Factors Influencing Properties of Spider Silk Coatings and Their Interactions within a Biological Environment
Abstract
:1. Spider Silk Coatings in Biomaterials Research
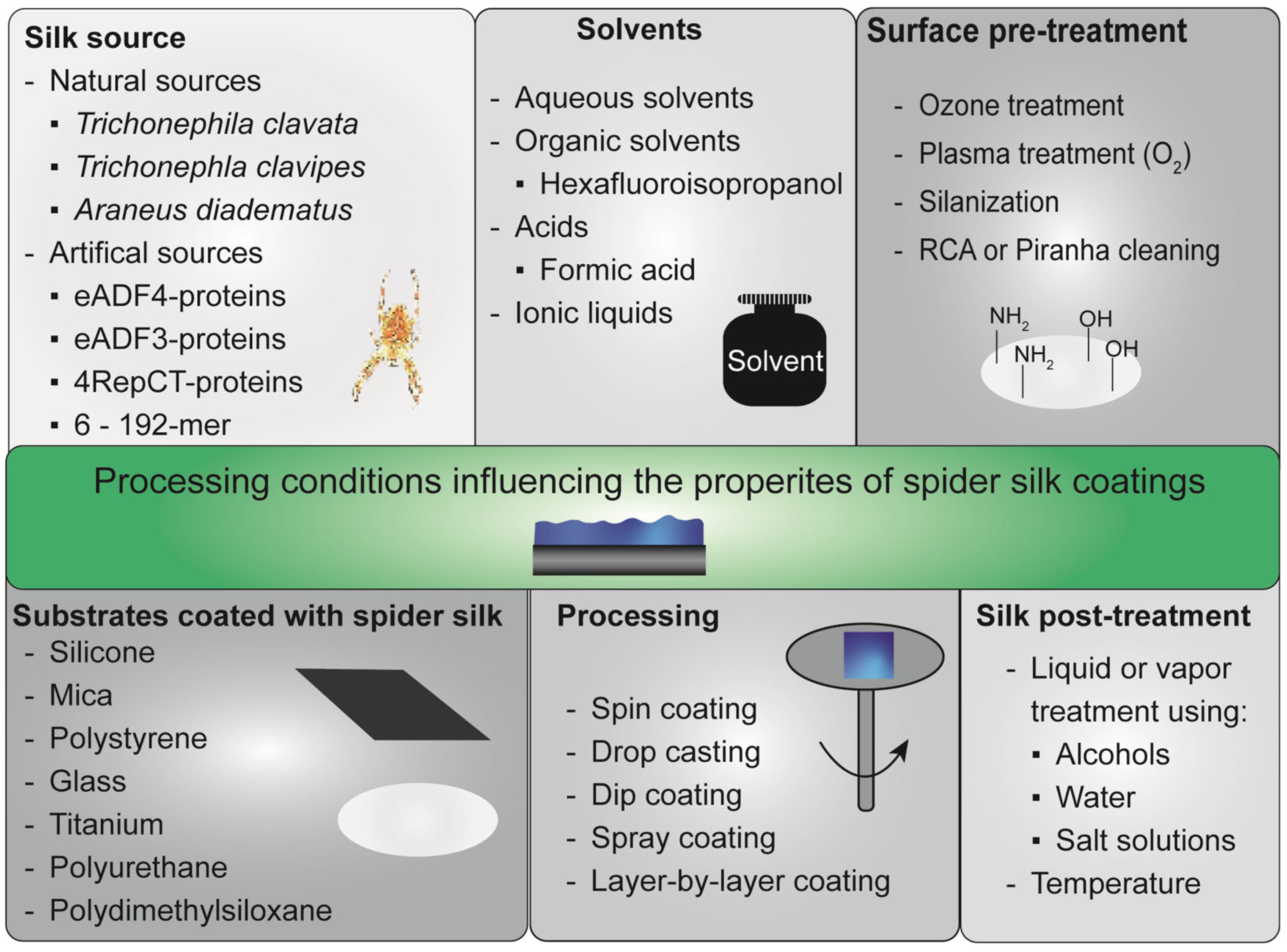
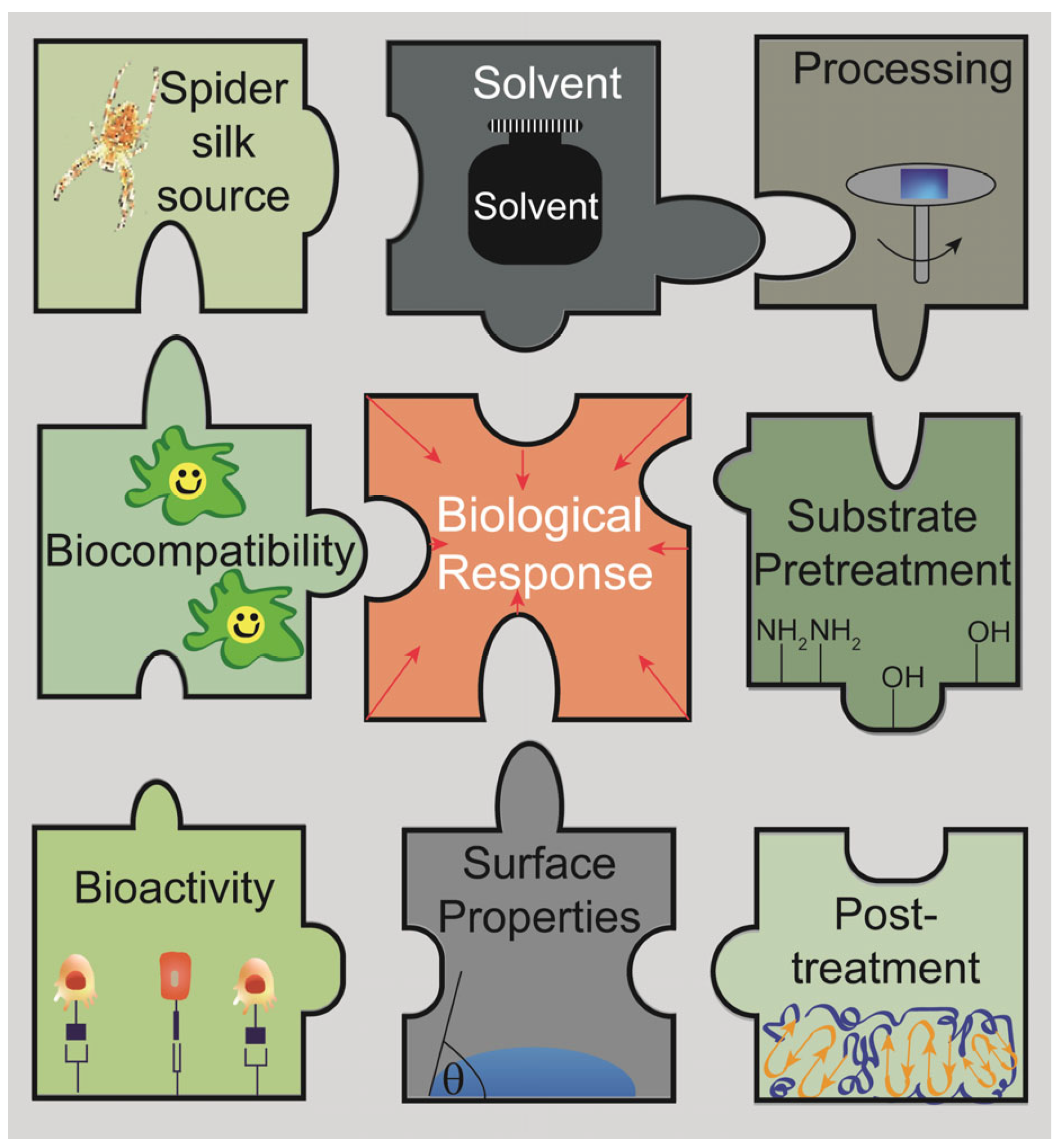
2. Silk Proteins and Processing Techniques to Gain Spider Silk Coatings
2.1. Spider Silk Source
2.2. Spider Silk Purification
2.3. Solubilization of Spider Silk Proteins
2.4. Protein Properties for Processing
2.5. Surfaces to Be Coated and Necessary Pre-Treatments
2.6. Coating Techniques
2.7. Post-Treatment Methods
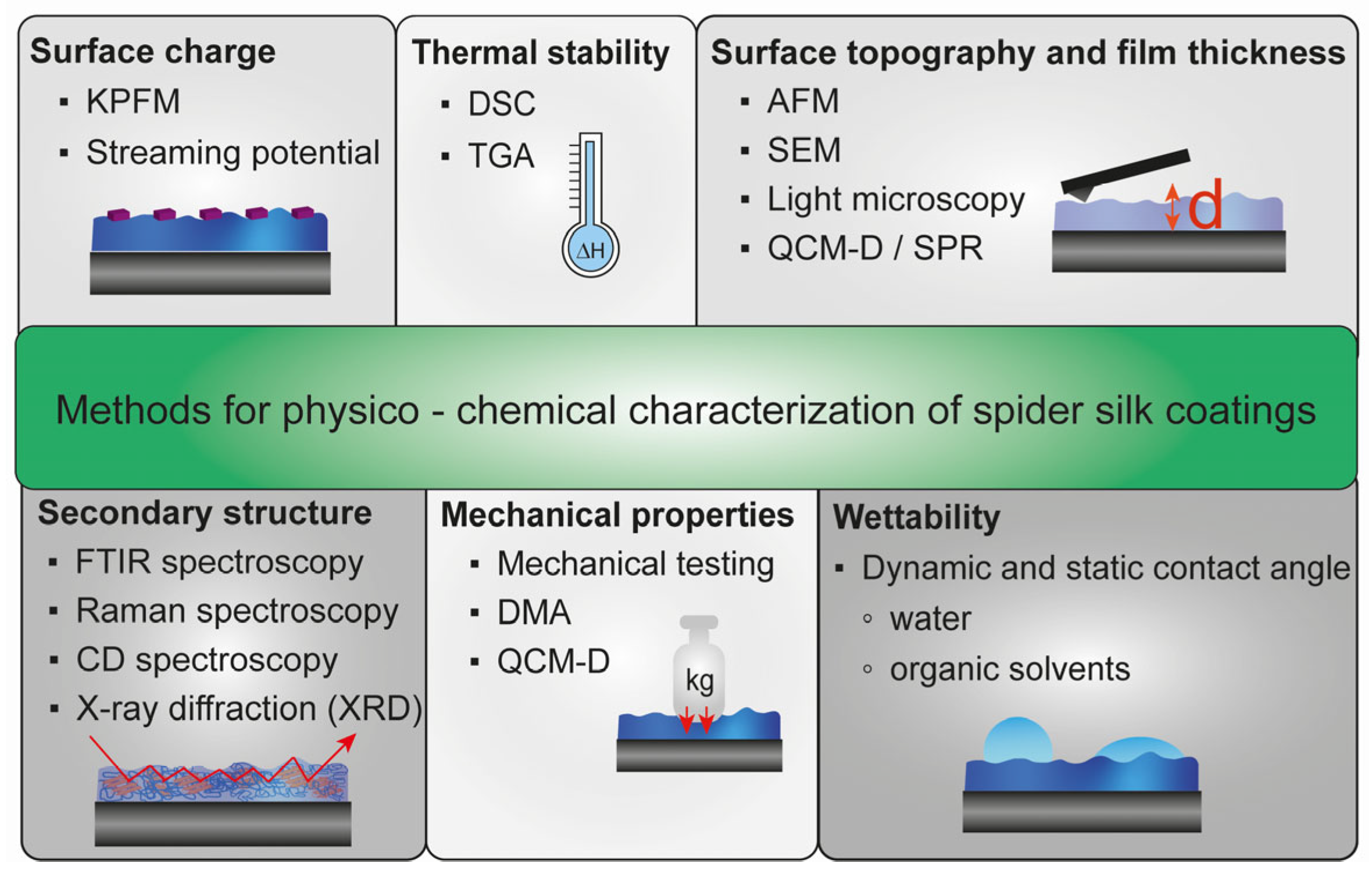
3. Surface Characterization Methods for Spider Silk Coatings
3.1. Surface Topography and Thickness of Spider Silk Films
3.2. Mechanical Properties of Spider Silk Films
3.3. Thermal Stability of Spider Silk Films
3.4. Secondary Structure of Spider Silk Films
3.5. Wettability of Spider Silk Films
3.6. Surface and Zeta Potential of Spider Silk Films
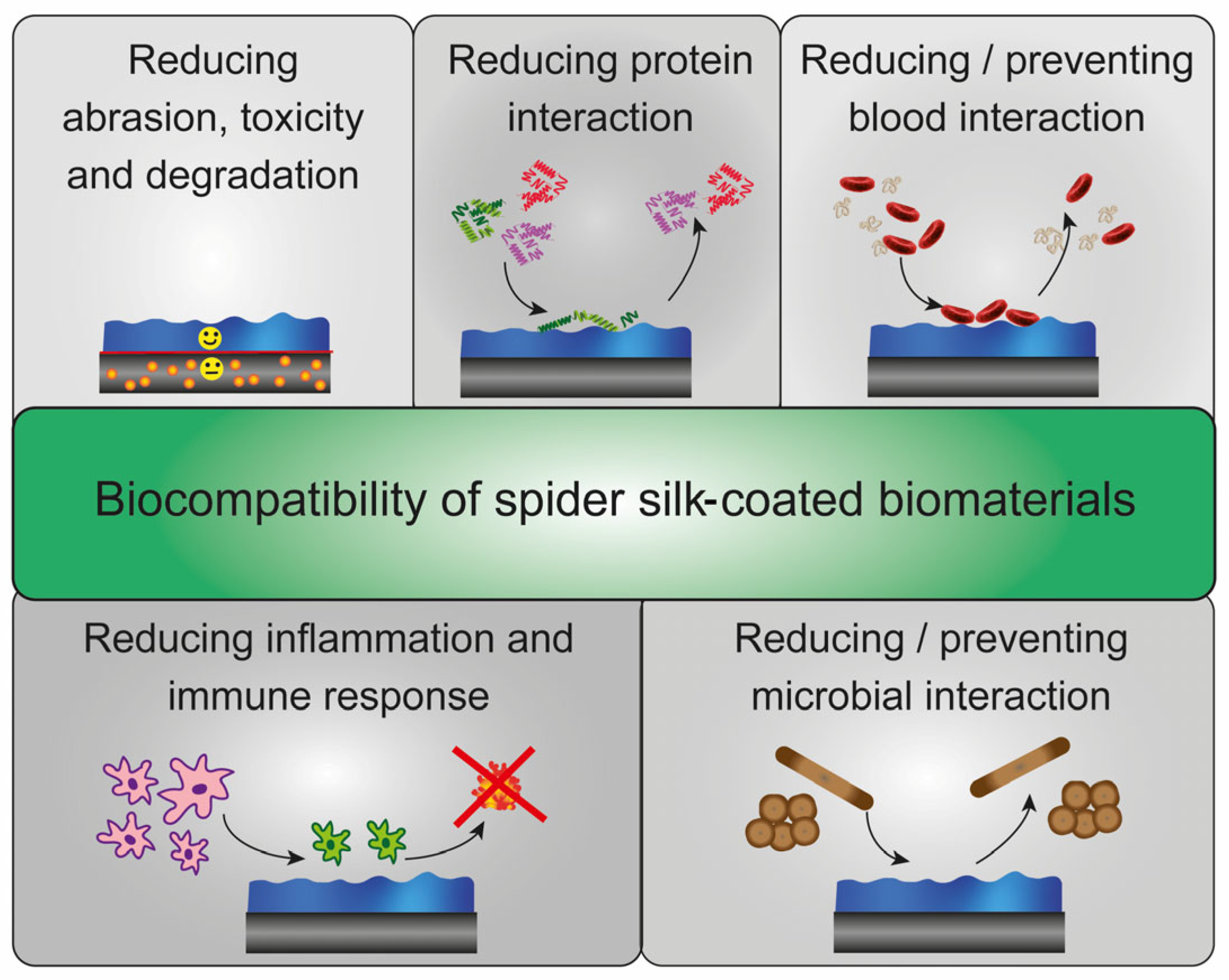
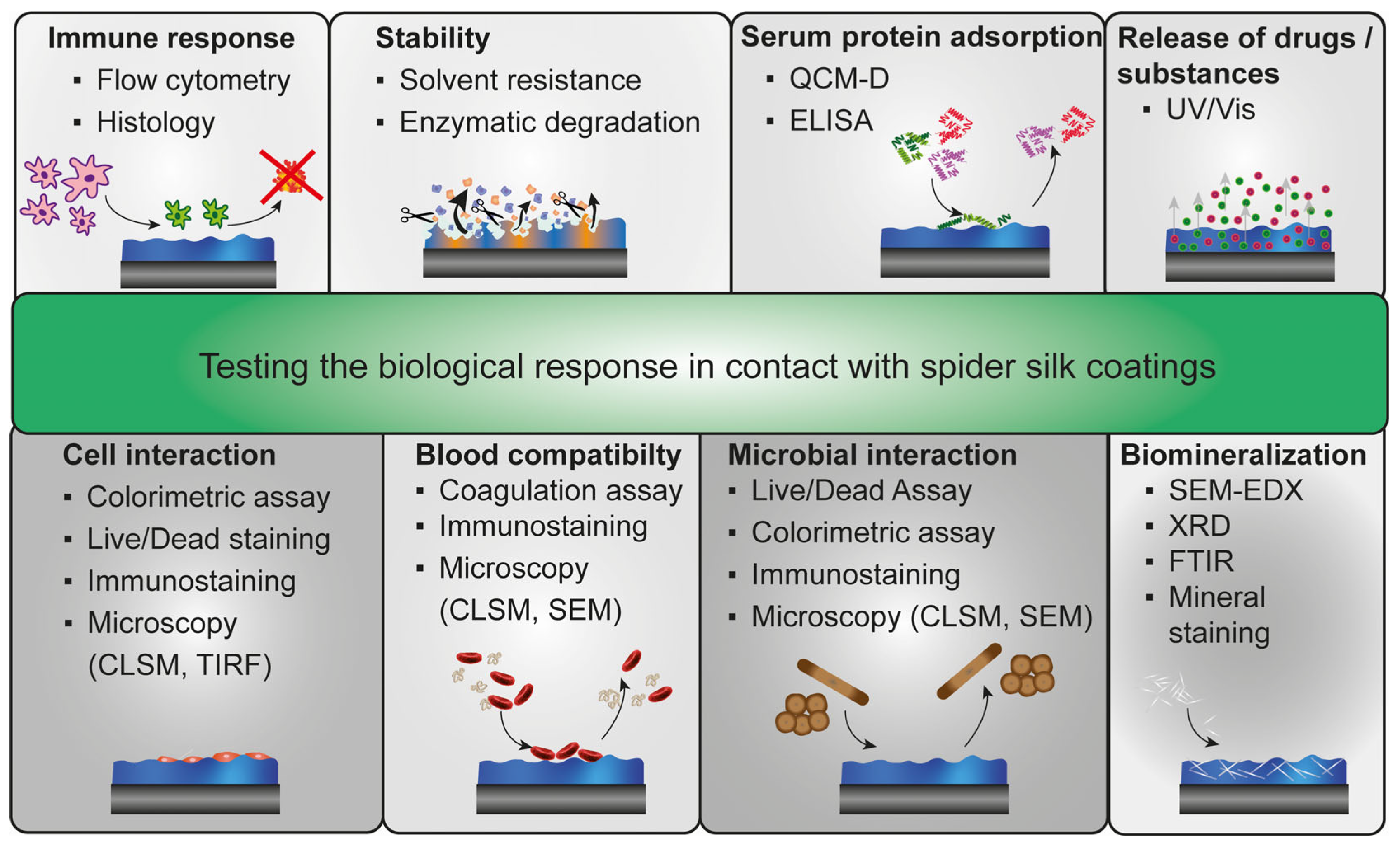
4. Biological Effects on Spider Silk Coatings
4.1. Immune Response against Spider Silk Coatings
4.2. Solvent Stability and Enzymatic Degradation of Spider Silk Coatings
4.3. Serum Protein Adsorption on Spider Silk Surfaces
4.4. Blood Interaction and Hemocompatibility of Spider Silk Surfaces
4.5. Biomineralization of Spider Silk Films
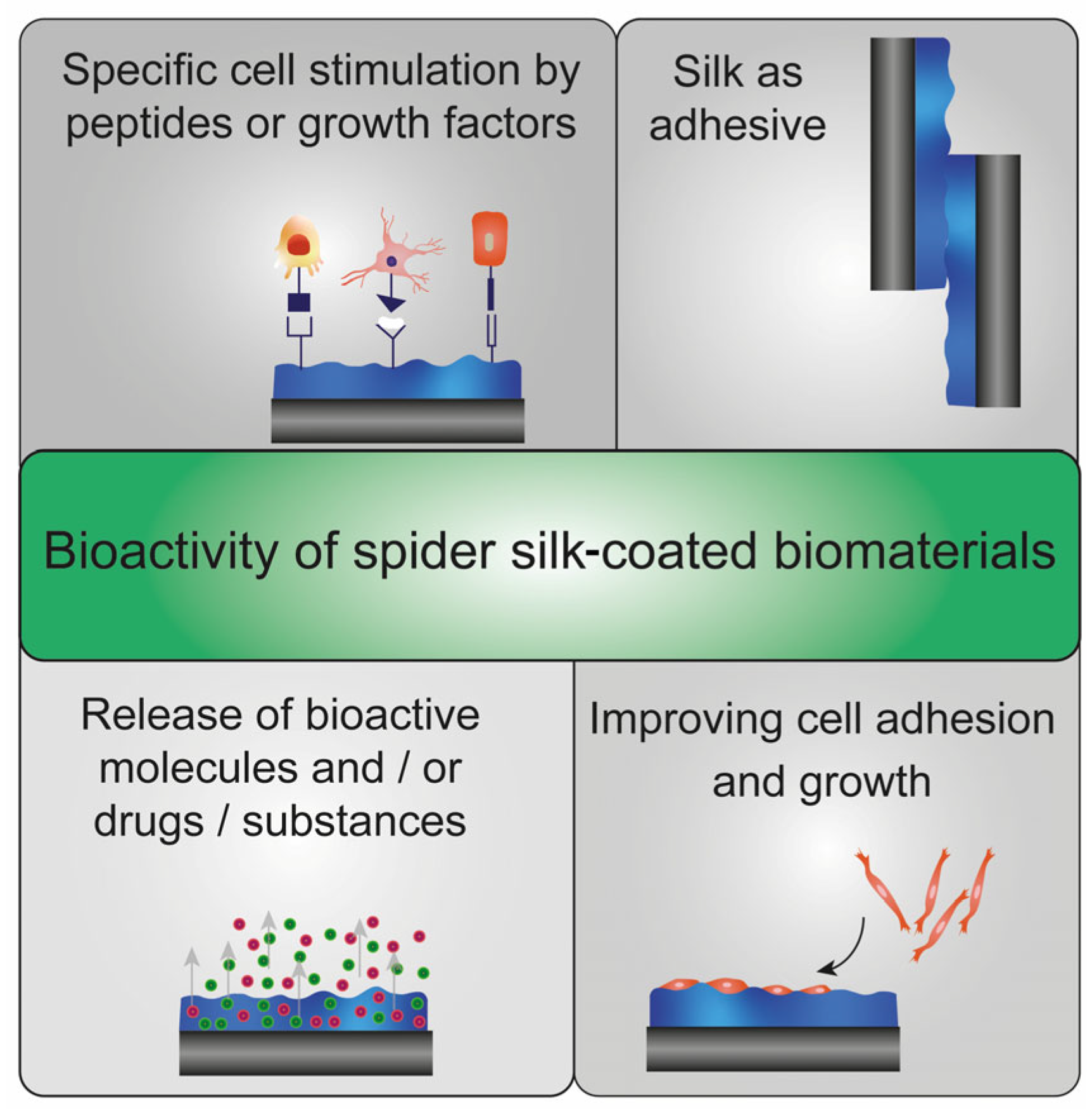
4.6. Cell Interaction with Spider Silk Surfaces
4.7. Microbial Interaction with Spider Silk Surfaces
4.8. Release of Substances from Spider Silk Films
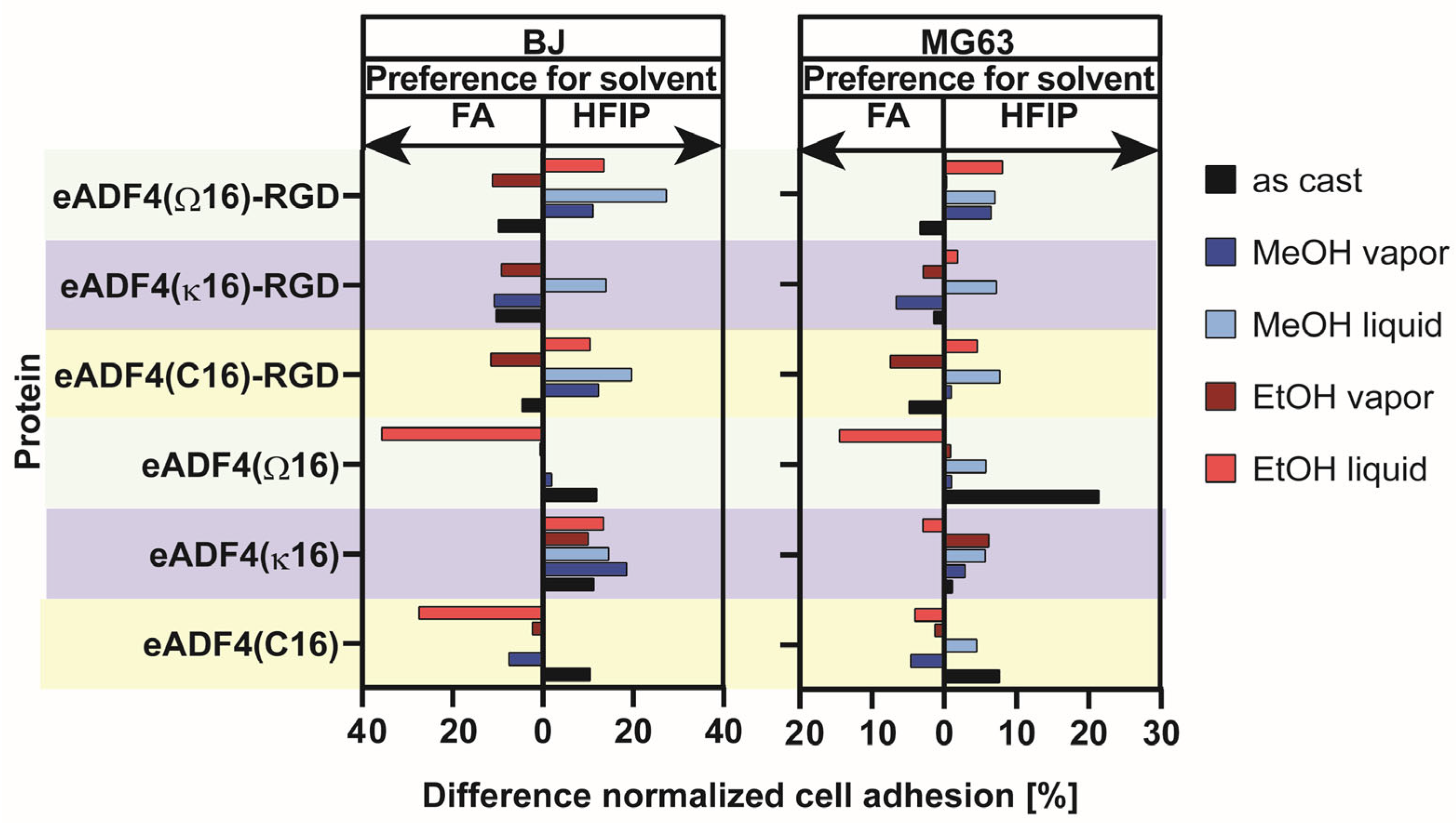
5. Influence of Solvent, Silk Protein and Post-Treatment Method on Cell Adhesion on Spider Silk Films
6. Materials and Methods
6.1. Spider Silk Protein Production
6.2. Spider Silk Film Casting
6.3. Post-Treatment of Spider Silk Films
6.4. Cell Adhesion on Spider Silk Films
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gobbi, S. Requirements for Selection/Development of a Biomaterial. Biomed J. Sci. Tech. Res. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Chapter 2—Biomaterials: Design, Development and Biomedical Applications. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 21–44. [Google Scholar]
- Kiradzhiyska, D.D.; Mantcheva, R.D. Overview of Biocompatible Materials and Their Use in Medicine. Folia Med. 2019, 61, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, S.J.; Reinke, G.; Gobbi, V.J.; Rocha, Y.; Sousa, T.P.; Coutinho, M.M. Biomaterial: Concepts and basics properties. Eur. Int. J. Sci. Technol. 2020, 9, 23–42. [Google Scholar]
- Borcherding, K.; Schmidmaier, G.; Hofmann, G.O.; Wildemann, B. The rationale behind implant coatings to promote osteointegration, bone healing or regeneration. Injury 2021, 52 (Suppl. S2), S106–S111. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R.; Gadegaard, N. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases WJCC 2015, 3, 52. [Google Scholar] [CrossRef]
- Kanerva, L.; Sipiläinen-Malm, T.; Estlander, T.; Zitting, A.; Jolanki, R.; Tarvainen, K. Nickel release from metals, and a case of allergic contact dermatitis from stainless steel. Contact Dermat. 1994, 31, 299–303. [Google Scholar] [CrossRef]
- Öztürk, O.; Türkan, U.u.; Eroğlu, A.E. Metal ion release from nitrogen ion implanted CoCrMo orthopedic implant material. Surf. Coat. Technol. 2006, 200, 5687–5697. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Filgueira, L. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening—Current concepts. J. Biomed. Mater. Res. Part A 2009, 91, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yang, K. 8—Antibacterial design for metal implants. In Metallic Foam Bone; Wen, C., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 203–216. [Google Scholar]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Tsoi, J.K.H.; Rodrigues, F.P.; Leprince, J.G.; Palin, W.M. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int. J. Adhes. Adhes. 2016, 69, 58–71. [Google Scholar] [CrossRef]
- Speranza, G.; Gottardi, G.; Pederzolli, C.; Lunelli, L.; Canteri, R.; Pasquardini, L.; Carli, E.; Lui, A.; Maniglio, D.; Brugnara, M.; et al. Role of chemical interactions in bacterial adhesion to polymer surfaces. Biomaterials 2004, 25, 2029–2037. [Google Scholar] [CrossRef]
- Jansen, B.; Peters, G.; Pulverer, G. Mechanisms and clinical relevance of bacterial adhesion to polymers. J. Biomater. Appl. 1987, 2, 520–543. [Google Scholar] [CrossRef] [PubMed]
- Barone, D.T.-J.; Raquez, J.-M.; Dubois, P. Bone-guided regeneration: From inert biomaterials to bioactive polymer (nano)composites. Polym. Adv. Technol. 2011, 22, 463–475. [Google Scholar] [CrossRef]
- Pagel, M.; Beck-Sickinger, A.G. Multifunctional biomaterial coatings: Synthetic challenges and biological activity. Biol. Chem. 2017, 398, 3–22. [Google Scholar] [CrossRef]
- Mikhalovsky, S.V.; Santin, M.; Mikhalovska, L.I.; Lloyd, A.W.; Denyer, S.P. Current Trends in Biomaterial Coatings. In Nanostructured Materials and Coatings for Biomedical and Sensor Applications; Gogotsi, Y.G., Uvarova, I.V., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 15–26. [Google Scholar]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Nouri, A.; Wen, C. Introduction to surface coating and modification for metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Wen, C., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 3–60. [Google Scholar]
- Spiller, S.; Clauder, F.; Bellmann-Sickert, K.; Beck-Sickinger, A.G. Improvement of wound healing by the development of ECM-inspired biomaterial coatings and controlled protein release. Biol. Chem. 2021, 402, 1271–1288. [Google Scholar] [CrossRef]
- Wang, X.; Kim, H.J.; Xu, P.; Matsumoto, A.; Kaplan, D.L. Biomaterial Coatings by Stepwise Deposition of Silk Fibroin. Langmuir 2005, 21, 11335–11341. [Google Scholar] [CrossRef]
- Hum, J.; Boccaccini, A.R. Collagen as Coating Material for 45S5 Bioactive Glass-Based Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1807. [Google Scholar] [CrossRef]
- Westhauser, F.; Senger, A.-S.; Obert, D.; Ciraldo, F.E.; Schuhladen, K.; Schmidmaier, G.; Moghaddam, A.; Boccaccini, A.R. Gelatin coating increases in vivo bone formation capacity of three-dimensional 45S5 bioactive glass-based crystalline scaffolds. J. Tissue Eng. Regen. Med. 2019, 13, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Borkner, C.B.; Elsner, M.B.; Scheibel, T. Coatings and Films Made of Silk Proteins. ACS Appl. Mater. Interfaces 2014, 6, 15611–15625. [Google Scholar] [CrossRef] [PubMed]
- Bougas, K.; Stenport, V.F.; Currie, F.; Wennerberg, A. Laminin Coating Promotes Calcium Phosphate Precipitation on Titanium Discs in vitro. J. Oral Maxillofac. Res. 2012, 2, e5. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Durand, C.; Pauthe, E.; Gand, A. Fibronectin-Enriched Biomaterials, Biofunctionalization, and Proactivity: A Review. Appl. Sci. 2021, 11, 12111. [Google Scholar] [CrossRef]
- Zeplin, P.H.; Maksimovikj, N.C.; Jordan, M.C.; Nickel, J.; Lang, G.; Leimer, A.H.; Römer, L.; Scheibel, T. Spider Silk Coatings as a Bioshield to Reduce Periprosthetic Fibrous Capsule Formation. Adv. Funct. Mater. 2014, 24, 2658–2666. [Google Scholar] [CrossRef]
- Deptuch, T.; Dams-Kozlowska, H. Silk Materials Functionalized via Genetic Engineering for Biomedical Applications. Materials 2017, 10, 1417. [Google Scholar] [CrossRef]
