Challenges and Future Perspectives for Additively Manufactured Polylactic Acid Using Fused Filament Fabrication in Dentistry
Abstract
1. Introduction
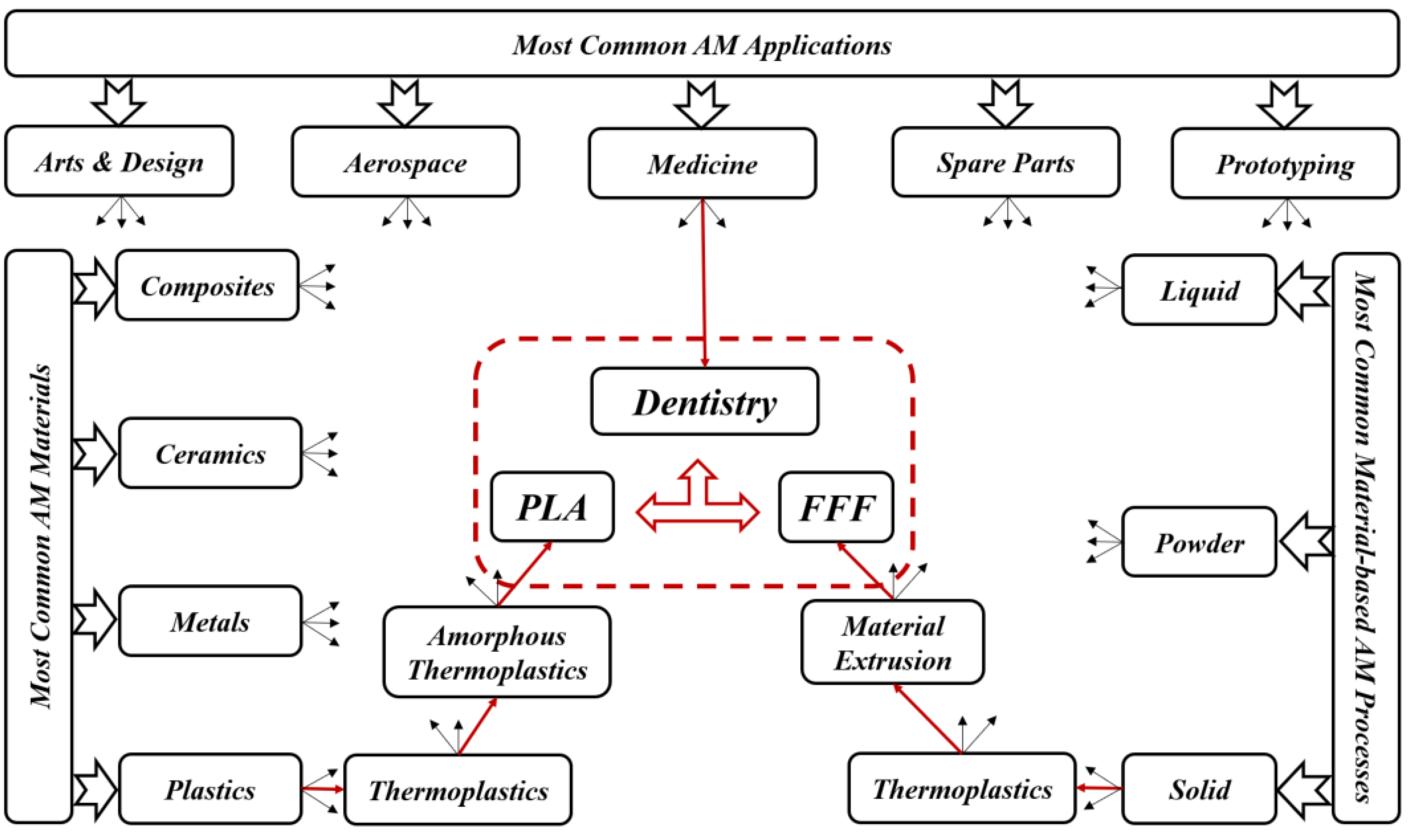
2. AM Techniques Used in Dentistry
3. AM Materials Used in Dentistry
4. AM-PLA Material and Its Application to Dentistry
5. The FFF Technique and Its Application to Dentistry
6. AM-PLA Using FFF for Dentistry with Considerations for Sustainability
7. AM-PLA Using FFF for Dentistry with Consideration for Uncertainty
8. AM-PLA Using FFF for Dentistry with Consideration for Artificial Intelligence
9. Challenges, Issues, and Future Perspectives
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Acrylonitrile butadiene styrene |
| AI | Artificial Intelligence |
| AM | Additive Manufacturing |
| AM-PLA | Additively Manufactured Polylactic acid |
| CIP | Cold Isostatic Pressing |
| DML | Deep Machine Learning |
| DMLS | Direct Metal Laser Sintering |
| DTO | Deterministic Topology Optimization |
| EBM | Electron Beam Melting |
| FDM | Fused Deposition Modeling |
| FFF | Fused Filament Fabrication |
| LOM | Laminated Object Manufacturing |
| ML | Machine Learning |
| PC | Polycarbonate |
| PCL | Polycaprolactone |
| PEI | Polyetherimide |
| PLA | Polylactic acid |
| PVA | Polyvinyl alcohol |
| RBTO | Reliability-Based Topology Optimization |
| RML | Reinforcement Machine Learning |
| SLA | Stereolithography |
| SLM | Selective Laser Melting |
| SLS | Selective Laser Sintering |
References
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Vikayavenkataraman, S.; Jerry, Y.H.F.; Wen, F.L. 3D Printing and 3D Bioprinting in Pediatrics. Bioengineering 2017, 4, 1–11. [Google Scholar]
- Low, Z.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef]
- Jadhav, A.; Jadhav, V.S. A review on 3D printing: An additive manufacturing technology. Mater. Today Proc. 2022, 62, 2094–2099. [Google Scholar] [CrossRef]
- Pradeep, K.; Pal, B. Chapter 24—Selected biomedical applications of additive manufacturing techniques. In Advances in Additive Manufacturing: Artificial Intelligence, Nature-Inspired, and Biomanufacturing; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Rouf, S.; Malik, A.; Singh, N.; Raina, A.; Naveed, N.; Siddiqui, M.I.H.; Haq, M.I.U. Additive manufacturing technologies: Industrial and medical applications. Sustain. Oper. Comput. 2022, 3, 258–274. [Google Scholar] [CrossRef]
- Bourell, D.; Kruth, J.P.; Leu, M.; Levy, G.; Rosen, D.; Beese, A.M.; Clare, A. Materials for additive manufacturing. CIRP Ann. 2017, 66, 659–681. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Abdul Rahman, W.A.W. Polylactic Acid, A Volume in Plastics Design Library; Elsevier: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Sin, L.T.; Tueen, B.S. Polylactic Acid, A Practical Guide for the Processing, Manufacturing, and Applications of PLA, A Volume in Plastics Design Library, 2nd ed.; Elsevier: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Massey, L.K. Chapter 1—Acrylonitrile-Butadiene-Styrene. In Plastics Design Library, The Effects of UV Light and Weather on Plastics and Elastomers, 2nd ed.; Massey, L.K., Ed.; William Andrew Publishing: Norwich, NY, USA, 2007; pp. 13–32. ISBN 9780815515258. [Google Scholar] [CrossRef]
- Peterson, A.M. Review of acrylonitrile butadiene styrene in fused filament fabrication: A plastics engineering-focused perspective. Addit. Manuf. 2019, 27, 363–371. [Google Scholar] [CrossRef]
