Influence of Individual Bracket Base Design on the Shear Bond Strength of In-Office 3D Printed Brackets—An In Vitro Study
Abstract
1. Introduction
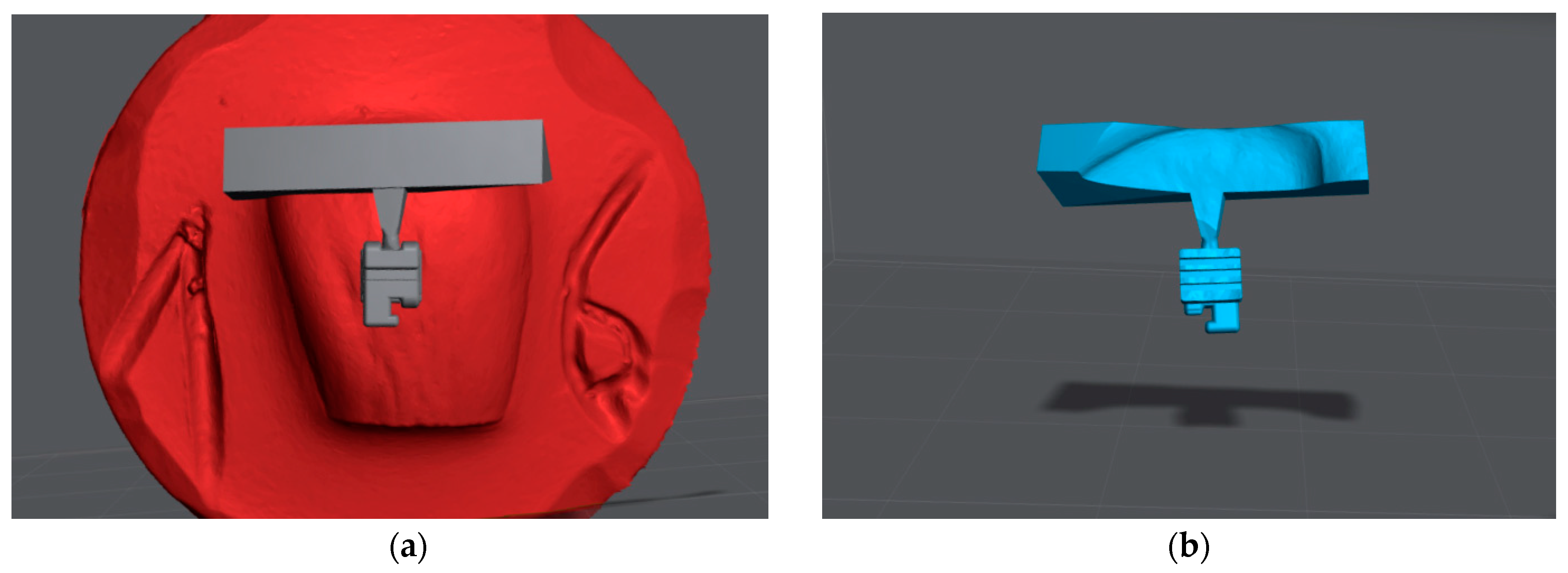
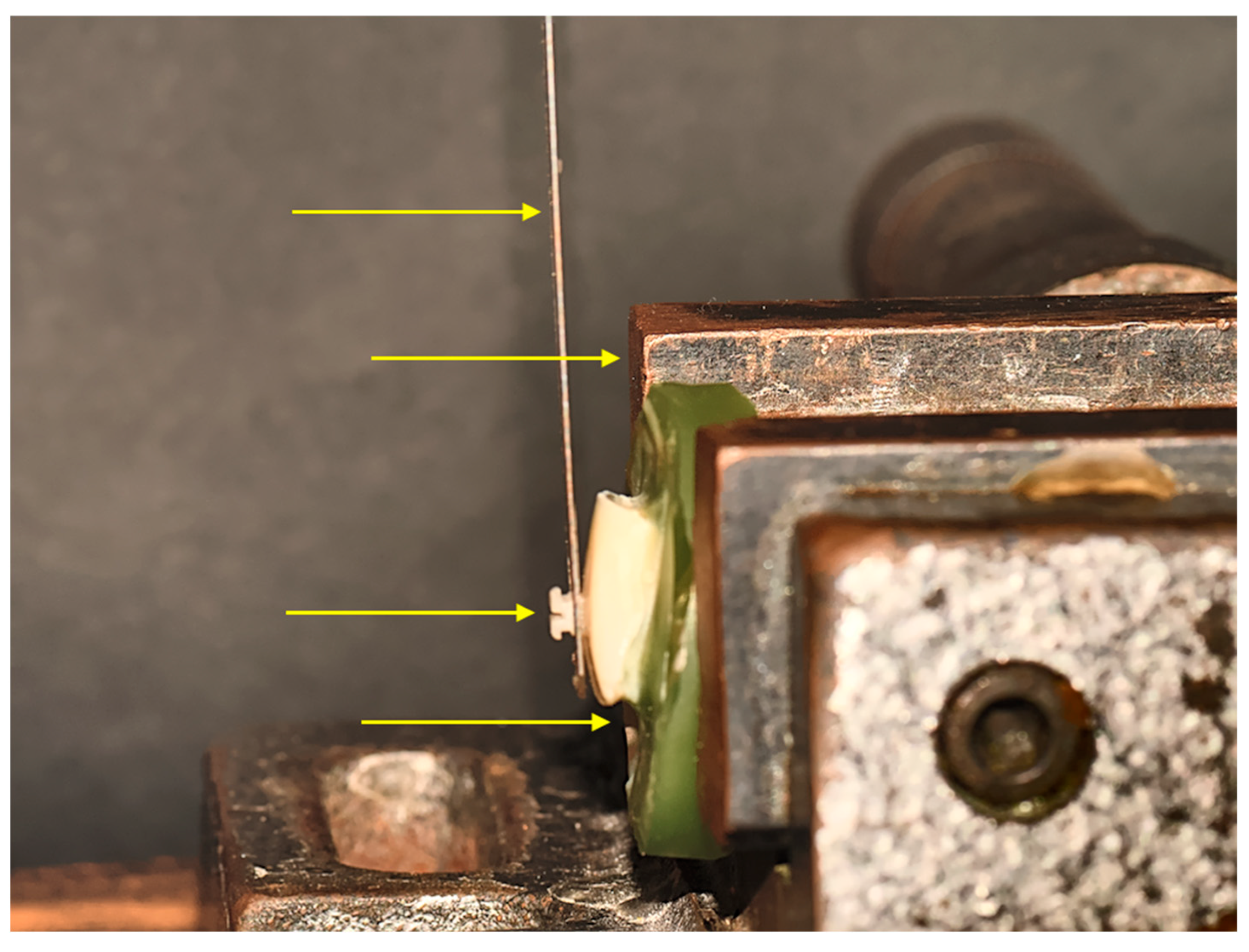
2. Materials and Methods
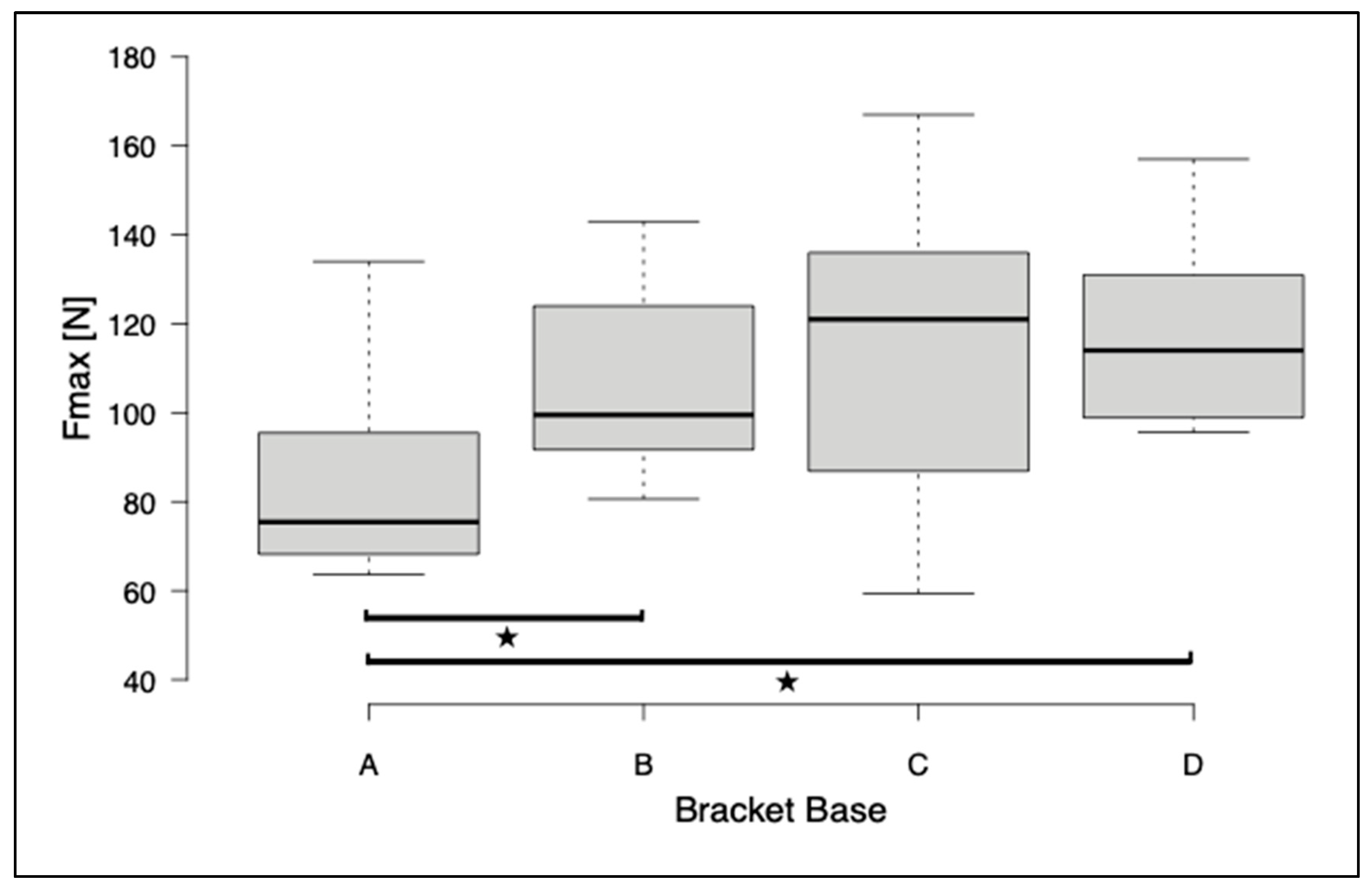
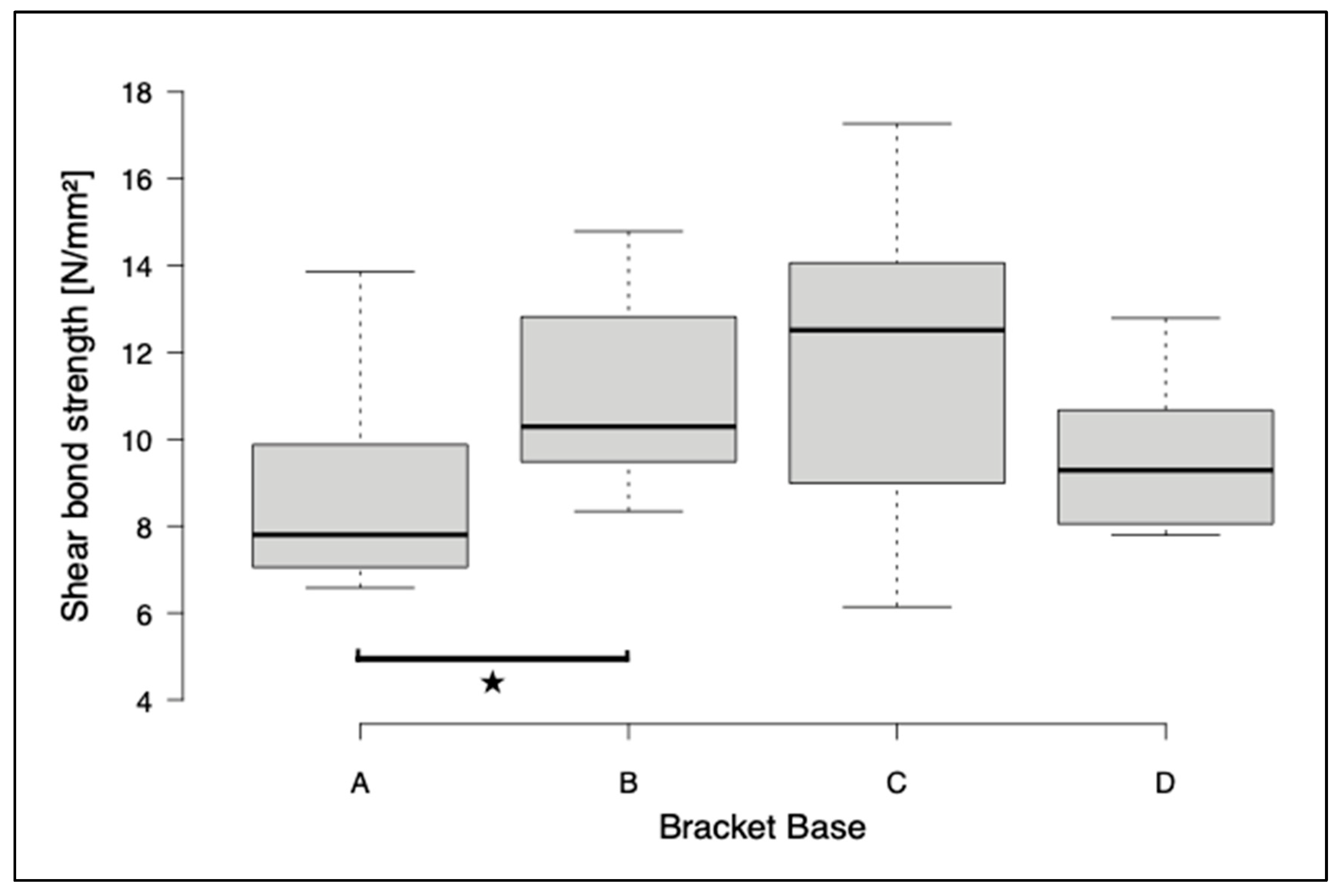
3. Results
4. Discussion
5. Conclusions
- Conventional metal brackets show the highest shear bond strength values in a standardized in vitro test procedure. Low loss rates and, consequently, fewer unscheduled appointments in clinical practice can be expected.
- Innovative 3D-printed bracket systems can meet the high clinical requirements if a base individualized to the tooth surface has a macro-retentive pattern.
- Scaling the cross-sectional area to model the bond strength between the tooth and bracket provides a further degree of individualization, in addition to color, prescription, and geometry, and can represent a further step towards the personalization of fixed orthodontic therapy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Försch, M.; Krull, L.; Hechtner, M.; Rahimi, R.; Wriedt, S.; Wehrbein, H.; Jacobs, C.; Jacobs, C. Perception of esthetic orthodontic appliances: An eye tracking and cross-sectional study. Angle Orthod. 2020, 90, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Gkantidis, N.; Zinelis, S.; Karamolegkou, M.; Eliades, T.; Topouzelis, N. Comparative assessment of clinical performance of esthetic bracket materials. Angle Orthod. 2012, 82, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Hiroce, M.; Fernandes, D.J.; Elias, C.N.; Miguel, J.A. Sliding resistance of polycarbonate self-ligating brackets and stainless steel esthetic archwires. Prog. Orthod. 2012, 13, 148–153. [Google Scholar] [CrossRef]