- Debabov, V.G.; Bogush, V.G. Recombinant Spidroins as the Basis for New Materials. ACS Biomater. Sci. Eng. 2020, 6, 3745–3761. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.I.; Gaztambide, D.A.; Day, B.A.; Brock, C.L.; Ruben, A.L.; Jones, J.A.; Lewis, R.V. Sticky Situation: An Investigation of Robust Aqueous-Based Recombinant Spider Silk Protein Coatings and Adhesives. Biomacromolecules 2016, 17, 3761–3772. [Google Scholar] [CrossRef]
- Liu, X.; Shi, L.; Wan, X.; Dai, B.; Chen, Y.; Wang, S. Recent Progress of Spider-Silk-Inspired Adhesive Materials. ACS Mater. Lett. 2021, 3, 1453–1467. [Google Scholar] [CrossRef]
- Baoyong, L.; Jian, Z.; Denglong, C.; Min, L. Evaluation of a new type of wound dressing made from recombinant spider silk protein using rat models. Burns 2010, 36, 891–896. [Google Scholar] [CrossRef]
- Gomes, S.; Gallego-Llamas, J.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. In Vivo Biological Responses to Silk Proteins Functionalized with Bone Sialoprotein. Macromol. Biosci. 2013, 13, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Gallego-Llamas, J.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. Biological responses to spider silk–antibiotic fusion protein. J. Tissue Eng. Regen. Med. 2012, 6, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.; Trossmann, V.T.; Borkner, C.B.; Beyersdorfer, V.; Rottmar, M.; Scheibel, T. Structure–Property Relationship Based on the Amino Acid Composition of Recombinant Spider Silk Proteins for Potential Biomedical Applications. ACS Appl. Mater. Interfaces 2022, 14, 31751–31766. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.C.G.; Herold, H.M.; Lentz, S.; Faria, M.; Besford, Q.A.; Ang, C.-S.; Caruso, F.; Scheibel, T. Surface Modification of Spider Silk Particles to Direct Biomolecular Corona Formation. ACS Appl. Mater. Interfaces 2020, 12, 24635–24643. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.R.; Fernandes, E.M.; Rodrigues, M.T.; Rodrigues, F.J.; Gomes, M.E.; Leonor, I.B.; Kaplan, D.L.; Reis, R.L. Antimicrobial coating of spider silk to prevent bacterial attachment on silk surgical sutures. Acta Biomater. 2019, 99, 236–246. [Google Scholar] [CrossRef]
- Mulinti, P.; Kalita, D.; Hasan, R.; Quadir, M.; Wang, Y.; Brooks, A. Development and Processing of Novel Heparin Binding Functionalized Modified Spider Silk Coating for Catheter Providing Dual Antimicrobial and Anticoagulant Properties. Materialia 2020, 14, 100937. [Google Scholar] [CrossRef]
- Mulinti, P.; Diekjürgen, D.; Kurtzeborn, K.; Balasubramanian, N.; Stafslien, S.J.; Grainger, D.W.; Brooks, A.E. Anti-Coagulant and Antimicrobial Recombinant Heparin-Binding Major Ampullate Spidroin 2 (MaSp2) Silk Protein. Bioengineering 2022, 9, 46. [Google Scholar] [CrossRef]
- Nilebäck, L.; Hedin, J.; Widhe, M.; Floderus, L.S.; Krona, A.; Bysell, H.; Hedhammar, M. Self-Assembly of Recombinant Silk as a Strategy for Chemical-Free Formation of Bioactive Coatings: A Real-Time Study. Biomacromolecules 2017, 18, 846–854. [Google Scholar] [CrossRef]
- Kumari, S.; Lang, G.; DeSimone, E.; Spengler, C.; Trossmann, V.T.; Lücker, S.; Hudel, M.; Jacobs, K.; Krämer, N.; Scheibel, T. Engineered spider silk-based 2D and 3D materials prevent microbial infestation. Mater. Today 2020, 41, 21–33. [Google Scholar] [CrossRef]
- Nilebäck, L.; Chouhan, D.; Jansson, R.; Widhe, M.; Mandal, B.B.; Hedhammar, M. Silk–Silk Interactions between Silkworm Fibroin and Recombinant Spider Silk Fusion Proteins Enable the Construction of Bioactive Materials. ACS Appl. Mater. Interfaces 2017, 9, 31634–31644. [Google Scholar] [CrossRef]
- Chouhan, D.; Thatikonda, N.; Nilebäck, L.; Widhe, M.; Hedhammar, M.; Mandal, B.B. Recombinant Spider Silk Functionalized Silkworm Silk Matrices as Potential Bioactive Wound Dressings and Skin Grafts. ACS Appl. Mater. Interfaces 2018, 10, 23560–23572. [Google Scholar] [CrossRef]
- Nilebäck, L.; Widhe, M.; Seijsing, J.; Bysell, H.; Sharma, P.K.; Hedhammar, M. Bioactive Silk Coatings Reduce the Adhesion of Staphylococcus aureus while Supporting Growth of Osteoblast-like Cells. ACS Appl. Mater. Interfaces 2019, 11, 24999–25007. [Google Scholar] [CrossRef] [PubMed]
- Trossmann, V.T.; Scheibel, T. Design of Recombinant Spider Silk Proteins for Cell Type Specific Binding. Adv. Healthc. Mater. 2023, 12, 2202660. [Google Scholar] [CrossRef] [PubMed]
- Bini, E.; Foo, C.W.P.; Huang, J.; Karageorgiou, V.; Kitchel, B.; Kaplan, D.L. RGD-Functionalized Bioengineered Spider Dragline Silk Biomaterial. Biomacromolecules 2006, 7, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. Spider silk-bone sialoprotein fusion proteins for bone tissue engineering. Soft Matter 2011, 7, 4964–4973. [Google Scholar] [CrossRef]
- Widhe, M.; Johansson, U.; Hillerdahl, C.-O.; Hedhammar, M. Recombinant spider silk with cell binding motifs for specific adherence of cells. Biomaterials 2013, 34, 8223–8234. [Google Scholar] [CrossRef]
- Widhe, M.; Shalaly, N.D.; Hedhammar, M. A fibronectin mimetic motif improves integrin mediated cell biding to recombinant spider silk matrices. Biomaterials 2016, 74, 256–266. [Google Scholar] [CrossRef]
- Dinjaski, N.; Plowright, R.; Zhou, S.; Belton, D.J.; Perry, C.C.; Kaplan, D.L. Osteoinductive recombinant silk fusion proteins for bone regeneration. Acta Biomater. 2017, 49, 127–139. [Google Scholar] [CrossRef]
- Petzold, J.; Aigner, T.B.; Touska, F.; Zimmermann, K.; Scheibel, T.; Engel, F.B. Surface Features of Recombinant Spider Silk Protein eADF4(κ16)-Made Materials are Well-Suited for Cardiac Tissue Engineering. Adv. Funct. Mater. 2017, 27, 1701427. [Google Scholar] [CrossRef]
- Chouhan, D.; Lohe, T.-u.; Thatikonda, N.; Naidu, V.G.M.; Hedhammar, M.; Mandal, B.B. Silkworm Silk Scaffolds Functionalized with Recombinant Spider Silk Containing a Fibronectin Motif Promotes Healing of Full-Thickness Burn Wounds. ACS Biomater. Sci. Eng. 2019, 5, 4634–4645. [Google Scholar] [CrossRef]
- Neubauer, V.J.; Scheibel, T. Spider Silk Fusion Proteins for Controlled Collagen Binding and Biomineralization. ACS Biomater. Sci. Eng. 2020, 6, 5599–5608. [Google Scholar] [CrossRef] [PubMed]
- Tasiopoulos, C.P.; Petronis, S.; Sahlin, H.; Hedhammar, M. Surface Functionalization of PTFE Membranes Intended for Guided Bone Regeneration Using Recombinant Spider Silk. ACS Appl. Bio Mater. 2020, 3, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Esser, T.U.; Trossmann, V.T.; Lentz, S.; Engel, F.B.; Scheibel, T. Designing of spider silk proteins for human induced pluripotent stem cell-based cardiac tissue engineering. Mater. Today Bio 2021, 11, 100114. [Google Scholar] [CrossRef] [PubMed]
- Thatikonda, N.; Nilebäck, L.; Kempe, A.; Widhe, M.; Hedhammar, M. Bioactivation of Spider Silk with Basic Fibroblast Growth Factor for in Vitro Cell Culture: A Step toward Creation of Artificial ECM. ACS Biomater. Sci. Eng. 2018, 4, 3384–3396. [Google Scholar] [CrossRef]
- Agostini, E.; Winter, G.; Engert, J. Water-based preparation of spider silk films as drug delivery matrices. J. Control. Release 2015, 213, 134–141. [Google Scholar] [CrossRef]
- Jansson, R.; Thatikonda, N.; Lindberg, D.; Rising, A.; Johansson, J.; Nygren, P.-Å.; Hedhammar, M. Recombinant Spider Silk Genetically Functionalized with Affinity Domains. Biomacromolecules 2014, 15, 1696–1706. [Google Scholar] [CrossRef]