- Kyriacos, D. Chapter 17—Polycarbonates. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2007; pp. 457–485. ISBN 9780323358248. [Google Scholar] [CrossRef]
- Zhang, B.B.; Chen, Y.; Wang, F.; Hong, R.Y. Surface modification of carbon black for the reinforcement of polycarbonate/acrylonitrile–butadiene–styrene blends. Appl. Surf. Sci. 2015, 351, 280–288. [Google Scholar] [CrossRef]
- Trivedi, P.D. Chapter 3—Polyetherimides (PEI). In Specialty Thermoplastics; Trivedi, P.D., Ed.; Hanser: New York, NY, USA, 2023; pp. 77–114. ISBN 9781569907009. [Google Scholar] [CrossRef]
- Shoeb, M.; Kumar, L.; Haleem, A.; Javaid, M. Chapter 2—Trends in additive manufacturing: An exploratory study. In Additive Manufacturing Materials and Technologies, Advances in Additive Manufacturing; Kumar, A., Mittal, R.K., Haleem, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 15–25. ISBN 9780323918343. [Google Scholar] [CrossRef]
- Müller, A.; Karevska, S. How will 3D printing make your company the strongest link in the value chain, EY’s Global 3D printing Report 2016; EY: London, UK; 72p.
- Yap, Y.L.; Tan, Y.S.E.; Tan, H.K.J.; Peh, Z.K.; Low, X.Y.; Yeong, W.Y.; Tan, C.S.H.; Laude, A. 3D printed bio-models for medical applications. Rapid Prototyp. J. 2017, 23, 227–235. [Google Scholar] [CrossRef]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Kharmanda, G. A Review on Uncertainty Cases in Additively Manufactured Polylactic Acid Using Fused Filament Fabrication Technique. Int. J. Addit. Manuf. Struct. 2023, 2, 1. [Google Scholar] [CrossRef]
- Arafa, K.A.O. Comparing the effects of titanium alloy and chrome cobalt in removable partial denture connectors on tooth mobility, bone loss and tissue reaction. Saudi J. Dent. Res. 2016, 7, 112–117. [Google Scholar] [CrossRef]
- Dhakshyani, R.; Nukman, Y.; Osman, A. Preliminary report: Rapid prototyping models for dysplastic hip surgery. Cent. Eur. J. Med. 2011, 6, 266–270. [Google Scholar] [CrossRef]
- Pettersson, A.B.V.; Salmi, M.; Vallittu, P.; Serlo, W.; Tuomi, J.; Mäkitie, A.A. Main clinical use of additive manufacturing (three-dimensional printing) in Finland restricted to the head and neck area in 2016–2017. Scand. J. Surg. 2020, 109, 166–173. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs; Springer: New York, NY, USA, 2017. [Google Scholar]
- Singh, S.; Singh, G.; Prakash, C.; Seeram Ramakrishna, S. Current status and future directions of fused filament fabrication. J. Manuf. Process. 2020, 55, 288–306. [Google Scholar] [CrossRef]
- Tikhomirov, E.; Åhlén, M.; Strømme, M.; Lindh, J. In situ thermal image analysis of selective laser sintering for oral dosage form manufacturing. J. Pharm. Biomed. Anal. 2023, 231, 115396. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.K.; Rahman, M.H.; Hart, D.; Hughes, B.; Saldana, D.A.; Zollars, C.; Rajak, D.K.; Menezes, P.L. Chapter 3—Fundamentals of stereolithography: Techniques, properties, and applications. In Elsevier Series on Tribology and Surface Engineering, Tribology of Additively Manufactured Materials; Kumar, P., Misra, M., Menezes, P.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 87–106. ISBN 9780128213285. [Google Scholar] [CrossRef]
- Ahn, D.; Kweon, J.H.; Choi, J.; Lee, S. Quantification of surface roughness of parts processed by laminated object manufacturing. J. Mater. Process. Technol. 2012, 212, 339–346. [Google Scholar] [CrossRef]