- Reicheneder, C.A.; Baumert, U.; Gedrange, T.; Proff, P.; Faltermeier, A.; Muessig, D. Frictional properties of aesthetic brackets. Eur. J. Orthod. 2007, 29, 359–365. [Google Scholar] [CrossRef]
- Nakano, T.; Nakajima, A.; Watanabe, H.; Osada, A.; Namura, Y.; Yoneyama, T.; Tanaka, E.; Motoyoshi, M. Evaluation of torque moment in esthetic brackets from bendable alloy wires. Angle Orthod. 2021, 91, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Malkiewicz, K.; Jastrzebska, A.; Janas-Naze, A.; Boryczko, W.; Bartczak, J. Assessment of the Susceptibility of Aesthetic Orthodontic Brackets to Discoloration. Coatings 2022, 12, 1080. [Google Scholar] [CrossRef]
- Lee, Y.K. Changes in the reflected and transmitted color of esthetic brackets after thermal cycling. Am. J. Orthod. Dentofac. 2008, 133, 641.e1–641.e6. [Google Scholar] [CrossRef]
- Faltermeier, A.; Behr, M.; Müssig, D. In vitro colour stability of aesthetic brackets. Eur. J. Orthod. 2007, 29, 354–358. [Google Scholar] [CrossRef]
- Knox, J.; Hubsch, P.; Jones, M.L.; Middleton, J. The influence of bracket base design on the strength of the bracket-cement interface. J. Orthod. 2000, 27, 249–254. [Google Scholar] [CrossRef]
- Khowassah, M.A.; Bishara, S.E.; Francis, T.C.; Henderson, W. Effect of temperature and humidity on the adhesive strength of orthodontic direct bonding materials. J. Dent. Res. 1975, 54, 146–151. [Google Scholar] [CrossRef]
- Guan, G.; Takano-Yamamoto, T.; Miyamoto, M.; Hattori, T.; Ishikawa, K.; Suzuki, K. Shear bond strengths of orthodontic plastic brackets. Am. J. Orthod. Dentofacial Orthop. 2000, 117, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Makou, M.; Papadopoulos, T.; Eliades, G. Laboratory evaluation of modern plastic brackets. Eur. J. Orthod. 