- Spieß, K.; Wohlrab, S.; Scheibel, T. Structural characterization and functionalization of engineered spider silk films. Soft Matter 2010, 6, 4168–4174. [Google Scholar] [CrossRef]
- Hardy, J.G.; Leal-Egaña, A.; Scheibel, T.R. Engineered Spider Silk Protein-Based Composites for Drug Delivery. Macromol. Biosci. 2013, 13, 1431–1437. [Google Scholar] [CrossRef]
- Herold, H.M.; Döbl, A.; Wohlrab, S.; Humenik, M.; Scheibel, T. Designed Spider Silk-Based Drug Carrier for Redox- or pH-Triggered Drug Release. Biomacromolecules 2020, 21, 4904–4912. [Google Scholar] [CrossRef]
- Jones, J.A.; Harris, T.I.; Tucker, C.L.; Berg, K.R.; Christy, S.Y.; Day, B.A.; Gaztambide, D.A.; Needham, N.J.C.; Ruben, A.L.; Oliveira, P.F.; et al. More Than Just Fibers: An Aqueous Method for the Production of Innovative Recombinant Spider Silk Protein Materials. Biomacromolecules 2015, 16, 1418–1425. [Google Scholar] [CrossRef]
- Borkner, C.B.; Wohlrab, S.; Möller, E.; Lang, G.; Scheibel, T. Surface Modification of Polymeric Biomaterials Using Recombinant Spider Silk Proteins. ACS Biomater. Sci. Eng. 2017, 3, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Dinjaski, N.; Ebrahimi, D.; Ling, S.; Shah, S.; Buehler, M.J.; Kaplan, D.L. Integrated Modeling and Experimental Approaches to Control Silica Modification of Design Silk-Based Biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Cheng, X.; Tang, Q.; Chen, L. The antigenicity of silk-based biomaterials: Sources, influential factors and applications. J. Mater. Chem. B 2021, 9, 8365–8377. [Google Scholar] [CrossRef]
- Mortimer, B.; Holland, C. 12—The use of spider silk as a biomaterial. In Advances in Silk Science and Technology; Basu, A., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 233–260. [Google Scholar]
- Wohlrab, S.; Spieß, K.; Scheibel, T. Varying surface hydrophobicities of coatings made of recombinant spider silk proteins. J. Mater. Chem. 2012, 22, 22050–22054. [Google Scholar] [CrossRef]
- Välisalmi, T.; Roas-Escalona, N.; Meinander, K.; Mohammadi, P.; Linder, M.B. Highly Hydrophobic Films of Engineered Silk Proteins by a Simple Deposition Method. Langmuir 2023, 39, 4370–4381. [Google Scholar] [CrossRef]
- Spiess, K.; Lammel, A.; Scheibel, T. Recombinant Spider Silk Proteins for Applications in Biomaterials. Macromol. Biosci. 2010, 10, 998–1007. [Google Scholar] [CrossRef]
- Koop, F.; Strauß, S.; Peck, C.-T.; Aper, T.; Wilhelmi, M.; Hartmann, C.; Hegermann, J.; Schipke, J.; Vogt, P.M.; Bucan, V. Preliminary application of native Nephila edulis spider silk and fibrin implant causes granulomatous foreign body reaction in vivo in rat’s spinal cord. PLoS ONE 2022, 17, e0264486. [Google Scholar] [CrossRef]
- Kornfeld, T.; Nessler, J.; Helmer, C.; Hannemann, R.; Waldmann, K.H.; Peck, C.T.; Hoffmann, P.; Brandes, G.; Vogt, P.M.; Radtke, C. Spider silk nerve graft promotes axonal regeneration on long distance nerve defect in a sheep model. Biomaterials 2021, 271, 120692. [Google Scholar] [CrossRef]
- Kuhbier, J.W.; Coger, V.; Mueller, J.; Liebsch, C.; Schlottmann, F.; Bucan, V.; Vogt, P.M.; Strauss, S. Influence of direct or indirect contact for the cytotoxicity and blood compatibility of spider silk. J. Mater. Sci. Mater. Med. 2017, 28, 127. [Google Scholar] [CrossRef]
- Radtke, C.; Allmeling, C.; Waldmann, K.-H.; Reimers, K.; Thies, K.; Schenk, H.C.; Hillmer, A.; Guggenheim, M.; Brandes, G.; Vogt, P.M. Spider Silk Constructs Enhance Axonal Regeneration and Remyelination in Long Nerve Defects in Sheep. PLoS ONE 2011, 6, e16990. [Google Scholar] [CrossRef]
- Yazawa, K.; Hidaka, K.; Negishi, J. Cell Adhesion Behaviors on Spider Silk Fibers, Films, and Nanofibers. Langmuir 2022, 38, 7766–7774. [Google Scholar] [CrossRef] [PubMed]
- Renault, A.; Rioux-Dubé, J.-F.; Lefèvre, T.; Beaufils, S.; Vié, V.; Paquet-Mercier, F.; Pézolet, M. Structure and Mechanical Properties of Spider Silk Films at the Air–Water Interface. Langmuir 2013, 29, 7931–7938. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Cabrejos, W. An experimental confirmation of thermal transitions in native and regenerated spider silks. Mater. Sci. Eng. C 2013, 33, 1432–1437. [Google Scholar] [CrossRef]
- Allmeling, C.; Jokuszies, A.; Reimers, K.; Kall, S.; Vogt, P.M. Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit. J. Cell. Mol. Med. 2006, 10, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Allmeling, C.; Jokuszies, A.; Reimers, K.; Kall, S.; Choi, C.Y.; Brandes, G.; Kasper, C.; Scheper, T.; Guggenheim, M.; Vogt, P.M. Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Prolif. 2008, 41, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Fließ, M.; Strauß, S. Spider Silk as Biomaterial for Medical Applications and Tissue Engineering. In Biomimetics and Bionic Applications with Clinical Applications; Israelowitz, M., Weyand, B., von Schroeder, H.P., Vogt, P., Reuter, M., Reimers, K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 61–69. [Google Scholar]
- Gellynck, K.; Verdonk, P.; Forsyth, R.; Almqvist, K.F.; Van Nimmen, E.; Gheysens, T.; Mertens, J.; Van Langenhove, L.; Kiekens, P.; Verbruggen, G. Biocompatibility and biodegradability of spider egg sac silk. J. Mater. Sci. Mater. Med. 2008, 19, 2963–2970. [Google Scholar] [CrossRef] [PubMed]
- Hernando, A.; Saputri, D.H.A.; Tan, M.I.; Barlian, A. Directing the chondrogenic differentiation of human Wharton’s jelly mesenchymal stem cells using spider silk-based micropattern. AIP Conf. Proc. 2021, 2346, 020001. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Wang, X. New Secrets of Spider Silk: Exceptionally High Thermal Conductivity and Its Abnormal Change under Stretching. Adv. Mater. 2012, 24, 1482–1486. [Google Scholar] [CrossRef]
- Li, X.; Zong, L.; Wu, X.; You, J.; Li, M.; Li, C. Biomimetic engineering of spider silk fibres with graphene for electric devices with humidity and motion sensitivity. J. Mater. Chem. C 2018, 6, 3212–3219. [Google Scholar] [CrossRef]
- Liebsch, C.; Bucan, V.; Menger, B.; Köhne, F.; Waldmann, K.-H.; Vaslaitis, D.; Vogt, P.M.; Strauss, S.; Kuhbier, J.W. Preliminary investigations of spider silk in wounds in vivo—Implications for an innovative wound dressing. Burns 2018, 44, 1829–1838. [Google Scholar] [CrossRef]
- Kuhbier, J.W.; Allmeling, C.; Reimers, K.; Hillmer, A.; Kasper, C.; Menger, B.; Brandes, G.; Guggenheim, M.; Vogt, P.M. Interactions between Spider Silk and Cells—NIH/3T3 Fibroblasts Seeded on Miniature Weaving Frames. PLoS ONE 2010, 5, e12032. [Google Scholar] [CrossRef]
- Millesi, F.; Weiss, T.; Mann, A.; Haertinger, M.; Semmler, L.; Supper, P.; Pils, D.; Naghilou, A.; Radtke, C. Defining the regenerative effects of native spider silk fibers on primary Schwann cells, sensory neurons, and nerve-associated fibroblasts. FASEB J. 2021, 35, e21196. [Google Scholar] [CrossRef] [PubMed]
- Strauß, S.; Reimers, K.; Allmeling, C.; Kuhbier, J.W.; Radtke, C.; Schäfer-Nolte, F.; Wendt, H.; Vogt, P.M. Spider Silk—A Versatile Biomaterial for Tissue Engineering and Medical Applications. Biomed. Tech. 2013, 58 (Suppl. S1), 000010151520134068. [Google Scholar] [CrossRef] [PubMed]