- Zaharia, C.; Gabor, A.G.; Gavrilovici, A.; Stan, A.T.; Idorasi, L.; Sinescu, C.; Negruțiu, M.L. Digital dentistry-3D printing applications. J. Interdiscip. Med. 2017, 2, 50–53. [Google Scholar] [CrossRef]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing Encompassing the facets of dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef]
- Dikova, T.; Dzhendov, D.A.; Ivanov, D.; Bliznakova, K. Dimensional accuracy and surface roughness of polymeric dental bridges produced by different 3D printing processes. Arch. Mater. Sci. Eng. 2018, 94, 65–75. [Google Scholar] [CrossRef]
- Liu, J.; Hwang, H.H.; Wang, P.; Whang, G.; Chen, S. Direct 3D-printing of cell-laden constructs in microfluidic architectures. Lab Chip 2016, 16, 1430–1438. [Google Scholar] [CrossRef]
- Chang, S.L.; Lo, C.H.; Jiang, C.P.; Juan, D.J. The fit consideration of the denture manufactured by 3D printing and sintering. Int. J. Pharma Med. Biol. Sci. 2015, 4, 184–187. [Google Scholar] [CrossRef]
- Carrel, J.P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin. Oral Implant. Res. 2016, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.B.; Farhan, B.A. Practical analysis of direct metal laser sintering process. Mater. Today Proc. 2021, 45 Pt 6, 5469–5475. [Google Scholar] [CrossRef]
- Ratanajanchai, M.; Kanchanavasita, W.; Suputtamongkol, K.; Wonglamsam, A.; Thamapipol, S.; Sae-Khow, O. Heat-cured poly(methyl methacrylate) resin incorporated with different food preservatives as an anti-microbial denture base material. J. Dent. Sci. 2021, 16, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, U.; Schwarzer, E.; Richter, H.J.; Moritz, T. Thermoplastic 3D printing—An additive manufacturing method for producing dense ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, 26–31. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Chhaya, M.P.; Wunner, F.M.; De-Juan-Pardo, E.M.; Schilling, A.F.; Schantz, J.-T.; van Griensven, M.; Hutmacher, D.W. Polylactides in additive biomanufacturing. Adv. Drug Deliv. Rev. 2016, 107, 228–246. [Google Scholar] [CrossRef]
- Myers, D.; Abdel-Wahab, A.; Hafeez, F.; Kovacev, N.; Essa, K. Optimisation of the additive manufacturing parameters of polylactic acid (PLA) cellular structures for biomedical applications. J. Mech. Behav. Biomed. Mater. 2022, 136, 105447. [Google Scholar] [CrossRef]
- Shengwei, H.; Zhiyong, W.; Qingang, H.; Wei, H. Combined use of an anterolateral thigh flap and rapid prototype modeling to reconstruct maxillary oncologic resections and midface defects. J. Craniofac. Surg. 2014, 25, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Saijo, H.; Igawa, K.; Kanno, Y.; Mori, Y.; Kondo, K.; Shimizu, K.; Suzuki, S.; Chikazu, D.; Iino, M.; Anzai, M. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J. Artif. Organs 2009, 12, 200–205. [Google Scholar] [CrossRef]
- Sun, Y.; Luebbers, H.T.; Agbaje, J.O.; Schepers, S.; Vrielinck, L.; Lambrichts, I.; Politis, C. Accuracy of upper jaw positioning with intermediate splint fabrication after virtual planning in bimaxillary orthognathic surgery. J. Craniofac. Surg. 2013, 24, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, J.; Khadka, A.; Hsu, Y.; Li, W.; Hu, J. CAD/CAM and rapid prototyped titanium for reconstruction of ramus defect and condylar fracture caused by mandibular reduction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.J.; Jeong, I.D.; Kim, W.C.; Kim, J.H. A comparative study of additive and subtractive manufacturing for dental restorations. J. Prosthet. Dent. 2017, 118, 187–193. [Google Scholar] [CrossRef]