2012, 34, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, P. Enamel alterations from bracket bonding and debonding: A study with the scanning electron microscope. Am. J. Orthod. 1981, 79, 500–522. [Google Scholar] [CrossRef] [PubMed]
- Joseph, V.P.; Rossouw, E. The shear bond strengths of stainless steel and ceramic brackets used with chemically and light-activated composite resins. Am. J. Orthod. Dentofacial Orthop. 1990, 97, 121–125. [Google Scholar] [CrossRef]
- Reynolds, I.R. A Review of Direct Orthodontic Bonding. Br. J. Orthod. 1975, 2, 171–178. [Google Scholar] [CrossRef]
- Mandall, N.A.; Hickman, J.; Macfarlane, T.V.; Mattick, R.C.; Millett, D.T.; Worthington, H.V. Adhesives for fixed orthodontic brackets. Cochrane Database Syst. Rev. 2018, 4, CD002282. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, V.; Reimann, S.; Jäger, A.; Bourauel, C. Comparison of shear bond strength of plastic and ceramic brackets. J. Orofac. Orthop. 2014, 75, 345–357. [Google Scholar] [CrossRef]
- Daratsianos, N.; Schütz, B.; Reimann, S.; Weber, A.; Papageorgiou, S.N.; Jäger, A.; Bourauel, C. The influence of enamel sandblasting on the shear bond strength and fractography of the bracket-adhesive-enamel complex tested in vitro by the DIN 13990:2017-04 standard. Clin. Oral. Investig. 2019, 23, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Kilponen, L.; Varrela, J.; Vallittu, P.K. Priming and bonding metal, ceramic and polycarbonate brackets. Biomater. Investig. Dent. 2019, 6, 61–72. [Google Scholar] [CrossRef]
- Reimann, S.; Mezey, J.; Daratsianos, N.; Jäger, A.; Bourauel, C. The influence of adhesives and the base structure of metal brackets on shear bond strength. J. Orofac. Orthop. 2012, 73, 184–193. [Google Scholar] [CrossRef]