- Hafner, K.; Montag, D.; Maeser, H.; Peng, C.; Marcotte, W.R.; Dean, D.; Kennedy, M.S. Evaluating adhesion and alignment of dental pulp stem cells to a spider silk substrate for tissue engineering applications. Mater. Sci. Eng. C 2017, 81, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ramezaniaghdam, M.; Nahdi, N.D.; Reski, R. Recombinant Spider Silk: Promises and Bottlenecks. Front. Bioeng. Biotechnol. 2022, 10, 835637. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, T.Y.; Lee, S.Y. Recent advances in production of recombinant spider silk proteins. Curr. Opin. Biotechnol. 2012, 23, 957–964. [Google Scholar] [CrossRef]
- Bittencourt, D.M.d.C.; Oliveira, P.; Michalczechen-Lacerda, V.A.; Rosinha, G.M.S.; Jones, J.; Rech Filho, E.L. Bioengineering of spider silks for the production of biomedical materials. Front. Bioeng. Biotechnol. 2022, 10, 958486. [Google Scholar] [CrossRef]
- Lewis, R.V. Spider Silk: Ancient Ideas for New Biomaterials. Chem. Rev. 2006, 106, 3762–3774. [Google Scholar] [CrossRef]
- Hsia, Y.; Gnesa, E.; Jeffery, F.; Tang, S.; Vierra, C. Spider silk composites and applications. Met. Ceram. Polym. Compos. Var. Uses 2011, 2, 303–324. [Google Scholar]
- Tokareva, O.; Jacobsen, M.; Buehler, M.; Wong, J.; Kaplan, D.L. Structure–function–property–design interplay in biopolymers: Spider silk. Acta Biomater. 2014, 10, 1612–1626. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Zhou, P.; Xu, M. Mechanical properties and application analysis of spider silk bionic material. e-Polymers 2020, 20, 443–457. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Meng, M.; Chen, M.; Cao, C. Progress in the application of spider silk protein in medicine. J. Biomater. Appl. 2021, 36, 859–871. [Google Scholar] [CrossRef]
- Ortlepp, C.S.; Gosline, J.M. Consequences of forced silking. Biomacromolecules 2004, 5, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yarger, J.L.; Holland, G.P. Exploring the backbone dynamics of native spider silk proteins in Black Widow silk glands with solution-state NMR spectroscopy. Polymer 2014, 55, 3879–3885. [Google Scholar] [CrossRef]
- Parent, L.R.; Onofrei, D.; Xu, D.; Stengel, D.; Roehling, J.D.; Addison, J.B.; Forman, C.; Amin, S.A.; Cherry, B.R.; Yarger, J.L.; et al. Hierarchical spidroin micellar nanoparticles as the fundamental precursors of spider silks. Proc. Natl. Acad. Sci. USA 2018, 115, 11507–11512. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, Z.; Starrett, J.; Hayashi, C.; Wang, X. Cross-plane thermal transport in micrometer-thick spider silk films. Polymer 2014, 55, 1845–1853. [Google Scholar] [CrossRef]
- Peng, X.; Shao, Z.; Chen, X.; Knight, D.P.; Wu, P.; Vollrath, F. Further Investigation on Potassium-Induced Conformation Transition of Nephila Spidroin Film with Two-Dimensional Infrared Correlation Spectroscopy. Biomacromolecules 2005, 6, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Dicko, C.; Vollrath, F.; Kenney, J.M. Spider Silk Protein Refolding Is Controlled by Changing pH. Biomacromolecules 2004, 5, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, A.P.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Recent Advances in Development of Functional Spider Silk-Based Hybrid Materials. Front. Chem. 2020, 8, 554. [Google Scholar] [CrossRef]
- Whittall, D.R.; Baker, K.V.; Breitling, R.; Takano, E. Host Systems for the Production of Recombinant Spider Silk. Trends Biotechnol. 2021, 39, 560–573. [Google Scholar] [CrossRef]
- Bhattacharyya, G.; Oliveira, P.; Krishnaji, S.T.; Chen, D.; Hinman, M.; Bell, B.; Harris, T.I.; Ghazitabatabaei, A.; Lewis, R.V.; Jones, J.A. Large scale production of synthetic spider silk proteins in Escherichia coli. Protein Expr. Purif. 2021, 183, 105839. [Google Scholar] [CrossRef] [PubMed]
- Poddar, H.; Breitling, R.; Takano, E. Towards engineering and production of artificial spider silk using tools of synthetic biology. Eng. Biol. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Jansson, R.; Lau, C.H.; Ishida, T.; Ramström, M.; Sandgren, M.; Hedhammar, M. Functionalized silk assembled from a recombinant spider silk fusion protein (Z-4RepCT) produced in the methylotrophic yeast Pichia pastoris. Biotechnol. J. 2016, 11, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Heppner, R.; Weichert, N.; Schierhorn, A.; Conrad, U.; Pietzsch, M. Low-Tech, Pilot Scale Purification of a Recombinant Spider Silk Protein Analog from Tobacco Leaves. Int. J. Mol. Sci. 2016, 17, 1687. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Henggeler, D.; Viviani, A.; Conrad, U. Purification of spider silk-elastin from transgenic plants and application for human chondrocyte proliferation. Transgenic Res. 2004, 13, 51–57. [Google Scholar] [CrossRef]
- Scheller, J.; Gührs, K.H.; Grosse, F.; Conrad, U. Production of spider silk proteins in tobacco and potato. Nat. Biotechnol. 2001, 19, 573–577. [Google Scholar] [CrossRef]
- Revkova, V.A.; Sidoruk, K.V.; Kalsin, V.A.; Melnikov, P.A.; Konoplyannikov, M.A.; Kotova, S.; Frolova, A.A.; Rodionov, S.A.; Smorchkov, M.M.; Kovalev, A.V.; et al. Spidroin Silk Fibers with Bioactive Motifs of Extracellular Proteins for Neural Tissue Engineering. ACS Omega 2021, 6, 15264–15273. [Google Scholar] [CrossRef]
- Teulé, F.; Cooper, A.R.; Furin, W.A.; Bittencourt, D.; Rech, E.L.; Brooks, A.; Lewis, R.V. A protocol for the production of recombinant spider silk-like proteins for artificial fiber spinning. Nat. Protoc. 2009, 4, 341–355. [Google Scholar] [CrossRef]
- Tokareva, O.; Michalczechen-Lacerda, V.A.; Rech, E.L.; Kaplan, D.L. Recombinant DNA production of spider silk proteins. Microb. Biotechnol. 2013, 6, 651–663. [Google Scholar] [CrossRef]
- Venkatesan, H.; Chen, J.; Hu, J. Fibers Made of Recombinant Spidroins—A Brief Review. AATCC J. Res. 2019, 6, 37–40. [Google Scholar] [CrossRef]
- Gomes, V.; Salgueiro, S.P. From small to large-scale: A review of recombinant spider silk and collagen bioproduction. Discov. Mater. 2022, 2, 3. [Google Scholar] [CrossRef]
- Xia, X.X.; Qian, Z.G.; Ki, C.S.; Park, Y.H.; Kaplan, D.L.; Lee, S.Y. Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proc. Natl. Acad. Sci. USA 2010, 107, 14059–14063. [Google Scholar] [CrossRef] [PubMed]
- Bowen, C.H.; Dai, B.; Sargent, C.J.; Bai, W.; Ladiwala, P.; Feng, H.; Huang, W.; Kaplan, D.L.; Galazka, J.M.; Zhang, F. Recombinant Spidroins Fully Replicate Primary Mechanical Properties of Natural Spider Silk. Biomacromolecules 2018, 19, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos-Pinto, J.R.A.; Arcuri, H.A.; Priewalder, H.; Salles, H.C.; Palma, M.S.; Lubec, G. Structural Model for the Spider Silk Protein Spidroin-1. J. Proteome Res. 2015, 14, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos-Pinto, J.R.A.; Arcuri, H.A.; Esteves, F.G.; Palma, M.S.; Lubec, G. Spider silk proteome provides insight into the structural characterization of Nephila clavipes flagelliform spidroin. Sci. Rep. 2018, 8, 14674. [Google Scholar] [CrossRef]
- Santos-Pinto, J.R.A.d.; Arcuri, H.A.; Lubec, G.; Palma, M.S. Structural characterization of the major ampullate silk spidroin-2 protein produced by the spider Nephila clavipes. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 1444–1454. [Google Scholar] [CrossRef]
- Mattanovich, D.; Branduardi, P.; Dato, L.; Gasser, B.; Sauer, M.; Porro, D. Recombinant protein production in yeasts. Methods Mol. Biol. 2012, 824, 329–358. [Google Scholar] [CrossRef]
- Sidoruk, K.V.; Davydova, L.I.; Kozlov, D.G.; Gubaidullin, D.G.; Glazunov, A.V.; Bogush, V.G.; Debabov, V.G. Fermentation optimization of a Saccharomyces cerevisiae strain producing 1F9 recombinant spidroin. Appl. Biochem. Microbiol. 2015, 51, 766–773. [Google Scholar] [CrossRef]