- Muta, S.; Ikeda, M.; Nikaido, T.; Sayed, M.; Sadr, A.; Suzuki, T.; Tagami, J. Chairside fabrication of provisional crowns on FDM 3D-printed PVA model. J. Prosthodont. Res. 2020, 64, 401–407. [Google Scholar] [CrossRef]
- Arnesano, A.; Padmanabhan, S.K.; Notarangelo, A.; Montagna, F.; Licciulli, A. Fused deposition modeling shaping of glass infiltrated alumina for dental restoration. Ceram. Int. 2020, 46, 2206–2212. [Google Scholar] [CrossRef]
- Revilla-León, M.; Husain, N.A.H.; Methani, M.M.; Özcan, M. Chemical composition, surface roughness, and ceramic bond strength of additively manufactured cobalt-chromium dental alloys. J. Prosthet. Dent. 2021, 125, 825–831. [Google Scholar] [CrossRef]
- Baciu, E.R.; Cimpoe, R.; Vi, A.; Baciu, C.; Cimpoe, N.; Sodor, A.; Zegan, G.; Murariu, A. Surface analysis of 3D (SLM) CO–CR–W dental metallic materials. Appl. Sci. 2021, 11, 255. [Google Scholar] [CrossRef]
- Avérous, L. Chapter 21—Polylactic Acid: Synthesis, Properties and Applications. In Alessandro Gandini, Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 433–450. ISBN 9780080453163. [Google Scholar] [CrossRef]
- Jin, F.L.; Hu, R.R.; Park, S.J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. Part B Eng. 2019, 164, 287–296. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sirohi, R.; Upadhyay, S.; Mishra, M.; Kumar, V.; Singh, L.K.; Pandey, A. Chapter 12—Production and applications of polylactic acid. In Biomass, Biofuels, Biochemicals; Binod, P., Raveendran, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 309–357. ISBN 9780128218884. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Vahabi, H.; Janbaz, S.; Darafsheh, A.; Mazur, T.R.; Seeram Ramakrishna, S. 4D printing of shape memory polylactic acid (PLA). Polymer 2021, 230, 124080. [Google Scholar] [CrossRef]
- Popescu, D.; Zapciu, A.; Amza, C.; Baciu, F.; Marinescu, R. FDM process parameters influence over the mechanical properties of polymer specimens: A review. Polym. Test. 2018, 69, 157–166. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, T.L.; Júnior, J.E.M.; de Queiroz, L.P.; Souza Ricardo, A.D.; Ponte Rocha, M.V. Polylactic acid production from biotechnological routes: A review. Int. J. Biol. Macromol. 2021, 186, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013, 5, 015001. [Google Scholar] [CrossRef]
- Yue, J.; Zhao, P.; Gerasimov, J.Y.; Marieke, V.D.L.; Grotenhuis, A. 3D-printableantimicrobial composite resins. Adv. Funct. Mater. 2015, 25, 6756–6767. [Google Scholar] [CrossRef]
- Mustufa, H.; Ali, A.; Saber, D.; Wadea, A.; Abidi, A.; Al-Ahmari, M. Rapid prototyping for assembly training and validation. IFAC 2015, 48, 412–417. [Google Scholar] [CrossRef]
- Goh, G.D.; Agarwala, S.; Goh, G.L.; Dikshit, V.; Sing, S.L.; Yeong, W.Y. Additive manufacturing in unmanned aerial vehicles (UAVs): Challenges and potential. Aerosp. Sci. Technol. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Montero, M.; Roundy, S.; Odell, D.; Ahn, S.H.; Wright, P.K. Material characterization of fused deposition modeling (FDM) ABS by designed experiments. Soc. Manuf. Eng. 2001, 10, 1–21. [Google Scholar]
- Alhubail, M. Statistical-Based Optimization of Process Parameters of Fused Deposition Modelling For Improved Quality. Ph.D. Thesis, University of Portsmouth, Portsmouth, UK, 2012. [Google Scholar]
- Fatimatuzahraa, A.W.; Farahaina, B.; Yusoff, W.A.Y. The effect of employing different raster orientations on the mechanical properties and microstructure of fused deposition modeling parts. In Proceedings of the 2011 IEEE Symposium on Business, Engineering and Industrial Applications (ISBEIA), Langkawi, Malaysia, 25–28 September 2011; pp. 22–27. [Google Scholar]
