.O.; Bumgardner, J.D. Chitosan coatings deliver antimicrobials from titanium implants: A preliminary study. Implant Dent. 2011, 20, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Lin, S.-H.; Chien, C.-S.; Kung, J.-C.; Shih, C.-J. In vitro bioactivity and antibacterial effects of a silver-containing mesoporous bioactive glass film on the surface of titanium implants. Int. J. Mol. Sci. 2022, 23, 9291. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial property and biocompatibility of silver, copper, and zinc in titanium dioxide layers incorporated by one-step micro-arc oxidation: A review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Lan, D.; Liu, L.; Dang, R.; Yao, C.; Liu, P.; Ma, F.; Qi, S.; Chen, X. Antibacterial Activity and Bioactivity of Zn-Doped TiO2 Coating for Implants. Coatings 2022, 12, 1264. [Google Scholar] [CrossRef]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef]
- Choi, S.-H.; Jang, Y.-S.; Jang, J.-H.; Bae, T.-S.; Lee, S.-J.; Lee, M.-H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019847067. [Google Scholar] [CrossRef]
- Shen, X.; Hu, Y.; Xu, G.; Chen, W.; Xu, K.; Ran, Q.; Ma, P.; Zhang, Y.; Li, J.; Cai, K. Regulation of the biological functions of osteoblasts and bone formation by Zn-incorporated coating on microrough titanium. ACS Appl. Mater. Interfaces 2014, 6, 16426–16440. [Google Scholar] [CrossRef]
- Luo, Q.; Cao, H.; Wang, L.; Ma, X.; Liu, X. ZnO@ ZnS nanorod-array coated titanium: Good to fibroblasts but bad to bacteria. J. Colloid Interface Sci. 2020, 579, 50–60. [Google Scholar] [CrossRef]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res. Part A 2017, 105, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Guellmar, A.; Voelpel, A.; Lesser, T.; Tonndorf-Martini, S.; Schmidt, J.; Schrader, C.; Faucon, M.; Finger, U.; Pfister, W. Bactericidal and biocompatible properties of plasma chemical oxidized titanium (TiOB®) with antimicrobial surface functionalization. Materials 2019, 12, 866. [Google Scholar] [CrossRef]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C 2020, 108, 110319. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Clapper, J.C. Antibacterial Activity of Electrospun Polyacrylonitrile Copper Nanoparticle Nanofibers on Antibiotic Resistant Pathogens and Methicillin Resistant Staphylococcus aureus (MRSA). Nanomaterials 2022, 12, 2139. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wu, J.; Xu, Y.; Zhang, Y.; Wang, R.; Li, K.; Xu, Y. Chemical stability and antimicrobial activity of plasma-sprayed cerium oxide–incorporated calcium silicate coating in dental implants. Implant Dent. 2019, 28, 564–570. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Li, Y.; Gu, Y.-X.; Zhang, C.-N.; Lai, H.-C.; Shi, J.-Y. Ta-coated titanium surface with superior bacteriostasis and osseointegration. Int. J. Nanomed. 2019, 14, 8693–8706. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.; Pan, H.; Wu, S. Construction of poly (lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater. Sci. Eng. C 2017, 79, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Luo, J.; Zhang, J.; Huang, H.; Xie, Z.; Xie, X. Silver-releasing micro-/nanoporous coating on additively manufactured macroporous Ti-Ta-Nb-Zr scaffolds with high osseointegration and antibacterial properties. Coatings 2021, 11, 716. [Google Scholar] [CrossRef]
- Kuo, Y.-J.; Chen, C.-H.; Dash, P.; Lin, Y.-C.; Hsu, C.-W.; Shih, S.-J.; Chung, R.-J. Angiogenesis, osseointegration, and antibacterial applications of polyelectrolyte multilayer coatings incorporated with silver/strontium containing mesoporous bioactive glass on 316L stainless steel. Front. Bioeng. Biotechnol. 2022, 10, 818137. [Google Scholar] [CrossRef]
- Jamali, R.; Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M.; Kolahi, A. Effects of co-incorporated ternary elements on biocorrosion stability, antibacterial efficacy, and cytotoxicity of plasma electrolytic oxidized titanium for implant dentistry. Mater. Chem. Phys. 2022, 276, 125436. [Google Scholar] [CrossRef]
- Liu, L.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Preparation and antibacterial properties of ZnSr-doped micro-arc oxidation coatings on titanium. Surf. Coat. Technol. 2023, 462, 129469. [Google Scholar] [CrossRef]