- Fahnestock, S.R.; Bedzyk, L.A. Production of synthetic spider dragline silk protein in Pichia pastoris. Appl. Microbiol. Biotechnol. 1997, 47, 33–39. [Google Scholar] [CrossRef]
- Widhe, M.; Johansson, J.; Hedhammar, M.; Rising, A. Current progress and limitations of spider silk for biomedical applications. Biopolymers 2012, 97, 468–478. [Google Scholar] [CrossRef]
- Jenkins, N.; Parekh, R.B.; James, D.C. Getting the glycosylation right: Implications for the biotechnology industry. Nat. Biotechnol. 1996, 14, 975–981. [Google Scholar] [CrossRef]
- Lazaris, A.; Arcidiacono, S.; Huang, Y.; Zhou, J.-F.; Duguay, F.; Chretien, N.; Welsh, E.A.; Soares, J.W.; Karatzas, C.N. Spider Silk Fibers Spun from Soluble Recombinant Silk Produced in Mammalian Cells. Science 2002, 295, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.R.; Palma Kimmerling, E.; Silva, C.; Rodrigues, F.J.; Leonor, I.B.; Reis, R.L.; Kaplan, D.L. Silk-Based Antimicrobial Polymers as a New Platform to Design Drug-Free Materials to Impede Microbial Infections. Macromol. Biosci. 2018, 18, 1800262. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, Y.; Lu, L.; Shao, H.; Qin, K.; Hu, X.; Xia, X. Recombinant spider silk from aqueous solutions via a bio-inspired microfluidic chip. Sci. Rep. 2016, 6, 36473. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cui, Y.; Chen, J.; Gao, C.; Yang, Y.; Yu, W.; Rai, K.; Zhang, M.; Nian, R.; Bao, Z.; et al. High-Strength Collagen-Based Composite Films Regulated by Water-Soluble Recombinant Spider Silk Proteins and Water Annealing. ACS Biomater. Sci. Eng. 2022, 8, 3341–3353. [Google Scholar] [CrossRef] [PubMed]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Self-Assembly of Genetically Engineered Spider Silk Block Copolymers. Biomacromolecules 2009, 10, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Kaplan, D.L. Silk-Based Gene Carriers with Cell Membrane Destabilizing Peptides. Biomacromolecules 2010, 11, 3189–3195. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Mieszawska-Czajkowska, A.J.; Kvenvold, L.A.; Kaplan, D.L. Silk-Based Nanocomplexes with Tumor-Homing Peptides for Tumor-Specific Gene Delivery. Macromol. Biosci. 2012, 12, 75–82. [Google Scholar] [CrossRef]
- Numata, K.; Reagan, M.R.; Goldstein, R.H.; Rosenblatt, M.; Kaplan, D.L. Spider Silk-Based Gene Carriers for Tumor Cell-Specific Delivery. Bioconjugate Chem. 2011, 22, 1605–1610. [Google Scholar] [CrossRef]
- Wong Po Foo, C.; Patwardhan, S.V.; Belton, D.J.; Kitchel, B.; Anastasiades, D.; Huang, J.; Naik, R.R.; Perry, C.C.; Kaplan, D.L. Novel nanocomposites from spider silk–silica fusion (chimeric) proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 9428–9433. [Google Scholar] [CrossRef]
- Huang, J.; Wong, C.; George, A.; Kaplan, D.L. The effect of genetically engineered spider silk-dentin matrix protein 1 chimeric protein on hydroxyapatite nucleation. Biomaterials 2007, 28, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Meirovitch, S.; Shtein, Z.; Ben-Shalom, T.; Lapidot, S.; Tamburu, C.; Hu, X.; Kluge, J.; Raviv, U.; Kaplan, D.; Shoseyov, O. Spider Silk-CBD-Cellulose Nanocrystal Composites: Mechanism of Assembly. Int. J. Mol. Sci. 2016, 17, 1573. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.C.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. Antimicrobial functionalized genetically engineered spider silk. Biomaterials 2011, 32, 4255–4266. [Google Scholar] [CrossRef]
- Tucker, C.L.; Jones, J.A.; Bringhurst, H.N.; Copeland, C.G.; Addison, J.B.; Weber, W.S.; Mou, Q.; Yarger, J.L.; Lewis, R.V. Mechanical and Physical Properties of Recombinant Spider Silk Films Using Organic and Aqueous Solvents. Biomacromolecules 2014, 15, 3158–3170. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Tang-Schomer, M.; Huang, W.; He, J.; Jones, J.; Lewis, R.V.; Kaplan, D.L. Physical and biological regulation of neuron regenerative growth and network formation on recombinant dragline silks. Biomaterials 2015, 48, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Deptuch, T.; Penderecka, K.; Kaczmarek, M.; Molenda, S.; Dams-Kozlowska, H. In vivo study of the immune response to bioengineered spider silk spheres. Sci. Rep. 2022, 12, 13480. [Google Scholar] [CrossRef]
- Florczak, A.; Jastrzebska, K.; Mackiewicz, A.; Dams-Kozlowska, H. Blending two bioengineered spider silks to develop cancer targeting spheres. J. Mater. Chem. B 2017, 5, 3000–3011. [Google Scholar] [CrossRef]
- Kozlowska, A.K.; Florczak, A.; Smialek, M.; Dondajewska, E.; Mackiewicz, A.; Kortylewski, M.; Dams-Kozlowska, H. Functionalized bioengineered spider silk spheres improve nuclease resistance and activity of oligonucleotide therapeutics providing a strategy for cancer treatment. Acta Biomater 2017, 59, 221–233. [Google Scholar] [CrossRef]
- Jastrzebska, K.; Florczak, A.; Kucharczyk, K.; Lin, Y.; Wang, Q.; Mackiewicz, A.; Kaplan, D.L.; Dams-Kozlowska, H. Delivery of chemotherapeutics using spheres made of bioengineered spider silks derived from MaSp1 and MaSp2 proteins. Nanomedicine 2018, 13, 439–454. [Google Scholar] [CrossRef]
- Malay, A.D.; Suzuki, T.; Katashima, T.; Kono, N.; Arakawa, K.; Numata, K. Spider silk self-assembly via modular liquid-liquid phase separation and nanofibrillation. Sci. Adv. 2020, 6, eabb6030. [Google Scholar] [CrossRef]
- Arcidiacono, S.; Mello, C.; Kaplan, D.; Cheley, S.; Bayley, H. Purification and characterization of recombinant spider silk expressed in Escherichia coli. Appl. Microbiol. Biotechnol. 1998, 49, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-F.; Qian, Z.-G.; Peng, Q.; Zhang, Y.; Xia, X.-X. Unconventional Spidroin Assemblies in Aqueous Dope for Spinning into Tough Synthetic Fibers. ACS Biomater. Sci. Eng. 2021, 7, 3608–3617. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Qian, Z.-G.; Zhong, J.-J.; Xia, X.-X. Hyper-production of large proteins of spider dragline silk MaSp2 by Escherichia coli via synthetic biology approach. Process Biochem. 2016, 51, 484–490. [Google Scholar] [CrossRef]
- Kim, E.; Jeon, J.; Zhu, Y.; Hoppe, E.D.; Jun, Y.-S.; Genin, G.M.; Zhang, F. A Biosynthetic Hybrid Spidroin-Amyloid-Mussel Foot Protein for Underwater Adhesion on Diverse Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 48457–48468. [Google Scholar] [CrossRef]
- Krishnaji, S.T.; Bratzel, G.; Kinahan, M.E.; Kluge, J.A.; Staii, C.; Wong, J.Y.; Buehler, M.J.; Kaplan, D.L. Sequence–Structure–Property Relationships of Recombinant Spider Silk Proteins: Integration of Biopolymer Design, Processing, and Modeling. Adv. Funct. Mater. 2013, 23, 241–253. [Google Scholar] [CrossRef]
- Plowright, R.; Dinjaski, N.; Zhou, S.; Belton, D.J.; Kaplan, D.L.; Perry, C.C. Influence of silk–silica fusion protein design on silica condensation in vitro and cellular calcification. RSC Adv. 2016, 6, 21776–21788. [Google Scholar] [CrossRef]
- Belton, D.J.; Mieszawska, A.J.; Currie, H.A.; Kaplan, D.L.; Perry, C.C. Silk-silica composites from genetically engineered chimeric proteins: Materials properties correlate with silica condensation rate and colloidal stability of the proteins in aqueous solution. Langmuir 2012, 28, 4373–4381. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, W.; Belton, D.J.; Simmons, L.O.; Perry, C.C.; Wang, X.; Kaplan, D.L. Control of silicification by genetically engineered fusion proteins: Silk-silica binding peptides. Acta Biomater. 2015, 15, 173–180. [Google Scholar] [CrossRef]