- Sood, A.K.; Ohdar, R.K.; Mahapatra, S.S. Parametric appraisal of mechanical property of fused deposition modelling processed parts. Mater. Des. 2010, 31, 287–295. [Google Scholar] [CrossRef]
- Górski, F.; Kuczko, W.; Wichniarek, R. Influence of process parameters on dimensional accuracy of parts manufactured using fused deposition modelling technology. Adv. Sci. Technol. Res. J. 2013, 7, 27–35. [Google Scholar] [CrossRef]
- Onwubolu, G.C.; Rayegani, F. Characterization and optimization of mechanical properties of ABS parts manufactured by the fused deposition modelling process. Int. J. Manuf. Eng. 2014, 2014, 598531. [Google Scholar] [CrossRef]
- Mohd Halidi, S.N.A.; Abdullah, J. Moisture and humidity effects on the ABS used in fused deposition modeling machine. Adv. Mater. Res. 2012, 576, 641–644. [Google Scholar] [CrossRef]
- Lederle, F.; Meyer, F.; Brunotte, G.P.; Kaldun, C.; Hübner, E.G. Improved mechanical properties of 3D-printed parts by fused deposition modeling processed under the exclusion of oxygen. Prog. Addit. Manuf. 2016, 1, 3–7. [Google Scholar] [CrossRef]
- Kim, E.; Shin, Y.J.; Ahn, S.H. The effects of moisture and temperature on the mechanical properties of additive manufacturing components: Fused deposition modeling. Rapid Prototyp. J. 2016, 22, 887–894. [Google Scholar] [CrossRef]
- Peng, T.; Kellens, K.; Tang, R.; Chen, C.; Chen, G. Sustainability of additive manufacturing: An overview on its energy demand and environmental impact. Addit. Manuf. 2018, 21, 694–704. [Google Scholar] [CrossRef]
- Jayawardane, H.; Davies, I.J.; Gamage, J.R.; John, M.; Biswas, W.K. Sustainability perspectives—A review of additive and subtractive manufacturing. Sustain. Manuf. Serv. Econ. 2023, 2, 100015. [Google Scholar] [CrossRef]
- Jawahir, I.; Rouch, K.; Dillon, J.O.; Holloway, L.; Hall, A. Design for sustainability (DFS): New challenges in developing and implementing a curriculum for next generation design and manufacturing engineers. Int. J. Eng. Educ. 2007, 23, 1053–1064. [Google Scholar]
- Calignano, F.; Mercurio, V. An overview of the impact of additive manufacturing on supply chain, reshoring, and sustainability. Clean. Logist. Supply Chain 2023, 7, 100103. [Google Scholar] [CrossRef]
- Hegab, H.; Khanna, N.; Monib, N.; Salem, A. Design for sustainable additive manufacturing: A review. Sustain. Mater. Technol. 2023, 35, e00576. [Google Scholar] [CrossRef]
- Jin, Y.; Du, J.; He, Y.; Fu, G. Modeling and process planning for curved layer fused deposition. Int. J. Adv. Manuf. Technol. 2017, 91, 273–285. [Google Scholar] [CrossRef]
- Allwood, J.M.; Ashby, M.F.; Gutowski, T.G.; Worrell, E. Material efficiency: A white paper. Resour. Conserv. Recycl. 2011, 55, 362–381. [Google Scholar] [CrossRef]
- Achillas, C.; Aidonis, D.; Iakovou, E.; Thymianidis, M.; Tzetzis, D. A methodological framework for the inclusion of modern additive manufacturing into the production portfolio of a focused factory. J. Manuf. Syst. 2015, 37 Pt 1, 328–339. [Google Scholar] [CrossRef]
- Singh, M.M.; Rajpal, S.; Priya, N.; Ali, M.G.; Akhtar, S. CAP splint: An armour to safeguard developing dentition in paediatric mandibular fractures—A case series. IP Indian J. Orthod. Dentofac. Res. 2021, 7, 77–81. [Google Scholar] [CrossRef]
- Wilkinson, T. Chapter 20—Occlusal Splints and Management of the Occlusion. In Functional Occlusion in Restorative Dentistry and Prosthodontics; Klineberg, I., Eckert, S.E., Eds.; Mosby: London, UK, 2016; pp. 245–252. ISBN 9780723438090. [Google Scholar] [CrossRef]