| Physical Surface Modifications (Subtractive) | Chemical Surface Modifications (Additive) | Biological Surface Modifications (Biomimetic) |
|---|---|---|
| Plasma spraying Low-pressure plasma spraying High-velocity oxy-fuel spraying Sputter deposition Magnetron sputtering Ion beam-assisted sputtering Pulsed laser deposition | Sol–gel deposition Electrophoretic deposition Electrochemical deposition Acid etching Anodization Peroxidation Alkaline treatment Fluoride treatment Vacuum treatment Plasma coating | Extracellular matrix Peptides Growth factors (BMPs, PDGF, and FGF) Drugs (antibiotic, statin, and bisphosphonate) |
| Properties of Bioactive Coatings | Studies | Findings |
|---|---|---|
| Bioactivity and osseointegration | Mackovic et al., 2012 [32] | With their extremely quick kinetics for bone-like hydroxyapatite mineralization and non-toxic effects on osteoblast cells, nanoscaled bioactive glass particles are a potentially useful material for bone-tissue engineering. |
| Li et al., 2018 [33] | Carbonated hydroxyapatite (CHA) bioceramic coating with synergistic surface chemistry and topography alteration has a bright future as an implant coating, to promote optimal osseointegration. | |
| Cellular response | Yu and Wei, 2013 [34] | Cell adhesion on distinct biomaterial surfaces is directly influenced by substrate surface qualities, which in turn influence cell proliferation and differentiation. |
| Ion dissolution and osteogenesis | Wu et al., 2020 [35] | Researchers used biological coating and surface topography modification to make biomimetic titanium implants with good-quality osteogenic potential. |
| Mechanical performance | Sebdani and Fathi, 2011 [36] | The elastic modulus, hardness, and fracture toughness of produced composite coatings increased as forsterite concentrations rose. |
| Erol-Tygun et al., 2013 [37] | Modified bioglasses (such as nanoparticles) may increase the mechanical characteristics of these materials (hardness, elastic modulus, and tensile strength). |
| Studies | Methodology | Findings |
|---|---|---|
| Ripamonti et al., 2012 [47] | Plasma sprayed with crystalline hydroxyapatite | The findings in nonhuman primates suggest that geometrically built plasma-sprayed titanium implants are intrinsically osteogenic, with the concavities creating an ideal microenvironment for inducing bone development. |
| Alghamdi et al., 2013 [11] | Calcium phosphate (CaP) coating sprayed by radio frequency magnetrons | In both healthy and osteoporotic situations, dental implants modified with a thin layer of calcium pseudophosphate (CaP) coating efficiently enhance osseointegration. |
| Jing et al., 2015 [45] | HA coating by micro-arc oxidation approach | Bone ingrowth and the strength of the bone–implant interface will be significantly improved by this coating process. |
| Carradò et al., 2017 [48] | Sodium titanate/hydroxyapatite nanoporous bilayer | Osteointegration and osteoconduction in vivo are enhanced by a nanoporous hydroxyapatite/sodium titanate bilayer. It avoids delamination during screwing and may strengthen the durability of HA-coated dental implants without adhesive failures. |
| Łukaszewska-Kuska et al., 2018 [49] | HA coating using a direct electrochemical method | Potential advantage in chemical and physical properties that promote osseointegration. |
| Hu et al., 2018 [50] | Nanostructured HA coating on Ti-6Al-4V implants | Ti-6Al-4V implants covered with nanostructured HA may enhance osteointegration in diabetes animals by increasing angiogenesis and osteogenesis and addressing pathological bone loss. |
| Fang et al., 2019 [51] | Nanocrystalline hydroxyapatites with SDF-1 | Biomimetic HA microsphere can promote alveolar bone repair. |
| Eawsakul et al., 2020 [52] | Double layers of gold nanoparticles | The coating possessed homogeneity and good biocompatibility, promoted osteoblast cell proliferation and had good stability. |