- Numata, K.; Subramanian, B.; Currie, H.A.; Kaplan, D.L. Bioengineered silk protein-based gene delivery systems. Biomaterials 2009, 30, 5775–5784. [Google Scholar] [CrossRef]
- Numata, K.; Hamasaki, J.; Subramanian, B.; Kaplan, D.L. Gene delivery mediated by recombinant silk proteins containing cationic and cell binding motifs. J. Control. Release 2010, 146, 136–143. [Google Scholar] [CrossRef]
- Mieszawska, A.J.; Nadkarni, L.D.; Perry, C.C.; Kaplan, D.L. Nanoscale control of silica particle formation via silk-silica fusion proteins for bone regeneration. Chem. Mater. 2010, 22, 5780–5785. [Google Scholar] [CrossRef] [PubMed]
- Oktaviani, N.A.; Matsugami, A.; Hayashi, F.; Numata, K. Ion effects on the conformation and dynamics of repetitive domains of a spider silk protein: Implications for solubility and β-sheet formation. Chem. Commun. 2019, 55, 9761–9764. [Google Scholar] [CrossRef]
- Pereira, A.M.; Machado, R.; da Costa, A.; Ribeiro, A.; Collins, T.; Gomes, A.C.; Leonor, I.B.; Kaplan, D.L.; Reis, R.L.; Casal, M. Silk-based biomaterials functionalized with fibronectin type II promotes cell adhesion. Acta Biomater. 2017, 47, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Aranko, A.S.; Lemetti, L.; Cenev, Z.; Zhou, Q.; Virtanen, S.; Landowski, C.P.; Penttilä, M.; Fischer, W.J.; Wagermaier, W.; et al. Phase transitions as intermediate steps in the formation of molecularly engineered protein fibers. Commun. Biol. 2018, 1, 86. [Google Scholar] [CrossRef]
- Mohammadi, P.; Aranko, A.S.; Landowski, C.P.; Ikkala, O.; Jaudzems, K.; Wagermaier, W.; Linder, M.B. Biomimetic composites with enhanced toughening using silk-inspired triblock proteins and aligned nanocellulose reinforcements. Sci. Adv. 2019, 5, eaaw2541. [Google Scholar] [CrossRef]
- Tormo, J.; Lamed, R.; Chirino, A.J.; Morag, E.; Bayer, E.A.; Shoham, Y.; Steitz, T.A. Crystal structure of a bacterial family-III cellulose-binding domain: A general mechanism for attachment to cellulose. EMBO J. 1996, 15, 5739–5751. [Google Scholar] [CrossRef] [PubMed]
- Bencharit, S.; Cui, C.B.; Siddiqui, A.; Howard-Williams, E.L.; Sondek, J.; Zuobi-Hasona, K.; Aukhil, I. Structural insights into fibronectin type III domain-mediated signaling. J. Mol. Biol. 2007, 367, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Keeble, A.H.; Banerjee, A.; Ferla, M.P.; Reddington, S.C.; Anuar, I.; Howarth, M. Evolving Accelerated Amidation by SpyTag/SpyCatcher to Analyze Membrane Dynamics. Angew. Chem. Int. Ed. Engl. 2017, 56, 16521–16525. [Google Scholar] [CrossRef]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef]
- Stark, M.; Grip, S.; Rising, A.; Hedhammar, M.; Engström, W.; Hjälm, G.; Johansson, J. Macroscopic Fibers Self-Assembled from Recombinant Miniature Spider Silk Proteins. Biomacromolecules 2007, 8, 1695–1701. [Google Scholar] [CrossRef]
- Hedhammar, M.; Rising, A.; Grip, S.; Martinez, A.S.; Nordling, K.; Casals, C.; Stark, M.; Johansson, J. Structural Properties of Recombinant Nonrepetitive and Repetitive Parts of Major Ampullate Spidroin 1 from Euprosthenops australis: Implications for Fiber Formation. Biochemistry 2008, 47, 3407–3417. [Google Scholar] [CrossRef] [PubMed]
- Lewicka, M.; Hermanson, O.; Rising, A.U. Recombinant spider silk matrices for neural stem cell cultures. Biomaterials 2012, 33, 7712–7717. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, C.; Hedhammar, M.; Feinstein, R.; Nordling, K.; Kratz, G.; Johansson, J.; Huss, F.; Rising, A. Tissue response to subcutaneously implanted recombinant spider silk: An in vivo study. Materials 2009, 2, 1908–1922. [Google Scholar] [CrossRef]
- Lewicka, M.; Rebellato, P.; Lewicki, J.; Uhlén, P.; Rising, A.; Hermanson, O. Recombinant Spider Silk Protein Matrices Facilitate Differentiation of Neural Stem Cells Into Mature and Functional Neurons. Front. Mater. 2021, 7, 560372. [Google Scholar] [CrossRef]
- Grip, S.; Johansson, J.; Hedhammar, M. Engineered disulfides improve mechanical properties of recombinant spider silk. Protein Sci. 2009, 18, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Johansson, U.; Ria, M.; Åvall, K.; Dekki Shalaly, N.; Zaitsev, S.V.; Berggren, P.-O.; Hedhammar, M. Pancreatic Islet Survival and Engraftment Is Promoted by Culture on Functionalized Spider Silk Matrices. PLoS ONE 2015, 10, e0130169. [Google Scholar] [CrossRef]
- Shalaly, N.D.; Ria, M.; Johansson, U.; Åvall, K.; Berggren, P.-O.; Hedhammar, M. Silk matrices promote formation of insulin-secreting islet-like clusters. Biomaterials 2016, 90, 50–61. [Google Scholar] [CrossRef]
- Tasiopoulos, C.P.; Widhe, M.; Hedhammar, M. Recombinant Spider Silk Functionalized with a Motif from Fibronectin Mediates Cell Adhesion and Growth on Polymeric Substrates by Entrapping Cells During Self-Assembly. ACS Appl. Mater. Interfaces 2018, 10, 14531–14539. [Google Scholar] [CrossRef]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Takase, M.; Tomita, M. Lactoferricin derived from milk protein lactoferrin. Curr. Pharm. Des. 2003, 9, 1277–1287. [Google Scholar] [CrossRef]
- Peyre, J.; Humblot, V.; Méthivier, C.; Berjeaud, J.-M.; Pradier, C.-M. Co-Grafting of Amino–Poly(ethylene glycol) and Magainin I on a TiO2 Surface: Tests of Antifouling and Antibacterial Activities. J. Phys. Chem. B 2012, 116, 13839–13847. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.M.; Franco, O.L.; Oliveira, M.D.L.; Andrade, C.A.S. Evaluation of Magainin I interactions with lipid membranes: An optical and electrochemical study. Chem. Phys. Lipids 2012, 165, 537–544. [Google Scholar] [CrossRef]
- Thatikonda, N.; Delfani, P.; Jansson, R.; Petersson, L.; Lindberg, D.; Wingren, C.; Hedhammar, M. Genetic fusion of single-chain variable fragments to partial spider silk improves target detection in micro- and nanoarrays. Biotechnol. J. 2016, 11, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Jansson, R.; Courtin, C.M.; Sandgren, M.; Hedhammar, M. Rational Design of Spider Silk Materials Genetically Fused with an Enzyme. Adv. Funct. Mater. 2015, 25, 5343–5352. [Google Scholar] [CrossRef]
- Harvey, D.; Bardelang, P.; Goodacre, S.L.; Cockayne, A.; Thomas, N.R. Antibiotic Spider Silk: Site-Specific Functionalization of Recombinant Spider Silk Using “Click” Chemistry. Adv. Mater. 2017, 29, 1604245. [Google Scholar] [CrossRef]
- Liu, F.Y.C. Silk-based antimicrobial peptide mixed with recombinant spidroin creates functionalized spider silk. bioRxiv 2021. bioRxiv:2021.2003.2026.437269. [Google Scholar] [CrossRef]
- Leppert, A.; Chen, G.; Lama, D.; Sahin, C.; Railaite, V.; Shilkova, O.; Arndt, T.; Marklund, E.G.; Lane, D.P.; Rising, A.; et al. Liquid–Liquid Phase Separation Primes Spider Silk Proteins for Fiber Formation via a Conditional Sticker Domain. Nano Lett. 2023, 23, 5836–5841. [Google Scholar] [CrossRef]
- Andersson, M.; Jia, Q.; Abella, A.; Lee, X.-Y.; Landreh, M.; Purhonen, P.; Hebert, H.; Tenje, M.; Robinson, C.V.; Meng, Q.; et al. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nat. Chem. Biol. 2017, 13, 262–264. [Google Scholar] [CrossRef]
- Arndt, T.; Greco, G.; Schmuck, B.; Bunz, J.; Shilkova, O.; Francis, J.; Pugno, N.M.; Jaudzems, K.; Barth, A.; Johansson, J.; et al. Engineered Spider Silk Proteins for Biomimetic Spinning of Fibers with Toughness Equal to Dragline Silks. Adv. Funct. Mater. 2022, 32, 2200986. [Google Scholar] [CrossRef]