- Carr, A.B.; Brown, D.T. CHAPTER 17—Occlusal Relationships for Removable Partial Dentures. In McCracken’s Removable Partial Prosthodontics, 12th ed.; Carr, A.B., Brown, D.T., Eds.; Mosby: London, UK, 2011; pp. 242–252. ISBN 9780323069908. [Google Scholar] [CrossRef]
- Mishra, S.; Datta-Gupta, A. Chapter 6—Uncertainty Quantification. In Applied Statistical Modeling and Data Analytics; Mishra, S., Datta-Gupta, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 119–167. ISBN 9780128032794. [Google Scholar] [CrossRef]
- Kharmanda, G. Additive manufacturing of polylactic acid (PLA) material considering preheating uncertainty effect. Uncertainties Reliab. Multi-Phys. Syst. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Verma, A.K.; Ajit, S.; Karanki, D.R. Uncertainty Analysis in Reliability/Safety Assessment, Chapter 13: Reliability and Safety Engineering, 2nd ed.; Springer: London, UK, 2016. [Google Scholar]
- Heidari-Rarani, M. Residual stresses in additive manufacturing of polymers and polymer matrix composites, Chapter 14. In Woodhead Publishing Series in Composites Science and Engineering, Residual Stresses in Composite Materials, 2nd ed.; Shokrieh, M.M., Ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 421–436. ISBN 9780128188170. [Google Scholar] [CrossRef]
- Liu, J.; Gaynor, A.T.; Chen, S.; Kang, Z.; Suresh, K.; Takezawa, A.; Li, L.; Kato, J.; Tang, J.; Wang, C.C.L.; et al. Current and future trends in topology optimization for additive manufacturing. Struct. Multidisc. Optim. 2018, 57, 2457–2483. [Google Scholar] [CrossRef]
- Li, Z.; Tsavdaridis, K.D. A Review of Optimised Additively Manufactured Steel Connections for Modular Building Systems. Ind. Addit. Manuf. 2021, 1, 357–373. [Google Scholar]
- Ribeiro, T.P.; Bernardo, L.F.A.; Andrade, J.M.A. Topology Optimisation in Structural Steel Design for Additive Manufacturing. Appl. Sci. 2021, 11, 2112. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Shi, J.; Wu, D. Prediction of surface roughness in extrusion-based additive manufacturing with machine learning. Robot. Comput. Integr. Manuf. 2019, 57, 488–495. [Google Scholar] [CrossRef]
- Zhu, Z.; Anwer, N.; Huang, Q.; Mathieu, L. Machine learning in tolerancing for additive manufacturing. CIRP Ann. 2018, 67, 157–160. [Google Scholar] [CrossRef]
- Baştanlar, Y.; Ozuysal, M. Introduction to machine learning second edition. In Methods in Molecular Biology; Clifton, N.J., Ed.; Humana Press: New York, NY, USA, 2014. [Google Scholar]
- Sharma, P.; Vaid, H.; Vajpeyi, R.; Shubham, P.; Agarwal, K.M.; Bhatia, D. Predicting the dimensional variation of geometries produced through FDM 3D printing employing supervised machine learning. Sens. Int. 2022, 3, 100194. [Google Scholar] [CrossRef]
- King, A.P.; Aljabar, P. (Eds.) Machine learning, Chapter 14. In Matlab® Programming for Biomedical Engineers and Scientists, 2nd ed.; Academic Press: New York, NY, USA, 2023; pp. 343–372. ISBN 9780323857734. [Google Scholar] [CrossRef]
- López-Monroy, A.P.; García-Salinas, J.S. Chapter 9—Neural networks and deep learning. In Biosignal Processing and Classification Using Computational Learning and Intelligence; Torres-García, A.A., Reyes-García, C.A., Villaseñor-Pineda, L., Mendoza-Montoya, O., Eds.; Academic Press: New York, NY, USA, 2022; pp. 177–196. ISBN 9780128201251. [Google Scholar] [CrossRef]