- Urichianu, M.; Makowka, S.; Covell, D., Jr.; Warunek, S.; Al-Jewair, T. Shear Bond Strength and Bracket Base Morphology of New and Rebonded Orthodontic Ceramic Brackets. Materials 2022, 15, 1865. [Google Scholar] [CrossRef] [PubMed]
- Artun, J.; Bergland, S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am. J. Orthod. 1984, 85, 333–340. [Google Scholar] [CrossRef] [PubMed]
- DIN 13990:2017-04; Zahnheilkunde—Prüfverfahren für die Scherhaftfestigkeit Von Adhäsiven für kieferorthopädische Befestigungselemente. Beuth Verlag GmbH: Berlin, Germany, 2017. [CrossRef]
- DIN ISO 3696:1991-06; Water for Analytical Laboratory Use. Specification and Test Methods; Beuth Verlag GmbH: Berlin, Germany, 1991. [CrossRef]
- Grzebieluch, W.; Kowalewski, P.; Grygier, D.; Rutkowska-Gorczyca, M.; Kozakiewicz, M.; Jurczyszyn, K. Printable and Machinable Dental Restorative Composites for CAD/CAM Application-Comparison of Mechanical Properties, Fractographic, Texture and Fractal Dimension Analysis. Materials 2021, 14, 4919. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.A.J.; Scheurer, M.; Bourauel, C.; Kretzer, J.P.; Roser, C.J.; Lux, C.J.; Hodecker, L.D. Precision of slot widths and torque transmission of in-office 3D printed brackets: An in vitro study. J. Orofac. Orthop. 2023, 67, 1–13. [Google Scholar] [CrossRef]
- DIN EN ISO 10650:2018-12; Dentistry—Powered Polymerization Activators. Beuth Verlag GmbH: Berlin, Germany, 2018. [CrossRef]
- Richter, C.; Jost-Brinkmann, P.G. Shear bond strength of different adhesives tested in accordance with DIN 13990-1/-2 and using various methods of enamel conditioning. J. Orofac. Orthop. 2015, 76, 175–187. [Google Scholar] [CrossRef]