| Yu et al., 2021 [53] | Polydopamine nanoparticles functionalized with hydroxyapatite (HA/nPDAs) coated in three dimensions on implant surfaces | The coating’s ability to prevent reactive oxygen species (ROS) and encourage osteogenesis in both normal and high ROS environments (like diabetes, periodontitis, and osteoporosis) showed great promise for enhancing implant osteointegration, particularly in situations where high ROS levels are brought on by diseases. |
| Su et al., 2022 [54] | Composite multifunctional coating of polydopa-mine/hydroxyapatite/gelatin (PHG) prepared using gelatin and polydopa-mine/hydroxyapatite nano-particles | The proposed PHG coating may increase soft tissue sealing and bone bonding. |
| Alcudia et al., 2022 [55] | Porous silver nanoparticle/polycaprolactone/polyvinyl alcohol coatings | This coatings exhibited excellent adherence and a honeycomb-like surface structure that could facilitate vascularization of the implant and improve osseointegration. |
| Mokobia et al., 2023 [56] | ZnO-NPs-Coated implants | Implant fixation was improved by ZnO-NPs coating on metal surfaces because it promoted osteogenesis and soft tissue integration. Furthermore, to achieve a strong biological attachment for implants, osteoconductive nanoparticles formed a chemical relationship with bone. There is little doubt that implants with ZnO-NPs placed to their surfaces exhibit superior clinical outcomes due to a decreased risk of infection. |
| Studies | Methodology | Outcomes |
|---|---|---|
| Lee et al., 2010 [69] | Titanium implants covered with a biodegradable polymer and basic fibroblast growth factor (bFGF). | The study’s findings suggest that electrospraying polylactic-co-glycolic acid (PLGA) and beta-fibroblast growth factor (bFGF) onto a titanium implant may promote bone formation adjacent to the implant’s surface. |
| Kim et al., 2013 [66] | Anodized implants covered in a mixture of human BMP-2 recombinant and human VEGFs. | Encourage the growth of vertical alveolar bone, yet it is unknown how rhBMP-2 and rhVEGF work together. |
| Schliephake et al., 2015 [70] | Oligodeoxynucleotides (ODNs) were anchored to the surface of sandblasted acid-etched (SAE) titanium screw implants and were hybridized with complementary strands of ODN conjugated to rhVEGF165 | Accelerate the bone-implant contact of titanium implants that have been sandblasted and etched to a certain point. The growth factor appears to have a limited effect on the tissue right next to the surface of the implant. |
| Guang et al., 2017 [59] | Coating the implant with VEGF in vivo | Experiments could help osteoblasts and endothelial cells grow. |
| Yang et al., 2017 [71] | Titanium disc and screw types coated with human bone morphogenetic protein-2 (hBMP-2) and human growth and differentiation factor-5 (hGDF-5) to allow for the controlled release of the growth factors. | Enhance the clinical characteristics of implants for use in dentistry and orthopedics. |
| Al-Jarsha et al., 2018 [68] | Poly-ethyl acrylate (PEA)-coated titanium discs were adsorbed with human bone morphogenetic protein 7 (BMP-7). | Cell adhesion, proliferation, mineralization, and the production of osteogenic markers (osteopontin and osteocalcin) demonstrated that, in the absence of PEA coatings, the system was more effective in promoting osteodifferentiation of mesenchymal cells than combinations of titanium and BMP-7. |
| Keceli et al., 2020 [72] | PDGF and BMP-6 are loaded into the titanium implant after anodization. | There is a considerable probability that the early osseointegration phase will be prolonged as a result of a more favorable factor release and its role in the mineralization, proliferation, and related gene expression in osteoblastic cells. |
| Eawsakul et al., 2021 [52] | Creating BMP-2 immobilization on titanium that has been altered using the layer-by-layer method (LBL). | Enhanced osteoblast cell proliferation and exhibited an increase in stability. |
| Palermo et al., 2022 [60] | Using concentrated growth factor (CGF) permeated dental implants. | Improved osseointegration and post-surgical problems. |