- Pearson, C.; Ginsburg, B. Deep learning, Chapter 16. In Programming Massively Parallel Processors, 4th ed.; Hwu, W.-m.W., Kirk, D.B., El Hajj, I., Eds.; Morgan Kaufmann: San Francisco, CA, USA, 2023; pp. 355–389. ISBN 9780323912310. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Li, W.; Guo, Q.; Du, Z.; Xu, Z. (Eds.) Chapter 2—Fundamentals of neural networks. In AI Computing Systems; Morgan Kaufmann: San Francisco, CA, USA, 2023; pp. 17–51. ISBN 9780323953993. [Google Scholar] [CrossRef]
- Daw, N.D. Chapter 16—Advanced Reinforcement Learning. In Neuroeconomics, 2nd ed.; Glimcher, P.W., Fehr, E., Eds.; Academic Press: New York, NY, USA, 2014; pp. 299–320. ISBN 9780124160088. [Google Scholar] [CrossRef]
- Morales, E.F.; Escalante, H.J. Chapter 6—A brief introduction to supervised, unsupervised, and reinforcement learning, In Biosignal Processing and Classification Using Computational Learning and Intelligence; Torres-García, A.A., Reyes-García, C.A., Villaseñor-Pineda, L., Mendoza-Montoya, O., Eds.; Academic Press: New York, NY, USA, 2022; pp. 111–129. ISBN 9780128201251. [Google Scholar] [CrossRef]
- Osinenko, P.; Dobriborsci, D.; Aumer, W. Reinforcement learning with guarantees: A review. IFAC-PapersOnLine 2022, 55, 123–128. [Google Scholar] [CrossRef]
- Yüksel, N.; Börklü, H.R.; Sezer, H.K.; Canyurt, O.E. Review of artificial intelligence applications in engineering design perspective. Eng. Appl. Artif. Intell. 2023, 118, 105697. [Google Scholar] [CrossRef]
- Zohuri, B.; McDaniel, P. (Eds.) Chapter 8—Artificial intelligence driven by machine learning & deep learning. In Transcranial Magnetic and Electrical Brain Stimulation for Neurological Disorders; Academic Press: New York, NY, USA, 2022; pp. 317–334. ISBN 9780323954167. [Google Scholar] [CrossRef]
- Tahvili, S.; Hatvani, L. (Eds.) Chapter Six—Benefits, results, and challenges of artificial intelligence. In Uncertainty, Computational Techniques, and Decision Intelligence, Artificial Intelligence Methods for Optimization of the Software Testing Process; Academic Press: New York, NY, USA, 2022; pp. 161–172. ISBN 9780323919135. [Google Scholar] [CrossRef]
- Guerrero, J.M. (Ed.) Chapter 5—The history of modern artificial intelligence. In Mind Mapping and Artificial Intelligence; Academic Press: New York, NY, USA, 2023; pp. 131–161. ISBN 9780128201190. [Google Scholar] [CrossRef]
- Dumas, J.; Hergel, J.; Lefebvre, S. Bridging the Gap: Automated Steady Scaffoldings for 3D Printing. ACM Trans. Graph. 2014, 33, 98. [Google Scholar] [CrossRef]
- Vanek, J.; Jag, G.; Benes, B. Clever Support: Efficient Support Structure Generation for Digital Fabrication. Comput. Graph. Forum 2014, 33, 117–125. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, X.; Stringer, J. Support Structures for Additive Manufacturing: A Review. J. Manuf. Mater. Process. 2018, 2, 64. [Google Scholar] [CrossRef]
- Turner, B.; Gold, S. A review of melt extrusion additive manufacturing processes: II. Materials, dimensional accuracy, and surface roughness. Rapid Prototyp. J. 2015, 21, 250–261. [Google Scholar] [CrossRef]
- Amirruddin, M.S.; Ismail, K.I.; Yap, T.C. Effect of layer thickness and raster angle on the tribological behavior of 3D printed materials. Mater. Today Proc. 2021, 48, 1821–1825. [Google Scholar] [CrossRef]
- Erick, O.; Lopez, C.; Pal, A.; Arturo, U.; Wu, R.; Misra, M.; Mielewski, D.F.; Kiziltas, A.; Mohanty, A. Recycled poly (lactic acid)-based 3D printed sustainable biocomposites: A comparative study with injection molding. Materials Today Sustainability 2019, 7, 100027. [Google Scholar] [CrossRef]