- Biadsee, A.; Rosner, O.; Khalil, C.; Atanasova, V.; Blushtein, J.; Levartovsky, S. Comparative evaluation of shear bond strength of orthodontic brackets bonded to three-dimensionally-printed and milled materials after surface treatment and artificial aging. Korean J. Orthod. 2023, 53, 45–53. [Google Scholar] [CrossRef]
- Roser, C.J.; Rückschloß, T.; Zenthöfer, A.; Rammelsberg, P.; Lux, C.J.; Rues, S. Orthodontic shear bond strength and ultimate load tests of CAD/CAM produced artificial teeth. Clin. Oral Investig. 2022, 26, 7149–7155. [Google Scholar] [CrossRef]
- Nespoli, A.; Passaretti, F.; Szentmiklósi, L.; Maróti, B.; Placidi, E.; Cassetta, M.; Yada, R.Y.; Farrar, D.H.; Tian, K.V. Biomedical NiTi and β-Ti Alloys: From Composition, Microstructure and Thermo-Mechanics to Application. Metals 2022, 12, 406. [Google Scholar] [CrossRef]
- Bishara, S.E.; Soliman, M.M.; Oonsombat, C.; Laffoon, J.F.; Ajlouni, R. The Effect of Variation in Mesh-Base Design on the Shear Bond Strength of Orthodontic Brackets. Angle Orthod. 2004, 74, 400–404. [Google Scholar]
- Sharma-Sayal, S.K.; Rossouw, P.E.; Kulkarni, G.V.; Titley, K.C. The influence of orthodontic bracket base design on shear bond strength. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 74–82. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Agarwal, D.K.; Gupta, A.; Bhattacharya, P.; Ansar, J.; Bhandari, R. Shear Bond Strength of Ceramic Brackets with Different Base Designs: Comparative In-vitro Study. J. Clin. Diagn. Res. 2016, 10, ZC64–ZC68. [Google Scholar] [CrossRef] [PubMed]
- Eliades, T.; Bourauel, C. Intraoral aging of orthodontic materials: The picture we miss and its clinical relevance. Am. J. Orthod. Dentofacial Orthop. 2005, 127, 403–412. [Google Scholar] [CrossRef]