| Maekawa et al., 2022 [73] | The first study to use BMP gene delivery combined with chemical vapor deposition (CVD) technology on titanium to encourage in vivo bone-to-implant contact and repair. | Enhances alkaline phosphatase activity and osteoblast cell development in vitro; enhances alveolar bone regeneration and bone-to-implant contact in a manner akin to high exogenous BMP-7 dosages in vivo. This new method of targeted gene distribution on implant surfaces provides an alternative to alveolar bone rebuilding. |
| Studies | Methodology | Findings |
|---|---|---|
| Morra et al., 2010 [79] | Collagen’s biochemical surface alteration in reaction to acid-etched titanium surfaces. | Results suggest that surface topography (morphological) and surface linkage of bioactive chemicals (biochemical) signals might work in concert to produce multifunctional implant surfaces. |
| Alghamdi et al., 2013 [80] | Comparison of three types of implants: uncoated, nano-CaP-coated, and coated with type 1 collagen. | Results failed to demonstrate a consistent beneficial effect of the collagen covering on bone growth throughout a three-month period, following implantation. |
| Lee et al., 2014 [81] | The development of peri-implant bone in implant groups that were uncoated (UC) and coated with HA, collagen plus HA (CH), and collagen, HA, and bone morphogenetic protein-2 (BMP-2). | Compared to the other groups, the BIC and new bone formation were significantly higher in the CH group. There were no notable variations observed in the other groups. |
| Korn et al., 2014 [82] | Collagen was combined with sulfated hyaluronan (sHya) or chondroitin sulfate (CS) in the coatings. | Implant surface coatings made of the selected organic ECM components demonstrated some potential to affect in vivo osseointegration. |
| de Barros et al., 2015 [83] | The implant surfaces underwent sandblasting and acid etching, and a portion of them were also coated with chondroitin sulfate and collagen type II (collagen/CS). | The width of the peri-implant gap affects the formation of peri-implant bone. There was not enough newly formed bone to completely fill in all the gaps surrounding each surface. The coating had a beneficial effect on bone growth when it was close to the surface. |
| Raphel et al., 2016 [84] | Elastin-like protein (ELP) that undergoes chemical modification to allow for new photocrosslinking and solution processing techniques to create stable coatings on the surfaces of titanium-based orthopedic and dental implants. | ELP coatings facilitate early implant loading, and may lessen micromotion, which may lead to aseptic loosening and early implant failure. They are also resistant to surgical implantation and accelerate osseointegration. |
| Yin et al., 2019 [85] | TNS-MAP is the designation given to titanium that has been alkali-treated and has nanonetwork structures (TNSs) covered with mussel adhesive protein (MAP). | TNS-MAP, a novel biocomposite implant material, with potential applications in orthopedics and practical dentistry. |
| Wu et al., 2020 [35] | TiO2 nanotubes or sandblasting and acid etching the surface of titanium were used to modify it. Mineralized extracellular matrix (ECM) made from cultured bone-marrow mesenchymal stromal cells was then applied. | The results demonstrated a viable strategy for producing biomimetic titanium implants with good osteogenic capacity, by combining surface topographical alteration with biological coating. |
| Syam et al., 2021 [86] | Dip-coating titanium (IDCT-Ti) implants with tetrapeptide Gly-Arg-Gly-Asp (GRGD). | The topography, hemocompatibility, and wettability of the implant surface—all of which are linked to enhanced osteoblast-cell adherence to implant surfaces and osseointegration—were positively impacted. |
| Rappe et al., 2022 [87] | The metallic foams were treated with an inorganic alkali thermochemical process and grafted with a cell adhesive tripeptide (RGD), in order to create a bioactive surface. | Combining these two techniques may be beneficial in improving the stability and osteointegration of porous metallic implants. |
| Studies | Methodology | Findings |
|---|---|---|
| [131] | AgNPs with polydopamine (PDA) coating applied to titanium. | May successfully prevent the growth of microorganisms against S. mutans and P. gingivalis. |