- Schmuck, B.; Greco, G.; Bäcklund, F.G.; Pugno, N.M.; Johansson, J.; Rising, A. Impact of physio-chemical spinning conditions on the mechanical properties of biomimetic spider silk fibers. Commun. Mater. 2022, 3, 83. [Google Scholar] [CrossRef]
- Rat, C.; Heiby, J.C.; Bunz, J.P.; Neuweiler, H. Two-step self-assembly of a spider silk molecular clamp. Nat. Commun. 2018, 9, 4779. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Holm, L.; Ridderstråle, Y.; Johansson, J.; Rising, A. Morphology and Composition of the Spider Major Ampullate Gland and Dragline Silk. Biomacromolecules 2013, 14, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Saric, M.; Eisoldt, L.; Döring, V.; Scheibel, T. Interplay of Different Major Ampullate Spidroins during Assembly and Implications for Fiber Mechanics. Adv. Mater. 2021, 33, 2006499. [Google Scholar] [CrossRef]
- Kronqvist, N.; Otikovs, M.; Chmyrov, V.; Chen, G.; Andersson, M.; Nordling, K.; Landreh, M.; Sarr, M.; Jörnvall, H.; Wennmalm, S.; et al. Sequential pH-driven dimerization and stabilization of the N-terminal domain enables rapid spider silk formation. Nat. Commun. 2014, 5, 3254. [Google Scholar] [CrossRef]
- Rising, A.; Johansson, J. Toward spinning artificial spider silk. Nat. Chem. Biol. 2015, 11, 309–315. [Google Scholar] [CrossRef]
- Sponner, A.; Vater, W.; Rommerskirch, W.; Vollrath, F.; Unger, E.; Grosse, F.; Weisshart, K. The conserved C-termini contribute to the properties of spider silk fibroins. Biochem. Biophys. Res. Commun. 2005, 338, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Sarr, M.; Kitoka, K.; Walsh-White, K.-A.; Kaldmäe, M.; Metlāns, R.; Tārs, K.; Mantese, A.; Shah, D.; Landreh, M.; Rising, A.; et al. The dimerization mechanism of the N-terminal domain of spider silk proteins is conserved despite extensive sequence divergence. J. Biol. Chem. 2022, 298, 101913. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, K.; Felcyn, E.; Kozak, M.; Szybowicz, M.; Buchwald, T.; Pietralik, Z.; Jesionowski, T.; Mackiewicz, A.; Dams-Kozlowska, H. The method of purifying bioengineered spider silk determines the silk sphere properties. Sci. Rep. 2016, 6, 28106. [Google Scholar] [CrossRef]
- Stephens, J.S.; Fahnestock, S.R.; Farmer, R.S.; Kiick, K.L.; Chase, D.B.; Rabolt, J.F. Effects of Electrospinning and Solution Casting Protocols on the Secondary Structure of a Genetically Engineered Dragline Spider Silk Analogue Investigated via Fourier Transform Raman Spectroscopy. Biomacromolecules 2005, 6, 1405–1413. [Google Scholar] [CrossRef]
- Moisenovich, M.M.; Pustovalova, O.L.; Yu Arhipova, A.; Vasiljeva, T.V.; Sokolova, O.S.; Bogush, V.G.; Debabov, V.G.; Sevastianov, V.I.; Kirpichnikov, M.P.; Agapov, I.I. In vitro and in vivo biocompatibility studies of a recombinant analogue of spidroin 1 scaffolds. J. Biomed. Mater. Res. Part A 2011, 96A, 125–131. [Google Scholar] [CrossRef]
- Mello, C.M.; Soares, J.W.; Arcidiacono, S.; Butler, M.M. Acid Extraction and Purification of Recombinant Spider Silk Proteins. Biomacromolecules 2004, 5, 1849–1852. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, S.; Mello, C.M.; Butler, M.; Welsh, E.; Soares, J.W.; Allen, A.; Ziegler, D.; Laue, T.; Chase, S. Aqueous Processing and Fiber Spinning of Recombinant Spider Silks. Macromolecules 2002, 35, 1262–1266. [Google Scholar] [CrossRef]
- Dams-Kozlowska, H.; Majer, A.; Tomasiewicz, P.; Lozinska, J.; Kaplan, D.L.; Mackiewicz, A. Purification and cytotoxicity of tag-free bioengineered spider silk proteins. J. Biomed. Mater. Res. Part A 2013, 101A, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Florczak, A.; Mackiewicz, A.; Dams-Kozlowska, H. Functionalized Spider Silk Spheres As Drug Carriers for Targeted Cancer Therapy. Biomacromolecules 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Rybka, J.D.; Hilgendorff, M.; Krupinski, M.; Slachcinski, M.; Mackiewicz, A.; Giersig, M.; Dams-Kozlowska, H. Composite spheres made of bioengineered spider silk and iron oxide nanoparticles for theranostics applications. PLoS ONE 2019, 14, e0219790. [Google Scholar] [CrossRef] [PubMed]
- Spiess, K.; Ene, R.; Keenan, C.D.; Senker, J.; Kremer, F.; Scheibel, T. Impact of initial solvent on thermal stability and mechanical properties of recombinant spider silk films. J. Mater. Chem. 2011, 21, 13594–13604. [Google Scholar] [CrossRef]
- Decker, R.E.; Harris, T.I.; Memmott, D.R.; Peterson, C.J.; Lewis, R.V.; Jones, J.A. Method for the Destruction of Endotoxin in Synthetic Spider Silk Proteins. Sci. Rep. 2018, 8, 12166. [Google Scholar] [CrossRef]
- Singh, A.; Upadhyay, V.; Upadhyay, A.K.; Singh, S.M.; Panda, A.K. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb. Cell Fact. 2015, 14, 41. [Google Scholar] [CrossRef]
- Ryan, B.J.; Kinsella, G.K. Differential Precipitation and Solubilization of Proteins. Methods Mol. Biol. 2017, 1485, 191–208. [Google Scholar] [CrossRef]
- Cai, H.; Chen, G.; Yu, H.; Tang, Y.; Xiong, S.; Qi, X. One-step heating strategy for efficient solubilization of recombinant spider silk protein from inclusion bodies. BMC Biotechnol. 2020, 20, 37. [Google Scholar] [CrossRef]
- Singh, S.M.; Panda, A.K. Solubilization and refolding of bacterial inclusion body proteins. J. Biosci. Bioeng. 2005, 99, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Hedhammar, M.; Bramfeldt, H.; Baris, T.; Widhe, M.; Askarieh, G.; Nordling, K.; von Aulock, S.; Johansson, J. Sterilized Recombinant Spider Silk Fibers of Low Pyrogenicity. Biomacromolecules 2010, 11, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Numata, K.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. AFM Study of Morphology and Mechanical Properties of a Chimeric Spider Silk and Bone Sialoprotein Protein for Bone Regeneration. Biomacromolecules 2011, 12, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Agostini, E.; Winter, G.; Engert, J. Scale-up of water-based spider silk film casting using a film applicator. Int. J. Pharm. 2017, 532, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kluge, J.A.; Rabotyagova, O.; Leisk, G.G.; Kaplan, D.L. Spider silks and their applications. Trends Biotechnol. 2008, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Huemmerich, D.; Slotta, U.; Scheibel, T. Processing and modification of films made from recombinant spider silk proteins. Appl. Phys. A 2006, 82, 219–222. [Google Scholar] [CrossRef]
- Gronau, G.; Qin, Z.; Buehler, M.J. Effect of sodium chloride on the structure and stability of spider silk’s N-terminal protein domain. Biomater. Sci. 2013, 1, 276–284. [Google Scholar] [CrossRef]
- Taha, M.; Lee, M.-J. Interactions of TRIS [tris(hydroxymethyl)aminomethane] and related buffers with peptide backbone: Thermodynamic characterization. Phys. Chem. Chem. Phys. 2010, 12, 12840–12850. [Google Scholar] [CrossRef]
- Bai, J.; Ma, T.; Chu, W.; Wang, R.; Silva, L.; Michal, C.; Chiao, J.C.; Chiao, M. Regenerated spider silk as a new biomaterial for MEMS. Biomed. Microdevices 2006, 8, 317–323. [Google Scholar] [CrossRef]
- Gustafsson, L.; Jansson, R.; Hedhammar, M.; van der Wijngaart, W. Structuring of Functional Spider Silk Wires, Coatings, and Sheets by Self-Assembly on Superhydrophobic Pillar Surfaces. Adv. Mater. 2018, 30, 1704325. [Google Scholar] [CrossRef]