- Eliades, T.; Eliades, G.; Watts, D.C. Structural conformation of in vitro and in vivo aged orthodontic elastomeric modules. Eur. J. Orthod. 1999, 21, 649–658. [Google Scholar] [CrossRef] [PubMed]
- İşman, E.; Karaarslan, E.S.; Okşayan, R.; Tunçdemır, A.R.; Üşümez, S.; Adanir, N.; Cebe, M.A. Inadequate shear bond strengths of self-etch, self-adhesive systems for secure orthodontic bonding. Dent. Mater. J. 2012, 31, 947–953. [Google Scholar] [CrossRef]
- Sung, J.W.; Kwon, T.Y.; Kyung, H.M. Debonding forces of three different customized bases of a lingual bracket system. Korean J. Orthod. 2013, 43, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Sfondrini, M.F.; Fraticelli, D.; Malfatto, M.; Gandini, P. Adhesive systems for CAD-CAM customised lingual orthodontic brackets: Which one is better? Eur. J. Paediatr. Dent. 2017, 18, 188–192. [Google Scholar] [CrossRef]
- Bakhadher, W.; Halawany, H.; Talic, N.; Abraham, N.; Jacob, V. Factors Affecting the Shear Bond Strength of Orthodontic Brackets—A Review of In Vitro Studies. Acta Med. 2015, 58, 43–48. [Google Scholar] [CrossRef]
- Liu, J.K.; Chuang, S.F.; Chang, C.Y.; Pan, Y.J. Comparison of initial shear bond strengths of plastic and metal brackets. Eur. J. Orthod. 2004, 26, 531–534. [Google Scholar] [CrossRef]
- Hofmann, E.; Elsner, L.; Hirschfelder, U.; Ebert, T.; Hanke, S. Effects of enamel sealing on shear bond strength and the adhesive remnant index: Study of three fluoride-releasing adhesives in combination with metal and ceramic brackets. J. Orofac. Orthop. 2017, 78, 1–10. [Google Scholar] [CrossRef]
- Saito, H.; Miyagawa, Y.; Endo, T. Effects of plastic bracket primer on the shear bond strengths of orthodontic brackets. J. Dent. Sci. 2021, 16, 424–430. [Google Scholar] [CrossRef]