| [132] | Spin-coating technology was used to manufacture a series of Zn-incorporated coatings on micro rough titanium (Micro-Ti) using the sol–gel process. | Encourages osseointegration and prevents gram-positive and gram-negative germs from adhering to surfaces. |
| [133] | A two-step hydrothermal process was used to create nanorod-array structured coatings with a controlled-release feature of zinc (Zn) based on the in situ conversion of ZnO to ZnO@ZnS. This method gave titanium surface cell selectivity. | Maintained a strong antimicrobial effect against S. aureus and E. coli |
| [134] | Zinc ions and fluoride integrated into calcium phosphate coatings. | Possess bactericidal effects, particularly efficient at preventing the proliferation, colonization, and adherence of P. gingivalis. |
| [135] | TiOB® (chemically oxidized titanium) coating containing ionic zinc. | Revealed that TiOB® functionalization with ionic zinc demonstrates bactericidal characteristics similar to a coating containing gentamicin. |
| [136] | Zinc oxide (ZnO) nanoparticles. | Displayed antimicrobial properties |
| [137] | Copper nanoparticles (CuNPs). | Can release copper ions, which are thought to have a dual function in aiding in the development of new bone and avoiding infection. |
| [138] | Calcium silicate coatings containing cerium oxide (CeO2-CS). | Promoted osteoblast differentiation, demonstrated significant antibacterial efficacy against E. faecalis while maintaining acceptable biocompatibility. |
| [139] | Tantalum-based implant. | Coated surface performed significant antibacterial action against F. nucleatum and P. gingivalis. |
| [140] | Poly (lactic-co-glycolic acid)/Ag/ZnO nanorods coating. | Provided a strong antibacterial activity and high degree of cytocompatibility. |
| [141] | Using plasma electrolytic oxidation (PEO), selective laser melting (SLM) produced volume-porous Ti-Ta-Nb-Zr scaffolds with a surface biofunctionalized. | Provided robust osteogenic stimulation and antimicrobial activity, without causing cytotoxicity in mammalian cells. |
| [142] | Silver/strontium glass integrated polyelectrolyte multilayer coatings on 316L stainless steel. | Angiogenesis, osseointegration, and antibacterial activity were all improved by the PEM/AgSrMBG coating’s prolonged release of silver and strontium ions. |
| [143] | Titanium substrates were treated with phosphorus, calcium, and copper co-incorporated titanium oxide (TiO2) layers, using plasma electrolytic oxidation. | Bactericidal action against E. coli. The biological reaction to the phosphorus-, calcium-, and copper-containing layer has improved MG-63 osteoblastic cell integration, proliferation, and viability. |
| [144] | By using one-step micro-arc oxidation (MAO) technology, zinc and strontium were added to the surface coating of implants in different concentrations. | Bone marrow mesenchymal stem cells (BMSCs) can be effectively promoted to proliferate and differentiate when exposed to S. aureus and P. gingivalis; exhibits good antibacterial activity against these bacteria, and greater proliferation is seen in the cells on the coating with a higher strontium level. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulghafor, M.A.; Mahmood, M.K.; Tassery, H.; Tardivo, D.; Falguiere, A.; Lan, R. Biomimetic Coatings in Implant Dentistry: A Quick Update. J. Funct. Biomater. 2024, 15, 15. https://doi.org/10.3390/jfb15010015
Abdulghafor MA, Mahmood MK, Tassery H, Tardivo D, Falguiere A, Lan R. Biomimetic Coatings in Implant Dentistry: A Quick Update. Journal of Functional Biomaterials. 2024; 15(1):15. https://doi.org/10.3390/jfb15010015
Chicago/Turabian StyleAbdulghafor, Mohammed Aso, Mohammed Khalid Mahmood, Herve Tassery, Delphine Tardivo, Arthur Falguiere, and Romain Lan. 2024. "Biomimetic Coatings in Implant Dentistry: A Quick Update" Journal of Functional Biomaterials 15, no. 1: 15. https://doi.org/10.3390/jfb15010015
APA StyleAbdulghafor, M. A., Mahmood, M. K., Tassery, H., Tardivo, D., Falguiere, A., & Lan, R. (2024). Biomimetic Coatings in Implant Dentistry: A Quick Update. Journal of Functional Biomaterials, 15(1), 15. https://doi.org/10.3390/jfb15010015