- Koga, T.; Morishita, T.; Harumoto, Y.; Nishimura, S.-n.; Higashi, N. Spider silk-inspired peptide multiblock hybrid copolymers for self-healable thin film materials. Mater. Adv. 2021, 2, 7851–7860. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Ishii, T.; Masunaga, H.; Numata, K. Spider dragline silk composite films doped with linear and telechelic polyalanine: Effect of polyalanine on the structure and mechanical properties. Sci. Rep. 2018, 8, 3654. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Harris, T.I.; Bell, B.E.; Oliveira, P.F. Material Formation of Recombinant Spider Silks through Aqueous Solvation using Heat and Pressure. J. Vis. Exp. 2019, 147, e59318. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Sun, L.; Liu, Z.; Xia, X.; Tao, T.H. “Genetically Engineered” Biofunctional Triboelectric Nanogenerators Using Recombinant Spider Silk. Adv. Mater. 2018, 30, 1805722. [Google Scholar] [CrossRef]
- Nilebäck, L.; Arola, S.; Kvick, M.; Paananen, A.; Linder, M.B.; Hedhammar, M. Interfacial Behavior of Recombinant Spider Silk Protein Parts Reveals Cues on the Silk Assembly Mechanism. Langmuir 2018, 34, 11795–11805. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Nateghi, S.S.; Gazani, M.M.; Dehghani, Z.; Mohammadzadeh, F. A review on advances in the applications of spider silk in biomedical issues. Int. J. Biol. Macromol. 2021, 192, 258–271. [Google Scholar] [CrossRef]
- Kyriakides, T.R. Molecular Events at Tissue–Biomaterial Interface. In Host Response to Biomaterials; Badylak, S.F., Ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Lentz, S.; Trossmann, V.T.; Scheibel, T. Selective Topography Directed Cell Adhesion on Spider Silk Surfaces. Adv. Mater. Interfaces 2022, 10, 2201936. [Google Scholar] [CrossRef]
- Guerette, P.A.; Ginzinger, D.G.; Weber, B.H.; Gosline, J.M. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science 1996, 272, 112–115. [Google Scholar] [CrossRef]
- Yarger, J.L.; Cherry, B.R.; van der Vaart, A. Uncovering the structure–function relationship in spider silk. Nat. Rev. Mater. 2018, 3, 18008. [Google Scholar] [CrossRef]
- Slotta, U.; Tammer, M.; Kremer, F.; Koelsch, P.; Scheibel, T. Structural Analysis of Spider Silk Films. Supramol. Chem. 2006, 18, 465–471. [Google Scholar] [CrossRef]
- Widhe, M.; Bysell, H.; Nystedt, S.; Schenning, I.; Malmsten, M.; Johansson, J.; Rising, A.; Hedhammar, M. Recombinant spider silk as matrices for cell culture. Biomaterials 2010, 31, 9575–9585. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, L.; Tasiopoulos, C.P.; Jansson, R.; Kvick, M.; Duursma, T.; Gasser, T.C.; van der Wijngaart, W.; Hedhammar, M. Recombinant Spider Silk Forms Tough and Elastic Nanomembranes that are Protein-Permeable and Support Cell Attachment and Growth. Adv. Funct. Mater. 2020, 30, 2002982. [Google Scholar] [CrossRef]
- Johari, N.; Khodaei, A.; Samadikuchaksaraei, A.; Reis, R.L.; Kundu, S.C.; Moroni, L. Ancient fibrous biomaterials from silkworm protein fibroin and spider silk blends: Biomechanical patterns. Acta Biomater. 2022, 153, 38–67. [Google Scholar] [CrossRef]
- McGill, M.; Holland, G.P.; Kaplan, D.L. Experimental Methods for Characterizing the Secondary Structure and Thermal Properties of Silk Proteins. Macromol. Rapid Commun. 2019, 40, 1800390. [Google Scholar] [CrossRef]
- Xu, L.-C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef]
- Rodrigues, S.N.; Gonçalves, I.C.; Martins, M.C.L.; Barbosa, M.A.; Ratner, B.D. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials 2006, 27, 5357–5367. [Google Scholar] [CrossRef]
- Sperling, C.; Maitz, M.F.; Grasso, S.; Werner, C.; Kanse, S.M. A Positively Charged Surface Triggers Coagulation Activation Through Factor VII Activating Protease (FSAP). ACS Appl. Mater. Interfaces 2017, 9, 40107–40116. [Google Scholar] [CrossRef]
- Kalaskar, D.M.; Alshomer, F. Chapter 8—Micro- and Nanotopographical Cues Guiding Biomaterial Host Response. In In Situ Tissue Regeneration; Lee, S.J., Yoo, J.J., Atala, A., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 137–163. [Google Scholar]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef]
- Nikkhah, M.; Edalat, F.; Manoucheri, S.; Khademhosseini, A. Engineering microscale topographies to control the cell–substrate interface. Biomaterials 2012, 33, 5230–5246. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Sathe, S.R.; Yim, E.K.F. From nano to micro: Topographical scale and its impact on cell adhesion, morphology and contact guidance. J. Phys. Condens. Matter 2016, 28, 183001. [Google Scholar] [CrossRef] [PubMed]
- Alicea-Serrano, A.M.; Bender, K.; Jurestovsky, D. Not all spider silks are antimicrobial. J. Arachnol. 2020, 48, 84–89. [Google Scholar] [CrossRef]
- Szymkowiak, P.; Tsiareshyna, M.; Koczura, R. Spider silk of Linothele fallax and Linothele megatheloides (Mygalomorphae, Dipluridae) does not affect the growth of bacteria. Biologia 2020, 75, 1679–1683. [Google Scholar] [CrossRef]
- Abraham, A.; Joseph, M.M.; Minu, M. Antimicrobial activities of natural and recombinant spider silk—A review. Uttar Pradesh J. Zool. 2020, 41, 106–112. [Google Scholar]
- Amaley, A.; Gawali, A.; Akarte, S. Antibacterial nature of dragline silk of Nephila pilipes (Fabricius, 1793). Indian Soc. Arachnol. 2014, 3, 8–11. [Google Scholar]
- Wright, S.; Goodacre, S.L. Evidence for antimicrobial activity associated with common house spider silk. BMC Res. Notes 2012, 5, 326. [Google Scholar] [CrossRef]
- Tahir, H.; Sattar, A.; Qamar, S.; Mukhtar, M.; Liaqat, I.; Ali, M.; Zaheer, A.; Arshad, N.; Yaqoob, R.; Naseem, S. Anti-bacterial potential of silk recovered from Eriovixia excelsa (Simon, 1889) spider. JAPS J. Anim. Plant Sci. 2019, 29, 1–4. [Google Scholar]
- Roozbahani, H.; Asmar, M.; Ghaemi, N.; Issazadeh, K. Evaluation of antimicrobial activity of spider silk Pholcus phalangioides against two bacterial pathogens in food borne. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 2197–2199. [Google Scholar]
- Zhang, S.; Piorkowski, D.; Lin, W.-R.; Lee, Y.-R.; Liao, C.-P.; Wang, P.-H.; Tso, I.-M. Nitrogen inaccessibility protects spider silk from bacterial growth. J. Exp. Biol. 2019, 222, jeb214981. [Google Scholar] [CrossRef]
- Urruticoechea, A.; Alemany, R.; Balart, J.; Villanueva, A.; Viñals, F.; Capellá, G. Recent advances in cancer therapy: An overview. Curr. Pharm. Des. 2010, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trossmann, V.T.; Lentz, S.; Scheibel, T. Factors Influencing Properties of Spider Silk Coatings and Their Interactions within a Biological Environment. J. Funct. Biomater. 2023, 14, 434. https://doi.org/10.3390/jfb14080434
Trossmann VT, Lentz S, Scheibel T. Factors Influencing Properties of Spider Silk Coatings and Their Interactions within a Biological Environment. Journal of Functional Biomaterials. 2023; 14(8):434. https://doi.org/10.3390/jfb14080434
Chicago/Turabian StyleTrossmann, Vanessa T., Sarah Lentz, and Thomas Scheibel. 2023. "Factors Influencing Properties of Spider Silk Coatings and Their Interactions within a Biological Environment" Journal of Functional Biomaterials 14, no. 8: 434. https://doi.org/10.3390/jfb14080434
APA StyleTrossmann, V. T., Lentz, S., & Scheibel, T. (2023). Factors Influencing Properties of Spider Silk Coatings and Their Interactions within a Biological Environment. Journal of Functional Biomaterials, 14(8), 434. https://doi.org/10.3390/jfb14080434