- Bednar, J.R.; Gruendeman, G.W. The influence of bracket design on moment production during axial rotation. Am. J. Orthod. Dentofac. Orthop. 1993, 104, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Dumbryte, I.; Vebriene, J.; Linkeviciene, L.; Malinauskas, M. Enamel microcracks in the form of tooth damage during orthodontic debonding: A systematic review and meta-analysis of in vitro studies. Eur. J. Orthod. 2018, 40, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Lossdörfer, S.; Bieber, C.; Schwestka-Polly, R.; Wiechmann, D. Analysis of the torque capacity of a completely customized lingual appliance of the next generation. Head Face Med. 2014, 10, 4. [Google Scholar] [CrossRef]
- Vlasa, A.; Eremie, L.Y.; Lazăr, L.; Bud, A.; Păcurar, M.; Bud, E.; Biriș, C. Correlation between orthodontic forces and root resorp-tion—A systematic review of the literature. J. Interdiscip. Med. 2016, 1, 142–145. [Google Scholar] [CrossRef]
- Julien, K.C.; Buschang, P.H.; Campbell, P.M. Prevalence of whitespot lesion formation during orthodontic treatment. Angle Orthod. 2013, 83, 641–647. [Google Scholar] [CrossRef]
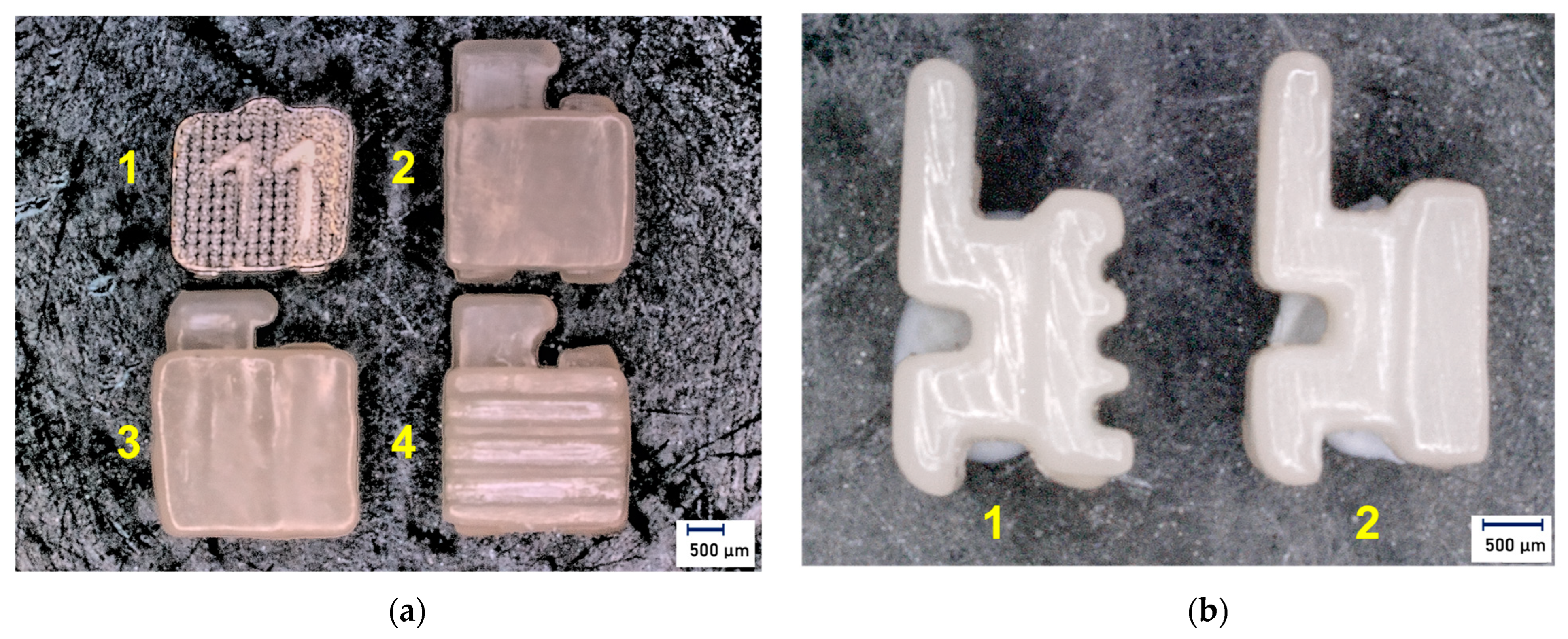

| Score | Definition |
|---|---|
| 0 | No adhesive remained on the enamel. |
| 1 | Less than 50% of adhesive remained on the enamel. |
| 2 | More than 50% of adhesive remained on the enamel. |
| 3 | All adhesive remained on the enamel. |
| Group | Bracket Type | Material | Base Morphology | Base Retention | Base Size (In mm2) |
|---|---|---|---|---|---|
| A | In-office printed | PCR | Individual | Micro-retention | 9.67 |
| B | In-office printed | PCR | Individual | Macro-retention | 9.67 |
| C | Discovery® | Metal | Standardized | Patented laser structured | 9.67 |