- Passeri, L.A.; Ellis, E., 3rd; Sinn, D.P. Relationship of substance abuse to complications with mandibular fractures. J. Oral Maxillofac. Surg. 1993, 51, 22–25. [Google Scholar] [CrossRef]
- Stacey, D.H.; Doyle, J.F.; Mount, D.L.; Snyder, M.C.; Gutowski, K.A. Management of mandible fractures. Plast Reconstr. Surg. 2006, 117, 48e–60e. [Google Scholar] [CrossRef] [PubMed]
- Goodday, R.H. Management of fractures of the mandibular body and symphysis. Oral Maxillofac. Surg. Clin. N. Am. 2013, 25, 601–616. [Google Scholar] [CrossRef]
- Oruç, M.; Işik, V.M.; Kankaya, Y.; Gürsoy, K.; Sungur, N.; Aslan, G.; Koçer, U. Analysis of Fractured Mandible Over Two Decades. J. Craniofac. Surg. 2016, 27, 1457–1461. [Google Scholar] [CrossRef]
- Hayes, A.C.; Träff, E.A.; Sørensen, C.V.; Willems, S.V.; Aage, N.; Sigmund, O.; Whiting, G.L. Topology optimization for structural mass reduction of direct drive electric machines. Sustain. Energy Technol. Assess. accepted/in press. [CrossRef]
- Kharmanda, G.; Mulki, H. Two decades review of reliability-based topology optimization developments. Uncertainties Reliab. Multi-Phys. Syst. 2022, 22. [Google Scholar] [CrossRef]
- Kharmanda, G.; Kharma, M.Y.; Ristinmaa, M.; Wallin, M. Structural optimization of mini-plates in fixation of human mandible fractures. In Proceedings of the 27th Nordic Seminar on Computational Mechanics—NSCM-27, Stockholm, Sweden, 22–24 October 2014. [Google Scholar]
- Kharmanda, G.; Kharma, M.Y. Evaluating the Effect of Minimizing Screws on Stabilization of Symphysis Mandibular Fracture by 3D Finite Element Analysis. J. Maxillofac. Oral Surg. 2017, 16, 205–211. [Google Scholar] [CrossRef] [PubMed]


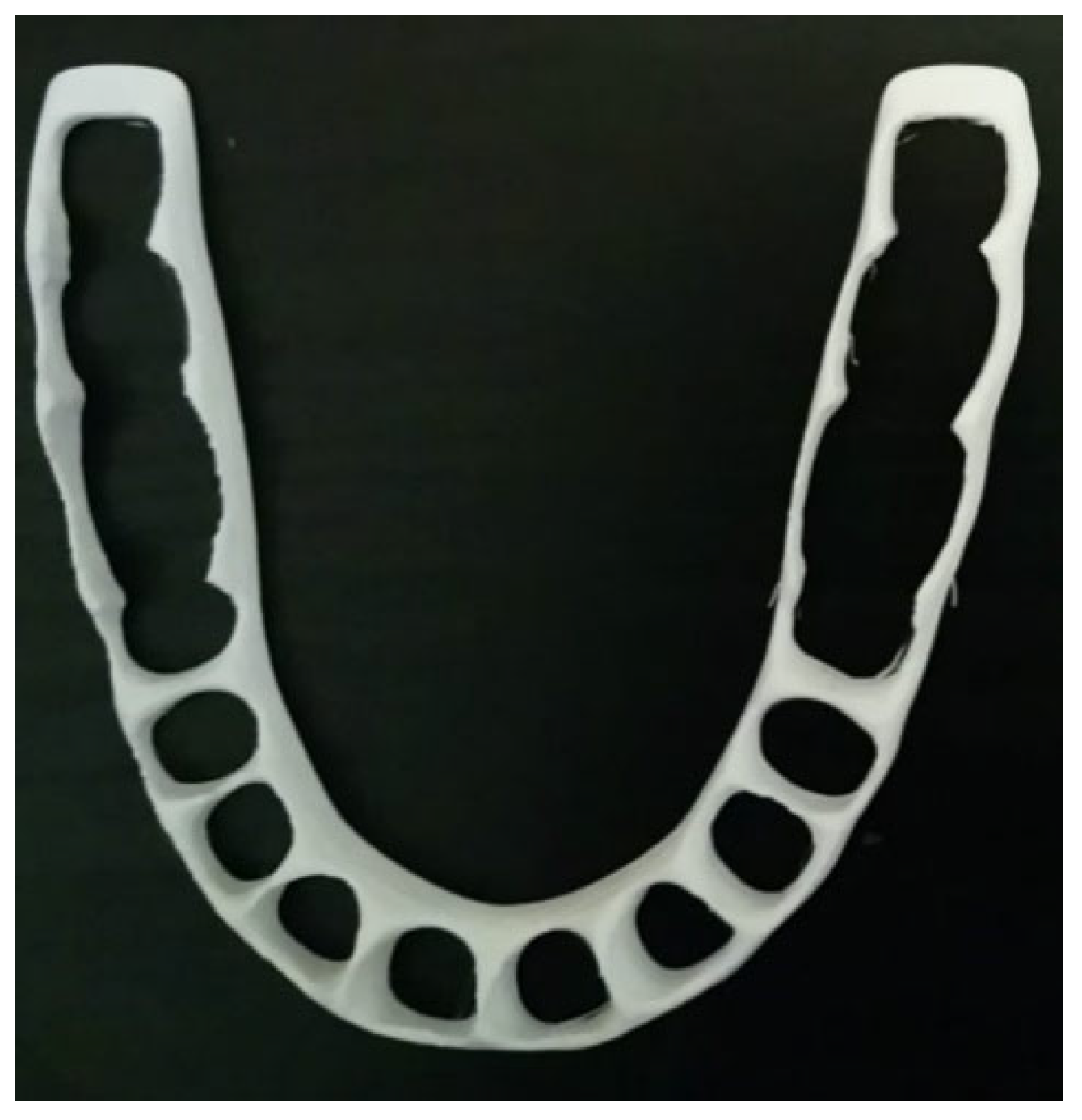



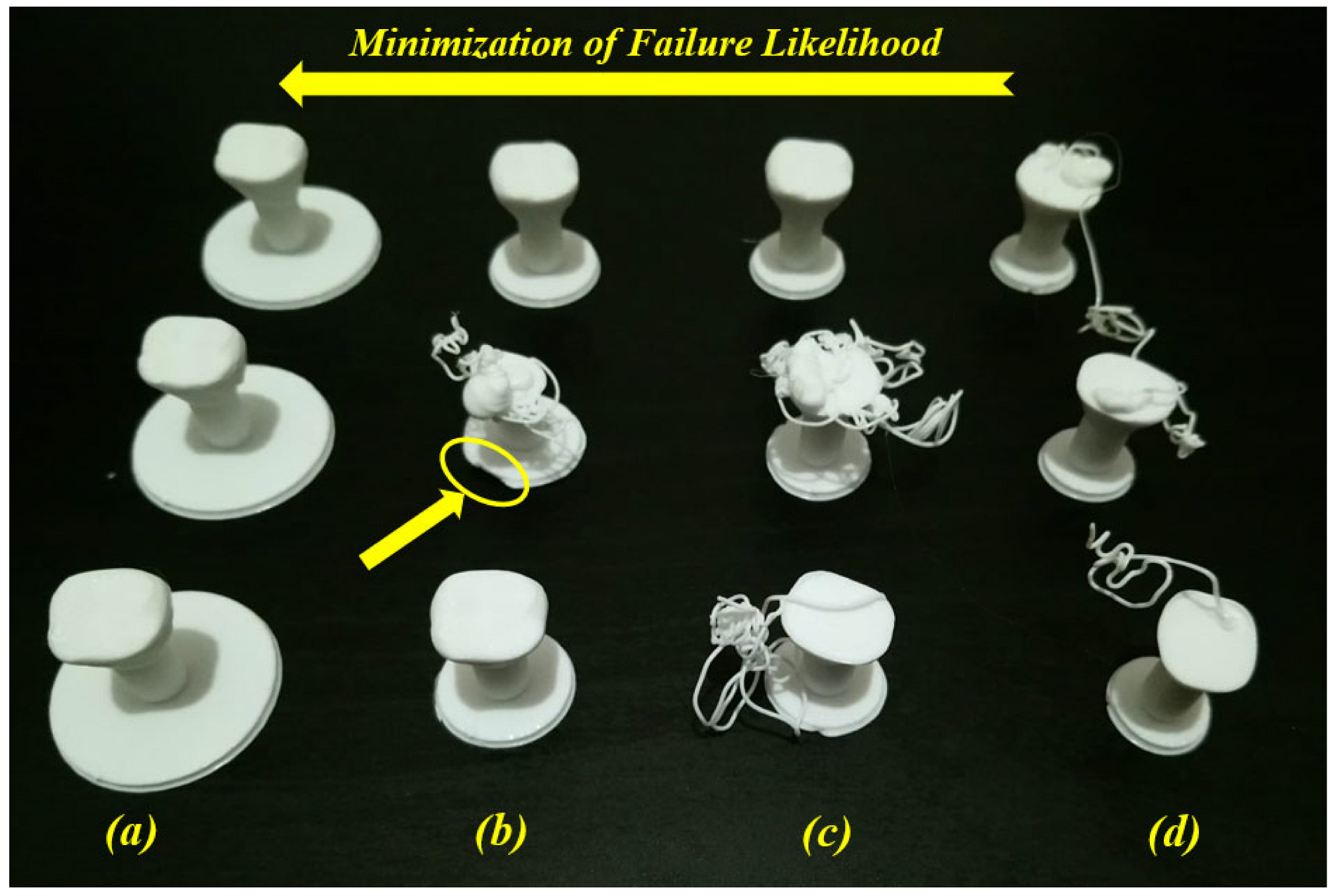
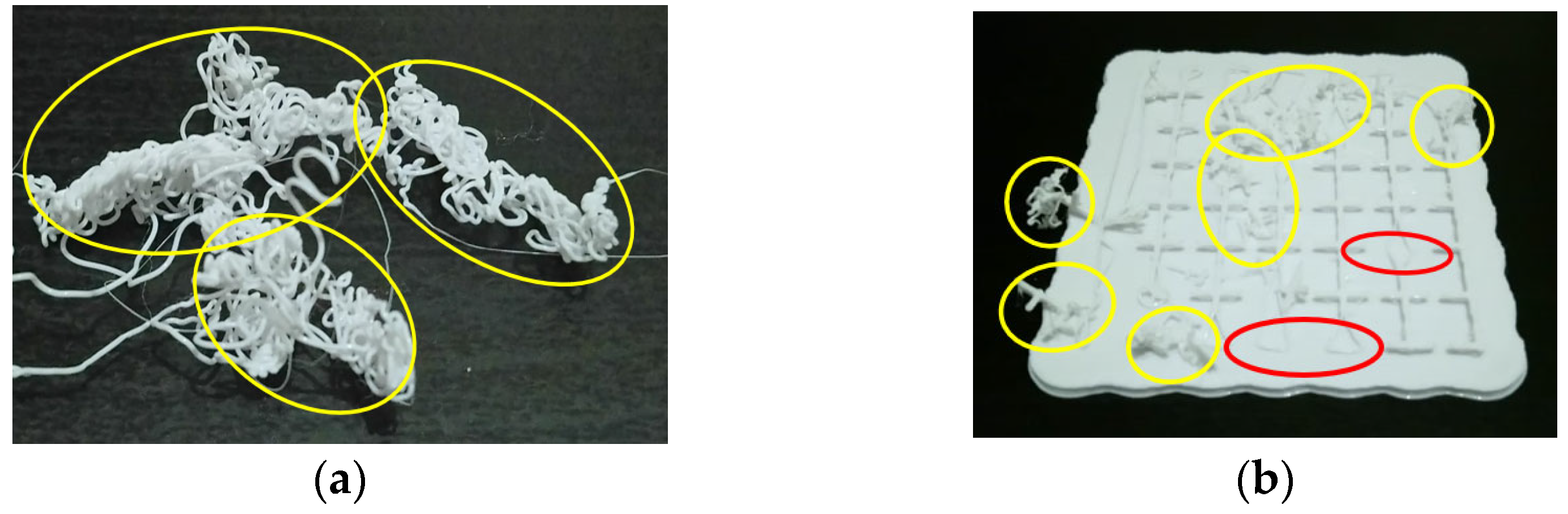






| Authors | Material | AM Technique | Dental Application | Results |
|---|---|---|---|---|
| Bae et al. [43] | 3Y-TZP ceramics | SLM + CIP | Dental crown, prostheses, restoration. | Foundation of SLS/CIP technology for 3Y-TZP dental ceramics |
| Muta et al. [44] | PVA | FDM | Provisional dental crown | Good accuracy |
| Arnesano et al. [45] | Alumina-Ceramic | FDM | Dental crown | Energy efficiency |
| Revilla-León et al. [46] | Co-Cr alloy | SLM + CM | Dental prostheses | Improved roughness with SLM process |
| Baciu et al. [47] | Co–Cr–W alloy | SLM | Dental inlays and bridges | Increased hardness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharmanda, G. Challenges and Future Perspectives for Additively Manufactured Polylactic Acid Using Fused Filament Fabrication in Dentistry. J. Funct. Biomater. 2023, 14, 334. https://doi.org/10.3390/jfb14070334
Kharmanda G. Challenges and Future Perspectives for Additively Manufactured Polylactic Acid Using Fused Filament Fabrication in Dentistry. Journal of Functional Biomaterials. 2023; 14(7):334. https://doi.org/10.3390/jfb14070334
Chicago/Turabian StyleKharmanda, Ghais. 2023. "Challenges and Future Perspectives for Additively Manufactured Polylactic Acid Using Fused Filament Fabrication in Dentistry" Journal of Functional Biomaterials 14, no. 7: 334. https://doi.org/10.3390/jfb14070334
APA StyleKharmanda, G. (2023). Challenges and Future Perspectives for Additively Manufactured Polylactic Acid Using Fused Filament Fabrication in Dentistry. Journal of Functional Biomaterials, 14(7), 334. https://doi.org/10.3390/jfb14070334