| D | In-office printed | PCR | Individual | Micro-retention | 12.28 |
| Groups | N | ARI = 0 (%) | ARI = 1 (%) | ARI = 2 (%) | ARI = 3 (%) |
|---|---|---|---|---|---|
| Base A | 10 | 0 (0) | 0 (0) | 4 (40) | 6 (60) |
| Base B | 10 | 0 (0) | 9 (90) | 0 (0) | 1 (10) |
| Base C | 10 | 1 (10) | 8 (80) | 0 (0) | 1 (10) |
| Base D | 10 | 0 (0) | 7 (70) | 2 (20) | 1 (10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodecker, L.D.; Scheurer, M.; Scharf, S.; Roser, C.J.; Fouda, A.M.; Bourauel, C.; Lux, C.J.; Bauer, C.A.J. Influence of Individual Bracket Base Design on the Shear Bond Strength of In-Office 3D Printed Brackets—An In Vitro Study. J. Funct. Biomater. 2023, 14, 289. https://doi.org/10.3390/jfb14060289
Hodecker LD, Scheurer M, Scharf S, Roser CJ, Fouda AM, Bourauel C, Lux CJ, Bauer CAJ. Influence of Individual Bracket Base Design on the Shear Bond Strength of In-Office 3D Printed Brackets—An In Vitro Study. Journal of Functional Biomaterials. 2023; 14(6):289. https://doi.org/10.3390/jfb14060289
Chicago/Turabian StyleHodecker, Lutz D., Mats Scheurer, Sven Scharf, Christoph J. Roser, Ahmed M. Fouda, Christoph Bourauel, Christopher J. Lux, and Carolien A. J. Bauer. 2023. "Influence of Individual Bracket Base Design on the Shear Bond Strength of In-Office 3D Printed Brackets—An In Vitro Study" Journal of Functional Biomaterials 14, no. 6: 289. https://doi.org/10.3390/jfb14060289
APA StyleHodecker, L. D., Scheurer, M., Scharf, S., Roser, C. J., Fouda, A. M., Bourauel, C., Lux, C. J., & Bauer, C. A. J. (2023). Influence of Individual Bracket Base Design on the Shear Bond Strength of In-Office 3D Printed Brackets—An In Vitro Study. Journal of Functional Biomaterials, 14(6), 289. https://doi.org/10.3390/jfb14060289







